Abstract
The efficacy of drug delivery systems can be significantly enhanced by making them target-specific via the attachment of various ligands to their surface. We attempted to enhance tumor accumulation and therapeutic effect of doxorubicin-loaded long-circulating liposomes (Doxil®, ALZA Corp.) by coupling to their surface the anti-cancer monoclonal antibody 2C5 (mAb 2C5) with nuclesome (NS)-restricted activity, that can recognize the surface of various tumor but not normal cells via the surface-bound nucleosomes released from the apoptotically dying neighboring tumor cells and specifically targets pharmaceutical carriers to tumor cells in vitro and in vivo. Antibody coupling to PEGylated doxorubicin-liposomes was performed by the “post-insertion” technique. The pharmacokinetics of plain and immuno-targeted Doxil®-mimicking liposomes, as well as their accumulation in primary Lewis Lung Carcinoma (LLC) tumors in mice was followed by real-time gamma-scintigraphy upon liposomal membrane labeling with 111In. Therapeutic action of various liposomal formulations was followed by registering primary tumor growth, determining tumor weigh upon mice sacrifice, and by counting the number of metastases in the liver and lungs. 2C5 antibody-targeted liposomes demonstrate significantly enhanced accumulation in LLC tumors. Targeted doxorubicin-loaded PEG-liposomes were significantly more effective in inhibiting tumor growth and metastatic process in the LLC tumor models in mice. Our results clearly show the remarkable capability of 2C5-targeted Doxil® to specifically deliver its cargo into various tumor manifestations (solid and metastatic) significantly increasing the efficacy of therapy.
Keywords: Tumor targeting, Long-circulating liposomes, Doxorubicin, Anticancer antibody, γ-Scintigraphy, Tumor growth inhibition, Metastases
INTRODUCTION
Liposomal formulations of various anticancer drugs are widely used in experimental and clinical oncology with some of them, such as Doxil® (doxorubicin incorporated into long-circulating PEGylated liposomes), becoming drugs of choice under certain conditions 1–3. The use of the liposomal carrier allows for decreased side effects of cancer chemotherapeutics, such as non-specific toxicity, and for enhanced drug delivery into tumors 4, 5. Thus, Doxil® demonstrates decreased cardiotoxicity and improved tumor accumulation via the enhanced permeability and retention (EPR) mechanism 6, 7. Clinically, EPR (“passively”)-targeted PEG-liposomes have been successfully used to deliver doxorubicin to skin, cervical or breast cancers and have been shown to enhance therapeutic activity compared with the same drug administered in the free form or entrapped in conventional liposomes 6, 8, 9. The effective delivery of doxorubicin in PEG-liposomes (Doxil®/Caelyx®) in the treatment of solid tumors in patients with breast-carcinoma metastases has resulted in a marked increase in survival 2, 10. Doxil®/Caelyx® is also in phase II clinical trials for patients with squamous cell cancer of head and neck and ovarian cancer 2. The encapsulation of doxorubicin into PEG-liposomes has not only increased the tumor concentration of the drug via the EPR effect, but also decreased its volume of distribution, and consequently the therapy-associated toxicity. Still, Doxil® is responsible for some non-specific toxicity (cardiomyopathy and myelosuppression) 11, 12, and additionally provokes certain mucocutaneous reactions 12, 13. Further decrease in its non-specific toxicity could be achieved by active targeting of the nanoencapsulated drug into tumors, since one can hypothesize that the active targeting in addition to the EPR-mediated accumulation, will accelerate drug accumulation in the tumor, which should result in lesser exposure of normal tissues to the drug, and the presence of the protein molecule (antibody or its fragment) on the preparation will enhance its clearance from the blood decreasing this exposure still further.
Active targeting of long-circulating lipososmes to the tumor tissue involves the use of specific “vector” molecules that show affinity toward certain components characteristic for target tumor tissues 14. Although the active targeting may further diminish unwanted interactions with normal tissues and cells, its main advantage over the passive targeting is the ability to deliver a larger drug payload directly into the tumor. The use of a targeting moiety could be especially important for tumors with immature vasculature, such as tumors in the early stages of their development, and for delocalized tumors. Vector molecules capable of recognizing tumors include antibodies 15, peptides 16, saccharides 17, hormones, transferrin 18, and some low molecular weight compounds like folate and some vitamins 19.
Several attempts have been made to further improve the anticancer efficiency of the Doxil® by targeting it with vector molecules specific to receptors typical for cancer cells. Thus, an attachment of folate residues to the liposomal surface improved the ability of liposomes to selectively recognize cancer cells that over-express folate receptors 20. These folate-targeted liposomes loaded with doxorubicin demonstrated a substantial increase in cytotoxicity towards target cells in vitro 21 and in vivo 20. Modification with folate promoted the internalization and nuclear delivery of liposomes and overcame multiple drug resistance 19. Similar notable results were obtained with doxorubicin-loaded long-circulating liposomes modified with RGD-peptide motif targeting the neovasculature of the angiogenic tumors 22. Using a small cell lung cancer cell line, it was shown that targeted liposomes were internalized much faster, delivered doxorubicin to the cell nuclei more efficiently, and were more cytotoxic compared to non-targeted liposomes 23. Furthermore, doxorubicin-loaded liposomes additionally modified with Fab′ fragments of anti-disialoganglioside antibodies selectively and almost completely inhibited the metastatic growth of human neuroblastoma in nude mouse model 24. The modification of Doxil® with anti-HER2 monoclonal antibody fragments resulted in a formulation that demonstrated marked anticancer efficiency against tumor lines over-expressing HER2 far superior to that of control non-targeted liposomes both in cell-culture and in in vivo models 15.
Earlier, we have identified a family of natural antibodies with nucleosome-restricted specificity capable of effective recognition and binding of a broad variety of cancer cells (but not normal ones) via the cancer cell surface-bound nucleosomes released from apoptotically dying neighboring cancer cells 25, 26. Extracellular and tumor cell-bound NSs were found in tumor cell cultures 27, and in patients with tumors 28, where they arise from apoptotic cells present in every in vivo developing tumor 29. Some antibodies belonging to the group of antinuclear autoantibodies (ANA) demonstrate a NS-restricted specificity being evidently produced as a result of NS uptake by B cells, which, upon the antigen processing display NS histone peptides recognized by appropriate receptors on T helper cells in turn, inducing the presenting B cells to produce ANA with NS-restricted specificity 30.
The ability to recognize the surface of tumor but not normal cells was observed for some monoclonal ANAs demonstrating 26, and tumor cell surface-bound NSs were proposed to be their target on tumor cell surface 25. The binding of extracellular NSs to tumor cell surface was hypothesized to be mediated by specific NS receptors reported by several investigators to be present on the surface of tumor cells. Thus, a 94 KD protein was recognized as a NS receptor in human B-lymphoblastoid Raji cell line, monkey CVI cells, and rat pancreas islet tumoral cell line RINm 31, while another 50 KD protein domain, calreticulin, was identified as a NS-binding site in a different study 32. For antibody-mediated tumor targeting purposes, it is especially important that NSs are exposed on the cell surface of tumor cells 33.
Functionally, extracellular chromatin fragments have been shown to inhibit the tumor cell killing by NK cells in vitro 28, 34. These findings in fact suggest considering the NS release by dying tumor cells as a tumor self-defense mechanism that protects the surviving tumor cells from host immune attack. In this case, the increased production of NS-specific cytotoxic autoantibodies by a tumor-bearing organism may be considered a response that counteracts tumor self-defense 33.
In addition to their own anticancer activity 30, 33, these antibodies and their typical representative, the monoclonal antibody 2C5 (mAb 2C5), when used in sub-therapeutic quantities, can serve as effective targeting moieties for the tumor-specific delivery of various drug-loaded pharmaceutical nanocarriers 35, 36. Earlier, we have obtained encouraging data on the increased in vitro cytotoxicity of Doxil® modified with mAb 2C5 37, 38. To attach the mAb 2C5 to Doxil® liposomes on top of the protective layer of PEG, we have used earlier developed protocol of antibody pre-modification with p-nitrophenyl-carbonyl-PEG-phosphatidyl ethanolamine (pNP-PEG-PE) conjugate 39 with the subsequent spontaneous “micelle transfer” incorporation of the modified antibody into the membrane of PEGylated liposomes via the hydrophobic PE moiety 40. As a result, mAb 2C5-modified Doxil® demonstrated significantly higher cytotoxicity towards various cancer cells, including those resistant to doxorubicin, than all control preparations. Our previous data indicated that the specific internalization of the mAb 2C5-Doxil® into cytosol, along with the nuclear localization of their drug load, inside the target cancer cells were mainly responsible the superior anticancer activity 38.
We present here the results of our extended in vivo studies on the specific tumor-targeting capacity of mAb 2C5-modified doxorubicin-loaded PEGylated liposomes, and their significantly enhanced therapeutic efficacy against both primary and metastatic tumor growths.
MATERIALS AND METHODS
Materials
Cholesterol (Chol), fully hydrogenated soy phosphatidylcholine (HSPC), N-(carbonyl-methoxy-poly(ethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (MPEG2000-DSPE), and phosphatidylehtanolamine (PE) were from Avanti Polar Lipids, Inc. (Alabaster, AL) and used without further purification. Doxil® was purchased from Pharmaceutics Inc. (West Roxbury, MA). Doxorubicin was purchased from Sigma Chem., Inc. (St. Louis, MO). Triethylamine (TEA), octyl glycoside (OG) and diethylenetriaminepentaacetic acide anhydride (DTPA) were products of Sigma. Poly-oxyethylene3400-bis(p-nitrophenyl carbonate) (PEG(pNP)2) was purchased from SunBio (Orinda, CA). Cell culture media, DMEM, fetal bovine serum (FBS) and concentrated solutions of Na-pyruvate, non-essential amino acids, L-glutamine and penicillin/streptomycin stock solutions were from CellGro (Kansas City, MO). Female BALB/c and C57/BL mice were purchased from Charles River Laboratories (Cambridge, MA). All solvents and other chemicals were analytical grade preparations. The mAb 2C5 was produced in ascites via the I.P injection of 1.5×106 hybridoma cells/ml into pristine primed BALB/c 4 week old male mice. The production and the purification of the mAb 2C5 were carried out by Harlan Bioproducts (Indiannapolis, IL) using the cell line from our laboratory. Control IgG2a isotype-matching antibody clone UPC-10 was from Sigma.
Methods
Attachment of antibody to liposomes
To prepare antibody (mAb 2C5 or non-specific IgG) conjugates with PEG3400-PE, an excess of pNP-PEG-PE dispersed in 10 mg/ml micellar solution of octyl glucoside in 5 mM Na-citrate, 150 mM NaCl, pH 5.0, was added to an equal volume of 1 mg/ml solution of a protein (mAb 2C5 or IgG) in Tris-buffered saline (TBS), pH 8.5. The mixtures were incubated for 24 hr at pH 8.5 at 4°C 39. To obtain doxorubicin-loaded liposomes modified with mAb 2C5 or non-specific IgG, the reaction mixtures form the conjugation reactions (see above) were mixed in equal volumes with Doxil® and incubated for 5 h at 4°C. Then, the remaining octyl glucoside and free, non-incorporated mAb 2C5 were removed by dialysis using cellulose ester dialysis tubes with a cutoff size of 300,000 Da. The complete removal of the non-incorporated mAb 2C5 (if such fraction did exist at all) was confirmed by the presence of only one peak of liposomes on the gel-chromatogram of the final sample (no peaks of free mAb 2C5-PEG-PE or mAb 2C5-PEG-PE micelles) 37. The absence of the protein band after dialysis in the control sample consisting of a simple mixture of free mAb 2C5 and Doxil® liposomes (without the presence of the cross-linker pNP-PEG-PE), indicates that the extensive dialysis process was efficient in removing virtually all antibodies, which could be non-specifically adsorbed on the liposomal surface, and the modification of the antibodies with the pNP-PEG-PE corss-linker is essential for stable incorporation of the antibody-conjugates onto the lipsomal membrane surface 37, 41. Earlier, it was also shown that the procedure used does not cause any significant loss of doxorubicin by liposomes 37, 38, 42.
Preparation of control liposomes
Control PEGylated liposomes mimicking Doxil® composition but containing no doxorubicin were prepared using the same lipid components and in the same concentrations as in Doxil®. A lipid film was obtained from N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (3.19 mg/ml), fully hydrogenated soy phosphatidylcholine (HSPC, 9.58 mg/ml), and cholesterol (3.19 mg/ml). The lipid film was suspended in HEPES-buffered saline (HBS), pH 7.4, and sonicated with a probe-type sonicator at 10 Watts power for 15 min, followed by several passages through mini-extruder with 100 nm pore size polycarbonate filter, until approximately 100 nm liposomes with narrow size distribution were obtained 37, 38.
All liposomal preparation were controlled for their size (via the dynamic light scattering) and for zeta potential using a Zeta PLUS particle size analyzer with ZETA PALS System, Brookhaven Corp. (Holtsville, NY) at 25 °C.
Cell cultures
Murine Lewis lung carcinoma (LLC) were purchased from the American Type Culture Collection (Manassas, VA), and were maintained in DMEM cell culture medium supplemented with FBS to 10%, Na pyruvate to 1 mM, and penicillin and streptomycin to 50 units/ml and 50 μg/ml, respectively.
111In Radiolabeling of liposomes
Doxil®-mimicking liposomes containing the amphiphilic chelate DTPA-PE (HSPC:CHOL:MPEG2000-DSPE:DTPA-PE in 3:2:0.3:0.3 molar ratios, respectively) were prepared along with the IgG- and 2C5-immuno-analogues. The loading of the liposome-incorporated DTPA-PE with 111In was performed via the transchelation mechanism. DTPA-PE-containing liposomes were incubated for 1 h with 111In chloride in 1M citrate buffer at RT, and then dialyzed overnight against HBS at 4°C to remove free label 40.
Tumor growth in mice
Using subcutaneous injection into fat pads in the lower abdominal region., LLC tumors were implanted in 8 week old C57/BL mice (5×104 cells/mouse) and in 6–8 weeks-old BALB/c mice (3×105 cells/mouse), for therapeutic and gamma-imaging studies, respectively. The time for the appearance of the tumor varies from one cell line to another and usually takes 10–14 days. Mice were constantly monitored and allowed for free access to food and water (following the animal care protocol no. R01210 approved by Northeastern University Institutional Animal Care and Use Committee, in accordance with the “Principles of laboratory animal care”, NIH publication no. 85–23, revised in 1985) and tumor volumes were calculated using the formula 0.5 (length × width2) by measuring the dimensions of the tumor at regular time intervals.
Tumor accumulation of 111In-labeled liposomes in mice
When the tumor diameter reached 5–8 mm, mice were injected with 0.1 ml of 4 mg/ml 111In-radiolabeled Doxil®-mimicking liposomal formulations via the lateral tail vein. The accumulation of 111In-radiolabeled Doxil®-mimicking liposomal formulations in the developed tumor was also visualized using an Ohio Nuclear 400 radio-isotope camera (Ohio-Nuclear Inc., solon, OH) equipped with a high energy collimator and NU Mac computer (NC systems, Boulder, CO) at 2, 4 and 6 hrs post injection after anesthetizing the mice by injecting a mixture of xylazine and ketamine intraperitoneally. Digital pictures of the tumor-bearing mouse were taken using a Kodak digital camera (Eastman Kodak Company, Rochester, NY) 35. Images were analyzed using ImageJ software (U. S. National Institutes of Health, Bethesda, MD) for calculation of mean signal intensity per equivalent area of selected tumor location.
Single-dose pharmacokinetics of 111In-labeled Doxil®-mimicking liposomes
In vivo biodistributon studies of 111In-radiolabeled Doxil®-mimicking liposomal formulation and their IgG and mAb 2C5 analogues were performed in healthy 8 week-old female BALB/c mice in two separate experiments. In each experiment, seventy two BALB/c mice were injected with 0.1 mL of 4 mg/mL 111In radiolabeled Doxil®-mimicking liposomal formulations via the lateral tail vein. At time points of 15, 30, 120, 360, 720, 1440 minutes post injection, blood was collected using a Pasteur pipette from the retro-orbital plexus of the eye, and then, the mice were euthanized with carbon dioxide followed by organ collection. Each time point had at least 4 mice and the organs collected were liver, kidney, spleen, lung, muscle and tail. The amount of the radioactivity was quantified as CPM using a Beckman 5500B gamma-counter (Beckman Instruments, Columbia, MD) and the amount of the accumulated radioactivity per gram of tissue was calculated followed by calculation of the pharmacokinetic parameters like elimination rate constant (Kel), area under the curve (AUMC), and half-life (MRT) using non-compartmental statistical analysis of the blood data, using PK Solutions 2.0 (Summit research Services, Montrose, CO). 35, 40
In vivo therapeutic efficacy of 2C5-modified Doxil® in murine tumor models
Therapeutic treatment of LLC tumor-bearing mice started 5 days after tumor implantation with the dose intensity of 2 mg doxorubicin/kg/every 5 days. Each treatment group consisting of 8–10 mice received the corresponding doxorubicin liposomal formulation for 4 times. Tumor diameters were measured and approximate tumor volume was calculated as 0.5 (length × width2) 24 every 3 days during the treatment period to monitor tumor growth. 35 days after the first injection, mice were sacrificed and tumors were excised and weighed.
Histological evaluation
After 35 days of initiation of treatment, lungs and livers were surgically removed and weighed, then fixed for 24 hr in Bouins solution (15:5:1 saturated picric acid: neutral buffer formalin: acetic acid). Afterwards, samples were submerged in 95% alcohol for 24 hr. Finally, metastatic nodules/foci per mouse were visually counted and the mean value was estimated for each sample group. Then, metastasis percentage, determined by percent of mice showing any metastasis in each group, was calculated 43, 44.
Statistics
Differences in the apparent tumor volumes during treatment and postmortem tumor weights were compared using the Student’s t-test for two independent samples and Kruskal-Wallis analysis with Tukey’s HSD Post-Hoc test for all pair mean comparisons, for three or more independent samples. These tests were analyzed using kaliedagraph software, ver. 3.6 (Synergy Software, Reading, PA). For easy representation of the difference in tumor volumes during the treatment between the control and treated groups, the average tumor volume (mm3) of the group vs. days was plotted. Tumor weights were plotted as box plots, which represent the median, quartiles and extremes in a group.
RESULTS
The method of antibody attachment to liposomes by transferring PEG-PE-modified antibodies from their loose micelles onto the liposome surface used in this study results in attaching approximately 70–80 antibody molecules per single 100 nm liposome 37, 38. The antibody incorporation was not accompanied by any significant loss of the liposomal doxorubicin, and virtually no difference in drug release was registered between the original Doxil® and the immuno-Doxil® formulations (modified with either the IgG-PEG-PE or the mAb 2C5-PEG-PE) in 48 h in vitro release experiment 37, 38, 42. Immuno-modification of Doxil® did not noticeably change the liposome size (remained in the range of approx. 90 to 120 nm) or the net charge at the liposomes surface (zeta potential of −25 to −23) 37, 38, 40. The physicochemical characterization of the immunoliposomes similar to the used in this study were reported by us earlier, and it could be useful to mention here that the number of liposome-bound antibodies was estimated as approx. 80 molecules per single liposome 37, 38, 40.
It was also repeatedly shown that liposomes, including doxorubicin-containing PEGylated liposomes, modified with mAb 2C5-PEG-PE demonstrate strong and specific binding with mAb 2C5 antigen, nucleosomes, while non-specific IgG-modified liposomes, and non-modified liposomes do not bind with the nucleosome monolayer 37,38.
In vivo tumor accumulation of mAb 2C5-modified liposomes
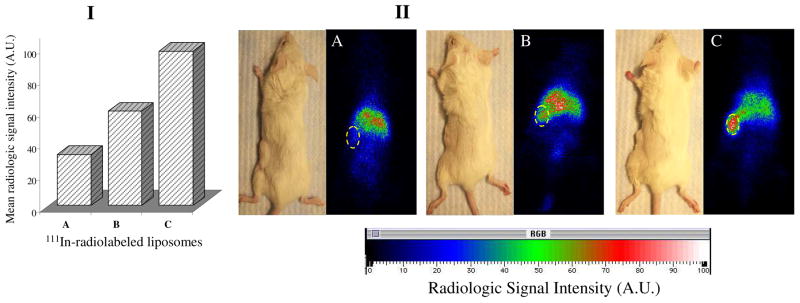
The investigation of tumor accumulation of 111In-labeled Doxil®-mimicking liposomes and their IgG and mAb 2C5 immuno-analogues in LLC murine tumor model clearly demonstrated an enhanced tumor accumulation of 2C5-modified liposomes. The intensity of the radiologic signal accumulated in primary S.C. tumor corresponding to mAb 2C5-modified liposomes, was scaled almost 2–3 times that of the non-specific IgG-bearing or plain liposomes formulations, measured at the same time (see Figure 1). The whole body direct gamma-imaging of tumor-bearing mice, starting only 4 h post-injection time-point, clearly confirmed the enhanced accumulation of mAb 2C5-targeted preparations in both tumors.
Figure 1.
Whole body imaging of LLC-tumor-bearing mice 4 hr after injection with 111In-labeled liposomes. Panel I - calculated radiologic signal intensity. Panel II – Gamma-scintigraphic images of mice injected with: (A) Control Doxil®-mimicking liposomes; (B) Non-specific IgG-modified Doxil®-mimicking liposomes; (C) mAb 2C5-modified Doxil®-mimicking liposomes. Circles indicate tumor locations.
Single dose pharmacokinetics of 111In-labeled Doxil-mimicking liposomes
The pharmacokinetic behavior of various liposomal preparations was studied in healthy BALB/c mice (Figure 2). Similar to the kinetic profile of analogous preparation in 4T1-tumour bearing mice 40, blood clearance data show that both IgG and mAb 2C5 immuno-analogues of the 111In-labeled Doxil®-mimicking liposomes expectedly cleared somewhat faster from the body compared to plain liposomes, yet both formulations retained the ability to long circulate in the blood, with half-lives (MRT values) of about 16.2 (Kel= 0.065 h−1) and 15.8 (Kel= 0.067 h−1) hours, respectively, compared to 21.6 hours (Kel= 0.045h−1) in case of the non-modified formulation. This corresponds well to results reported earlier with other targeted liposomes 24. Furthermore, the decrease noticed in the calculated area under the curves (AUMC) for the Doxil®-mimicking liposomes (AUMC = 13630 % cpm.h2/g) was less than 2-fold in case of immuno-modification with either IgG (AUMC= 6930 % cpm.h2/g) or mAb 2C5 (AUMC= 6380 % cpm.h2/g).
Figure 2.
Single dose pharmacokinetics of various 111In-labeled Doxil®-mimicking liposomal preparations in healthy BALB/C mice. (n = 5, results indicated ± SD)
The observed slight increase in the clearance of the antibody-modified liposomes from the blood could be also explained by the presence of whole antibodies on the liposomal surface, which makes them more liable to be taken up by RES via the Fc-mediated uptake. The use of smaller mAb 2C5 fragments, such as Fab′, to prepare cancer-targeted immunoliposomes, could effectively prevent this Fc receptor-mediated clearance by RES, although this was not specifically studied in our set of experiments.
Previous work with LLC-tumor bearing mice for the biodistribution of the radiolabeled liposomes, in various organs over 48 h, indicated that while immuno(IgG- or mAb 2C5-modified)-liposomes are mainly excreted by liver and spleen; they do have negligible accumulation in non-target tissues 40.
In vivo therapeutic and anitmetastatic activity of 2C5-modified Doxil® in murine Lewis lung carcinoma model
Based on previous therapeutic studies of Doxil® in murine tumor models, 45, the dose of 2.2 mg of doxorubicin/Kg/every 5 days (corresponds to about 6 half-lives for the original Doxil® and approximately 7 half-lives of the antibody-modified Doxil® formulations, which means almost complete elimination of drug by the time for the following dose) for four consecutive administrations was chosen (i.e. total dose given is just about 9 mg/Kg). Some previous studies with Doxil® in similar models used similar dosing intensity of 9 mg/kg/every two weeks for two doses or 4.5 mg/kg/every 3 days for four doses (i.e. total dose equals 18 mg/kg) 45. Using the selected treatment regimen, the therapeutic efficacy of mAb 2C5-modified Doxil® was compared to that of both the non-specific IgG-modified Doxil® and the original Doxil® formulations, in addition to the negative control PBS injections. The effect was assessed in terms of the average tumor volume observed during treatment period of 36 days and in terms of the average postmortem tumor weight at the end of the treatment period.
In this study, murine Lewis lung carcinoma (LLC) tumor model, which produces lung and liver metastasis when implanted subcutaneously, was used, and the assessment for the therapeutic efficacy against the primary tumor growth followed the criteria as mentioned above. Considering the primary tumors, the results show that compared to all controls, the mAb 2C5-modified Doxil®, as with other tumors, demonstrated a significantly enhanced therapeutic efficacy in mice expressed as smaller tumor volumes starting at 20 days after tumor implantation (p≤ 0.05). The final average tumor weight in animals treated with this preparation was only ca. 0.52 g compared to ca. 1.6 g in animals treated with IgG-modified Doxil® or original Doxil® and ca. 2.3 g in animals receiving only the buffer injections (Figure 3A and B).
Figure 3.
Therapeutic activity, expressed as tumor volumes (A) and post-mortem tumor weight (B) of 2C5-modified Doxil® against control preparations in LLC-implanted mice. Arrows indicate treatment schedule, 2 mg/kg/q 5 days (n= 8–10), * (P = 0.05, Vs. corresponding IgG-Doxil®), non-parametric Kruskal-Wallis with Tukey’s HSD Post-Hoc test. (Results presented as mean± SD).
As follows from Tables 1 and 2, the metastatic growth in lungs and livers of host animals was also most significantly inhibited in the case of mAb 2C5-modified Doxil®. There was a significant difference in the number of lung and liver metastases between the targeted preparation-treated group and control groups – untreated and treated with unmodified Doxil®. Although, the unmodified Doxil® significantly inhibits the metastatic process compared to untreated controls, its specific targeting to tumors with mAb 2C5 significantly enhanced this inhibition.
Table 1.
Lung metastases in LLC tumor-bearing mice (at a,b,c; P≤0.05)
| Treatment | Number of lung metastatic foci | % of metastases ( no. mice with metastases/gp) |
|---|---|---|
| PBS control | 43±11a,b | 80% (8/10) |
| Doxil® | 24±8a,c | 45% (4/9) |
| mAb 2C5-modified Doxil® | 11±1.5b,c | 22% (2/9) |
Table 2.
Liver metastases in LLC tumor-bearing mice (at a, b; P≤0.05)
| Treatment | Relative liver weight (%) | Number of liver metastatic foci | % of metastases ( no. mice with metastases/gp) |
|---|---|---|---|
| PBS control | 5.1±0.12 | 52±17a,b | 60% (6/10) |
| Doxil® | 4.94±0.07 | 33±10.5a | 22% (2/9) |
| mAb 2C5-modified Doxil® | 4.76±0.05 | 18b | 11% (1/9) |
One has to note here that although the high accumulation of imuunoliposomes in liver and spleen as the ultimate consequence of the RES-mediated clearance is a well known phenomenon, it is not very likely that liposomes residing in the liver for metabolism can exert as significant therapeutic effect systemically serving as a secondary drug depot. One observation supporting the absence of the depot effect is the rapid therapeutic effect of the treatment on the implanted tumors, where within about a week of therapy and right after the second dose, the tumor volumes of Doxil®-treated animals starts to markedly decrease, with clearly superior tumor size reduction in case of targeted Doxil®. This indeed corresponds well with the earlier observation of the fast high tumor-specific accumulation of the immuno-targeted liposomes rather than suggesting any organ-based depot effect. At the same time, one cannot reject the possibility of a certain effect of the drug escaping cellular metabolism to affect neighboring metastatic foci in close vicinity within liver tissues, especially if these cancerous metastases were transformed/derived from the original Kupffer cells.
DISCUSSION
The toxic side-effects of anti cancer drugs, resulting from the lack of specificity of conventional therapies, usually limit the increase in dose intensity often required to eradicate the cancerous growth. One of the most evolved strategies that have been developed in the last two decades was the enhancement of the tumor specific delivery of the chemotherapeutic drug, mainly anthracyclins, through actively targeting them via monoclonal antibodies capable of selectively binding antigens that are over-expressed on cancer cells. At the same time, the clinical support received for several liposomal formulations of doxorubicin (Doxil®/Caelyx®) and daunorubicin (Daunoxome) was entirely based on their unique ability to improve the pharmacokinetics of the liposome-laden drugs. Yet, despite the advances with prolonged circulation and liposomes accumulation in the targeted tumor interstituim, the current clinically approved Doxil® formulation have resulted in only a moderate increased antitumor efficacy. Moreover, while this formulation have shown a substantial reduction of the toxicity profile of the free doxorubicin, these long-circulating liposomes suffered from the introduction of new side effects, mainly skin toxicity manifested as hand-foot syndrome and mucositis 46, 47. These adverse effects are virtually inherent to the Stealth™ liposomal carriers and can be overcome by modification of the liposome composition. The active targeting of these liposomes via monoclonal antibodies and their fragments has received extensive research attention in the last decade, in order to render them more tumor-avid, hence facilitating the delivery of these liposomes with their drug cargos into the cellular site of drug action and evading undesired side effects. This line of research stimulated our attempts to utilize the mAb 2C5 with its unique ability to specifically recognize a broad variety of tumors, as a targeting ligand to actively target Doxil®/Caelyx®, toward aggressive tumors in vivo 42.
From our earlier in vitro experiments 35, 37, 38, the remarkable anticancer activity of mAb 2C5-Doxil® against the various tumor cell lines of diverse origins was evident, mainly due to mAb 2C5 proven superior specific surface cell binding and subsequent specific uptake inside the cancer cells. These experiments naturally led us to a comprehensive set of in vivo studies.
Using the LLC murine in vivo model, the biodistribution data demonstrated that tumor accumulation ratios of 111In radiolabeled 2C5-modified Doxil®-mimicking liposomes (prepared by adding 0.5 mol % of amphiphilic chelate, DTPA-PE to lipid composition), compared to the neighboring muscle were almost double that of the non-targeted formulations after 24hr, and even after 48 hr, despite their relatively rapid clearance 40. Moreover, the whole body gamma-imaging of the LLC tumor-bearing mice models, at 4 hours post administration, confirms the tumor-preferential distribution of the 2C5-modified Doxil®-mimicking liposomes compared to control formulations. Based on the pharmacokinetics and the biodistribution profile of the immunoliposomes, it is warranted to assume that this mAb 2C5-modificatoion of the Doxil®-mimicking liposomes have made them significantly less EPR-dependent, and a much fewer number of passages of the 2C5-liposomes in a relatively short period of time (approx. 4–6 hours) has resulted in a great increase in the tumor accumulation of these liposomes, and consequently a markedly stronger tumor-specific signal compared to that of the EPR-dependent, plain Doxil®-mimicking liposomes.
The pharmacokinetic analysis indicated that both IgG and mAb 2C5 immuno-analogues of the 111In-labeled Doxil®-mimicking liposomes suffered form faster clearance from the body, mainly through the uptake by RES organs - liver and spleen. In spite of that, both immuno-formulations retained sufficient ability to circulate long in the blood for about 16 hours, compared to 21 hours in the case of the non-modified formulation. This fairly hassled clearance of the antibody-modified liposomes from the body was expected, owing to the presence of whole antibodies on the liposomal surface, which makes them susceptible to the uptake by the RES. The use of smaller mAb 2C5 fragments, such as Fab′ to prepare cancer-targeted immuno-liposomes should also be possible and will probably improve the blood residence time of the mAb 2C5-modified liposomes to be close to that of the plain liposomes, as reported with other groups 48. The gamma-tracking of the radiolabeled liposomal formulations along with the biodistribution data, in fact allow for considering the decrease in circulation half-life of the radiolabeled liposomes due to the immuno-modification with mAb 2C5 as having an only minor effect on the development of a sharper and faster tumor-specific signal this preparations actually produces.
The selection of the suitable dose regimen, 2.2 mg/kg/q 5 days for 4 doses with total dose of 9 mg/kg, was based upon both the pharmacokinetic data of the liposomal formulations and the effective Doxil® therapeutic regimen used in similar tumor models (total therapeutic dose of 18 mg/Kg). It was chosen as a sub-therapeutic dose of Doxil® in order to be able to investigate the statistical significance of the therapeutic difference between the various Doxil® formulations. Considering the primary S.C. solid tumor growth in the tested LLC lung tumor model, compared to all controls, the mAb 2C5-modified Doxil® demonstrated a significantly improved therapeutic efficacy in mice, resulting in markedly smaller tumor volumes starting less than three weeks after tumor implantation, and significantly lower final average tumor weights, compared to other treatments (p≤ 0.05). These impressive therapeutic results confirm the important role of the tumor-specific accumulation of the mAb 2C5-modified Doxil® in achieving high drug concentration in the tumor. Although the diffusion and deep tumor penetration are not expected for particles of the size of liposomes, especially in case of large tumors with high interstitial pressure, making our approach more applicable for smaller tumor, however it is quite likely that the encapsulated drug, being much more diffusible than the carrier itself, can reach distant tumor cells surrounding the area of extravasated liposomes, and exert such a significant anti-neoplastic effect.
One can assume that a significantly enhanced effect of the mAb 2C5-targeted formulation on the developments of metastases in the case of LLC liver and lung metastases compared to the original Doxil® (p≤ 0.05) is in a sense limited by the fact that most metastatic nodules are considerably smaller in size than primary tumors, and with no or very small necro-apoptotic cores, compared to solid primary LLC tumors. Hence, they may have limited amounts of NSs bound onto their surfaces and available as targets for the recognition by the mAb 2C5-modified Doxil®. Hypothetically, the pre-treatment of a cancerous conditions with pro-apoptotic drugs can increase the apoptotic death of cancer cells in the body, nucleosome release, and nucleosome attachment to cancer cells in metastatic sites making them better targets for mAb 2C5-modified drugs and drug delivery systems 49. General scheme of targeting primary tumors and metastatic cells with mAb 2C5-modified drug-loaded long-circulating liposomes is presented in Figure 4.
Figure 4.
Schematics of tumor targeting and uptake of nucleosome-specific monoclonal antibody 2C5-modified Doxil® liposomes, for both primary site and metastatic cells.
CONCLUSIONS
The present study provides clear evidence that the simple immuno-modification of Doxil® with the cancer-specific anti-nucleosome mAb 2C5 resulted in a successful developments of a strong tumor-targeted doxorubicin delivery system, demonstrating both prolonged circulation in the body and fast and specific accumulation in solid LLC tumors. This resulted in a significant inhibition of both primary and metastatic growths of this aggressive tumor in mice. The mAb 2C5-modified Doxil® provides a promising opportunity to further enhance the therapeutic index of the original formulation and broaden the spectrum of its anti-tumor activity.
Acknowledgments
This work was supported by the NIH grant 2R01 HL55519 to V. P. Torchilin
References
- 1.Skubitz KM. Phase II trial of pegylated-liposomal doxorubicin (Doxil) in sarcoma. Cancer Invest. 2003;21(2):167–176. doi: 10.1081/cnv-120016412. [DOI] [PubMed] [Google Scholar]
- 2.Tejada-Berges T, Granai CO, Gordinier M, Gajewski W. Caelyx/Doxil for the treatment of metastatic ovarian and breast cancer. Expert Rev Anticancer Ther. 2002;2(2):143–150. doi: 10.1586/14737140.2.2.143. [DOI] [PubMed] [Google Scholar]
- 3.Harris L, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94(1):25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 4.Gabizon AA, Lyass O, Berry GJ, Wildgust M. Cardiac safety of pegylated liposomal doxorubicin (Doxil/Caelyx) demonstrated by endomyocardial biopsy in patients with advanced malignancies. Cancer Invest. 2004;22(5):663–669. doi: 10.1081/cnv-200032899. [DOI] [PubMed] [Google Scholar]
- 5.Safra T, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11(8):1029–1033. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 6.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42(5):419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 7.Gabizon A, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54(4):987–992. [PubMed] [Google Scholar]
- 8.Gabizon A, et al. Dose dependency of pharmacokinetics and therapeutic efficacy of pegylated liposomal doxorubicin (DOXIL) in murine models. J Drug Target. 2002;10(7):539–548. doi: 10.1080/1061186021000072447. [DOI] [PubMed] [Google Scholar]
- 9.Alberts DS, et al. Efficacy and safety of liposomal anthracyclines in phase I/II clinical trials. Semin Oncol. 2004;31(6 Suppl 13):53–90. doi: 10.1053/j.seminoncol.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Ranson MR, et al. Treatment of advanced breast cancer with sterically stabilized liposomal doxorubicin: results of a multicenter phase II trial. J Clin Oncol. 1997;15(10):3185–3191. doi: 10.1200/JCO.1997.15.10.3185. [DOI] [PubMed] [Google Scholar]
- 11.Lyass O, et al. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer. 2000;89(5):1037–1047. doi: 10.1002/1097-0142(20000901)89:5<1037::aid-cncr13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Danesi R, et al. Pharmacokinetic-pharmacodynamic relationships of the anthracycline anticancer drugs. Clin Pharmacokinet. 2002;41(6):431–444. doi: 10.2165/00003088-200241060-00004. [DOI] [PubMed] [Google Scholar]
- 13.Charrois GJ, Allen TM. Drug release rate influences the pharmacokinetics, biodistribution, therapeutic activity, and toxicity of pegylated liposomal doxorubicin formulations in murine breast cancer. Biochim Biophys Acta. 2004;1663(1–2):167–177. doi: 10.1016/j.bbamem.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 15.Park JW, et al. Tumor targeting using anti-her2 immunoliposomes. J Control Release. 2001;74(1–3):95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 16.Schiffelers RM, et al. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J Control Release. 2003;91(1–2):115–122. doi: 10.1016/s0168-3659(03)00240-2. [DOI] [PubMed] [Google Scholar]
- 17.Eliaz RE, Szoka FC., Jr Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001;61(6):2592–2601. [PubMed] [Google Scholar]
- 18.Kobayashi T, et al. Effect of transferrin receptor-targeted liposomal doxorubicin in P-glycoprotein-mediated drug resistant tumor cells. Int J Pharm. 2007;329(1–2):94–102. doi: 10.1016/j.ijpharm.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Goren D, et al. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin Cancer Res. 2000;6(5):1949–1957. [PubMed] [Google Scholar]
- 20.Shmeeda H, et al. Intracellular uptake and intracavitary targeting of folate-conjugated liposomes in a mouse lymphoma model with up-regulated folate receptors. Mol Cancer Ther. 2006;5(4):818–824. doi: 10.1158/1535-7163.MCT-05-0543. [DOI] [PubMed] [Google Scholar]
- 21.Lee RJ, Low PS. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim Biophys Acta. 1995;1233(2):134–144. doi: 10.1016/0005-2736(94)00235-h. [DOI] [PubMed] [Google Scholar]
- 22.Xiong XB, et al. Enhanced intracellular uptake of sterically stabilized liposomal Doxorubicin in vitro resulting in improved antitumor activity in vivo. Pharm Res. 2005;22(6):933–939. doi: 10.1007/s11095-005-4588-x. [DOI] [PubMed] [Google Scholar]
- 23.Xiong XB, et al. Intracellular delivery of doxorubicin with RGD-modified sterically stabilized liposomes for an improved antitumor efficacy: in vitro and in vivo. J Pharm Sci. 2005;94(8):1782–1793. doi: 10.1002/jps.20397. [DOI] [PubMed] [Google Scholar]
- 24.Pastorino F, et al. Doxorubicin-loaded Fab′ fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 2003;63(1):86–92. [PubMed] [Google Scholar]
- 25.Iakoubov LZ, Torchilin VP. A novel class of antitumor antibodies: nucleosome-restricted antinuclear autoantibodies (ANA) from healthy aged nonautoimmune mice. Oncol Res. 1997;9(8):439–446. [PubMed] [Google Scholar]
- 26.Iakoubov LZ, Rokhlin O, Torchilin VP. Anti-nuclear autoantibodies of the aged reactive against the surface of tumor but not normal cells. Immunol Lett. 1995;47(1–2):147–149. doi: 10.1016/0165-2478(95)00066-e. [DOI] [PubMed] [Google Scholar]
- 27.Bell DA, Morrison B. The spontaneous apoptotic cell death of normal human lymphocytes in vitro: the release of, and immunoproliferative response to, nucleosomes in vitro. Clin Immunol Immunopathol. 1991;60(1):13–26. doi: 10.1016/0090-1229(91)90108-m. [DOI] [PubMed] [Google Scholar]
- 28.Le Lann AD, et al. In vitro inhibition of natural-killer-mediated lysis by chromatin fragments. Cancer Immunol Immunother. 1994;39(3):185–192. doi: 10.1007/BF01533385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyllie AH. Apoptosis (the 1992 Frank Rose Memorial Lecture) Br J Cancer. 1993;67(2):205–208. doi: 10.1038/bjc.1993.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torchilin VP, Iakoubov LZ, Estrov Z. Therapeutic potential of antinuclear autoantibodies in cancer. Cancer Therapy. 2003;1:179–190. [Google Scholar]
- 31.Jacob L, et al. A monoclonal anti-double-stranded DNA autoantibody binds to a 94-kDa cell-surface protein on various cell types via nucleosomes or a DNA-histone complex. Proc Natl Acad Sci U S A. 1989;86(12):4669–4673. doi: 10.1073/pnas.86.12.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seddiki N, et al. Calreticulin, a potential cell surface receptor involved in cell penetration of anti-DNA antibodies. J Immunol. 2001;166(10):6423–6429. doi: 10.4049/jimmunol.166.10.6423. [DOI] [PubMed] [Google Scholar]
- 33.Chakilam AR, Pabba S, Mongayt D, Iakoubov LZ, Torchilin VP. A single monoclonal antinuclear autoantibody with nucleosome-restricted specificity inhibits the growth of diverse human tumors in nude mice. Cancer Therapy. 2004;2(4):353–364. [Google Scholar]
- 34.Le Lann-Terrisse AD, Fournie GJ, Benoist H. Nucleosome-dependent escape of tumor cells from natural-killer-mediated lysis: nucleosomes are taken up by target cells and act at a postconjugation level. Cancer Immunol Immunother. 1997;43(6):337–344. doi: 10.1007/s002620050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elbayoumi TA, Pabba S, Roby A, Torchilin VP. Antinucleosome antibody-modified liposomes and lipid-core micelles for tumor-targeted delivery of therapeutic and diagnostic agents. J Liposome Res. 2007;17(1):1–14. doi: 10.1080/08982100601186474. [DOI] [PubMed] [Google Scholar]
- 36.Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: Targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci U S A. 2003;100(10):6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J Control Release. 2004;100(1):135–144. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Elbayoumi TA, Torchilin VP. Enhanced cytotoxicity of monoclonal anticancer antibody 2C5-modified doxorubicin-loaded PEGylated liposomes against various tumor cell lines. Eur J Pharm Sci. 2007;32(3):159–168. doi: 10.1016/j.ejps.2007.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torchilin VP, et al. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim Biophys Acta. 2001;1511(2):397–411. doi: 10.1016/s0005-2728(01)00165-7. [DOI] [PubMed] [Google Scholar]
- 40.Elbayoumi TA, Torchilin VP. Enhanced accumulation of long-circulating liposomes modified with the nucleosome-specific monoclonal antibody 2C5 in various tumours in mice: gamma-imaging studies. Eur J Nucl Med Mol Imaging. 2006;33(10):1196–1205. doi: 10.1007/s00259-006-0139-x. [DOI] [PubMed] [Google Scholar]
- 41.Elbayoumi TA, Torchilin VT. Tumor-targeted immuno-liposomes for delivery of chemotherapeutics and diagnostics. J Pharmaceutical Innovation. 2008;3(1):51–58. [Google Scholar]
- 42.Elbayoumi TA, Torchilin VP. Tumor-specific antibody-mediated targeted delivery of Doxil® reduces the manifestation of auricular erythema side effect in mice. Int J Pharm. 2008;357(1–2):272–279. doi: 10.1016/j.ijpharm.2008.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu DY, Xu B, Ding J. Antitumor effects of two bisdioxopiperazines against two experimental lung cancer models in vivo. BMC Pharmacol. 2004;4(1):32. doi: 10.1186/1471-2210-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura K, et al. Inhibitory effect of Cordyceps sinensis on spontaneous liver metastasis of Lewis lung carcinoma and B16 melanoma cells in syngeneic mice. Jpn J Pharmacol. 1999;79(3):335–341. doi: 10.1254/jjp.79.335. [DOI] [PubMed] [Google Scholar]
- 45.Charrois GJ, Allen TM. Multiple injections of pegylated liposomal Doxorubicin: pharmacokinetics and therapeutic activity. J Pharmacol Exp Ther. 2003;306(3):1058–1067. doi: 10.1124/jpet.103.053413. [DOI] [PubMed] [Google Scholar]
- 46.Uziely B, et al. Liposomal doxorubicin: antitumor activity and unique toxicities during two complementary phase I studies. J Clin Oncol. 1995;13(7):1777–1785. doi: 10.1200/JCO.1995.13.7.1777. [DOI] [PubMed] [Google Scholar]
- 47.Lotem M, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136(12):1475–1480. doi: 10.1001/archderm.136.12.1475. [DOI] [PubMed] [Google Scholar]
- 48.Brignole C, et al. Development of Fab′ fragments of anti-GD(2) immunoliposomes entrapping doxorubicin for experimental therapy of human neuroblastoma. Cancer Lett. 2003;197(1–2):199–204. doi: 10.1016/s0304-3835(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 49.Iakoubov LZ, Torchilin VP. Nucleosome-releasing treatment makes surviving tumor cells better targets for nucleosome-specific anticancer antibodies. Cancer Detect Prev. 1998;22(5):470–475. doi: 10.1046/j.1525-1500.1998.00055.x. [DOI] [PubMed] [Google Scholar]