SUMMARY
Background
An important contributing factor to the success of terrestrial flowering plants in colonizing the land was the evolution of a developmental strategy, termed skotomorphogenesis, whereby post-germinative seedlings emerging from buried seed grow vigorously upward in the subterranean darkness toward the soil surface.
Results
Here we provide genetic evidence that a central component of the mechanism underlying this strategy is the collective repression of premature photomorphogenic development in dark-grown seedlings by several members of the phytochrome (phy)-interacting factor (PIF) subfamily of bHLH transcription factors (PIF1, PIF3, PIF4 and PIF5). Conversely, evidence presented here and elsewhere, collectively indicates that a significant component of the mechanism by which light initiates photomorphogenesis upon first exposure of dark-grown seedlings to irradiation involves reversal of this repression by rapid reduction in the abundance of these PIF proteins, through degradation induced by direct interaction of the photoactivated phy molecule with the transcription factors.
Conclusions
We conclude that bHLH transcription factors PIF1, PIF3, PIF4 and PIF5 act as constitutive repressors of photomorphogenesis in the dark, action that is rapidly abrogated upon light exposure by phy-induced proteolytic degradation of these PIFs, allowing the initiation of photomorphogenesis to occur.
INTRODUCTION
Terrestrial flowering plants have evolved a developmental strategy termed skotomorphogenesis (etiolated, heterotrophic growth), whereby post-germinative seedlings emerging from buried seed grow vigorously upward in the subterranean darkness toward the soil surface. Upon reaching the surface, the etiolated growth is redirected by light toward the familiar photomorphogenic pattern of fully green plants. This dramatic developmental transition is termed deetiolation, and involves coordinate inhibition of hypocotyl elongation, unfolding of the apical hook, separation and expansion of the cotyledons, and chlorophyll accumulation.
The light signals triggering this transition are perceived by sensory photoreceptors, of which members of the phytochrome (phy) family (phyA through phyE in Arabidopsis) mediate the responses to red (R) and far-red (FR) wavelengths [1]. The photosensory function of the phys resides in their capacity to photoreversibly switch between two conformers upon R and FR photon absorption: the biologically inactive Pr (R-absorbing) form and the biologically active Pfr (FR-absorbing) form [2]. Upon light activation, the Pfr form translocates into the nucleus [3] where it triggers changes in gene expression [4], thereby implementing the photomorphogenic program. However, the primary molecular mechanism by which the phys transfer the light signal to initiate photomorphogenesis is still unknown.
Interest in defining the cellular and molecular mechanism by which the phys transduce their signaling information to responsive target genes has focused, in recent years, on the role of a subset of the basic helix-loop-helix (bHLH) superfamily of transcription factors. The photoactivated phy molecule has been shown to interact directly and conformer-specifically with several of these factors in subfamily 15 [5], defined as Phytochrome-Interacting Factors (PIFs) [5–8]. The data show that intranuclear binding of the Pfr form of phyA and/or phyB to several of these proteins, including PIFs 1, 3, 4 and 5, induces rapid (within minutes) phosphorylation and degradation of the transcription factors [9–15], suggesting that these may be primary molecular events in phy-signaling. The current evidence indicates, therefore, that these PIFs accumulate in young dark-grown seedlings, and that light-activated phy induces their rapid degradation. However the functional relevance of this degradation to phy signaling is still unclear.
Genetic analysis of the potential functional role of the PIF factors in early phy-induced seedling development has resulted in a complex picture. The data indicate that these factors can function either positively or negatively in a light-induced response depending on the parameter being measured [13, 15–27]. Evidence from visible-phenotype studies on light-grown seedlings using single and double pif mutants grown under prolonged irradiation indicates that the light-hypersensitive phenotype observed is the indirect result of feedback modulation of the global sensitivity of the seedling to light, caused by PIF-induced degradation of the phyB protein, rather than direct signal-relay activity of the PIF protein in the phyB signal-transduction chain [22, 24, 25].
On the other hand, investigations of seed and seedling responses in darkness have provided evidence that some PIFs act negatively in certain facets of early development, such as seed germination, chlorophyll biosynthesis, gravitropic sensitivity and apical development in the presumed absence of phy activation [14, 18, 22, 28, 29]. Together with the earlier observations in light-grown plants, these data led to the general hypothesis that the PIFs may act to repress light-induced seedling development, and that phy initiates the transition from skotomorphogenesis to photomorphogenesis by inducing their proteolytic degradation [30]. This hypothesis predicts that pif mutants might be expected to display a constitutive photomorphogenic (cop)-like phenotype when grown in the dark. However, no such robust cop-like phenotype has been documented for the single and double pif mutants thus far studied.
Here we have generated double, triple and quadruple mutant combinations of pif1, pif3, pif4 and pif5 and have examined their early seedling development. We show that simultaneous genetic removal of PIFs 1, 3, 4 and 5 in the pif1pif3pif4pif5 quadruple mutant leads to a robust cop-like phenotype in seedlings germinated and grown in darkness, indicating that these PIFs redundantly and constitutively repress seedling deetiolation in the emerging seedling. In addition, we discovered that pre-exposure of pre-germinative seeds to light enhances the cop-like phenotype of these pif mutants in subsequent darkness, indicating that light perceived by phy antagonizes the repressive effects of the PIFs. We show that Pfr formed in the ungerminated seed operates trans-developmentally in the emerging seedling in darkness by regulating the levels of PIF3, and possibly other PIFs. Together with previous reports, our data suggest that high levels of PIFs 1, 3, 4 and 5 are required to maintain the etiolated growth of the seedling in the dark, whereas phy-induced removal of these PIFs in response to light activation is a primary step in initiating the seedling deetiolation transition.
RESULTS
PIF1 and PIF3 act redundantly as negative regulators of seedling deetiolation in the dark
In order to examine the function of the PIF proteins in early visible photomorphogenic responses induced upon initial exposure of dark-grown seedlings to light, we focused primarily on cotyledon separation and hook unfolding as the most readily measurable parameters of early seedling deetiolation. In addition, we chose to work with two-day dark-grown seedlings, rather than the commonly-used four-day dark-grown seedlings [9, 14, 16–18, 21, 22, 24], because we noted in preliminary experiments that these younger seedlings exhibited more robust responses to the light-signal than older seedlings relative to the dark controls (Figure S1 in the Supplemental Data available with this article online). The ‘standard’ protocol for these experiments is shown schematically in Figure 1A.
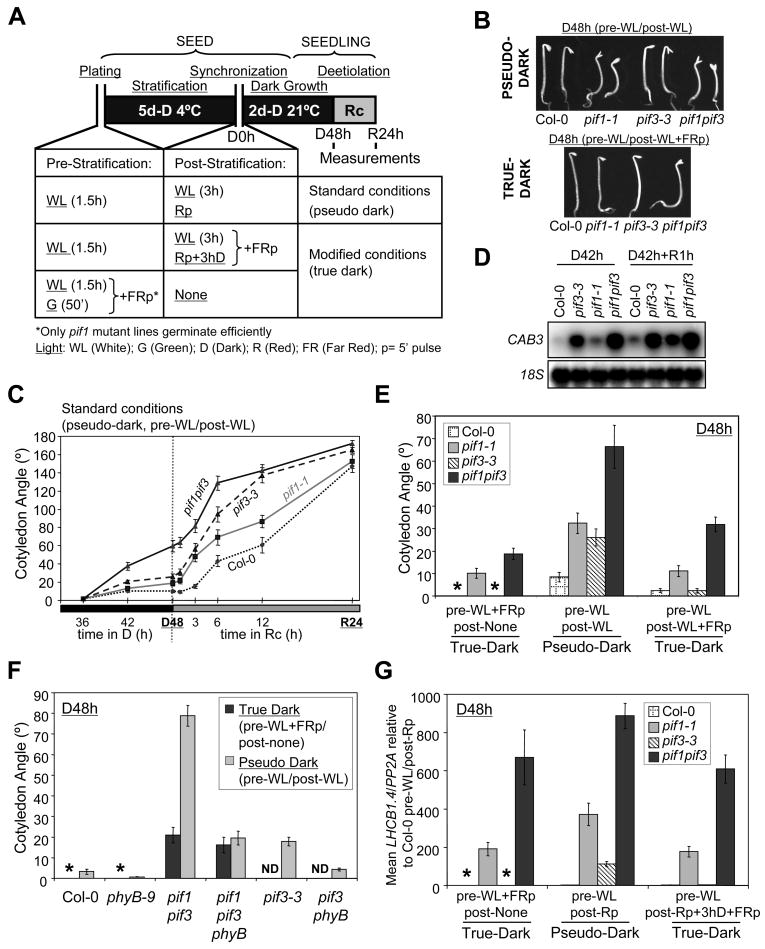
Figure 1. PIF1 and PIF3 act redundantly as constitutive and light-modulated repressors of seedling deetiolation in the dark.
A) Schematic representation of standard and modified protocols for seedling growth. Under standard conditions, seeds were exposed to 1.5h of white light (WL) during sterilization and plating (pre-stratification) and placed for 5d at 4°C in darkness (stratification). Three hours of WL were used to synchronize germination (post-stratification) before incubation for 45h at 21°C in the dark. A 5-min pulse of red light (Rp) was used as an alternative post-stratification treatment. Two-day-old dark-grown seedlings (D48h) were then transferred to Rc (7.2 μmol/m2/s) for 24h (R24h). Under the modified protocol conditions, a terminal 5-min pulse of far-red light (FRp) was provided after either pre- or post-stratification light treatments as indicated. Green light (G) was used as an alternative to WL during the pre-stratification treatment in one configuration of the modified conditions.
B) Visible phenotypes of 2d-old (D48h) dark-grown seedlings under standard protocol conditions (pre-WL/post-WL) (Top) and modified conditions (pre-WL/post-WL+FRp) (Bottom). Photos of representative wild-type (Col-0) and pif mutant seedlings are shown.
C) Time-course quantification of cotyledon separation in the dark and during the dark-tored light transition under standard protocol conditions (pre-WL/post-WL). D36h and D42h data-points were from an independent experiment.
D) Northern blot analysis of CAB3 gene expression under standard protocol conditions (pre-WL/post-WL), except that after the post-stratification WL, seeds were placed in the dark for 39h at 21°C. Dark-grown seedlings (D42h) were exposed to Rc (9 μmol/m2/s) for 1h (D42h+R1h). 18S was used as normalization control. A representative blot is shown.
E) Quantification of cotyledon separation in 2d-old (D48h) wild-type (Col-0) and pif mutant seedlings grown in darkness under the standard and the modified protocol conditions indicated in panel A. A more extensive analysis with additional treatments and measurement of hook angle and hypocotyl responses is shown in Figure S4.
F) phyB mediates most of the cotyledon separation pseudo-dark effects observed in pif3 and pif1pif3 mutants. The indicated genotypes were grown for 2days in the dark (D48h) under standard pseudo-dark conditions (pre-WL/post-WL) or under modified true-dark conditions (pre-WL+FRp/post-none). ND: Not determined. pif3-3 and pif3phyB data from an independent experiment are included for comparison.
G) True- and pseudo-dark regulation of LHCB1.4 marker gene expression by PIF1 and PIF3 in 2d-old dark-grown seedlings (D48h). Q-PCR analysis in wild-type (Col-0) and pif mutants was used to measure LHCB1.4 gene expression under the indicated pre- and post-stratification treatments, and PP2A was used as a normalization control. The LHCB1.4/PP2A ratio is represented as the mean and standard error of 3 independent biological replicates. Data are presented relative to the mean of Col-0 pre-WL/post-Rp set at unity.
Data represent the mean and standard error of at least 25 (C) or 30 (E, F) seedlings. Asterisks in E, F and G indicate low germination precluding reliable measurements.
Initially, we focused on the roles of PIF1 and PIF3 and their interactions using pif1-1 [20] and pif3-3 [19] single and double mutants. Consistent with previous reports [22], wild-type (WT) Col-0 seedlings maintained unseparated cotyledons and folded hooks over the 2d dark-growth period (Figure 1B, C and Figure S2A, B), whereas exposure to continuous R (Rc) induced relatively rapid cotyledon separation and hook unfolding, visible within 1–3 hours of the onset of irradiation (Figure 1C and Figure S2B). Although pif1-1 and pif3-3 single and double mutants appeared initially to respond more rapidly to light, closer inspection using detailed time-course analysis revealed that significant effects of the mutations were already apparent in the dark-grown seedlings before the onset of Rc irradiation at two days (Figures 1B, C and S2B). These dark-effects on hook unfolding were quantitatively variable for the single mutants, sometimes being absent or considerably less than shown in this experiment (see Figure S2C for two additional independent experiments). In contrast, the subtle cotyledon separation observed in pif1 and pif3 single mutants in the dark was always consistent (Figures 1C and S2C), although showing incomplete phenotypic penetration, since only a small percentage of the individuals displayed this phenotype (see Figure 1B for representative seedlings). The subtlety and partial nature of these dark-effects could be the reason why this phenotype was previously unnoticed in pif1 and pif3 single mutant seedlings grown under standard physiological conditions in [14, 17–20, 31].
In contrast to these minor effects displayed by the single mutants, pif1pif3 double mutants showed more obvious features of seedling deetiolation in the dark, with an almost fully unfolded hook and a robust separation of the cotyledons at two days in the dark (Figures 1B, C and S2B). The synergistic effect observed in the pif1pif3 double mutants suggests that PIF1 and PIF3 act as redundant repressors of hook unfolding and cotyledon separation in the dark. Consistent with this conclusion, pif1and pif3 single and double mutants also showed more expanded cotyledons (Figure S2D) and de-repression of photosynthetic gene expression in dark-grown seedlings (Figure 1D). We also observed that 2d-old dark-grown pif1 and pif1pif3, but not pif3 mutant seedlings, showed slightly shorter hypocotyls compared to WT (Figure S2E). Time-course analysis over the 48h (2d) dark-growth period indicates that obvious visible morphological differences are only observed after 36h of dark-growth (Figure 1C, Figure S2B and Figure S3).
Pre-germinative light-activation of phyB antagonizes the constitutive repressive action of PIF1 and PIF3 on seedling deetiolation in subsequent darkness
The standard protocol referred to above, is widely used for these types of experiments with Arabidopsis and involves the exposure of the ungerminated seed to two periods of (usually white) light before subsequent germination and early seedling growth in darkness. These two light exposures occur (a) initially during seed sterilization and plating (here a 1.5 h white light (WL) exposure, referred to as ‘Pre-stratification’ (pre)), and (b) after stratification (here a 3 h WL exposure, referred to as ‘Post-stratification’ (post)) intended to stimulate and synchronize germination (Figure 1A). This pre-germination exposure to light raises the possibility that the apparent ‘dark’ effects on seedling development are in fact wholly or partly the consequence of the residual effects of the pre-germinative light treatments, mediated through the retention of the Pfr form of phytochrome in the germinating embryo and developing seedling in the subsequent dark period. To explore this possibility, we grew WT, pif1 and pif3 single and double mutant seedlings in the dark for 2 days under a series of modified light-treatment schedules designed to circumvent or reverse any Pfr formation in the seed (Figure 1A and Figure S4A).
The data show that insertion of a terminal FR pulse (FRp) after the 3-h post-stratification WL treatment in the standard protocol (pre-WL/post-WL+FRp), strongly reduces cotyledon separation in all four genotypes in the subsequent two-day dark period (Figure 1B, E). This result shows that Pfr that was pre-formed in the seed by phy photoactivation during, or prior to, the post-stratification light treatment induces partial seedling deetiolation during subsequent germination and growth in the dark in the wild-type, and that this response is enhanced in the pif mutants. This conclusion is supported by the more extensive and diagnostic photobiological experiments in Figure S5 using a variety of R and FR light-pulse treatments. Thus, a significant proportion of the deetiolation response in dark-grown seedlings under standard-protocol conditions is a residual light effect mediated by one or more phytochromes, rather than a constitutive dark response. For convenience, we term this a “pseudo-dark” response (Figure 1A, B, E). Because the magnitude of this response is greater in the pif mutants than the wild type, the evidence indicates that both PIF1 and PIF3 act antagonistically to Pfr in eliciting this effect. Moreover, because the FRp completely reverses the WL-induced effect down to the WT level in the single pif3 mutant, the data indicate that the enhanced cotyledon separation in the absence of PIF3 is entirely a pseudo-dark response resulting from the removal of PIF3 antagonism of preformed Pfr activity (Figure 1B, E).
On the other hand, the FRp does not completely eliminate the cotyledon separation phenotype of the pif1 single mutant, and this FRp-refractory phenotype is strongly further enhanced in the additional absence of PIF3 in the pif1pif3 double mutant (Figure 1B, E). These data indicate either the retention of a constitutive-dark response in these two genotypes, or a pseudo-dark response to Pfr formed and acting either during, or prior to, the post-stratification light treatment, including the possible presence of Pfr pre-formed in the mature seed. A series of additional treatments were used to distinguish between these possibilities (Figure 1A, E and Figure S4A, B). The data show that pif1 and pif1pif3 mutants retained significant cotyledon-separation phenotypes under conditions where any seed Pfr formed either during seed maturation or the 1.5 h plating period in WL was removed by a FRp provided immediately after plating before stratification (pre-WL+FRp/post-none) (Figure 1E). Moreover, importantly, no pronounced differences were found between this treatment (plating in WL) and plating in green (G) “safelight” (pre-G+FRp/post-none) (see below, Figure 2C and S9), establishing that this pre-stratification FRp treatment (pre-WL+FRp/post-none) represents “true”-dark conditions for seedling development. The evidence indicates, therefore, that the cotyledon-separation phenotype is a constitutive (i.e. light-independent) dark-response in these genotypes. Only pif1 and pif1pif3 mutant seeds germinated under these conditions (Figure 1A, E), since PIF1 is a repressor of seed germination in the dark [18, 28]. In addition, across multiple experiments (see above, Figures 1A, E, and below, Figures 2C, S9 and S10), we observed that even delaying the FRp until after the post-stratification WL or R-pulse treatment (pre-WL/post-WL+FRp and pre-WL/post-Rp+3hD+FRp) is essentially as effective as the pre-stratification FRp (pre-WL+FRp/post-none) in suppressing the pseudo-dark effects (Figure 1A, E above, and Figures 2C, S9 and S10 below). Therefore, these post-stratification FRp treatments (pre-WL/post-WL+FRp and pre-WL/post-Rp+3hD+FRp) also represent “true”-dark conditions and have the advantage that they permit germination of all the genotypes, including those containing wild-type PIF1 (Figures 1A, B, E and S5C above, and Figure 2C below). Collectively, these data provide genetic evidence that PIF1 and PIF3 act as constitutive repressors of cotyledon separation in the etiolated seedling, with PIF1 being the main regulator of this response and PIF3 playing a redundant role only observed when PIF1 is absent. Moreover, importantly, the discovery of the pseudo-dark effect, reflecting the antagonistic stimulatory and repressive actions of light-activated phy and the two PIF proteins on cotyledon separation, provides evidence, in addition, that the Pfr form of phy acts to derepress the repressive action of the PIFs.
Figure 2. pif1pif3pif4pif5 quadruple mutant displays a robust pleiotropic constitutive-photomorphogenic phenotype in the dark.
A) Visible phenotypes of 2d-old (2dD or D48h) and 4d-old (4dD or D96h) dark-grown seedlings grown under the standard conditions indicated in Figure 1A (pre-WL/post-WL). Photos of representative wild-type (Col-0) and pif mutant seedlings are shown.
B) Visible phenotypes of 2d-old (D48h) seedlings grown in darkness under the modified schedules indicated in Figure 1A. Photos of representative seedlings for each genotype are shown.
C) Quantification of cotyledon separation (Top) and hypocotyl elongation (Bottom) phenotypes under the indicated modified schedules. Data represent the mean and standard error of at least 20 seedlings. Asterisk indicates low germination precluding reliable measurements.
In order to determine whether the pseudo-dark (i.e. light-induced) responses are mediated by phyB, we generated a pif1pif3phyB triple mutant. The data show that the phyB mutation suppressed a major part of the cotyledon separation phenotype displayed by the pif1pif3 mutant under pseudo-dark conditions (Figure 1F). In fact, under our standard protocol conditions (pre-WL/post-WL) the cotyledon separation phenotype of pif1pif3phyB mutants was similar to that of the pif1pif3 parental mutant grown under true-dark conditions (preWL+FRp/post-none) (Figure 1F). These results indicate that: 1) phyB is the main, if not exclusive, photoreceptor mediating the pseudo-dark responses of the pif1pif3 mutant; and 2) PIF1 and PIF3 repress cotyledon separation under true-dark conditions independently of phyB. Also consistent with the results presented in Figure 1E, we also observed that the phyB mutation suppressed the phenotype of the pif3 single mutant (Figure 1F). These results indicate that the pif3 single-mutant phenotype in the dark (where PIF1 is present) is due essentially entirely to the pseudo-dark (i.e. residual-light) effects that are mediated by phyB. We did not observe increased levels of phyB in the pif1pif3 mutant seeds or seedlings compared to wild type before or after the post-stratification WL treatment (Figure S6), indicating that the pseudo-dark effects are not due indirectly to enhanced light sensitivity conferred by greater photoreceptor abundance in the mutant, as observed under prolonged irradiation conditions [24, 25]. Instead, the data support a direct action of PIF1 and PIF3 proteins in repressing the phyB-induced pseudo-dark responses.
Interestingly, our analysis, of the hook-unfolding and hypocotyl-length responses in the dark-grown seedlings under the modified schedule (Figures 1A and S4A) showed that, in contrast to cotyledon separation, the dark-phenotypes observed were predominantly due to pseudo-dark effects (Figure S4) mainly mediated by phyB (Figure S7). This observation suggests possible tissue- or organ-specific branching of the PIF regulatory pathways.
To determine whether molecular aspects of the seedling deetiolation phenotype are also subject to “true”- and “pseudo”-dark regulation, we examined whether the expression of a marker photosynthetic gene, light harvesting complex gene LHCB1.4 [31], was de-reperessed in dark-grown pif mutant seedlings under the indicated modified growth conditions (Figures 1A, G and S5). We observed that this gene was strongly de-repressed in the dark in pif1 and pif1pif3 compared to WT and pif3 under the true-dark conditions, where pseudo-dark effects were removed by a post-stratification FRp (pre-WL/post-Rp+3hD+FRp). This de-repression was highest in the pif1pif3 double mutants and was also observed at comparable levels in the alternative true-dark conditions, where only pif1 mutant seeds germinate (pre-WL+FRp/post-none) (Figure 1G), indicating that PIF1 and PIF3 (in the absence of PIF1) act in the dark as constitutive repressors of gene expression associated with seedling deetiolation. Parallel to the observed cotyledon separation phenotypes (Figures 1E and S5), the de-repression of LHCB1.4 gene expression in the pif mutants was further enhanced by the phy-activating light exposure under pseudo-dark conditions (pre-WL/post-Rp) (Figure 1G). The pseudo-dark effects were particularly striking in the pif3 single mutants, where most of the LHCB1.4 de-repression is reversed by the FRp.
PIF3, PIF4 and PIF5 act redundantly with PIF1 in repressing seedling deetiolation in the dark
The partial constitutive deetiolation phenotype of the pif1pif3 double mutant indicates simultaneously that PIF1 and PIF3 act synergistically to repress cotyledon separation in the dark, and, conversely, that other potential repressors are responsible for the residual skotomorphogenic phenotype of this mutant. Possible candidates for this residual repressor activity are other phy-interacting members of the bHLH transcription factor family. Because PIF4 and PIF5 are closely-related members of this family, with evidence of partly overlapping functions with PIF1 and PIF3 [16, 22], we generated a pif1pif3pif4pif5 quadruple mutant to explore this possibility. Strikingly, under our standard pseudo-dark protocol conditions (pre-WL/post-WL) (Figure 1A), 2d and 4d dark-grown pif1pif3pif4pif5 quadruple mutant seedlings displayed a pronounced cop-like phenotype (Figure 2A and Figure S8). This phenotype is significantly more pronounced and pleiotropic than for pif1pif3 in affecting multiple parameters of the deetiolation process, including hypocotyl inhibition and cotyledon separation and expansion, suggesting that PIFs 1,3,4 and 5 collectively regulate these responses.
To establish how much of this response is a true- (constitutive) or a pseudo-dark effect in response to light-activation of the phy in the pre-germinative seed, we grew WT and pif mutant seedlings under the modified light schedule (Figure 1A and Figure S9A), and measured cotyledon separation and hypocotyl length as representative morphological responses. For simplicity, the most relevant treatments are shown in Figure 2B, C, whereas the complete experiment is presented in Figure S9.
The data show that both constitutive- and pseudo-constitutive cotyledon separation effects are evident to a greater or lesser extent in all mutant genotypes examined (Figure 2B, C). However, notably, under true-dark conditions where the pseudo-dark (i.e. light-induced) effects are abrogated by a post-stratification FRp (pre-WL/post-Rp+3hD+FRp), the pif3pif4pif5 triple mutant has a closed cotyledon phenotype, indistinguishable from WT, whereas any mutant containing the pif1 mutation has separated cotyledons. Additional analysis under the alternative true-dark conditions (pre-G+FRp/post-none) where only seeds containing the pif1 mutation are able to germinate, shows that pif1-containing pif1pif3pif4 and pif1pif3pif5 triple mutants are similar to the pif1pif3 double mutant (Figure 2B, C). By contrast, the quadruple mutant has a more prominent cotyledon separation phenotype than any of these three mutants, consistent with the conclusion that PIF4 and PIF5 act redundantly with each other to repress cotyledon separation in the dark in the absence of PIF1 and PIF3, and that they collectively play a synergistic role with PIF1 and PIF3 as constitutive repressors of cotyledon separation in the dark (Figure 2B, C). Altogether, we conclude that PIF1 is the main player imposing constitutive repression of the cotyledon-separation response, because when PIF1 is present (WT and pif3pif4pif5) there is no such true-dark phenotype. However, this role is redundantly played by either PIF3, PIF4 or PIF5, in an apparently quantitatively synergistic fashion, as can be concluded from the effect of adding the pif3, pif4 and pif5 mutations in the absence of PIF1 (compare pif1 to pif1pif3pif4pif5).
In addition to the constitutive dark response, all pif-mutant genotypes displayed a pseudo-constitutive cotyledon-separation phenotype in response to a post-stratification Rp light treatment (pre-WL/post-Rp) (Figure 2B, C). In most cases, this Rp-induced response was reversed by a subsequent FRp (pre-WL/post-Rp+3hD+FRp) down to the level of the true-dark response for that genotype (pre-G+FRp/post-none), indicating that Pfr produced in the seed by the Rp is responsible for the enhancement of the cotyledon-separation phenotypes. This response was especially striking in pif3pif4pif5 triple mutant seedlings, in which all of the cotyledon phenotype appears to be due to residual Pfr (Figure 2B, C). Similar to PIF1 and PIF3 (Figure 1E), these data reflect apparent antagonistic stimulatory and repressive actions of light-activated phy on the one hand and PIF4 and PIF5 proteins on the other on cotyledon separation, thus providing evidence that the Pfr form of phy acts to de-repress the repressive action of PIF4 and PIF5. Similar constitutive and pseudo-constitutive phenotypes were observed in the hypocotyl-elongation response (Figure 2B, C) and in 4d-old seedlings (Figure S10), further reinforcing the conclusion that PIFs 1,3,4,5 redundantly, and reversibly, repress seedling deetiolation in the dark.
From the additional treatments presented in Figure S9, we observed that stable Pfr carry-over from plating in 1.5h WL (pre-WL) is able to induce a pronounced pseudo-dark effect in the pif1pif3pif5 mutant in contrast to the absent or sometimes marginal effect observed for the other pif mutants (Figure S4B and S9) (compare pre-WL/post-none with pre-WL+FRp/post-none). This Pfr carry-over can act after stratification because it can be reversed by a FRp delayed till after stratification (compare pre-WL/post-none with pre-WL/post-FRp). The reason for this difference between genotypes is not understood.
Pre-germinative-light-potentiated pseudo-dark responses are mediated by phy-regulated changes in PIF protein levels
As concluded above, the exaggerated light-potentiated pseudo-dark responses observed in the pif mutants apparently reflect an antagonistic interplay between phy and PIF activities in determining the deetiolated state of the emerging seedling. In order to test whether the molecular basis for this antagonism is the known photoreversible, phy-imposed repression of PIF protein levels [9–15, 19], we chose to measure PIF3 under the pseudo-dark conditions. We observed that the standardly used 3hWL treatment of the pre-germinative seeds does indeed induce the degradation of a H:PIF3:MYC fusion protein [11] during that irradiation (Figure 3A, B), whereas subsequent dark incubation results in its re-accumulation (Figure 3A, B). The rate of re-acumulation of PIF3 in the dark is accelerated by a phy-inactivating FRp at the end of the 3h WL treatment (Figure 3A, B), demonstrating that phy Pfr formed during the 3hWL treatment is acting during the dark-period to sustain reduced PIF3 levels. Similar data have been obtained for PIF1 in seeds [32]. Therefore, the light-induced pseudo-dark effects observed in the pif mutants may be explained by enhanced sensitivity to the light pretreatments, resulting from a synergistic effect of the genetic removal of one or more PIFs, superimposed on the light-induced removal of the remaining PIFs in the pre-germinative seeds by rate-limiting levels of phy.
Figure 3. Pre-germinative-light-potentiated pseudo-dark responses are mediated by phy-regulated changes in PIF protein levels.
A) Schematic representation of the modified growth protocol used. After the post-stratification WL treatment (3hWL) seeds were placed in darkness (3hWL+D(h)). Alternatively, a 5-min FRp was provided before the dark incubation (3hWL+FRp+D(h)).
B) Immunoblot analysis of H:PIF3:MYC fusion protein. Protein extracts were prepared from H:PIF3:MYC transgenic lines [11] and immunoblotted with an anti-MYC antibody (upper and middle panels). Tubulin was used as loading control. H:PIF3:MYC signal normalized to tubulin was quantified from the blots shown in the middle panel using Image J software as described [24], and the data are presented relative to D0 time point (lower panel).
C) Immunoblot analysis of endogenous PIF3 protein in wild-type (Col-0) and phyB-9 mutant seeds and seedlings. Protein extracts were immunoblotted with an anti-PIF3 antibody [11] (upper panel). Tubulin was used as loading control. PIF3 signal normalized to tubulin was quantified as in B), and the data are presented relative to Col-0 D0 time point (lower panel).
D) Immunoblot analysis of endogenous PIF3 protein in wild-type (Col-0) and phyB-9 mutant seeds grown under the indicated conditions. Tubulin was used as loading control.
E) Quantification of cotyledon separation phenotype of 2d-old dark-grown spa1spa2spa3 mutant seedlings grown under the standard protocol (3hWL+D45h). Data points represent the mean and standard error of at least 30 seedlings.
F) Immunoblot analysis of endogenous PIF3 protein in wild-type (Col-0) and spa1spa2spa3 mutant seedlings grown as in Figure 3E (3hWL+D45h). Tubulin was used as loading control.
Where indicated, protein extracts from pif3 and pif1pif3 mutants were used as controls. n.s.: non-specific band.
Because phyB is predominantly responsible for the pseudo-dark phenotypic responses observed in the pif mutants (Figure 1F), we wished to test whether phyB is the main photoreceptor regulating PIF levels in response to, and during the dark-incubation after, the post-stratification 3hWL treatment (Figure 3B). For this purpose, we analyzed PIF3 protein recovery in the dark in WT and phyB-9 mutant seeds after the 3hWL exposure (Figure 3A). We observed that PIF3 is degraded after 3hWL incubation in phyB-9 mutant seeds similar to WT (Figure 3C), indicating that other photoreceptors, such as phyA [11], are involved in this process. However, the phyB-9 mutants showed a transiently more rapid increase in PIF3 protein levels compared to WT when the irradiated seeds were returned to the dark (Figure 3C). Because the PIF3 levels in WT seeds treated with a FRp after the 3hWL reaccumulate similarly to those in the phyB-9 mutant without a FRp (Figure 3D), we conclude that phyB is the main photoreceptor activated during the 3hWL treatment responsible for maintaining reduced PIF3 levels during the subsequent dark-incubation. Together with similar data obtained for PIF1 [32], our results suggest that the balance between the reciprocal effects of the dark-accumulation of the PIF repressors, and the phy-induced degradation of the PIFs triggered by light, determine the final deetiolation state of the emerging seedling.
Since the maintenance of high levels of the PIF proteins in the emerging dark-grown seedling appears to be an important determinant in sustaining etiolated growth, we reasoned that other previously described constitutive photomorphogenic mutants might show reduced PIF levels in the dark. To test this idea, we chose the cop1-like spa1spa2spa3 triple mutant [33], which also showed a prominent deetiolated phenotype in the dark in our standard conditions (Figure 3E and Figure S11A). Immunoblot analysis showed that the spa triple mutant accumulates reduced levels of PIF3 in the dark (Figure 3F and Figure S11B). This result indicates that reduced PIF3, and possibly other PIFs, can partly explain the cop-like phenotypes of the dark-grown spa1spa2spa3 mutants. These data, together with the observed reduced PIF3 levels in the cop1 mutant [9], suggest that the COP1-SPA complex of proteins may repress photomorphogenesis in the dark, at least partly, by stabilizing PIF3 and possibly other PIFs. Altogether, these results further reinforce the notion that fine control of PIF levels (either genetic, light-induced or COP1/SPA-regulated removal of PIFs) is an important factor determining deetiolation in the emerging seedling.
DISCUSSION
The findings presented here have identified a major component of the molecular mechanism that terrestrial plants have evolved to enable utilization of a skotomorphogenic strategy of seedling development during early post-germinative growth in subterranean darkness. The evidence indicates that this mechanism involves repression of photomorphogenic development in darkness by the concerted action of a subset of phy-interacting bHLH transcription factors, including PIF1, PIF3, PIF4 and PIF5 (Figure 4A). Conversely, the data here and elsewhere also indicate that light reverses this repression by targeted degradation of these transcription factors, triggered by direct, light-induced, conformer-specific binding of the Pfr form of the phy molecule to the bHLH proteins, thereby initiating the photomorphogenic pathway.
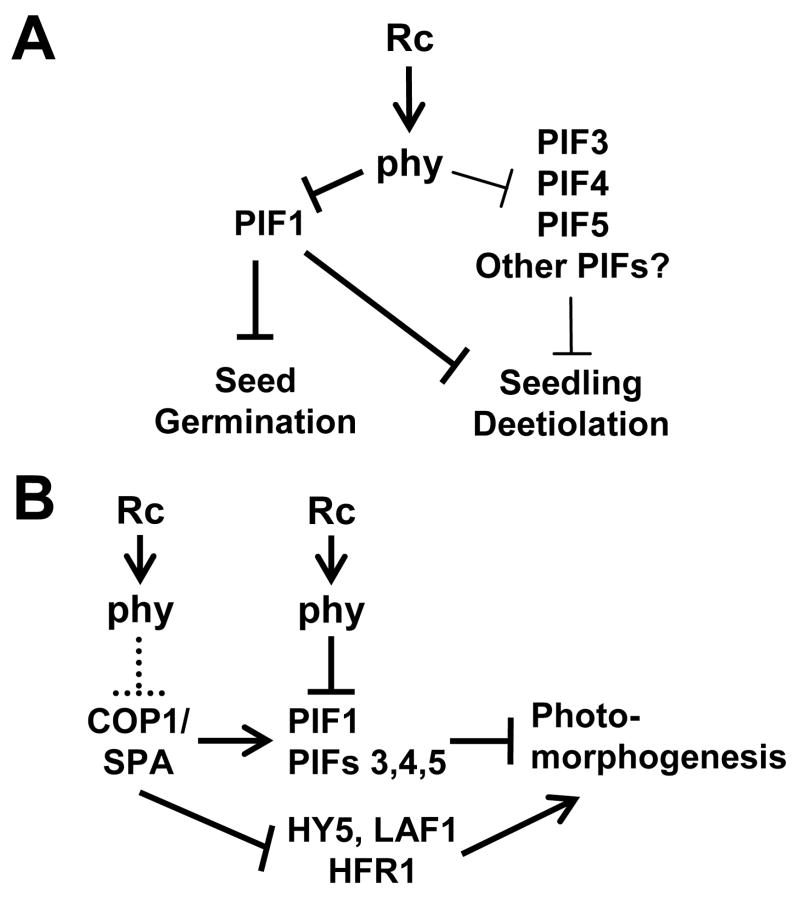
Figure 4. Model of phy-induced initiation of photomorphogenesis by direct removal of repressors PIF1, PIF3, PIF4 and PIF5.
A) PIFs 1, 3, 4 and 5 collectively promote skotomorphogenesis during early post-germinative seedling development in darkness. Experiments under true-dark conditions show that PIF1 has a dominant role in this process, whereas PIF3, PIF4 and PIF5 act redundantly with PIF1. Light-induced activation of the phy molecule triggers rapid degradation of these PIFs, as a consequence of direct physical interaction of the photoreceptor with the PIFs. The phy-induced removal of PIF repression, revealed here under the pseudo-dark experimental dark conditions, is proposed to initiate the developmental transition from skotomorphogenesis to photomorphogenesis.
B) Proteosome-pathway photomorphogenesis repressors COP1/SPA regulate the abundance of PIF3, and possibly other PIFs. It is proposed that part of COP1 and SPA action in repressing photomorphogenesis in the dark may be through promotion of PIF-protein accumulation, in addition to their established role in promoting the degradation of positive factors, such as HY5 [37, 38]. Light activates phy to remove the repressive action of the PIFs through direct molecular interaction (solid line), and to remove COP1/SPA activity through a possibly indirect mechanism (dotted line), thereby initiating seedling photomorphogenesis.
Although previous studies had implied the possible existence of such a repressive mechanism of action of these phy-interacting bHLH proteins in seedling development, this proposal was based on extrapolation from the behavior of fully-deetiolated light-grown plants [16–18, 20, 21]. In these studies, pif mutants displayed hypersensitivity to prolonged exposure to light, interpreted as indicating that the PIFs act negatively in the phy signaling pathway controlling seedling deetiolation. However, these data did not rule out alternative possibilities. Recent evidence renders this interpretation of the data uncertain by showing that the hypersensitivity under prolonged irradiation is due to PIF-controlled, feedback modulation of phyB-protein abundance via direct interaction with the activated photoreceptor molecule [19, 22, 24, 25]. This finding indicates that, under prolonged irradiation, the PIFs modulate global seedling sensitivity to light, indirectly, by regulating photoreceptor levels, with no current compelling evidence that they participate directly in the phy signaling pathway under these conditions.
By contrast, the genetic and photobiological data presented here for dark-grown seedlings do simultaneously provide evidence for the constitutive repressive action of the phy-interacting bHLH proteins in darkness, and for the highly photosensitive reversal of this activity by pre-exposure of seed embryos to light (Figure 4A). The key to these observations was the discovery of, and experimental differentiation between, the “pseudo”-and “true”-dark components of the deetiolation response observed in dark-grown seedlings under standard-protocol conditions. By using “true”-dark conditions that avoided or reversed light-induced, post-imbibition Pfr formation during plating and/or germination-synchronization pre-treatment (Figure 1A), we obtained evidence for light-independent seedling deetiolation in the pif mutants. The data show that, overall, the progressive genetic removal of the PIF proteins studied here, up to the level of a quadruple pif1pif3pif4pif5 mutant, culminated in a cop-like phenotype in darkness, establishing that PIF1, PIF3, PIF4 and PIF5 repress seedling deetiolation in a partially redundant manner (Figure 2B, C). PIF1 seems to be the dominant partner in this repression, since the pif1 single mutant and all pif1 mutant-containing combinations, show some degree of cop-like phenotype, whereas PIF3, PIF4 and PIF5 repressive action is only observed in the absence of PIF1 (compare pif3pif4pif5 with pif1pif3pif4pif5 under true dark conditions in Figures 2B, C). This central role of PIF1 is supported by a recent report that overexpression of a truncated version of PIF1 causes a cop-like phenotype, possibly by sequestering other PIFs in a dominant negative manner [14], thus behaving as a multiple pif mutant.
Concomitantly, our investigations of the “pseudo”-dark (i.e. light-induced) component of the deetiolation response have revealed that the deetiolation state of the seedling under these conditions is defined by a delicately-poised, dynamic antagonism between the positive and negative actions of phyB and the PIFs. The photobiological experiments show that phytochrome photoactivated in the ungerminated seed is stably retained and acts in the Pfr form to induce the “pseudo”-dark responses during subsequent growth of the post-germinative seedling in the dark (Figures 1E, 2C, and S9). Genetic analysis indicates that phyB is the predominant, if not exclusive photoreceptor responsible for this light-induced activity (Figure 1F). Trans-developmental storage of light signals, such as that observed here, was recognized in principle by Casal and colleagues [34, 35], but under their different experimental conditions, was attributed to factors other than stable activated phy.
The observation that genetic elimination of PIF expression substantially enhances the effectiveness of activated phyB in eliciting the “pseudo”-dark deetiolation response (Figure 1E and Figure 2C), provides strong evidence of the antagonistic interplay between the photoreceptor and transcription-factor activities mentioned above. This effect is not due to enhanced photosensitivity conferred by elevated phyB levels in the absence of the PIF proteins, as observed under prolonged irradiation conditions [19, 22, 24, 25], because no effect of the pif mutations on phyB abundance was detected here (Figure S6). Instead, the data are consistent with the model (Figure 4A) that the well-documented light-induced, phy-mediated degradation of the PIF proteins [9–15], acts synergistically with the effect of genetically removing the PIF factors in promoting the “pseudo”-dark response. Supporting this conclusion is our analysis of the dynamics of phyB-regulated PIF3 abundance. The data show that post-stratification light (i.e. pseudo-dark conditions) induces the degradation of the PIF3 protein in the ungerminated seeds, whereas subsequent dark-incubation then permits its re-accumulation (Figure 3B, C). The rate of this re-accumulation is determined by the presence or absence of residual phyB-Pfr during the dark period, photobiologically-controled in the wild type (Figure 3B, D) and genetically removed in the phyB mutant (Figure 3C). The data indicate, therefore, that light-activation of phyB under pseudo-dark conditions determines how much PIF3 protein is available to effectively repress deetiolation during the subsequent dark-growth period of the seedling. Because similar data have been obtained for PIF1 in seeds [32] and for PIF4 and PIF5 in seedlings [12, 13, 15], it is reasonable to suggest that pseudo-dark responses also operate by phy-modulation of PIF1, 4 and 5 protein levels.
On the other hand, the less-than-complete constitutive deetiolation in the quadruple pif mutant under “true”-dark conditions, coupled with the retention of residual responsiveness to “pseudo”-dark (i.e. transient light-exposure) conditions by this mutant (Figure 2), indicates that other phy-regulated repressive factors must be active in maintaining the etiolated state of the seedling. Additional phy-interacting bHLH factors, such as PIF6 [36] and PIF7 [24], are potential candidates for this activity. Alternatively, the established role of COP1 and the SPA family in maintaining the etiolated state of the seedling through degradation of positive regulators such as HY5 [37–39], might also account for at least some of this residual repression of photomorphogenesis. Our observation that SPA1, SPA2 and SPA3 appear to have a function in maintaining high PIF3 levels in the dark, similar to that reported for COP1 [9], suggests a possible regulatory interconnection with the PIF pathway worthy of further investigation (Figure 4B).
Taken together, the data suggest that, in dark-germinated, wild-type seedlings, PIF1, PIF3 PIF4, PIF5 and other unidentified factors act collectively at high levels, in at least partially-redundant fashion, to constitutively repress premature deetiolation in darkness, and that rapid, phy-induced degradation of the PIF proteins, upon initial exposure to light, triggers derepression of this process. Reversal of the repression of presumptive target genes of these bHLH transcription factors thus appears to be an integral component of the primary mechanism by which the activated photoreceptor switches the seedling from skotomorphogenic to photomorphogenic development. The proposed function of the dynamically-poised antagonism between the phys and PIFs in regulating growth responses of fully-deetiolated plants to fluctuating light environments, such as encountered under diurnal or shade-avoidance conditions [13, 15, 26, 27], is worthy of further investigation.
Supplementary Material
Experimental procedures and supplemental data including 11 additional figures, are available with this article online.
Acknowledgments
We thank Ute Hoecker for providing us the spa1spa2spa3 mutant and Wing-Chi Leung for her contribution in selecting pif triple and quadruple mutants. This work was supported by a postdoctoral fellowship from the Spanish Ministry of Education and Science to P.L., by a “Ramon y Cajal” contract and a Spanish Ministry of Education and Science Grant BIO2006-09254 to E.M., by a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists to Y.O., by National Science Foundation Grants IBN-0418653 and IOS-0822811 to E.H., and by National Institutes of Health Grant GM-47475, Department of Energy Grant DEFG03-87ER13742, and USDA Agricultural Research Service Current Research Information System Grant 5335-21000-017-00D to P.H.Q.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schafer E, Nagy F. Photomorphogenesis in Plants and Bacteria. Dordrecht, Netherlands: Springer; 2006. [Google Scholar]
- 2.Rockwell NC, Su YS, Lagarias JC. Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol. 2006;57:837–858. doi: 10.1146/annurev.arplant.56.032604.144208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagatani A. Light-regulated nuclear localization of phytochromes. Curr Opin Plant Biol. 2004;7:708–711. doi: 10.1016/j.pbi.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–230. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- 5.Toledo-Ortiz G, Huq E, Quail PH. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell. 2003;15:1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duek PD, Fankhauser C. bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 2005;10:51–54. doi: 10.1016/j.tplants.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Castillon A, Shen H, Huq E. Phytochrome Interacting Factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 2007;12:514–521. doi: 10.1016/j.tplants.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Quail PH. Phytochrome interacting factors. In: Whitelam GC, Halliday KJ, editors. Light and Plant Development. Oxford, UK: Blackwell Publishing; 2007. pp. 81–105. [Google Scholar]
- 9.Bauer D, Viczian A, Kircher S, Nobis T, Nitschke R, Kunkel T, Panigrahi KC, Adam E, Fejes E, Schafer E, Nagy F. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell. 2004;16:1433–1445. doi: 10.1105/tpc.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen H, Moon J, Huq E. PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize photomorphogenesis of seedlings in Arabidopsis. Plant J. 2005;44:1023–1035. doi: 10.1111/j.1365-313X.2005.02606.x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Sady B, Ni W, Kircher S, Schafer E, Quail PH. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell. 2006;23:439–446. doi: 10.1016/j.molcel.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Shen Y, Khanna R, Carle CM, Quail PH. Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol. 2007;145:1043–1051. doi: 10.1104/pp.107.105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448:358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Zhu L, Castillon A, Majee M, Downie B, Huq E. Light-Induced Phosphorylation and Degradation of the Negative Regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis Depend upon Its Direct Physical Interactions with Photoactivated Phytochromes. Plant Cell. 2008;20:1586–1602. doi: 10.1105/tpc.108.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008;53:312–323. doi: 10.1111/j.1365-313X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- 16.Huq E, Quail PH. PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. Embo J. 2002;21:2441–2450. doi: 10.1093/emboj/21.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Yi H, Choi G, Shin B, Song PS. Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell. 2003;15:2399–2407. doi: 10.1105/tpc.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh E, Kim J, Park E, Kim JI, Kang C, Choi G. PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell. 2004;16:3045–3058. doi: 10.1105/tpc.104.025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monte E, Tepperman JM, Al-Sady B, Kaczorowski KA, Alonso JM, Ecker JR, Li X, Zhang Y, Quail PH. The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc Natl Acad Sci U S A. 2004;101:16091–16098. doi: 10.1073/pnas.0407107101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huq E, Al-Sady B, Hudson M, Kim C, Apel K, Quail PH. Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science. 2004;305:1937–1941. doi: 10.1126/science.1099728. [DOI] [PubMed] [Google Scholar]
- 21.Fujimori T, Yamashino T, Kato T, Mizuno T. Circadian-controlled basic/helix-loop-helix factor, PIL6, implicated in light-signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 2004;45:1078–1086. doi: 10.1093/pcp/pch124. [DOI] [PubMed] [Google Scholar]
- 22.Khanna R, Shen Y, Marion CM, Tsuchisaka A, Theologis A, Schafer E, Quail PH. The basic helix-loop-helix transcription factor PIF5 acts on ethylene biosynthesis and phytochrome signaling by distinct mechanisms. Plant Cell. 2007;19:3915–3929. doi: 10.1105/tpc.107.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin J, Park E, Choi G. PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 2007;49:981–994. doi: 10.1111/j.1365-313X.2006.03021.x. [DOI] [PubMed] [Google Scholar]
- 24.Leivar P, Monte E, Al-Sady B, Carle C, Storer A, Alonso JM, Ecker JR, Quail PH. The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels. Plant Cell. 2008;20:337–352. doi: 10.1105/tpc.107.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Sady B, Kikis EA, Monte E, Quail PH. Mechanistic duality of transcription factor function in phytochrome signaling. Proc Natl Acad Sci U S A. 2008;105:2232–2237. doi: 10.1073/pnas.0711675105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lucas M, Daviere JM, Rodriguez-Falcon M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blazquez MA, Titarenko E, Prat S. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–484. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 27.Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S, Schafer E, Fu X, Fan LM, Deng XW. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–479. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penfield S, Josse EM, Kannangara R, Gilday AD, Halliday KJ, Graham IA. Cold and light control seed germination through the bHLH transcription factor SPATULA. Curr Biol. 2005;15:1998–2006. doi: 10.1016/j.cub.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Moon J, Zhu L, Shen H, Huq E. PIF1 directly and indirectly regulates chlorophyll biosynthesis to optimize the greening process in Arabidopsis. Proc Natl Acad Sci U S A. 2008;105:9433–9438. doi: 10.1073/pnas.0803611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae G, Choi G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu Rev Plant Biol. 2008;59:281–311. doi: 10.1146/annurev.arplant.59.032607.092859. [DOI] [PubMed] [Google Scholar]
- 31.Alabadi D, Gallego-Bartolome J, Orlando L, Garcia-Carcel L, Rubio V, Martinez C, Frigerio M, Iglesias-Pedraz JM, Espinosa A, Deng XW, Blazquez MA. Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J. 2008;53:324–335. doi: 10.1111/j.1365-313X.2007.03346.x. [DOI] [PubMed] [Google Scholar]
- 32.Oh E, Yamaguchi S, Kamiya Y, Bae G, Chung WI, Choi G. Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 2006;47:124–139. doi: 10.1111/j.1365-313X.2006.02773.x. [DOI] [PubMed] [Google Scholar]
- 33.Laubinger S, Fittinghoff K, Hoecker U. The SPA quartet: a family of WD-repeat proteins with a central role in suppression of photomorphogenesis in arabidopsis. Plant Cell. 2004;16:2293–2306. doi: 10.1105/tpc.104.024216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alconada Magliano T, Casal JJ. Pre-germination seed-phytochrome signals control stem extension in dark-grown Arabidopsis seedlings. Photochem Photobiol Sci. 2004;3:612–616. doi: 10.1039/b315511k. [DOI] [PubMed] [Google Scholar]
- 35.Mazzella MA, Arana MV, Staneloni RJ, Perelman S, Rodriguez Batiller MJ, Muschietti J, Cerdan PD, Chen K, Sanchez RA, Zhu T, Chory J, Casal JJ. Phytochrome control of the Arabidopsis transcriptome anticipates seedling exposure to light. Plant Cell. 2005;17:2507–2516. doi: 10.1105/tpc.105.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khanna R, Huq E, Kikis EA, Al-Sady B, Lanzatella C, Quail PH. A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell. 2004;16:3033–3044. doi: 10.1105/tpc.104.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoecker U. Regulated proteolysis in light signaling. Curr Opin Plant Biol. 2005;8:469–476. doi: 10.1016/j.pbi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Yi C, Deng XW. COP1 - from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15:618–625. doi: 10.1016/j.tcb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Zhu D, Maier A, Lee JH, Laubinger S, Saijo Y, Wang H, Qu LJ, Hoecker U, Deng XW. Biochemical Characterization of Arabidopsis Complexes Containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA Proteins in Light Control of Plant Development. Plant Cell. 2008 doi: 10.1105/tpc.107.056580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and supplemental data including 11 additional figures, are available with this article online.