Abstract
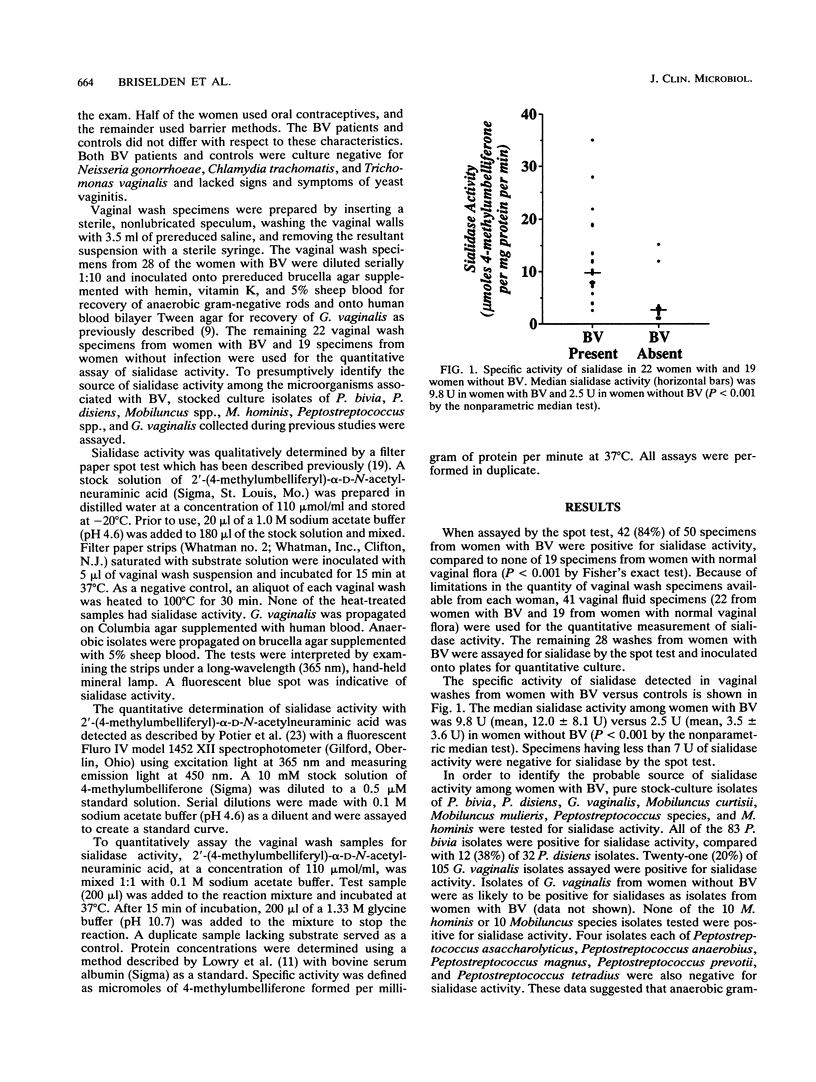
Bacterial vaginosis, Prevotella species, and Bacteroides species have been associated with prematurity and upper genital tract infection. Prevotella (Bacteroides) species and Bacteroides fragilis have also been associated with preterm birth. However, the mechanism by which lower genital tract infection causes upper genital tract disease remains poorly understood. Sialidases (neuraminidases) are enzymes which enhance the ability of microorganisms to invade and destroy tissue. Elevated levels of sialidase activity were detected in 42 (84%) of 50 vaginal fluid specimens from women with bacterial vaginosis and none of 19 vaginal fluids from women without bacterial vaginosis (P less than 0.001). Vaginal fluid from women with bacterial vaginosis had a median specific activity of 9.8 U compared to 2.5 U of sialidase in women without bacterial vaginosis (P less than 0.001). In order to determine the probable source of sialidases in vaginal fluid, the microorganisms recovered from women with bacterial vaginosis before and after treatment were assayed. Of 28 specimens from women with bacterial vaginosis, 27 (96%) yielded sialidase-positive bacteria, at a median concentration of 10(6.5) CFU/ml of vaginal fluid. Prevotella and Bacteroides species accounted for the sialidase activity in 26 of the vaginal fluids, and Gardnerella vaginalis accounted for the sialidase activity in the remaining fluid. After treatment, sialidase was detected in the vaginal fluid of 1 (5%) of 22 women who responded to therapy and in all of 6 women for whom therapy failed. These data suggest that vaginal fluid sialidase is highly correlated with bacterial vaginosis and that the probable sources for this enzyme activity are the Bacteroides and Prevotella species present in the vagina.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalto M., Rönnemaa T., Kulonen E. Effect of neuraminidase on the synthesis of collagen and other proteins. Biochim Biophys Acta. 1974 Apr 11;342(2):247–253. doi: 10.1016/0005-2795(74)90079-8. [DOI] [PubMed] [Google Scholar]
- Brown J. G., Straus D. C. Characterization of neuraminidases produced by various serotypes of group B streptococci. Infect Immun. 1987 Jan;55(1):1–6. doi: 10.1128/iai.55.1.1-6.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzeniek R. Viral and bacterial neuraminidases. Curr Top Microbiol Immunol. 1972;59:35–74. doi: 10.1007/978-3-642-65444-2_2. [DOI] [PubMed] [Google Scholar]
- Hillier S. L., Krohn M. A., Kiviat N. B., Watts D. H., Eschenbach D. A. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 1):955–961. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- Hillier S. L., Martius J., Krohn M., Kiviat N., Holmes K. K., Eschenbach D. A. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988 Oct 13;319(15):972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- Hillier S., Krohn M. A., Watts D. H., Wolner-Hanssen P., Eschenbach D. Microbiologic efficacy of intravaginal clindamycin cream for the treatment of bacterial vaginosis. Obstet Gynecol. 1990 Sep;76(3 Pt 1):407–413. [PubMed] [Google Scholar]
- Holst E., Wathne B., Hovelius B., Mårdh P. A. Bacterial vaginosis: microbiological and clinical findings. Eur J Clin Microbiol. 1987 Oct;6(5):536–541. doi: 10.1007/BF02014242. [DOI] [PubMed] [Google Scholar]
- Kelly R. T., Farmer S., Greiff D. Neuraminidase activities of clinical isolates of Diplococcus pneumoniae. J Bacteriol. 1967 Jul;94(1):272–273. doi: 10.1128/jb.94.1.272-273.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn M. A., Hillier S. L., Lee M. L., Rabe L. K., Eschenbach D. A. Vaginal Bacteroides species are associated with an increased rate of preterm delivery among women in preterm labor. J Infect Dis. 1991 Jul;164(1):88–93. doi: 10.1093/infdis/164.1.88. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambotte R., Uhlenbruck G. Amniomucoids--a new class of hexosamine-rich glycoproteins. Nature. 1966 Oct 15;212(5059):290–291. doi: 10.1038/212290a0. [DOI] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Studies on the nature of receptors involved in attachment of tissue culture cells to mycoplasmas. Br J Exp Pathol. 1969 Feb;50(1):66–75. [PMC free article] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Utilization of neuraminic acid receptors by mycoplasmas. J Bacteriol. 1969 Jun;98(3):914–919. doi: 10.1128/jb.98.3.914-919.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martius J., Krohn M. A., Hillier S. L., Stamm W. E., Holmes K. K., Eschenbach D. A. Relationships of vaginal Lactobacillus species, cervical Chlamydia trachomatis, and bacterial vaginosis to preterm birth. Obstet Gynecol. 1988 Jan;71(1):89–95. [PubMed] [Google Scholar]
- McDonald H. M., O'Loughlin J. A., Jolley P., Vigneswaran R., McDonald P. J. Vaginal infection and preterm labour. Br J Obstet Gynaecol. 1991 May;98(5):427–435. doi: 10.1111/j.1471-0528.1991.tb10335.x. [DOI] [PubMed] [Google Scholar]
- McGregor J. A., French J. I., Richter R., Franco-Buff A., Johnson A., Hillier S., Judson F. N., Todd J. K. Antenatal microbiologic and maternal risk factors associated with prematurity. Am J Obstet Gynecol. 1990 Nov;163(5 Pt 1):1465–1473. doi: 10.1016/0002-9378(90)90607-9. [DOI] [PubMed] [Google Scholar]
- McGregor J. A., Lawellin D., Franco-Buff A., Todd J. K. Phospholipase C activity in microorganisms associated with reproductive tract infection. Am J Obstet Gynecol. 1991 Feb;164(2):682–686. doi: 10.1016/s0002-9378(11)80046-3. [DOI] [PubMed] [Google Scholar]
- Minkoff H., Grunebaum A. N., Schwarz R. H., Feldman J., Cummings M., Crombleholme W., Clark L., Pringle G., McCormack W. M. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol. 1984 Dec 15;150(8):965–972. doi: 10.1016/0002-9378(84)90392-2. [DOI] [PubMed] [Google Scholar]
- Moncla B. J., Braham P. Detection of sialidase (neuraminidase) activity in Actinomyces species by using 2'-(4-methylumbelliferyl)alpha-D-N-acetylneuraminic acid in a filter paper spot test. J Clin Microbiol. 1989 Jan;27(1):182–184. doi: 10.1128/jcm.27.1.182-184.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncla B. J., Braham P., Hillier S. L. Sialidase (neuraminidase) activity among gram-negative anaerobic and capnophilic bacteria. J Clin Microbiol. 1990 Mar;28(3):422–425. doi: 10.1128/jcm.28.3.422-425.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piot P., Van Dyck E., Godts P., Vanderheyden J. The vaginal microbial flora in non-specific vaginitis. Eur J Clin Microbiol. 1982 Oct;1(5):301–306. doi: 10.1007/BF02019976. [DOI] [PubMed] [Google Scholar]
- Potier M., Mameli L., Bélisle M., Dallaire L., Melançon S. B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979 Apr 15;94(2):287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Shah H. N., Collins D. M. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int J Syst Bacteriol. 1990 Apr;40(2):205–208. doi: 10.1099/00207713-40-2-205. [DOI] [PubMed] [Google Scholar]
- Silver H. M., Sperling R. S., St Clair P. J., Gibbs R. S. Evidence relating bacterial vaginosis to intraamniotic infection. Am J Obstet Gynecol. 1989 Sep;161(3):808–812. doi: 10.1016/0002-9378(89)90406-7. [DOI] [PubMed] [Google Scholar]
- Sperling R. S., Newton E., Gibbs R. S. Intraamniotic infection in low-birth-weight infants. J Infect Dis. 1988 Jan;157(1):113–117. doi: 10.1093/infdis/157.1.113. [DOI] [PubMed] [Google Scholar]
- Watts D. H., Krohn M. A., Hillier S. L., Eschenbach D. A. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet Gynecol. 1990 Jan;75(1):52–58. [PubMed] [Google Scholar]
- von Nicolai H., Hammann R., Salehnia S., Zilliken F. A newly discovered sialidase from Gardnerella vaginalis. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Oct;258(1):20–26. doi: 10.1016/s0176-6724(84)80003-6. [DOI] [PubMed] [Google Scholar]



