Abstract
Borrelia burgdorferi (Bb), the causative agent of Lyme disease, is transmitted to mammalian hosts through an arthropod (tick) vector. To establish infection, Bb must acquire essential nutrients, including transition metals, from its mammalian and tick hosts. Thus far, no metal transporter has been identified in Bb. Here, we report the identification of the first metal transporter, BmtA (BB0219), in Bb. BmtA-deficient mutants of virulent Bb were readily generated, and the mutants grew slightly slower than wild-type Bb in vitro. However, BmtA mutants were sensitive to the chelating actions of EDTA, suggesting a role for BmtA in metal utilization. Intracellular accumulation of manganese (Mn) was substantially diminished in the bmtA mutant, indicating that BmtA was operative in Mn uptake. Given that BmtA lacks homology to any known Mn transporter, we postulate that BmtA is part of a novel mechanism for Mn acquisition by a bacterial pathogen. BmtA also was essential to the infectious life cycle of Bb in ticks and mammals, thereby qualifying BmtA as a new borrelial virulence factor. In addition, the bmtA mutant was sensitive to treatment with t-butyl hydroperoxide, implying that BmtA, and thus Mn, is important to Bb for detoxifying reactive oxygen species, including those potentially liberated by immune effector cells during the innate immune response. Our discovery of the first molecule involved in metal transport in Bb provides a foundation for further elucidating metal homeostasis in this important human pathogen, which may lead to new strategies for thwarting Lyme disease.
Keywords: metal transport, pathogenesis
Lyme disease, caused by the spirochetal bacterium Borrelia burgdorferi (Bb), remains the most common vector-borne disease in the United States (1). Bb has a complex life cycle involving an arthropod (Ixodes scapularis tick) vector and various mammalian hosts (2, 3). In recent years, extensive efforts have been directed toward elucidating the mechanisms by which Bb cycles, adapts, and sustains itself in these diverse niches. It is now well established that outer surface (lipo)protein C (OspC), OspA/B, decorin-binding protein B/A (DbpB/A), PncA, and BptA are required by Bb for efficient infection of ticks or mammalian hosts (4–10). It is also generally accepted that the recently discovered Rrp2-RpoN (σ54)-RpoS (σS) regulatory pathway plays prominently in Bb's virulence expression (11–14).
Transition metals, including iron (Fe), zinc (Zn), and manganese (Mn), are critical to both bacterial metabolism and virulence (15–18). However, transition metals are tightly sequestered within mammalian body fluids, making the scavenging of these cofactors by bacteria challenging. To overcome this, bacteria have evolved a number of elegant systems for acquiring transition metals from their environments. For example, Escherichia coli possesses multiple iron uptake systems (18, 19), the MntH Mn transporter (17, 20), and 2 Zn transporters (ZnuABC and ZupT) (16, 21). By tight regulation of these systems, bacteria maintain metal homeostasis.
Compared with other bacterial pathogens, little is known about transition metal acquisition and homeostasis in Bb. Genome sequence analysis has implied that Bb lacks genes encoding Fe-acquisition systems (22), suggesting unconventionally that Fe may not be required by this pathogen. This assertion was corroborated by an elegant study showing that Bb accumulates Mn, rather than Fe, when grown in a serum-free medium (23). However, evidence has been lacking regarding whether Bb requires Mn for growth and proliferation and, more importantly, to what extent Mn is needed by Bb to establish mammalian infection and how Bb acquires this element.
Blast analysis of the Bb genome has not revealed obvious homologs of known Mn transporters. However, the Bb chromosomal gene bb0219 encodes a GufA homolog with unknown function (22). Upon comprehensive sequence analysis, we discovered that BB0219 possesses the signature sequence (AxxxHNxxxGLAVG) for the ZIP family [zinc-regulated metal transporters (ZRTs) and iron-regulated metal transporters(IRTs)-like protein family] (24), whose members typically are competent for the uptake of Zn, Fe, or Mn (16, 21, 25, 26). Thus, we postulated that bb0219 may be involved in the uptake of one or more transition metals by Bb. To examine this possibility, we generated isogenic bb0219 mutants and complemented strains of virulent Bb and examined their phenotypes with respect to metal accumulation and their abilities to infect ticks and mice.
Results
In Silico Analysis of BB0219, a GufA Homolog.
Blast searches revealed that, in addition to Bb, functionally uncharacterized homologs of BB0219 exist in other bacteria, such as Borrelia garinii, Borrelia afzelii, Clostridium botulinum, Methanococcus maripaludis, Desulfotalea psychrophila, Staphylococcus epidermidis, and Listeria monocytogenes. Originally, bb0219 was annotated as GufA, a protein with unknown function (22, 27). GufA proteins have been placed as a subgroup of the larger ZIP family (25). In bacteria, some ZIP proteins transport not only Zn2+, but also other cations, such as Mn2+, Fe2+, and Cd2+ (16, 21, 25). All functionally characterized ZIP proteins contain a conserved topology with 8 transmembrane domains. This topology also was predicted to be in BB0219 when we analyzed BB0219 by using protein topology prediction programs PSORTb (28) and TMHMM 2.0 (26). Most ZIP proteins contain a metal-binding site (HxHxH) between spanners III and IV (24), and all ZIP proteins have a signature sequence (AxxxHNxxxGLAVG) in the most conserved spanner IV (of which the conserved central histidine provides an intramembranous heavy metal ion-binding site) (24). When we analyzed BB0219, we noted that although the metal-binding motif HxHxH was not apparent, the predicted protein possesses the signature sequence (AxxxHNxxxGLAVG) for the ZIP family (24) in the putative spanner IV region. These in silico analyses thus implicated BB0219 in metal uptake by Bb.
bb0219 Is Cotranscribed Within a 6-Gene Operon.
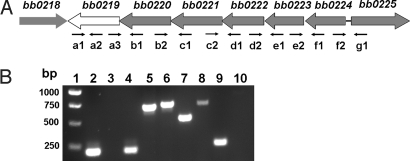
In the Bb genome, bb0219, bb0220, bb0221, bb0222, bb0223, and bb0224 are oriented in the same direction (Fig. 1A). In this region, bb0219 is separated from bb0220 by 40 bp, bb0223 is separated from bb0222 by 20 bp, and no intergenic regions are predicted between the other adjacent genes (22). To determine whether these genes are cotranscribed, RT-PCR was performed on RNA by using specific primers [supporting information (SI) Methods and Table S1]. As shown in Fig. 1B (lane 5), a fragment was amplified by using the primer pair spanning the junction of bb0219 and bb0220. Similar results were obtained for primer pairs spanning the junctions of bb0221 and bb0220 (lane 6), bb0222 and bb0221 (lane 7), bb0223 and bb0222 (lane 8), and bb0224 and bb0223 (lane 9), but not in the amplification using primers spanning bb0225 and bb0224 (lane 10). Results from all positive and negative controls were as expected (Fig. 1B, lanes 2–4). These data indicate that bb0219–bb0224 are cotranscribed.
Fig. 1.
bb0219 is cotranscribed with bb0220–bb0224. (A) Schematic representation of the putative bb0219–bb0224 operon in Bb. (B) Results from RT-PCR. Lane 1, molecular weight markers; lane 2, primer pair a1 and a2 in ordinary PCR using genomic DNA as template; lane 3, primer pair a1 and a2 in ordinary PCR using RNA as template (no RT control); lane 4, primer pair a1 and a2; lane 5, primer pair a3 and b1; lane 6, primer pair b2 and c1; lane 7, primer pair c2 and d1; lane 8, primer pair d2 and e1; lane 9, primer pair e2 and f1; and lane 10, primer pair f2 and g1.
Inactivation and Complementation of bb0219 in Bb.
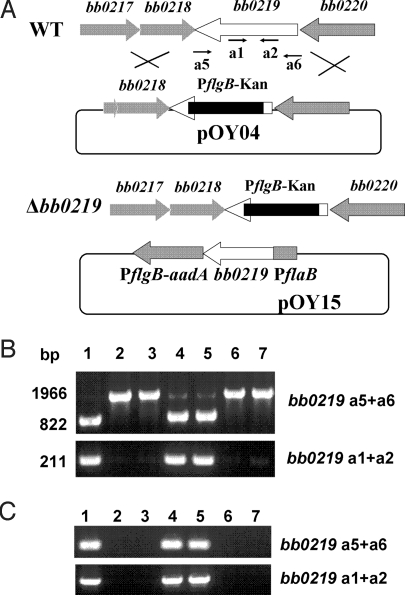
bb0219 disruption mutants were constructed via homologous recombination (Fig. 2A). Through allelic exchange, a 740-bp internal fragment of the 822-bp ORF of bb0219 was replaced with the PflgB-kan cassette, yielding 2 kanamycin-resistant transformants, OY04/D4 and OY04/F42. To complement the bb0219 mutants, the shuttle vector pOY15 was created by fusing bb0219 to the Bb flaB promoter PflaB (Fig. 2A). After electroporation of pOY15 into OY04/D4 or OY04/F42, 2 corresponding complemented clones, OY06/D11 and OY06/F6, were created. In addition, 2 mock-complemented strains, OY07/F1 and OY07/F62, also were generated by transforming the empty shuttle vector pJD54 into OY04/D4 or OY04/F42, respectively. The inactivation and complementation of bb0219 in these strains were confirmed by using PCR amplification (Fig. 2B), and the insertion and orientation of the PflgB-kan cassette within the disrupted bb0219 gene were confirmed by sequence analysis. Complementation also was confirmed by recovery of the shuttle vector from the complemented strains.
Fig. 2.
Construction of bb0219 disruption mutants and complemented strains. (A) Schematic representation of bb0217–bb0220 genes in the Bb chromosome, insertion of the PflgB-kan gene cassette by homologous recombination, and the relevant complementation plasmid. Arrows indicate the approximate positions of the oligonucleotide primers used for subsequent PCR analyses. (B) PCR analysis of WT 297, bb0219 mutants, and the complemented strains. The bb0219-specific primer pairs used in PCR are indicated on the right. Lane 1, WT 297; lane 2, bb0219 mutant OY04/D4; lane 3, mutant OY04/F42; lane 4, complemented strain OY06/D11; lane 5, complemented strain OY06/F6; lane 6, mock-complemented strain OY07/F1; and lane 7, mock-complemented OY07/F62. (C) RT-PCR was used to determine the presence or absence of bb0219 transcripts. Lanes and primer pair designations are as in B.
To assess the expression of bb0219 in Bb strains, RT-PCR employing specific primers was performed to detect transcripts. As expected, bb0219 transcripts were detected in both WT 297 and the complemented strains, but not in the mutants and the mock-complemented clones (Fig. 2C). Borrelia plasmids, especially those that are virulence-associated (such as lp25 and lp28–1), are easily lost during in vitro genetic manipulation, culminating in a loss of borrelial virulence (29). Hence, PCR-based plasmid profiling was performed to ensure that all plasmids were retained in the mutant and complemented strains. As shown in Fig. S1, the bb0219 mutant OY04/D4 contained the same plasmid profile as that of WT 297. In addition, the bb0219 mutant clone OY04/F42, the complemented strains (OY06/D11 and OY06/F6), and the mock-complemented strains (OY07/F1 and OY07/F62) also contained all plasmids present in WT 297.
Involvement of BB0219 in Metal Uptake.
Under darkfield microscopy, the bb0219 mutant exhibited spirochetal morphology identical to that of the 297 parental strain. Mutant spirochetes grew slightly slower than wild type in Barbour-Stoenner-Kelly II (BSK-II) medium (approximately a 3.6-fold difference in spirochete numbers between the WT and mutant after 9 days; Fig. S2). When the bb0219 mutation was complemented by using pOY15, spirochetal growth was restored to the same level as WT 297. To determine whether the mutant had a defect in growth in a mammalian environment, borreliae were cultivated in dialysis membrane chambers (DMCs) implanted into the peritoneal cavities of rats. After 12 days in DMCs, spirochetes of Bb strains 297, the bb0219 mutant, and the complemented strain increased comparably from 103/mL to ≈107/mL. These combined findings suggest that bb0219 is not essential for Bb growth either in vitro or in a surrogate mammalian model system.
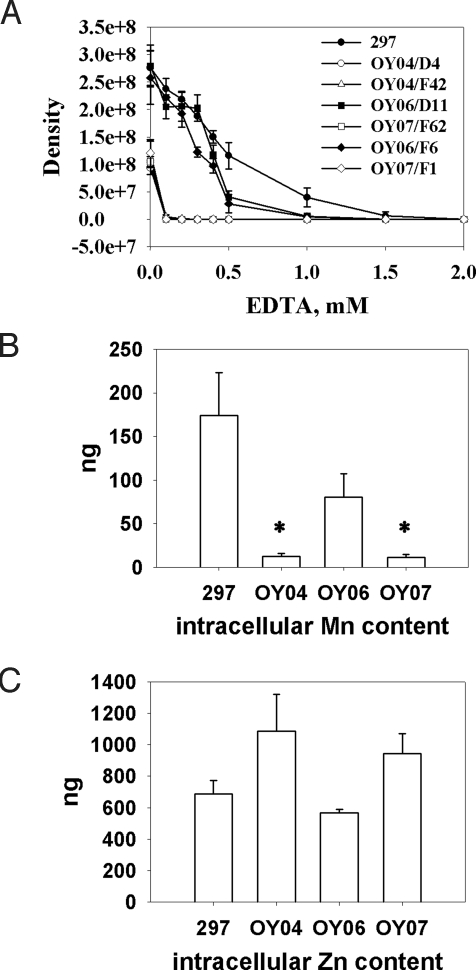
Because bb0219 was postulated to encode a metal transporter, we first examined the response of Bb to metal limitation by comparing the growth of WT 297, bb0219 mutants, and complemented strains under metal-restricted conditions. As shown in Fig. 3A, the growth of the bb0219 mutants (OY04/D4 and OY04/F42) was inhibited when bacteria were cultivated in the presence of EDTA. Growth of the bb0219 mutants was completely inhibited in the presence of 0.1 mM EDTA, whereas WT 297 and the complemented strains (OY06/F6 and OY06/D11) grew well under this condition. This metal-associated phenotype implies that BB0219 is related to metal acquisition by Bb, prompting us to rename bb0219 as bmtA for Borrelia metal transport protein A.
Fig. 3.
Role of metals in spirochete growth. In all experiments, BSK-II medium was inoculated with 1,000 spirochetes per mL. After growth at 37 °C for 9 days, spirochetes were collected and enumerated by darkfield microscopy. Data were derived from 3 independent experiments. Error bars indicate standard deviations, and the asterisk indicates statistical significance using Student's t test (P < 0.05). (A) Metal ions were depleted from BSK-II medium by adding varying amounts of EDTA; bacterial growth under these conditions was monitored. (B and C) Spirochetes from 200 mL of culture were collected, and the intracellular Mn (B) and Zn (C) contents were measured by inductively coupled plasma atomic emission spectrometry. Values are shown as nanograms per 2.5 × 1010 spirochetes of strains WT 297, bb0219 mutant OY04/D4, complemented strain OY06/D11, or mock-complemented strain OY07/F62. For Mn contents in OY04 and OY07, the detection limit (10 ng per 2.5 × 1010 spirochetes) is represented, as values were below this threshold.
To garner more direct evidence for the involvement of BmtA in metal transport, we measured the intracellular metal content of various Bb strains. We hypothesized that if BmtA indeed is a metal transporter, the accumulation of one or more metals that BmtA transports would be impaired in BmtA-deficient mutants. To assess this, borreliae grown in BSK-II medium were harvested and lysed, and the metal contents within the hydrolysates were determined. We found 174 or 81 ng of Mn in 2.5 × 1010 spirochetes of the WT or complemented strain, respectively (Fig. 3B). In contrast, Mn was not detected (below the detection limit of 10 ng) in the same numbers of spirochetes of the bmtA mutant or the mock-complemented strain. Because in silico analyses indicated that BmtA might be a Zn-transporting ZIP protein, we also compared the Zn contents among these isolates. Contrary to our expectations, intracellular Zn accumulation was not reduced in the BmtA-deficient mutants (Fig. 3C). In fact, the BmtA-deficient mutants appeared to accumulate more Zn than the WT or the complemented strains (Fig. 3C). The BmtA-deficient mutants also accumulated magnesium (Mg) at levels comparable to those of WT 297. The combined data indicate that bmtA encodes a protein involved in Mn transport, rather than in Zn or Mg transport, in Bb.
We also investigated Bb's sensitivity to Mn as a surrogate marker of Mn uptake. When spirochetes were grown in BSK-II medium containing exogenously added Mn, WT 297 was substantially more sensitive to Mn toxicity (60% growth inhibition at 50 μM Mn) than the BmtA-deficient mutant (no growth inhibition at 50 μM Mn), as we predicted (Fig. S3). These data were consistent with the conclusion that BmtA is involved in the uptake of Mn by Bb.
BmtA Is Essential for Bb to Establish Mammalian Infection.
To investigate the contribution of bmtA to Bb mammalian infectivity, C3H/HeN mice were infected intradermally with various Bb strains. After 4 weeks, skin, heart, and joint tissues were collected and transferred into fresh BSK-II medium. Cultures were monitored for 4 weeks for spirochete growth. Whereas motile spirochetes were recovered from tissues of all mice inoculated with 104 spirochetes of either the WT or complemented strains (OY06/F6 and OY06/D11), no bacterial growth was observed in any cultures of tissues from mice infected with either 104 or 107 of the bmtA mutants (OY04/D4 and OY04/F42) or mock-complemented strains (OY07/F1 and OY07/F62) (Table 1). These results demonstrate that bmtA is essential for mammalian infectivity by Bb.
Table 1.
Infectivity of B. burgdorferi clones in mice
| Strain, clone | Description | Dose | No. of cultures positive/total no. of specimens examined |
No. of mice infected/total no. of mice | |||
|---|---|---|---|---|---|---|---|
| Heart | Joint | Skin | All sites | ||||
| 297 | WT B. burgdorferi | 104 | 7/7 | 7/7 | 7/7 | 21/21 | 7/7 |
| OY04/D4 | 297, ΔbmtA* | 104 | 0/6 | 0/6 | 0/6 | 0/18 | 0/6 |
| OY04/F42 | 297, ΔbmtA | 104 | 0/6 | 0/6 | 0/6 | 0/18 | 0/6 |
| OY04/D4 | 297, ΔbmtA | 107 | 0/5 | 0/5 | 0/5 | 0/15 | 0/5 |
| OY04/F42 | 297, ΔbmtA | 107 | 0/5 | 0/5 | 0/5 | 0/15 | 0/5 |
| OY06/F6 | OY04/D4 transformed with pOY15 | 104 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 |
| OY06/D11 | OY04/F42 transformed with pOY15 | 104 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 |
| OY07/F1 | OY04/D4 transformed with pJD54 | 104 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 |
| OY07/F62 | OY04/F42 transformed with pJD54 | 104 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 |
| OY07/F1 | OY04/D4 transformed with pJD54 | 107 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 |
| OY07/F62 | OY04/F42 transformed with pJD54 | 107 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 |
*ΔbmtA: bmtA mutant.
BmtA Is Required by Bb to Infect Ticks.
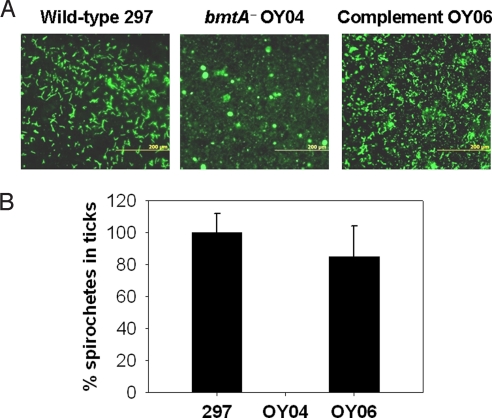
Because the bmtA mutant was unable to infect mice, it was not possible to examine the contribution of BmtA to the entire Bb infectious cycle between ticks and mammals (mice). Therefore, as an alternative means to assess the ability of the bmtA mutant to infect, multiply, and survive in ticks, we used a microinjection technique to introduce spirochetes into sterile nymphs. Microinjected ticks carrying various Bb strains then were allowed to feed on naïve mice, and naturally detached ticks were collected and dissected, and tick tissue contents were subjected to immunofluorescence assays. As shown in Fig. 4, in contrast to WT 297 and the complemented strain (OY06), the bmtA mutant (OY04) was unable to colonize and survive in ticks. Comparable results also were obtained when larval ticks were alternatively infected by using a tick immersion method (30). The combined data demonstrated that bmtA is required by Bb to infect and survive in its arthropod vector.
Fig. 4.
BmtA is required by Bb to infect and survive in ticks. Unfed I. scapularis nymphs were microinjected with various Bb strains and were allowed to feed to repletion on normal mice. After detachment, fed nymphs were dissected, and the tick tissue contents were subjected to immunofluorescence assays with FITC-labeled anti-Bb antibody. (A) Confocal immunofluorescence microscopy; spirochete morphology is evident from tissues of ticks infected with WT 297 or the complemented (OY06) strains. (B) Quantitative assessment (number of borreliae per microscope field) of the data shown in A; WT 297 was considered the 100% value.
BmtA Is Important for Protecting Bb Against Reactive Oxygen Species (ROS).
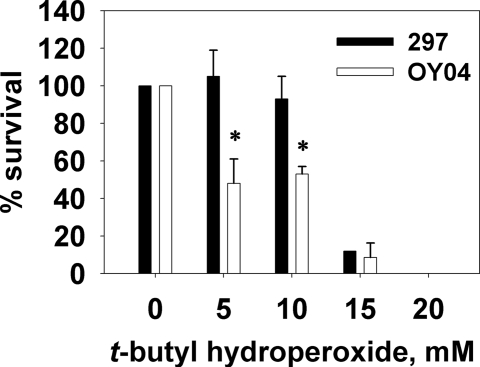
In many bacteria, Fe is involved in antioxidant activities, primarily through the ability of Fe2+ to reduce H2O2 and by functioning as a key cofactor of superoxide dismutase (SodA) (15). Given the facts that (i) Bb does not accumulate iron (23) but does accumulate Mn (Mn accumulation was severely impaired in the bmtA mutant), (ii) Mn is believed to be involved in the oxidative stress response of a number of pathogens (17, 31, 32), and (iii) Bb SodA (BB0153) has been predicted to be Mn-dependent (22, 33), we explored whether the inactivation of bmtA adversely impacted Bb's ability to detoxify intracellular superoxide. When WT 297 was exposed to 5 mM or 10 mM t-butyl hydroperoxide, ≈100% or 93%, respectively, of the cells survived (Fig. 5). In contrast, the bmtA mutant was significantly more sensitive to t-butyl hydroperoxide, with a survival of ≈50% at 5 mM t-butyl hydroperoxide. These data suggest that BmtA and Mn play important roles in the detoxification of ROS (i.e., oxidative stress response).
Fig. 5.
The bmtA mutant is sensitive to treatment with t-butyl hydroperoxide. Bb (WT 297 or bmtA mutant OY04/D4) was grown to a cell density of 1 × 107 cells/mL and then treated with varying amounts of t-butyl hydroperoxide. Averages with standard deviations are shown. The asterisk denotes a significant difference in t-butyl hydroperoxide sensitivity (P < 0.05).
Discussion
Analyses of genomic sequence information have suggested that the utilization of transition metals by Bb may be dramatically different from other bacteria. For example, in contrast to most bacterial pathogens, Bb does not appear to require iron to support its growth (23). Rather, it was reported that Bb accumulated Mn when grown in a serum-free medium (23), but the relevance of this finding to what occurs when Bb is cultivated in more nutrient-rich (e.g., serum-containing) environments, or what Bb encounters in ticks or mammalian hosts, has remained obscure. It additionally has been unknown to what extent Mn may be important for establishing mammalian infection by Bb.
Employing molecular genetics to create BmtA mutants and complemented strains, here we have identified what appears to be a novel Mn transporter in Bb. We derived this conclusion from 2 key observations. First, BmtA has a predicted topology similar to Zn transporters (ZIP proteins) (25); in our analyses, BmtA was predicted to be a cytoplasmic membrane protein containing 8 transmembrane domains (26, 28). Second, our data clearly showed that BmtA is critical for Mn uptake in Bb, at least when Bb is grown in BSK medium. Therefore, it is likely that bmtA encodes a transporter mediating the uptake of Mn across the cytoplasmic membrane of Bb.
Our study is significant in at least two major ways. First, to our knowledge, no metal transporter had thus far been reported in this important human pathogen. Second, 3 classes of Mn transporters have been characterized in prokaryotes, including the natural resistance-associated macrophage protein (Nramp) type Mn transporter, the ATP-binding cassette (ABC) Mn permease, and the P-type ATPase Mn transporter (15, 17, 34). Sequence similarity searching revealed that BmtA has no homology to any of these bacterial Mn transporters. Despite this, we found that the ZIP domain-carrying BmtA protein is involved in the transport of Mn but not Zn. This is particularly striking, given that ZIP domains typically are involved in Zn transport (25). Our study thus not only provides further insights into the physiological functions of bacterial ZIP proteins, but also suggests that BmtA and its homologs comprise a novel mechanism(s) for Mn acquisition.
Because of their similar ionic radius and chelate structures, Mn2+, Fe2+, Mg2+, and Zn2+ are interchangeable in the metal-binding site of many proteins. In this regard, Mn in place of iron has been proposed to be required by Bb (23). Unlike most pathogens that possess overlapping or redundant Mn transporters, Bb probably expresses only one Mn transporter, BmtA, at least when borreliae are grown in vitro. This contention is strongly supported by our data that Mn was not detected in the BmtA mutant. Regarding the fact that the mutant had no substantial growth defect in vitro or in DMCs, we propose that Mn is not an obligate requirement for Bb physiology, although it may facilitate optimal growth of borreliae in vitro. Because Zn may substitute for activities typically served by Fe or Mn, when Mn is not available in the environment, Bb may switch its requirement from Mn to Zn. Consistent with this assertion, our data showed that the bmtA mutant accumulated more Zn than the WT strain, ostensibly through the activation of one or more potential Zn transporter(s). Transcriptional profiling comparisons between WT, bmtA mutants, and complemented strains might shed added light on this possibility. Alternatively, in addition to the import of Mn, BmtA may also be involved in the efflux of Zn, in so much that this dual import–export function has been noted for other bacterial metal transporters (e.g., CorA) (35). Finally, it remains possible that the physiological functions of Mn also may be served by Mg because of their similar structure.
Inactivation of bmtA rendered Bb completely noninfectious for mice and unable to infect and proliferate in ticks, indicating that BmtA is essential for Bb's mammalian infectivity and colonization of its arthropod vector, even though BmtA and Mn are dispensable when Bb is cultivated in vitro. The contribution of Mn transporters and Mn to bacterial pathogenesis has been well established in many other pathogens, including in those where Mn is not an obligate requirement for bacterial growth in vitro (15, 17, 31). Mn typically is not a widely used cofactor in biology and serves as a physiological cofactor for only a handful of enzymes (15, 17). Therefore, if Mn is dispensable when bacteria are cultivated in vitro, why is it important for bacterial virulence? In the case of Bb, one possibility is that BSK medium does not recapitulate the physiological or metal conditions that Bb encounters in either ticks or mammals. However, there are a number of additional observations in our study that may address this question. First, in comparison with WT, the Bb bmtA mutant was sensitive to t-butyl hydroperoxide, suggesting that BmtA (and thus Mn) is probably involved in the detoxification of ROS. Akin to the situation for other bacterial pathogens (17, 31, 32), the most obvious role for BmtA and Mn likely is in the Bb oxidative stress response. For example, in the process of tick feeding or during the process of mammalian host dissemination, BmtA and Mn may be required by Bb to counter superoxide contained in mammalian blood (36, 37). Further, BmtA and Mn may also play a role in defending the spirochete from the initial host immune response, including the extremely deleterious effects of ROS generated during the innate host immune response to Lyme borreliosis (36). Although entirely speculative, borrelial Mn-dependent SodA also may participate in one or more of these events. Finally, in a wide variety of bacterial pathogens, Mn contributes to virulence by regulating the expression of many virulence determinants (15, 17, 31). Similarly, Mn, possibly via the Mn-dependent Fur homolog BosR (23, 33, 37, 38), might play a critical role in controlling Bb virulence. Future microarray studies may shed additional light on to what extent BmtA, and by inference Mn, regulates virulence expression in Bb.
Our study has demonstrated that the Lyme disease spirochete requires BmtA's Mn-associated transport function(s) to maintain its infectious cycle in nature. To date, only relatively few virulence factors, including OspC, DbpB/A, OspA/B, PncA, and BptA, have been identified in Bb, and the functions of most of these proteins remain obscure (4, 5, 7–10). In this regard, our identification of BmtA as a novel virulence factor in Bb provides further insights into molecular mechanisms that contribute to Bb's survival in nature and may lead to new strategies to interrupt the spirochete life cycle. In addition, our findings represent a first step in elucidating transition metal homeostasis in Bb. Our study also prompts a number of salient questions. First, considering the predicted topology of BmtA, we propose that the theoretical 8 transmembrane segments assemble a channel to allow Mn or other ions to pass through the cytoplasmic membrane. However, how the channel is opened or closed, how the metal ions are transported, or whether energy is required remain completely unknown. It also is unknown whether BmtA is involved in the transport of other cations, as the affinities and selectivity of BmtA for Mn and other cations have not yet been fully assessed. Biochemical experiments with recombinant BmtA and, possibly, reconstitution into liposomes may be strategic for addressing some of these questions. Second, what are the functional relationships between the other 5 members of the bmtA operon? Although none of these 5 proteins was predicted to be implicated in metal transport, it remains possible that they indeed contribute to Mn transport. Inactivation of each gene of the operon may shed additional light on this issue. Finally, what are the specific cellular processes in Bb for which Mn is required? In many pathogens, Mn transport is controlled by an Mn-specific transcription factor, MntR, as well as by Fur, PerR, or OxyR (15, 17, 31). Although BosR in Bb was predicted to be Mn-dependent (23), it is not clear whether bmtA is regulated by BosR or responses to the intracellular Mn pool. Examination of bmtA transcriptional control using reporter assays may help to clarify the regulation of bmtA.
Methods
Bacterial Strains and Culture Conditions.
Infectious Bb strain 297 (39) was used as the WT strain throughout this study. Bb was cultured at 37 °C in BSK-II medium (40) supplemented with 6% rabbit serum (Pel-Freeze). To assess the growth of Bb in a mammalian host-adapted state, borreliae were also cultivated within DMCs implanted into the peritoneal cavities of rats, as previously described (41). All animal experiments were approved by the Institutional Animal Care and Use Committee at UT Southwestern Medical Center (Dallas, TX).
Construction of bmtA Mutants and Complemented Strains.
The bmtA mutant strain OY04 was created by allelic exchange in Bb 297 using a suicide vector pOY04. The mutation in bmtA was complemented by transforming a shuttle vector, pOY15, based on the pJD54 plasmid, into the bmtA mutant, generating OY06. In addition, a mock-complemented mutant, OY07, was constructed by transforming pJD54 into Bb OY04. All plasmid constructs were characterized by PCR, restriction digestion, and sequence analysis (SI Methods). Transformation of Bb was performed as previously described (12, 14). Plasmid contents of all Bb strains were determined by PCR using specific primers (4).
Intracellular Metal Contents.
Intracellular metal contents of borreliae were measured as previously described (19). Briefly, bacterial cultures grown to stationary phase were harvested, and the pellets were lysed by using 65% HNO3 (TraceMetal grade; Sigma). The metal contents in the lysates were measured by using inductively coupled plasma atomic emission spectrometry by the Research Analytical Laboratory, University of Minnesota (St. Paul, MN; SI Methods). Three independent tests were performed, and the results were analyzed by using Student's t test.
Sensitivity of Bb to ROS.
This test was performed as previously described, with modifications (36, 37). Briefly, when spirochete growth reached a cell density of 5 × 107 cells/mL, the culture was divided into 5 aliquots and treated with varying concentrations of oxidants (0–20 mM t-butyl hydroperoxide) at 37 °C for 4 h. After incubation, cells were serially diluted in fresh BSK medium in a 96-well plate and incubated for 2 weeks. Percent survivability was calculated as the number of clones recovered from the treated group versus the number of clones recovered from the untreated group.
Bb Infection of Mice.
The infectivity of Bb clones was assessed by using the murine needle-challenge model of Lyme borreliosis (42). C3H/HeN mice (Charles River Laboratories) were infected via intradermal injection with various concentrations of Bb. At 4 weeks after inoculation, skin, heart, and joint tissues were collected and cultured in BSK supplemented with 1× Borrelia antibiotic mixture (Sigma). The outgrowth of spirochetes in these cultures was assessed by using darkfield microscopy.
Artificial Infection of Ticks with Bb.
I. scapularis nymphs or larvae (obtained from the Tick Rearing Facility at Oklahoma State University, Stillwater, OK) were artificially infected with various strains of Bb by using a microinjection technique (11, 43) or an immersion method (30) (see SI Methods). After infection, ticks were fed to repletion on separate naïve C3H/HeN mice. Subsets of ticks were dissected immediately and analyzed by immunofluoresence assay (SI Methods). Spirochetes were stained with the BacTrace FITC-conjugated goat anti-Bb antibody (Kirkegaard and Perry Laboratories) and observed by using an Olympus BX50 fluorescence microscope.
Supplementary Material
Acknowledgments.
We thank Farol Tomson and Eric Hansen for critical reading of the manuscript. This work was supported by National Institutes of Health Public Health Service Grant AI-059062 from the National Institute of Allergy and Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812999106/DCSupplemental.
References
- 1.CDC. Lyme disease–United States, 2003–2005. MMWR Morb Mortal Wkly Rep. 2007;56:573–576. [PubMed] [Google Scholar]
- 2.Burgdorfer W, et al. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 4.Blevins JS, Hagman KE, Norgard MV. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 2008;8:82. doi: 10.1186/1471-2180-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimm D, et al. Outer-surface protein C of the Lyme disease spirochete: A protein induced in ticks for infection of mammals. Proc Natl Acad Sci USA. 2004;101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelakanta G, et al. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog. 2007;3:e33. doi: 10.1371/journal.ppat.0030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purser JE, et al. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 8.Revel AT, et al. bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc Natl Acad Sci USA. 2005;102:6972–6977. doi: 10.1073/pnas.0502565102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Xu Q, McShan K, Liang FT. Both decorin-binding proteins A and B are critical for overall virulence of Borrelia burgdorferi. Infect Immun. 2008;76:1239–1246. doi: 10.1128/IAI.00897-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boardman BK, et al. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun. 2008;76:3844–3853. doi: 10.1128/IAI.00467-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubner A, et al. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci USA. 2001;98:12724–12729. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouyang Z, Blevins JS, Norgard MV. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology. 2008;154:2641–2658. doi: 10.1099/mic.0.2008/019992-0. [DOI] [PubMed] [Google Scholar]
- 14.Yang XF, Alani SM, Norgard MV. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2003;100:11001–11006. doi: 10.1073/pnas.1834315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakubovics NS, Jenkinson HF. Out of the iron age: New insights into the critical role of manganese homeostasis in bacteria. Microbiology. 2001;147:1709–1718. doi: 10.1099/00221287-147-7-1709. [DOI] [PubMed] [Google Scholar]
- 16.Hantke K. Bacterial zinc transporters and regulators. Biometals. 2001;14:239–249. doi: 10.1023/a:1012984713391. [DOI] [PubMed] [Google Scholar]
- 17.Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- 18.Wandersman C, Delepelaire P. Bacterial iron sources: From siderophores to hemophores. Annu Rev Microbiol. 2004;58:611–647. doi: 10.1146/annurev.micro.58.030603.123811. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang Z, Isaacson R. Identification and characterization of a novel ABC iron transport system, fit, in Escherichia coli. Infect Immun. 2006;74:6949–6956. doi: 10.1128/IAI.00866-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patzer SI, Hantke K. Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J Bacteriol. 2001;183:4806–4813. doi: 10.1128/JB.183.16.4806-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grass G, Wong MD, Rosen BP, Smith RL, Rensing C. ZupT is a Zn(II) uptake system in Escherichia coli. J Bacteriol. 2002;184:864–866. doi: 10.1128/JB.184.3.864-866.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser CM, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 23.Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- 24.Eng BH, Guerinot ML, Eide D, Saier MH., Jr Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J Membr Biol. 1998;166:1–7. doi: 10.1007/s002329900442. [DOI] [PubMed] [Google Scholar]
- 25.Guerinot ML. The ZIP family of metal transporters. Biochim Biophys Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 26.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 27.McGowan SJ, Gorham HC, Hodgson DA. Light-induced carotenogenesis in Myxococcus xanthus: DNA sequence analysis of the carR region. Mol Microbiol. 1993;10:713–735. doi: 10.1111/j.1365-2958.1993.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 28.Gardy JL, et al. PSORTb v. 2.0: Expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics. 2005;21:617–623. doi: 10.1093/bioinformatics/bti057. [DOI] [PubMed] [Google Scholar]
- 29.Rosa PA, Tilly K, Stewart PE. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev. 2005;3:129–143. doi: 10.1038/nrmicro1086. [DOI] [PubMed] [Google Scholar]
- 30.Policastro PF, Schwan TG. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J Med Entomol. 2003;40:364–370. doi: 10.1603/0022-2585-40.3.364. [DOI] [PubMed] [Google Scholar]
- 31.Horsburgh MJ, et al. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol. 2002;44:1269–1286. doi: 10.1046/j.1365-2958.2002.02944.x. [DOI] [PubMed] [Google Scholar]
- 32.Wu HJ, et al. PerR controls Mn-dependent resistance to oxidative stress in Neisseria gonorrhoeae. Mol Microbiol. 2006;60:401–416. doi: 10.1111/j.1365-2958.2006.05079.x. [DOI] [PubMed] [Google Scholar]
- 33.Boylan JA, Posey JE, Gherardini FC. Borrelia oxidative stress response regulator, BosR: A distinctive Zn-dependent transcriptional activator. Proc Natl Acad Sci USA. 2003;100:11684–11689. doi: 10.1073/pnas.2032956100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archibald F. Manganese: Its acquisition by and function in the lactic acid bacteria. Crit Rev Microbiol. 1986;13:63–109. doi: 10.3109/10408418609108735. [DOI] [PubMed] [Google Scholar]
- 35.Lunin VV, et al. Crystal structure of the CorA Mg2+ transporter. Nature. 2006;440:833–837. doi: 10.1038/nature04642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boylan JA, Lawrence KA, Downey JS, Gherardini FC. Borrelia burgdorferi membranes are the primary targets of reactive oxygen species. Mol Microbiol. 2008;68:786–799. doi: 10.1111/j.1365-2958.2008.06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seshu J, et al. A conservative amino acid change alters the function of BosR, the redox regulator of Borrelia burgdorferi. Mol Microbiol. 2004;54:1352–1363. doi: 10.1111/j.1365-2958.2004.04352.x. [DOI] [PubMed] [Google Scholar]
- 38.Katona LI, Tokarz R, Kuhlow CJ, Benach J, Benach JL. The fur homologue in Borrelia burgdorferi. J Bacteriol. 2004;186:6443–6456. doi: 10.1128/JB.186.19.6443-6456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes CA, Kodner CB, Johnson RC. DNA analysis of Borrelia burgdorferi NCH-1, the first northcentral U.S. human Lyme disease isolate. J Clin Microbiol. 1992;30:698–703. doi: 10.1128/jcm.30.3.698-703.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollack RJ, Telford SR, III, Spielman A. Standardization of medium for culturing Lyme disease spirochetes. J Clin Microbiol. 1993;31:1251–1255. doi: 10.1128/jcm.31.5.1251-1255.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 43.Narasimhan S, et al. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc Natl Acad Sci USA. 2004;101:1141–1146. doi: 10.1073/pnas.0307669100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.