Abstract
Corpora amylacea (CA) are a frequent microscopic finding in radical prostatectomy specimens from men undergoing treatment for prostate cancer. Although often observed histologically to be associated with inflammation, the contribution of CA to prostatitis-related symptoms of unknown etiology or to prostate carcinogenesis remains unclear. Prostatic calculi (PC), which potentially represent calcified forms of CA, are less common but can cause urological disease including urinary retention and prostatitis. We conducted a comprehensive compositional analysis of CA/PC to gain insight into their biogenesis. Infrared spectroscopy analysis of calculi collected from 23 patients confirmed a prevalence of calcium phosphate in the form of hydroxyapatite. This result sets PC apart from most urinary stones, which largely are composed of calcium oxalate. Tandem mass spectrometry-based proteomic analysis of CA/PC revealed that lactoferrin is the predominant protein component, a result that was confirmed by Western blot analysis. Other proteins identified, including calprotectin, myeloperoxidase, and α-defensins, are proteins contained in neutrophil granules. Immunohistochemistry (IHC) suggested the source of lactoferrin to be prostate-infiltrating neutrophils as well as inflamed prostate epithelium; however, IHC for calprotectin suggested prostate-infiltrating neutrophils as a major source of the protein, because it was absent from other prostate compartments. This study represents a definitive analysis of the protein composition of prostatic CA and calculi and suggests that acute inflammation has a role in their biogenesis—an intriguing finding, given the prevalence of CA in prostatectomy specimens and the hypothesized role for inflammation in prostate carcinogenesis.
Keywords: acute inflammation, lactoferrin, neutrophil, hydroxyapatite, prostatic calculi
Prostatic corpora amylacea (CA) are tiny, seemingly laminated bodies that are a frequent histologic finding, numbering from few to several thousands, in the prostate of the adult male (1). Prostatic calculi (PC) are calcified “stones” that also form in the human prostate. Although PC can be microscopic (i.e., < 200 μM in diameter), they generally are larger than corpora amylacea and tend to vary in size from millimeters to more than several centimeters in diameter in rare cases (2). CA and PC are assumed to be related and perhaps to represent different stages in the same formation process (i.e., PC represent calcified forms of CA); however, evidence for this supposition is based solely on morphology (3). The incidence of CA/PC is believed to begin after puberty and increase with age, although cases of symptomatic calculi have been reported in boys as young as 4 years old (4). Both CA and calculi occur in numerous other locations throughout the human body and are associated with disease pathologies, including several forms of cancer [supporting information (SI) Table S1]. Although PC typically are thought to be asymptomatic, sequelae associated with larger PC in the prostate include urinary retention and prostatitis/chronic pelvic pain syndrome (CPPS) (5–7). (The terms prostatitis and “CPPS” are used here according to the National Institutes of Health consensus classification for category I–IV prostatitis syndromes: I) acute bacterial prostatitis; II) chronic bacterial prostatitis; III) chronic prostatitis/CPPS; IV) asymptomatic inflammatory prostatitis.) CA often have been shown histologically to be associated with focal acute and chronic inflammation, epithelial trauma, and gland occlusion (8); however the potential involvement of CA in causing prostatitis/CPPS of unknown etiology remains largely undetermined. Inflammation and epithelial damage caused by prostatic CA also have been proposed to contribute to prostate carcinogenesis; however, studies have yielded disparate results (9–12). Many of these studies are compromised by a biased method of detection (primarily by x-ray), which favors the identification of only large calculi, and by failure to evaluate the presence of CA in the peripheral prostate where most cancers arise (i.e., examining only samples collected from the transition zone of the prostate during surgical treatment for benign prostatic hyperplasia). Intriguingly, ACI/Seg rats, which are among the few known rodent models of spontaneous prostate cancer, also have abundant CA in the prostate before the development of adenocarcinoma (13).
The mechanism of prostatic CA/PC formation is unknown. Proposed contributing factors include prostatic infections (14, 15), urinary retention or reflux into the prostate (16), penetration of spermatozoa into prostatic tissue (17), and desquamation of prostatic epithelium (3). We hypothesized that the determination of the crystalline and protein composition of prostatic CA and PC would give important insight into their biogenesis. The results of previous studies of the crystalline composition of PC using both x-ray diffraction and scanning electron microscopy are varied and indicate a prevalence of either i) carbonate apatite and calcium phosphate in the form of whitlockite (18, 19) or ii) calcium phosphate in the form of hydroxyapatite (20–22). These studies have focused almost exclusively on PC, and in the present study we sought to determine if CA also contain a crystalline component. Likewise, previous studies aiming to identify protein components of prostatic CA or PC have yielded disparate results. These studies have focused almost exclusively on CA, and methods of protein identification generally have involved either ultrastructural analysis with electron microscopy, x-ray diffraction, direct immunohistochemical staining of prostatic CA in formalin-fixed paraffin-embedded tissues, or Western blot analysis of extracted proteins with selected antibodies. Based on these studies, the most commonly accepted protein components include i) keratins and/or protein remnants of desquamated epithelial cells (3, 23), ii) components of prostatic secretions (24), or iii) byproducts of urine reflux and/or previously described components of amyloid (16, 25, 26). In the present study, we used infrared spectroscopy to identify the crystalline composition and high-performance liquid chromatography combined with tandem mass spectrometry (LC/MS/MS) for a comprehensive analysis of the protein composition of prostatic CA and PC.
Results
The Crystalline Component of Prostatic Calculi is Predominantly Calcium Phosphate in the Form of Hydroxyapatite.
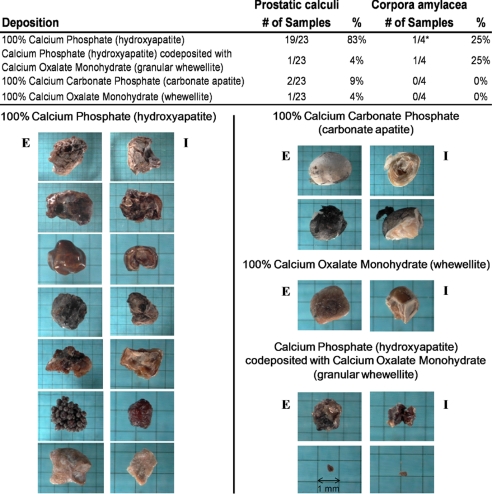
We collected PC from 23 radical prostatectomy specimens for stone analysis with infrared spectroscopy. These analyses revealed that the crystalline component of PC is predominantly calcium phosphate in the form of hydroxyapatite (Fig. 1). In all, 19 of the 23 PC samples (83%) were found to be composed entirely of hydroxyapatite, and 20 of the 23 samples (87%) contained hydroxyapatite. PC also were composed of 100% carbonate apatite in 2 of the 23 cases (9%), 100% whewellite in 1 of the 23 cases (4%), and a combination of hydroxyapatite and granular whewellite in 1 of the 23 cases (4%). The stones recovered from each prostate varied widely in size, shape, and color, even when stones were composed of identical crystalline components (Fig. 1). Four additional specimens that were comprised entirely of “concretions,” which were less than 1 mm in diameter and were determined after microscopic examination to represent CA rather than calculi (Fig. S1), also were analyzed with infrared spectroscopy. Interestingly, 2 of the CA samples contained no detectable crystalline component, whereas the other 2 were found to contain either 100% hydroxyapatite or a combination of hydroxyapatite and granular whewellite.
Fig. 1.
Crystalline composition of prostatic calculi and corpora amylacea. (Top) Results of stone analysis by infrared spectroscopy. *Two CA samples contained no detectable mineral deposition. (Bottom) Variation in size, color, and shape among different crystalline compositions. E = exterior of stone; I = interior of stone.
Proteomic Analysis of Prostatic Corpora Amylacea and Calculi Reveals Evidence of an Acute Inflammatory Process.
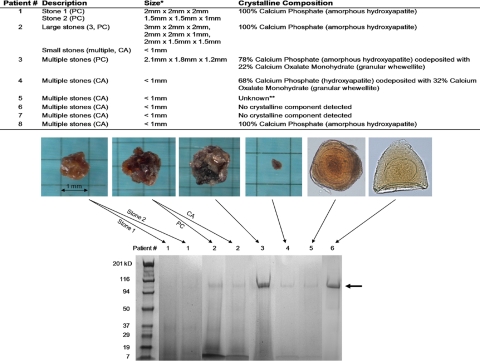
High-performance LC/MS/MS is a method that allows comprehensive identification of proteins in complex mixtures. We sought to use this “shotgun proteomics” approach to analyze total proteins extracted from prostatic CA and PC. After testing several extraction methods, we found that protein could be extracted readily from CA and PC by first crushing with a Dounce homogenizer and then heating in 1M Tris pH 8.0 at 70 °C for 30 min. When these extracts were analyzed by standard SDS/PAGE, a predominant band at ≈100 kDa and several fainter bands at lower molecular weights generally were observed (Fig. 2).
Fig. 2.
Protein extraction from prostatic corpora amylacea and calculi. (Top) Description and crystalline composition of samples subject to protein extraction. The sample set included 2 separate PC from 1 patient (“stone 1” and “stone 2”) and PC versus CA from a separate patient. In all, the protein composition of a total of 10 samples from 8 patients was determined. *Average size if multiple stones. **Sample not available for chemical stone analysis. (Bottom) Examples of SDS/PAGE of extracted proteins on 4%–20% gradient gels visualized by Coomassie blue stain. Samples from separate gels have been grouped together. Arrow indicates predominant protein band observed at ≈100 kDa.
By LC/MS/MS, the most prevalent proteins found (in 100% of patients) were identified as lactoferrin, myeloperoxidase, and S100 calcium-binding proteins A8 and A9 (which complex to form human calprotectin) (Table 1 and Table S2). All 3 of these proteins, as well as several others identified by the LC/MS/MS analysis, including α-defensins, secretory leukocyte peptidase inhibitor, lipocalin 2 (NGAL), β2-microglobulin, azurocidin 1, bactericidal/permeability-increasing protein, cathepsin G, lysozyme C, orosomucoid 1, and elastase 2, are proteins contained in neutrophil granules (27). The remaining proteins identified include components of human prostatic fluid such as prostate-specific antigen and prostatic acid phosphatase (PAP) as well as additional proteins involved in immunity (Ig heavy and light chains, RNases, and complement system components). A comparison between 2 separate PC from Patient 1 and PC versus CA from Patient 2 indicated that protein composition generally was consistent between different CA/PC from the same patient, especially for peptides with higher ions scores and multiple peptide hits (Table S3). Furthermore, we found that the protein extract of the 2 CA samples that did not contain any detectable crystalline component displayed the characteristic ≈100-kDa band upon SDS/PAGE analysis and yielded a LC/MS/MS profile similar to that of calcified samples, consistent with the idea that noncalcified CA are related to, or in fact are precursors of, PC.
Table 1.
Selecteda top peptide hits in total protein analysis of extracted CA/PC proteins as determined by LC/MS/MS
| Identified Protein | Ions Scoreb | Frequency |
|---|---|---|
| Lactoferrinc | 3519 | 8/8 (100%) |
| Myeloperoxidasec | 1418 | 8/8 |
| S100 calcium-binding protein A9c | 592 | 8/8 |
| S100 calcium-binding protein A8c | 223 | 8/8 |
| Prostate specific antigen | 519 | 6/8 (75%) |
| Serum albumin | 436 | 6/8 |
| Beta-hemoglobin | 1041 | 5/8 (63%) |
| Prostatic acid phosphatase | 313 | 5/8 |
| Defensin HNP2 or HNP3 (alpha)c | 187 | 5/8 |
| N-acylsphingosine amidohydrolase 1 | 182 | 5/8 |
| Secretory leukocyte peptidase inhibitor (SLPI)c | 74 | 5/8 |
| Lipocalin 2c | 501 | 4/8 (50%) |
| Beta-2 microglobulinc | 305 | 4/8 |
| Immunoglobulin heavy chain | 267 | 4/8 |
| Azurocidin 1c | 231 | 4/8 |
| Beta-microseminoprotein | 161 | 4/8 |
| Immunoglobulin kappa light chain | 223 | 3/8 (38%) |
| Bactericidal/permeability-increasing (BPI)c | 183 | 3/8 |
| Ribonuclease, RNase A family, 3 | 149 | 3/8 |
| Cathepsin Gc | 124 | 3/8 |
| Lysozyme Cc | 119 | 3/8 |
| Complement component C3 | 89 | 3/8 |
| Orosomucoid 1c | 340 | 2/8 (25%) |
| CD177c | 181 | 2/8 |
| Elastase 2, neutrophilc | 91 | 2/8 |
aSee Table S2 for complete list.
bFor multiple matches, the highest score observed in a single case is shown.
cProteins associated with and/or found in neutrophil granules.
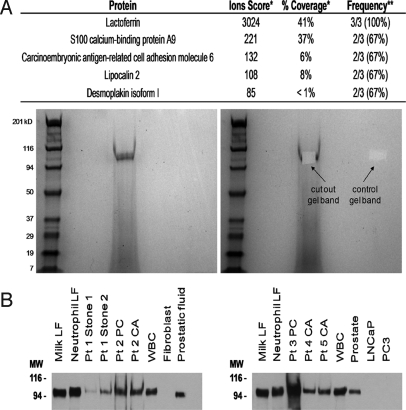
Identification of Predominant Protein Band as Lactoferrin.
To confirm the identity of the protein(s) that comprise the predominant band consistently observed in the protein extract of prostatic CA and PC, we excised the gel band observed at ≈100 kDa after SDS/PAGE for Patient 2 (PC), Patient 3 (PC), and Patient 4 (CA) (Fig. 2) for in-gel protein analysis by LC/MS/MS. The identification of the best match for peptide present in these excised gel bands was lactoferrin, which was identified in all 3 of the samples (Fig. 3A). Peptide hits also were observed in 2 of the 3 samples for S100 calcium-binding protein A9, carcinoembryonic antigen-related cell adhesion molecule 6, lipocalin 2, and desmoplakin; however, the ions scores and the number of peptide hits for these proteins were far lower than for lactoferrin. Whether these proteins are contained within the ≈100-kDa band along with lactoferrin, or, more likely, are caused by the minor “streaking” of the protein samples during electrophoresis could not be determined. To confirm further that the predominant band observed in the protein extract from prostatic CA and PC consisted of human lactoferrin, we performed Western blot analysis. Samples analyzed included human milk lactoferrin, human neutrophil lactoferrin, and peripheral blood white blood cell lysate (all as positive controls) and human skin fibroblast lysate and LNCaP and PC3 prostate cancer cell line lysates (all as negative controls, because lactoferrin previously has been shown to be downregulated significantly in prostate cancer) (28, 29). Samples also included a human prostate tissue lysate from tissue biopsy samples taken from a radical prostatectomy specimen, prostatic fluid collected from a prostatectomy specimen, and 7 of the CA/PC protein extract samples analyzed by LC/MS/MS. The results of Western blot analysis showed positive staining for lactoferrin in all the CA/PC protein extracts at ≈100 kDa (Fig. 3B), further confirming the presence of lactoferrin in the CA/PC extracts and localizing it to the predominant band observed by SDS/PAGE.
Fig. 3.
Identification of predominant protein band as lactoferrin. (A, Top) Results of in-gel peptide analysis by LC/MS/MS. (Bottom) Example of protein band excised from SDS/PAGE gel. *For multiple matches, the highest score observed in a single case is shown. **Peptide hit(s) also were observed in a single case for the following proteins: α-1-antichymotrypsin, TIMP-1, myeloperoxidase, uromodulin, plakophilin 1 isoform 1b, annexin A2, ubiquitin, ARG1, caspase 14 precursor, stratifin, and catalase. (B) Western blot analysis for human lactoferrin. Membranes were probed with rabbit anti-human lactoferrin (Sigma) at a dilution of 1:5000. Milk LF = human milk lactoferrin (0.5 μg), Neutrophil LF = human neutrophil lactoferrin (0.5 μg), WBC = human peripheral blood white blood cell lysate (5 μg), Fibroblast = human skin fibroblast lysate (5 μg), Prostatic fluid (5 μg), Prostate = human prostate tissue lysate (5 μg), LNCaP lysate (5 μg), PC3 lysate (5 μg).
Association Between Corpora Amylacea and Prostatic Inflammation.
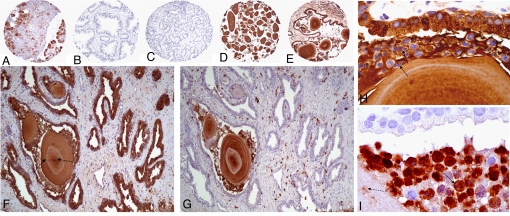
IHC on prostate tissue sections revealed that lactoferrin is present in prostate-infiltrating leukocytes and, as has been reported previously, in prostate epithelium in areas of inflamed prostate and areas of atrophy (Fig. 4, E and F) (30). Likewise, as has been reported previously, prostate cancer was found to be negative for lactoferrin production (Fig. 4C) (29). We observed that CA stained very strongly with the lactoferrin and calprotectin antibodies. Although this observation is subject to potential artifacts because various antibodies seem to bind nonspecifically to CA in the prostate (A.M. De Marzo, unpublished observation), the very strong intensity of staining, as well as the “ring” structures that were negative for staining, are a unique observation (Fig. 4 D–I), and, together with the results of LC/MS/MS and Western blot analyses, indicate that the staining indeed is likely to be authentic lactoferrin/calprotectin staining. IHC for calprotectin in prostate tissue sections showed positive staining predominantly in prostate-infiltrating leukocytes and in CA (Fig. 4, G and I). This finding, together with the identification of several other proteins contained in neutrophil granules (myeloperoxidase and α-defensins, among others), indicates that at least some fraction of the proteins found in prostatic CA are derived from neutrophils, as opposed to prostate epithelial secretions.
Fig. 4.
IHC for lactoferrin and calprotectin in human prostate tissues. Tissue sections were probed with rabbit anti-human lactoferrin at 1:2000 (A–F and H) or mouse anti-human calprotectin (Dako) at 1:300 (G and I). (A) Human salivary gland tissue (lactoferrin positive control). (B) Normal human prostate. (C) Prostate cancer. (D) CA. (E) Inflamed prostate tissue with CA inside atrophic gland. (F) Positive lactoferrin staining of atrophic prostate epithelium, acute inflammatory cells, and CA. (G) Calprotectin staining of section adjacent to (F) showing positive staining of infiltrating leukocytes and CA only. CA stain was positive for both lactoferrin (D–F) and calprotectin (G) with areas of dense staining (dashed arrow in F) and concentric rings that do not stain positive (solid arrow in F). (H) Example of epithelium, neutrophils (arrows, as evidenced by multilobed nuclei), and CA staining positive for lactoferrin. (I) Section adjacent to (H) showing negative epithelium, focal positive calprotectin staining of CA (dashed arrow) and strong calprotectin staining of neutrophils (solid arrow).
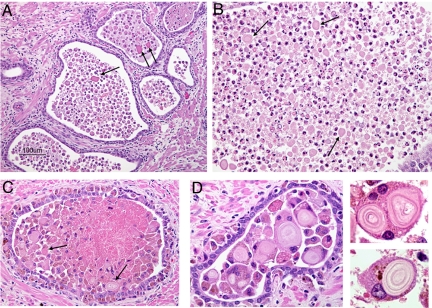
Because our data indicate that acute inflammation probably contributes to CA formation, we decided to examine radical prostatectomy specimens that contain inflammation for further evidence of this process. We observed that areas of acutely inflamed prostate, and specifically regions where gland lumens are infiltrated densely with neutrophils and/or macrophages, also can contain very tiny laminated bodies (< 100 μm) that probably represent very early forms of CA (Fig. 5). Intriguingly, we saw that these tiny corpora seem to be engulfed by macrophages and/or multinucleated giant cells in some instances (Fig. 5D). We do not know if this engulfment represents an immune response to CA or whether engulfment may contribute to CA enlargement.
Fig. 5.
Presence of CA within acutely inflamed prostate glands. (A) Mixed neutrophil and macrophage infiltrates and CA (arrows) within glands and stromal lymphocytes surrounding glands. (B) Dense population of primarily neutrophils and CA (arrows) within prostate gland. (C) Dense population of primarily macrophages and CA (arrows) within prostate gland. (D) Examples of engulfment of CA by macrophages and multinucleated giant cells.
Discussion
Whether the formation of CA and PC can be considered a benign process associated with aging or whether the inflammation, epithelial damage, and glandular occlusion observed histologically to be associated with prostatic CA/PC (8) contribute to symptomatic prostatitis/CPPS of unknown etiology or to prostate carcinogenesis remains largely undetermined. In the present study we have confirmed that the crystalline component of PC is predominantly calcium phosphate in the form of hydroxyapatite. We find this result to be intriguing because, unlike calcium oxalate calcification which is prevalent in most urinary stones (31), classical hydroxyapatite calcification in the context of bone formation requires specific protein(s) (e.g., collagen) along with “matrix vesicles” to mineralize. Furthermore, alkaline phosphatase also is required for the cleavage of phosphates from external substrates in the matrix mineralization process. In this respect, it is of interest that we detected PAP in most of the CA/PC protein samples analyzed by mass spectrometry. Whether this enzyme plays a role in the hydroxyapatite calcification of CA is yet to be determined, but an involvement of PAP could explain the prostate specificity of these concretions.
Although the crystalline component of prostatic CA/PC differs from that most commonly observed in urinary stones, several surprising correlations exist in the protein composition. For example, calprotectin has been shown previously to be a component of the organic matrix of calcium oxalate urinary stones (32–34). Calprotectin is a calcium ion-binding protein that plays a role in inflammatory responses and in the regulation of cell cycle progression and differentiation. Studies have shown that high concentrations of calcium can induce calprotectin aggregation and serve as a potential mechanism for amyloid formation (35). Conversely, studies also have indicated that calprotectin may serve as an inhibitor of stone formation (36). Interestingly, S100-binding proteins were identified in CA in the human brain (37). S100 calcium-binding proteins A8 and A9 also have been reported previously to be up-regulated in prostate intraepithelial neoplasia and carcinoma (38). We did not confirm these findings when we performed IHC for calprotectin. On the contrary, we found that calprotectin staining was restricted to infiltrating immune cells and areas of inflamed prostate. Our results could explain why serum and urinary levels of MRP-14 (S100 calcium-binding protein A9) recently were shown to be ineffective in discriminating between prostate cancer cases and controls (39). As with prostate-specific antigen (a widely used serum marker for prostate cancer), false-positive results may occur when the prostate is inflamed but contains no cancer.
In striking correlation to the present study, mass spectrometry analysis of proteins extracted from calcium oxalate urinary stones identified proteins that we identified in prostatic CA and PC, including calprotectin, neutrophil elastase 2, cathepsin G, azurocidin, ribonuclease 3 (eosinophil cationic protein), lysozyme C, and α-defensin HNP3 (32). Lactoferrin also was identified in a similarly conducted study of urinary stones but only as a low-abundance protein (33). Lactoferrin also has been implicated in pancreatic stone formation (40) and is thought to play an important role in pancreatic inflammation (or chronic pancreatitis) during this process.
Clearly, with such a preponderance of acute inflammation-related proteins identified in urinary stones and now in prostatic CA/PC, it is reasonable to propose that acute inflammation may play a role in the formation of these stones. Whether these proteins induce stone formation or are incorporated into the stone as a result of inflammation caused by the stone is unknown. Our finding that neutrophil-related proteins are contained in all forms of CA and PC, including noncalcified CA, which we predict are the earliest stage in a continuous formation process, strongly argues that these proteins contribute directly to stone formation. Cells involved in innate immunity (including macrophages, neutrophils, and mast cells) have been identified as contributing factors in inflammation-associated malignancy (41, 42). Both acute and chronic inflammation of unknown etiology is observed commonly in the prostates of prostate cancer patients, and a similar role for inflammation in the initiation and/or progression of prostate cancer has been proposed (8, 43). Our discovery that CA and PC in the human prostate contain multiple proteins involved in acute inflammatory pathways raises the intriguing possibility that these tiny concretions may, in fact, be indicative of past inflammatory events in the prostate. Along these lines, 2 of the most prevalent proteins identified in this study, lactoferrin and calprotectin, are critical in defense against bacterial infections and have been implicated in severe inflammatory conditions such as inflammatory bowel disease (where fecal levels of lactoferrin and calprotectin are, in fact, diagnostic of the disease) and in microabscesses (44, 45). Interestingly, several studies have correlated prostatic CA/PC with bacterial infections, and multiple groups have succeeded in culturing bacteria from PC (7, 9, 14, 15). Species cultured include Escherichia coli and Pseudomonas spp., species that have been identified in the prostates of cancer patients via 16S rDNA analysis and have been suggested as possibly playing a role in prostatic inflammation and/or carcinogenesis (46). Another indirect indication that prostatic CA may play a role in the development of prostate cancer is the finding that frequency of ejaculation is inversely related to prostate cancer risk (47, 48). Higher ejaculation frequency may result in “clearance” of prostatic CA before they can grow to a size that causes glandular obstruction or inflammation. An important follow-up study would be to determine if the number of CA in the prostate correlates with the risk of prostate cancer or with ejaculation frequency.
The requirement for a protein “nidus,” or organic matrix, is well described for calcification of stones in the urinary tract. This requirement is analogous to the requirement for an organic matrix (e.g., collagen fibrils) for bone mineralization by hydroxyapatite. CA may begin as protein aggregates of lactoferrin and/or calprotectin, both of which have been shown to aggregate or to form amyloid in vitro (35, 49), perhaps as a result of acute inflammation in the prostate. This possibility is supported further by the observation of lactoferrin secretion specifically in inflamed prostatic epithelium. Also, prostate-infiltrating Th17 cells have been shown to be present in cancer patients, and a primary role of IL-17 is in neutrophil recruitment (50). Many conditions associated with dense neutrophil infiltration and/or acute inflammation (ulcers, abscesses, fistulae) are not known to contain CA. Peptide fragments of PAP recently were shown to be capable of forming amyloid fibrils both in vitro and in vivo (51). These fibrils also were shown to increase the infectivity of HIV drastically. If PAP is involved in CA formation, this involvement might help explain the high prevalence of CA in the prostate (i.e., where PAP is produced almost exclusively), as opposed to other organs that suffer relapsing bouts of acute inflammation. In addition, other proteins identified, including β2-microglobulin and α-1-antichymotrypsin, also have been implicated in amyloid formation. The formation of CA may occlude prostatic ducts and lead to infection caused by stasis of prostatic secretions. The resulting inflammation could result in the deposition of calcium salts (e.g., calcium phosphate) that calcify CA. Additionally, many of the proteins we identified in CA and PC are known calcium-binding proteins (e.g., S100 calcium-binding proteins), and calcium binding may play an important role in the calcification process. The calcification of CA may occur very early in the formation process, as evidenced by the detection of calcium phosphate in samples less than 1 mm in diameter.
In the present study, we have confirmed a prevalence of calcium phosphate in the form of hydroxyapatite in PC. We also have conducted the most comprehensive analysis to date of the protein components of prostatic CA and calculi, showing that the most prevalent protein is lactoferrin and that several other proteins contained in CA and PC also are components of neutrophil granules. The results of this study provide intriguing evidence for a role for acute inflammation in the process of CA/PC formation in the prostate, an observation that may give important insight into the frequency of asymptomatic acute inflammation in the prostate and a potential contributing factor to prostate carcinogenesis.
Materials and Methods
Patient Population and Clinical Specimens.
All specimens were obtained under a protocol approved by the Johns Hopkins Medicine Institutional Review Board. CA (defined in this study as laminated bodies with the microscopic appearance of CA without calcification and a size of < 1 mm in diameter) and calculi were collected from radical prostatectomy specimens from patients undergoing treatment for adenocarcinoma of the prostate at the Johns Hopkins Hospital in Baltimore, MD as described in SI Text.
Stone Analysis.
Prostatic CA and PC from 27 patients were submitted for stone analysis by modern infrared spectroscopy at Beck Analytical Services (Indianapolis, IN). The “stones” varied in size from < 1 mm to 8.9 mm in diameter. In all but 3 cases, multiple stones from each patient were submitted.
Protein Analysis.
Protein could be extracted from all the samples tested by first crushing with a Dounce homogenizer and then heating the resulting suspension in 100 μl of 1M Tris pH 8.0 at 70 °C for 30 min. All samples submitted for mass spectrometry analysis were treated in this manner. We found that after any kind of treatment, a proportion of the sample still consisted of a fine “silt-like” material. The samples were centrifuged at 16,000 × g for 2 min to pellet the silt, and 30 μl of the supernatant was used for protein visualization by SDS/PAGE using Pierce Precise 4–20% polyacrylamide gradient gels (Pierce) followed by Coomassie staining (SimplyBlue SafeStain, Invitrogen). Gel bands excised for in-gel LC/MS/MS analysis were taken from these SDS/PAGE gels as described in SI Text.
For total peptide analysis, the silt was resuspended in the remaining sample, and 30 μl was removed for mass spectrometry analysis. Both the supernatant and the “silt” from this 30 μl were submitted for analysis by LC/MS/MS. Peptides were identified in the “silt” samples; generally, however, a greater number of peptides were identified in the supernatant.
LC/MS/MS analyses were performed at the Mass Spectrometry Core Facility at the Johns Hopkins Hospital (Baltimore, MD), as described in SI Text.
Western Blot Analysis and Immunohistochemistry.
The remaining protein extract from the prostatic CA/PC samples was used for Western blot analysis for human lactoferrin. After traditional SDS/PAGE using Pierce Precise 4%–20% polyacrylamide gradient gels (Pierce) followed by electrophoretic transfer to Hybond-ECL nitrocellulose membranes (Amersham Biosciences), the resulting membranes were blocked for 30 min at room temperature in 5% bovine milk. After blocking, the membranes were probed with polyclonal rabbit anti-human lactoferrin antibody (Sigma L3262) diluted at 1:5000 overnight at 4 °C. The membranes were washed in 0.1% Tween Buffered Saline (TBS) and probed with HRP-conjugated goat anti-rabbit secondary antibody (Pierce) at a 1:5000 dilution overnight at 4 °C. The Western blots were developed using ECL Detection Reagent (Pierce). Positive controls for Western blot analyses were prepared as described in SI Text.
IHC was performed with the PowerVision IHC detection system (ImmunoVision Technologies, Co.) as described in SI Text.
Supplementary Material
Acknowledgments.
We thank Jurga Sauvageot and Marta Gielzak for assistance with sample collection. We also thank Dr. Robert Cole of the Johns Hopkins Mass Spectrometry Core Facility, Dr. Donald S. Coffey, and Dr. Patrick C. Walsh for assistance and helpful discussion. The support of WT Gerrard, MA Duhon, and John and Jennifer Chalsty to W.B.I. is gratefully acknowledged. This work was funded by the National Institutes of Health-National Institute of Diabetes and Digestive and Kidney Diseases Grant 2T32DK007552 (to K.S.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810473106/DCSupplemental.
References
- 1.Thompson SH. The Diseases of the Prostate: Their Pathology and Treatment. London: J. & A. Churchill; 1883. [Google Scholar]
- 2.Shah SK, Chau MH, Schnepper GD, Lui PD. Open prostatolithotomy for the management of giant prostatic calculi. Urology. 2007;70:1008.e1009–1010. doi: 10.1016/j.urology.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Moore RA. Morphology of prostatic corpora amylacea and calculi. Arch Pathol. 1936;22:24–40. [Google Scholar]
- 4.Izzidien AY. Prostatic calcification in a 4-year-old boy. Arch Dis Child. 1980;55:963–964. doi: 10.1136/adc.55.12.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedir S, et al. Endoscopic treatment of multiple prostatic calculi causing urinary retention. International Journal of Urology. 2005;12:693–695. doi: 10.1111/j.1442-2042.2005.01133.x. [DOI] [PubMed] [Google Scholar]
- 6.Geramoutsos I, et al. Clinical correlation of prostatic lithiasis with chronic pelvic pain syndromes in young adults. European Urology. 2004;45:333–337. doi: 10.1016/j.eururo.2003.09.020. discussion 337–338. [DOI] [PubMed] [Google Scholar]
- 7.Thomas BA, Robert JT. Prostatic calculi. J Urol. 1927;18:470–493. [Google Scholar]
- 8.De Marzo AM, et al. Inflammation in prostate carcinogenesis. Nature Reviews: Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkle AL. The relationship of antecedent genito-urinary infections to the development of prostatic calculi and carcinoma. Bulletin of the New York Academy of Medicine. 1953;29:585–586. [PMC free article] [PubMed] [Google Scholar]
- 10.Golden M, Abeshouse BS. Prostatic calculi and neoplasm. Sinai Hospital Journal. 1952;1:20–28. [PubMed] [Google Scholar]
- 11.Cristol DS, Emmett JL. Incidence of coincidental prostate calculi, prostatic hyperplasia and carcinoma of the prostate. JAMA. 1944;124:646–652. [Google Scholar]
- 12.Kovi J, et al. Incidence of prostatic calcification in blacks in Washington, D.C., and selected African cities. Correlation of specimen roentgenographs and pathologic findings. Cooperative Prostatic Research Group. Urology. 1979;14:363–369. doi: 10.1016/0090-4295(79)90081-5. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs JT. The aging ACI/Seg versus Copenhagen male rat as a model system for the study of prostatic carcinogenesis. Cancer Res. 1984;44:5785–5796. [PubMed] [Google Scholar]
- 14.Eykyn S, Bultitude MI, Mayo ME, Lloyd-Davies RW. Prostatic calculi as a source of recurrent bacteriuria in the male. British Journal of Urology. 1974;46:527–532. doi: 10.1111/j.1464-410x.1974.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 15.Meares EM. Infection stones of prostate gland. Laboratory diagnosis and clinical management. Urology. 1974;4:560–566. doi: 10.1016/0090-4295(74)90490-7. [DOI] [PubMed] [Google Scholar]
- 16.Cross PA, Bartley CJ, McClure J. Amyloid in prostatic corpora amylacea. J Clin Pathol. 1992;45:894–897. doi: 10.1136/jcp.45.10.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein-Werblowky R. On the etiology of cancer of the prostate. European Urology. 1978;4:370–373. doi: 10.1159/000473996. [DOI] [PubMed] [Google Scholar]
- 18.Sutor DJ, Wooley SE. The crystalline composition of prostatic calculi. British Journal of Urology. 1974;46:533–535. doi: 10.1111/j.1464-410x.1974.tb03852.x. [DOI] [PubMed] [Google Scholar]
- 19.Torres Ramirez C, Aguilar Ruiz J, Zuluaga Gomez A, del Rio Samper S, de la Fuente Serr A. Ultrastructure of primary or endogenous prostatic calculi. Scanning electron microscopic study. Archivos españoles de urología. 1981;34:13–22. [PubMed] [Google Scholar]
- 20.Huggins C, Bear RS. The course of the prostatic ducts and the anatomy, chemical and x-ray diffraction analysis of prostatic calculi. J Urol. 1944;51:37–47. [Google Scholar]
- 21.Magura CE, Spector M. Scanning electron microscopy of human prostatic corpora amylacea and corpora calculi, and prostatic calculi. Scanning Electron Microscopy. 1979:713–720. [PubMed] [Google Scholar]
- 22.Kodaka T, et al. Fine structure and mineral components of primary calculi in some human prostates. Journal of Electron Microscopy. 2008;57:133–141. doi: 10.1093/jmicro/dfn013. [DOI] [PubMed] [Google Scholar]
- 23.Schrodt GR, Murray M. The keratin structure of corpora amylacea. Arch Pathol. 1966;82:518–525. [PubMed] [Google Scholar]
- 24.Gentile A. True prostatic calculus. J Urol. 1947;57:746–754. doi: 10.1016/S0022-5347(17)69696-4. [DOI] [PubMed] [Google Scholar]
- 25.Gueft B. The x-ray diffraction pattern of prostatic corpora amylacea. Acta Pathologica et Microbiologica Scandinavica. 1972;233(Supplement):132–134. [PubMed] [Google Scholar]
- 26.Horita S, Nitta K, Ozasa H, Nihei H, Toma H. Localization of beta-2-microglobulin in prostatic corpora amylacea of prostatic hypertrophy patients. Nephron. 1996;72:730–731. doi: 10.1159/000188981. [DOI] [PubMed] [Google Scholar]
- 27.Borregaard N, Sørensen OE, Theilgaard-Mönch K. Neutrophil granules: A library of innate immunity proteins. Trends in Immunology. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Shaheduzzaman S, et al. Silencing of lactotransferrin expression by methylation in prostate cancer progression. Cancer Biology & Therapy. 2007;6:1088–1095. doi: 10.4161/cbt.6.7.4327. [DOI] [PubMed] [Google Scholar]
- 29.van Sande M, Van Camp K. Lactoferrin in human prostate tissue. Urological Research. 1981;9:241–244. doi: 10.1007/BF00256894. [DOI] [PubMed] [Google Scholar]
- 30.Reese JH, McNeal JE, Goldenberg SL, Redwine EA, Sellers RG. Distribution of lactoferrin in the normal and inflamed human prostate: An immunohistochemical study. Prostate. 1992;20:73–85. doi: 10.1002/pros.2990200109. [DOI] [PubMed] [Google Scholar]
- 31.Saita A, Bonaccorsi A, Motta M. Stone composition: Where do we stand? Urologia Internationalis. 2007;79(Suppl 1):16–19. doi: 10.1159/000104436. [DOI] [PubMed] [Google Scholar]
- 32.Chen WC, et al. Mass spectroscopic characteristics of low molecular weight proteins extracted from calcium oxalate stones: Preliminary study. J Clin Lab Anal. 2008;22:77–85. doi: 10.1002/jcla.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merchant ML, et al. Proteomic analysis of renal calculi indicates an important role for inflammatory processes in calcium stone formation. Am J Physiol. Renal Physiology. 2008;295:F1254–F1258. doi: 10.1152/ajprenal.00134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mushtaq S, et al. Identification of myeloperoxidase, alpha-defensin and calgranulin in calcium oxalate renal stones. Clin Chim Acta. 2007;384:41–47. doi: 10.1016/j.cca.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Yousefi R, et al. Investigation on the surface hydrophobicity and aggregation kinetics of human calprotectin in the presence of calcium. Journal of Biochemistry and Molecular Biology. 2005;38:407–413. doi: 10.5483/bmbrep.2005.38.4.407. [DOI] [PubMed] [Google Scholar]
- 36.Pillay SN, Asplin JR, Coe FL. Evidence that calgranulin is produced by kidney cells and is an inhibitor of calcium oxalate crystallization. Am J Physiol. Renal Physiology. 1998;275:F255–261. doi: 10.1152/ajprenal.1998.275.2.F255. [DOI] [PubMed] [Google Scholar]
- 37.Hoyaux D, et al. S100 proteins in corpora amylacea from normal human brain. Brain Res. 2000;867:280–288. doi: 10.1016/s0006-8993(00)02393-3. [DOI] [PubMed] [Google Scholar]
- 38.Hermani A, et al. Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res. 2005;11:5146–5152. doi: 10.1158/1078-0432.CCR-05-0352. [DOI] [PubMed] [Google Scholar]
- 39.Müller H, Haug U, Rothenbacher D, Stegmaier C, Brenner H. Evaluation of serum and urinary myeloid related protein-14 as a marker for early detection of prostate cancer. J Urol. 2008;180:1309–1313. doi: 10.1016/j.juro.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 40.Jin CX, et al. Pancreatic stone protein of pancreatic calculi in chronic calcified pancreatitis in man. JOP: Journal of the Pancreas. 2002;3:54–61. [PubMed] [Google Scholar]
- 41.Coussens LM, Werb Z. Inflammatory cells and cancer: Think different! J Exp Med. 2001;193:23F–26. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gounaris E, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Nat Acad Sci USA. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 44.Otten CM, et al. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clinical Chemistry and Laboratory Medicine. 2008;46:1275–1280. doi: 10.1515/CCLM.2008.246. [DOI] [PubMed] [Google Scholar]
- 45.Corbin BD, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 46.Sfanos KS, et al. A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms. Prostate. 2008;68:306–320. doi: 10.1002/pros.20680. [DOI] [PubMed] [Google Scholar]
- 47.Leitzmann MF, Platz EA, Stampfer MJ, Willett WC, Giovannucci E. Ejaculation frequency and subsequent risk of prostate cancer. JAMA. 2004;291:1578–1586. doi: 10.1001/jama.291.13.1578. [DOI] [PubMed] [Google Scholar]
- 48.Giles GG, et al. Sexual factors and prostate cancer. BJU International. 2003;92:211–216. doi: 10.1046/j.1464-410x.2003.04319.x. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson MR, Dobson CM. In vitro characterization of lactoferrin aggregation and amyloid formation. Biochemistry. 2003;42:375–382. doi: 10.1021/bi0204746. [DOI] [PubMed] [Google Scholar]
- 50.Sfanos KS, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munch J, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.