Abstract
While studies in animal models have linked Toll-like receptor (TLR) 4 signaling to kidney injury induced by ischemia and reperfusion, the relevance of TLR4 activation to allograft injury in human kidney transplants is unknown. Here we show that TLR4 is constitutively expressed within all donor kidneys but is significantly higher in deceased-, compared with living-donor organs. Tubules from deceased- but not living-donor kidneys also stained positively for high-mobility group box-1 (HMGB1), a known endogenous TLR4 ligand. In vitro stimulation of human tubular cells with HMGB1, in a TLR4-dependent system, confirmed that HMGB1 can stimulate proinflammatory responses through TLR4. To assess the functional significance of TLR4 in human kidney transplantation, we determined whether TLR4 mutations that confer diminished affinity for HMGB1 influence intragraft gene-expression profiles and immediate graft function. Compared with kidneys expressing WT alleles, kidneys with a TLR4 loss-of-function allele contained less TNFα, MCP-1, and more heme oxygenase 1 (HO-1), and exhibited a higher rate of immediate graft function. These results represent previously undetected evidence that donor TLR4 contributes to graft inflammation and sterile injury following cold preservation and transplantation in humans. Targeting TLR4 signaling may have value in preventing or treating postischemic acute kidney injury after transplantation.
Keywords: delayed graft function, high mobility group box-1
Delayed graft function (DGF) occurs commonly following kidney transplantation, with an incidence as high as 50% in some series involving organs from deceased donors (1, 2). The consequences of developing DGF are significant. In addition to the acute complications related to renal failure and the associated economic impact of prolonged hospitalization, the development of DGF increases the risk of chronic allograft nephropathy and shortens allograft survival (3, 4). Current dogma is that DGF following kidney transplantation is primarily a consequence of ischemia/reperfusion (IR) injury, thus explaining the higher incidence in deceased donor allografts that routinely endure prolonged periods of ischemia.
Toll like receptors (TLRs) are a family of cell surface and intracellular proteins initially described as molecular sentinels capable of recognizing pathogen-associated molecular patterns (PAMPs) that activate innate immunity in response to invading pathogens. Humans express >10 distinct TLRs that, upon binding to PAMPs derived from the universe of pathogens, transmit signals through the adaptor Toll/IL-1R domain-containing adaptor, inducing IFN or myeloid differentiation-primary response protein (MyD88). Downstream effects include cytokine and chemokine release, as well as up-regulation of costimulatory molecules, which together initiate and amplify local inflammation (5, 6).
A growing body of literature suggests that select TLRs, including TLR2 and TLR4, function as detectors of sterile (not pathogen-associated) injury upon binding to endogenous ligands released by damaged cells (damage-associated molecular patterns, DAMPs) (7). Examples of putative endogenous ligands are heat-shock proteins, high-mobility group box 1 (HMGB1), heparan sulfate, hyaluronan fragments, and fibronectin (6). Studies from multiple laboratories have also revealed that TLR expression is not confined to cells of the innate immune system, but are also expressed on epithelial, endothelial, and mesenchymal cells of the kidney, heart, lung, and liver, among others. Kidney expression of TLRs is functionally important, as parenchymal cell deficiency of TLR2 or TLR4 dramatically limits kidney injury following IR, and diminishes the associated intrarenal inflammation (8, 9). Whether IR injury in humans, particularly in the context of kidney transplantation, is also impacted by ischemia-induced release of DAMPs and subsequent TLR signaling is not known. Herein we provide the previously undetected evidence that donor kidney-produced TLR4 and HMGB1 contribute to the development of IR injury following human kidney transplantation. The results have important implications, as they delineate TLR4 and its ligands as potential targets for novel therapies aimed at preventing DGF.
Results
TLR4 and HMGB1 Are Expressed in Donor Kidneys.
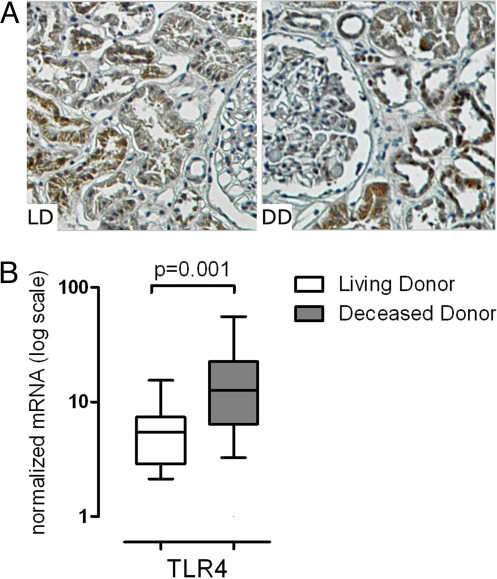
Although TLR4 expression in proximal and distal tubular epithelial cells of transplanted kidneys in the context of acute and chronic tubular injury was documented by others (10), the impact of brain death and ischemia on TLR4 expression in human kidneys has not been described. To address this, we examined TLR4 in preanastomosis kidney biopsies obtained from living (n = 15) and deceased donors (n = 9). Cold ischemia time was 38 ± 3.6 min in the living donors and 20 h ± 1.3 h in the deceased donors. Immunohistochemical analysis of tissue sections revealed TLR4 protein in proximal and distal tubular cells (Fig. 1A), with significantly higher expression in deceased donor kidneys (208 ± 7.0 vs. 243 ± 13.0, P = 0.016) and a strong positive correlation between protein staining and mRNA expression levels (P = 0.0014). Quantitative gene expression (qRT-PCR) analysis revealed 2.3-fold more TLR4 mRNA in the deceased (n = 28) versus the living donors (n = 18) (P = 0.001) (Fig. 1B).
Fig. 1.
TLR4 expression in implantation biopsies. (A) Preanastomosis biopsy sections (×200) from living- (n = 15) and deceased-donor kidneys (n = 9) were analyzed by immunohistochemistry for TLR4. TLR4 was expressed in proximal and distal tubuli, with higher expression in deceased-donor kidneys compared with living donors (P = 0.016). The amount of TLR4 protein quantified correlated with their respective mRNA expression levels (P = 0.0014). (B) TLR4 mRNA expression levels in preanastomosis biopsies was significantly higher in samples from deceased donors (n = 28) compared to samples from living donors (n = 18). Box and whisker blots show the medians, and the percentile values (10, 25, 75, 90) for normalized mRNA.
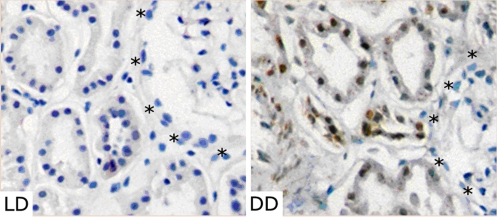
Because endogenously produced HMGB1 binds to and stimulates TLR4, and because HMGB1 blockade limits liver IR injury in animals (11), HMGB1 may function as an endogenous DAMP in the pathogenesis of kidney IR injury. We thus examined kidney tissue from deceased and living donors for HMGB1 expression (n = 5 each). In the deceased-donor kidneys we noted strong staining for HMGB1 in the proximal and distal tubules, as well as in the smooth muscle cells, but not in the endothelial or glomerular cells (Fig. 2). HMGB1 was localized in the nuclei as well as in the cytoplasm. In contrast, we did not detect HMGB1 staining in any of these segments in any of living-donor kidneys (see Fig. 2).
Fig. 2.
HMGB1 expression in implantation biopsies. HMGB1 was localized in distal and proximal tubules from deceased-donor kidneys (DD) obtained prior anastomosis. However, no HMGB1 staining was observed in living-donor kidneys (LD). Glomeruli (*indicates border) were negative for HMGB1 for both types of donors (n = 5 each group; ×200).
We also analyzed biglycan and HSP70 mRNA expression levels in pre- and postanastomosis biopsies of living donors (n = 18) and deceased donors (n = 28). Both genes were significantly up-regulated after reperfusion, whereas HSP70 was significantly lower in preanastomosis deceased-donor kidneys when compared with living donors (P = 0.02). There was no detectable relationship between the donor-kidney source (deceased vs. living) and either the preanastomosis HSP70 gene expression or the postanastomosis levels of HSP70 and biglycan (Fig. S1).
TLR4/HMGB1 Interactions Mediate Up-Regulation of Kidney Inflammatory Genes.
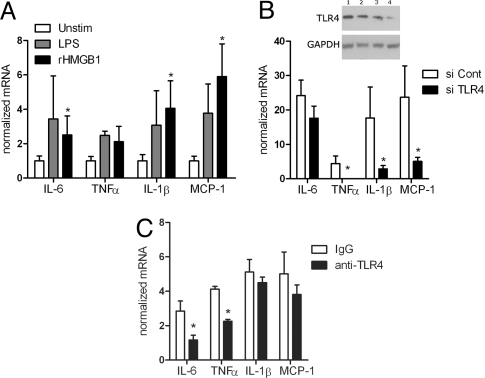
TLR4 engagement initiates a signaling cascade leading to activation of the transcription factor NFκB, which regulates the induction of multiple inflammatory genes. To directly test whether HMGB1 can mediate renal inflammation, we measured gene-expression patterns in TLR4-expressing, HK-2 human renal epithelial cells after addition of recombinant, LPS-free HMGB1 (Fig. 3A). The HMGB1 induced marked up-regulation of IL-6, IL-1β, and MCP-1, to a similar extent as stimulation with the positive control LPS, which activates HK-2 cells only via TLR4 (12). To specifically test whether the HMGB1-induced cytokine up-regulation was mediated through TLR4, we repeated the experiments using HK-2 cells after siRNA-mediated TLR4 down-regulation. Fig. 3B confirms that transfection with the TLR4-specific siRNA, but not the control, markedly down-regulated TLR4 expression. Remarkably, cytokine up-regulation was significantly diminished in the TLR4 siRNA-transfected HK-2 cells stimulated with HMGB1, compared with HMGB1-treated HK-2 cells transfected with control nonspecific siRNA (see Fig. 3B). We also tested the effect of an anti-human TLR4 antibody, known to inhibit LPS signaling via TLR4, on rHMGB1-induced cytokine up-regulation. Anti-TLR4 mAb (20 μg/ml) attenuated the stimulatory effect of rHMGB1 (5 μg/ml) (Fig. 3C).
Fig. 3.
HMGB1-induced TLR4-mediated inflammation. (A) qRT-PCR analysis of cytokine genes of proximal tubular cell line (HK-2). Cells were cultured without (Unstim) or with the TLR4-specific ligand LPS (1 μg/ml) or with rHMGB1 (5 μg/ml) (n = 3–7 per group; *P < 0.05 compared with unstimulated controls). (B) Western blot shows TLR4 expression of HK-2 cells transfected with control siRNA (lane 1: 62.5 nM; lane 2: 125 nM), or TLR4 siRNA (lane 3: 62.5 nM; lane 4: 125 nM). GAPDH and tubulin (not shown) staining was used as a loading control. HK-2 cells were transfected transiently with control siRNA or siRNA (125 nM each) against TLR4 and then stimulated with rHMGB1 as described in (A) (n = 3 each; *P < 0.05). (C) HK-2 cells were cultured for 30 min with monoclonal anti-TLR4 antibody or isotype (IgG) control (20 μg/ml each) and then stimulated with rHMGB1 (5 μg/ml) as described in (A) (n = 4 each; *P < 0.05).
Together with previously published observations in mice (13) and with our documentation that kidneys from diseased donors express TLR4 and HMGB1, these data support the conclusion that endogenously released HMGB1 can stimulate TLR4-expressing human kidney cells (including renal tubular epithelial cells), and thereby function as an intrinsic mechanism for initiating renal inflammation in response to IR injury.
Loss-of-Function TLR4 Mutations Are Associated with Improved Immediate Kidney Graft Function.
Studies of the TLR4 gene in humans have uncovered 2 loss of function SNPs, Asp299Gly and Thr399Ile, which diminish receptor binding of LPS (14) but do not affect TLR4 gene or protein expression (15). We reasoned that if TLR4 signaling impacts IR injury following transplantation, outcome should be improved in kidneys expressing at least one of these SNPs. To test this hypothesis, we determined donor TLR4 genotypes in a cohort of 267 patients with kidney transplants and correlated the presence or absence of at least 1 mutated SNP at 1 locus with graft function (both TLR4 SNPs are closely cosegregated and were analyzed together, and will be referred to hereafter as “mutated”). Allelic frequencies (299 G-allele: 10.4%, 399 T-allele: 8.6%) were in Hardy-Weinberg equilibrium corresponding to published data (16). The distribution of the donor ethnicity between WT vs. mutated-TLR4 donor kidneys was not different (Caucasian, 83% vs. 77%; African-American, 7% vs. 13%; Hispanics, 8% vs. 10%; others, 2% vs. 0%), excluding an effect of donor ethnicity on the measured outcomes. In addition, donor and recipient age and sex, cold-ischemia time, the percentage of deceased donor kidneys, and the percentage of kidneys defined as standard criteria donors were not different between the WT and TLR-mutated groups (Table 1).
Table 1.
Demographic data for donors and recipients according to the TLR4 mutation
| TLR4 wild type receiver (n = 237) | TLR4 mutant receiver* (n = 30) | P | |
|---|---|---|---|
| Recipient gender, male (%) | 60.8 | 50.0 | 0.38 |
| Donor gender, male (%) | 46.6 | 50.0 | 0.85 |
| Recipient age (y) | 45.3 ± 13 | 44.0 ± 14 | 0.53 |
| Donor age (y) | 38.2 ± 15 | 37.7 ± 18 | 0.60 |
| Deceased donors (%) | 64.6 | 56.7 | 0.42 |
| Standard criteria donors†,‡ (%) | 86.5 | 73.7 | 0.14 |
| Cold-ischemia time (min)‡ | 1,177 ± 466 | 1,139 ± 387 | 0.63 |
*Mutated TLR4 Asp299Gly and TLR4 Thr399Ile combined.
†Standard criteria donors vs. extended criteria/donation after cardiac death.
‡Deceased donors only.
Because the dialysis-based definition of DGF is a subjective, a clinician-dependent decision, and because even slow recovery of renal function after transplant has been associated with poor late outcomes, we chose to analyze our results with respect to a more stringently defined outcome, immediate graft function (IGF) (17). The overall incidence of IGF, as defined by drop in serum creatinine of ≥25% (18), was 42% (18% for deceased and 84% for living donors). In a univariate analysis for all patients, IGF was significantly more frequent in recipients of a kidney with a mutated TLR4 [66.7% vs. 38.8%; P = 0.005, odds ratio (OR) 3.15, 95% confidence interval (CI): 1.41–7.04]. This association remained significant in a multivariable analysis of risk factors known to impact IGF, including donor age, cold-ischemia time, and deceased- versus living-donor type (P = 0.007, OR 4.67, 95% CI 1.61–13.53). Notably, if only living donors were analyzed, there was no effect on donor TLR4 genotype on IGF rate (P = 0.12). In contrast, Table 2 shows that within the high-risk subpopulation of recipients who received deceased-donor kidneys, IGF was significantly more likely if the donor expressed a mutated, loss of function, TLR4 allele (41.2% vs. 15.7%; P = 0.017, OR 3.61, 95% CI: 1.26–10.39). This association remained significant after multivariable analysis (P = 0.018; hazard ratio 3.89, 95% CI 1.26–12.05).
Table 2.
Univariate and multivariate analysis for the deceased donors for the occurrence of immediate graft function
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |
| TLR4 mutant* | 0.017 | 3.61 (1.26–10.39) | 0.018 | 3.89 (1.26–12.05) |
| Donor age (y) | 0.019 | 0.97 (0.95–0.99) | 0.015 | 0.96 (0.93–0.99) |
| Donor type† | 0.99 | 0.99 (0.35–2.86) | 0.22 | 2.72 (0.55–13.45) |
| Cold-ischemia time‡ (min) | 0.15 | 0.99 (0.99–1.0) | 0.23 | 0.99 (0.99–1.0) |
| Center | 0.49 | 1.32 (0.60–2.94) | 0.78 | 1.14 (0.46–2.88) |
*Mutated TLR4 Asp299Gly and TLR4 Thr399Ile combined.
†Standard criteria donors vs. extended criteria/donation after cardiac death.
‡Deceased donors only.
To exclude the possibility that differences in immediate graft outcome were related to disparities in baseline histology between groups (19), we performed a histologic analysis on a subset of 51 preanastomosis deceased-donor kidney biopsies. The degree of fibrosis was not different in TLR4-mutated versus WT kidneys, making a significant variation in organ quality as a cause of the difference in IGF between these groups unlikely (data not shown).
In contrast to published data with a smaller cohort (20), we did not uncover a significant correlation between donor TLR4 genotype and the incidence of acute rejection, which was 14.7% in our population. Given the low incidence of acute rejection, our study did not have the power to detect a difference in this outcome.
Mutated TLR4 Was Linked to a Pattern of Altered Intragraft Gene Expression.
All samples were implantation biopsies and in all available samples we performed qRT-PCR for candidate proinflammatory genes (MCP-1 and TNFα), a marker of T-cell infiltration (CD3ε), and one anti-inflammatory/protective gene (HO-1) that previous literature implicates are differentially expressed based on the degree of IR injury in animal models (21–23) and humans (24).
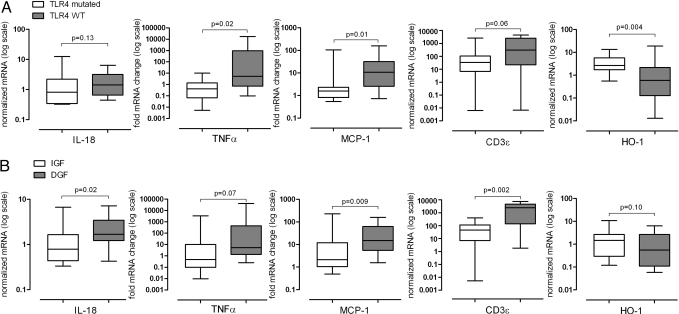
When we analyzed the results according the presence or absence of a mutated donor-TLR4 allele (Fig. 4A), we noted significantly less TNFα and MCP-1 and a trend toward lower CD3ε expression in kidneys with the TLR4 mutation. Moreover, we detected significantly more HO-1 gene expression in TLR4-mutated kidneys. This gene expression pattern was similar when analyzed based on clinical outcome (Fig. 4B). We found significantly higher expression of MCP-1, and CD3ε, and a trend for TNFα in DGF-kidneys compared to IGF-kidneys, consistent with published work documenting that IR injury is accompanied by proinflammatory gene expression in the kidney (see Fig. 4B).
Fig. 4.
Messenger RNA levels in kidney tissue in relation to TLR4 mutation and kidney function. (A) Gene expression in relation to the TLR4-donor genotype (WT n = 38; TLR4 mutated n = 10). (B) Expression levels of tubular injury marker IL-18, TNFα, MCP-1, T-cell marker (CD3ε), and HO-1 were analyzed in kidneys with IGF (n = 22) or DGF (n = 26). Box and whisker blots show the medians, and the percentile values (10, 25, 75, 90) for normalized mRNA.
As a control to test whether differences in immediate graft outcome were related to more (ischemic) tubular injury before, and independent of, the reperfusion, we measured IL-18 gene expression, which was noted by others to be markedly up-regulated in postischemic kidneys (25). Fig. 4B shows that IL-18 expression was correlated with DGF, but no difference in IL-18 gene expression was noted between TLR4-mutated and WT groups (see Fig. 4A). These results support our interpretation that TLR4-mediated signals influence IR injury after transplant and make it unlikely that a significant difference in tubular injury before reperfusion caused the difference in IGF between groups.
Discussion
Our results provide strong evidence that the pathogenesis of IR injury following kidney transplantation in humans involves signaling through TLR4 expressed in donor kidney cells. First, we showed that TLR4 is expressed in normal kidneys obtained from living donors and is significantly up-regulated by ischemic injury (see Fig. 1). Next, we show that ischemic injury is associated with up-regulated tubular cell expression of HMGB1, a putative endogenous ligand for TLR4 (see Fig. 2) and that HMGB1 stimulation of human tubular cells leads to TLR4-dependent proinflammatory responses (see Fig. 3). We then showed that loss of function TLR4 mutations are linked to less intragraft proinflammatory gene expression and to a higher rate of IGF (see Fig. 4 and Table 2).
Our findings are in accordance with animal data, demonstrating an important role of renal parenchymal TLR4 expression in mediating IR injury by promoting leukocyte accumulation, the production of proinflammatory molecules, and in mediating kidney damage (9). Although it is clear that TLR4 is critical, no specific TLR4 ligands were identified. In addition to IR injury, several models of sterile tissue injury identified a central role for TLR4 in mediating the early inflammatory response (13, 26–28). The importance of local TLR4 gene expression in human kidney transplantation was recently suggested (10, 29).
While TLR4 expression and regulation in the human kidney is not well understood, the current study supports animal data in vitro (9) and vivo (30), which showed TLR4 up-regulation under ischemic conditions. However, it is not known whether tubular TLR4 expression continues to increase after IR injury in the hours or days after human kidney transplantation.
The functional relevance of TLR4 in IR injury is demonstrated through association between IGF and the 2 cosegregating missense TLR4 mutants, Asp299Gly and Thr399Ile. After correction for risk factors, the occurrence of IGF was significantly higher in patients carrying a donor kidney with a mutated TLR4. Both SNPs studied are known to affect the LPS response of epithelial bronchial cells, as well as cells transfected with mutated TLR4, and are found in up to 10% of Caucasians and 15% of African Americans (14, 31).
The exact mechanism of the protective effect of TLR4 mutation in our study is unclear, but based on our data, it is not likely that differences in immediate graft outcomes were related to differences in baseline histology (19) or pretransplant tubular injury (25). We showed, however, that TLR4 mutants lead to less inflammation, further underscoring the relationship between TLR4 signaling and IR injury. Expression of HO-1, TNFα, MCP-1 and, although it did not reach statistical significance, CD3, were linked to the TLR4 mutation. Of note, the up-regulation of HO-1 was found to mediate the beneficial effect of TLR4 deficiency in a mouse model of IR liver injury (21), and TNFα (22) and MCP-1 (23) have been shown to contribute to ischemic kidney injury. In addition, high intraoperative CD3 expression in the renal allograft was associated with early acute rejection and lower long-term renal function in kidney transplant recipients (24), and animal data suggest an important role of T cells in the early steps of IR injury (32).
We extended the previous studies on the role of TLR4 in IR injury by studying potential ligands. HMGB1 is a chromatin-binding protein that regulates transcription and chromosome architecture. Its release from the cell nucleus into the extracellular environment can occur passively as cells undergo necrotic death, and actively in response to stressors when it functions as a proinflammatory danger signal (33). In our study, strong tubular immunostaining for HMGB1 was detected on the kidney sections obtained from deceased-donor kidneys. No positive staining was observed in the kidney specimens from living donors. Our documentation that ischemia up-regulates HMGB1 (see Fig. 2), which up-regulate proinflammatory genes via TLR4 signaling in kidney tissue (see Fig. 3) is unique, but is consistent with reports that implantation kidney biopsies contain apoptotic as well as necrotic cells (34). This data also extends previous findings that implicate HMGB1 as signaling through TLR2 and TLR4 (35–37) to the transplantation setting. Although our study is not able to conclusively link HMGB1 as a danger signal and ligand of TLR4 in vivo, two lines of evidence support this conclusion. First, HMGB1 was only present in deceased donor organs and not in living donors. Second, the loss of TLR4 function had only an impact on deceased- but not living-donor kidney transplants.
The studied TLR4 variants are situated within the extracellular domain and diminish receptor binding (14, 15), and these mutations were documented by others to diminish the binding of HMGB1 to TLR4 (37). Furthermore, hypoxia-induced HMGB1 release by hepatocytes is promoted by TLR4-dependent reactive oxygen species (ROS) production, and optimal production of ROS and HMGB1 release require intact TLR4 signaling (13), implicating a positive-feedback loop. These previous findings provide a putative explanation to account for the protective effect of the TLR4 mutations on IR injury. Suboptimal HMGB1/TLR4 binding limits signal transduction and diminishes the positive-feedback loop, preventing release of proinflammatory gene products and ROS that contribute to the injury.
It is important to note that while our data suggest that IR injury relates to TLR4 signals transduced through tubular cells (see Fig. 3), endothelial cells, tissue macrophages, and dendritic cells express TLR4 and could, via a similar mechanism, contribute to the inflammatory response that occurs following ischemic injury (1). Consistent with a possible role of leukocytes in this process is data that chimeric mice lacking TLR4 only on bone marrow-derived cells were partially protected from IR injury (9). Our study did not evaluate the role of recipient TLR4 mutation on IR injury. The involvement of recipient TLR signals in clinical kidney transplantation is supported by reports indicating improved kidney graft survival, lower risk of posttransplant atherosclerotic events, and acute cellular rejection of allografts in patients with TLR4 SNPs (38, 39). Notably, these studies did not assess the impact of these variants on the risk of DGF, and therefore the impact of recipient TLR4 mutation on IR injury is unknown.
Although our study indicates that TLR4 mediates human kidney IR injury, other TLRs may additionally contribute. Others have shown that targeted deletion of TLR2 or down-regulation of TLR2 with antisense oligonucleotides protect mice from IR injury (8, 40). Additional studies will be required to assess the role TLR2 and or other TLRs as mediators of IR injury in humans. Of note, the low frequency of functional TLR2 mutation would require a large sample size to conduct these studies (41).
Our unique observations support the hypothesis that HMGB1 is one relevant ligand for TLR4-mediated ischemic kidney injury, but other putative ligands may play contributory roles. Work from several groups have shown that other DAMPs, including heparan sulfate, fibrinogen, hyaluronan, fibronectin, and Tamm-Horsfall protein can function as endogenous activators of TLR4 signaling and are likely released peritransplantation (42–45). Consistent with the concept that HMGB1 is not a unique ligand, we found that 2 other putative TLR4 ligands, HSP70 and biglycan, were also significantly up-regulated after reperfusion (see Fig. S1) (7).
The recent expansion of criteria for acceptable deceased-donor kidneys, nonheart-beating and expanded-criteria donors will likely increase the DGF rate and its clinical impact. Thus, identifying organs at risk or molecules involved in its pathogenesis could improve kidney-transplant outcome. In the clinical context, blockade of TLR4 signaling is more likely to be successful than targeting individual ligands or downstream effectors, which often serve redundant functions. Targeting TLR signaling could have several potential implications beyond protecting against IR injury, as there is extensive evidence that the innate system interacts with the adaptive immune system and that reducing the IR-induced inflammation could increase the success of tolerogeneic protocols. Together with previous animal studies demonstrating the beneficial effects of TLR4 inhibition on IR injury, acute rejection, tolerance, and nephrotoxic tissue injury (reviewed in ref. 7), our study sets the stage for future work aimed at inhibiting TLR activation in a clinical setting to prevent IR injury after transplantation. In fact, TLR4 antagonists are in clinical development, and targeting this receptor may have value in both for preventing or treating postischemic acute kidney injury and for long-term maintenance of graft function and survival.
Methods
Study Population.
A total of 267 (156 from the Medical Center of Maine and 111 from Mount Sinai Medical Center in New York) renal transplant recipients were included in this study. Donor and recipients demographic and follow-up data were extracted from the hospital records. During the study period, immunosuppressive protocols were based on tacrolimus or cyclosporine, mycophenolate, and with or without steroids. Sixty percent of patients received depleting induction therapy and all patients received intraoperative 500 mg of solumedrol. The Internal Review Board of the Mount Sinai School of Medicine, New York, NY, and the Maine Medical Center, Portland, ME approved the study and written informed consent was obtained at the time of enrolment.
Collection of Kidney Biopsies and Histopathology.
In a subset of 51 patients, wedge biopsies were obtained at the end of the cold storage (also called preanastomisis biopsies) and 30-to 40 min after reperfusion. Tissue for molecular analysis was placed in RNAlater (Quiagen) stabilization solution and stored at –20 °C. A tissue specimen was placed in 10% formalin solution for the quantification of fibrosis. Three-micrometer sections were used for periodic acid-Schiff (2 sections) and Masson trichrome staining (2 sections). The Aperio virtual slide scanner (ScanScope CS) was used to digitize the image of the whole sections at 40×. The area of the entire cortical sample (excluding capsule), stained red in PAS (all basement membranes), was subtracted from the area stained blue in trichrome (fibrillar and basement membrane collagens).
Immunostaining for HMGB1 and TLR4.
Preanastomisis biopsies were stained for HMGB1, clone 1D5 (3 μg/ml) (Abnova,) and TLR4 (1:100; ZYMED Laboratories) according to the manufacturer's protocol. In brief, after deparaffinizing and antigen retrieval (10 mM citrate buffer, ph 6.0), samples were stained with the antibodies aforementioned overnight at 4 °C and visualized using the EnVision Detection Kit (HMGB1, DAKO) or the Vectastain Kit with DAB-staining (TLR4, Vectashield Laboratories). Slides where the primary antibody was omitted were used as negative controls. All samples were counterstained with hematoxylin. TLR4 protein level was quantified using MetaMorph software (Molecular Devices).
Quantitative RT-PCR.
Total RNA was extracted using phenol/guanidine isothiocyanate containing TriZol solution (Life Technologies BRL). RNA concentration was calculated using a Nanodrop ND1000 spectrophotometer (NanoDrop Technologies). For cDNA synthesis, total RNA was primed with oligo(dT) and PCR was either performed on a LightCycler (Roche Applied Science) or the ABI Prism 7900HT Sequence Detection System (Applied Biosystems), using the FastStart QuantiTect SYBR Green PCR kit (Quiagen) as described previously. For TLR4 (HS00152939_m1), 18S (Hs99999901_s1), gene expression assays by Applied Biosystems were used according to the manufacture's protocol. Expression levels were calculated using the 2−ΔΔCt method after normalization to the housekeeping genes 18S (total tissue) or AQP1 (tubular fraction). The positive correlation of these 2 housekeeping genes was highly significant (P < 0.0001). The primer sequences are available on request.
In Vitro TLR4 and rHMGB1 Stimulation.
The human proximal tubular cell line (HK-2, ATCC CRL-2190) was grown under regular conditions (95% air, 5% CO2) with keratinocyte serum-free medium supplemented with bovine pituitary extract and epidermal growth factor, and seeded out into 6-well plates. Cells were used in a state of submaximal confluence (80%). HK-2 cells were stimulated at 37 °C for 24 h in 1 ml of fresh serum-free medium in the presence or absence of purified LPS (1 μg/ml; E. coli 0111:B4, InvivoGene) or rHMGB1 (5 μg/ml, Sigma). The ultra-pure LPS used only activates the TLR4 pathway (12). Except for LPS-treated samples, polymyxin B (10 μg/ml, Fluka Chemie GmbH) was added to prevent the possible effect of contaminating endotoxin. Reagents are endotoxin-tested and rHMGB1 contained <0.01 EU/μg.
RNA Interference and Blocking Antibody.
HK-2 cells were plated at a density of 2 × 105 cells per well in 6-well dishes and transfected with 62.5 nM or 125 nM small interfering RNA (siRNA) oligonucleotides (Dharmacon) using X-treme gene siRNA transfection reagent (Roche) according to the manufacturer's protocol. Twenty-four hours after transfection, cells were stimulated for 24 h with rHMGB1 (5 μg/ml). Negative control siRNA (Dharmacon), which has no significant homology to any known gene sequences from mouse, rat, or human being, was used as a control. Functional grade purified mouse-anti-human TLR4 (clone HTA125) antibody (20 μg/ml) or IgG isotype contol (eBioscience) were added 30 min before 24-h exposure to rHMGB1 (5 μg/ml).
Western Blot Analysis.
Cells were lysed in CHAPS buffer (20 mM Tris, pH 7.5, 500 mM NaCl, 0.5% CHAPS) containing protease and phosphatase inhibitors. Lysates (30 μg per lane) were subjected to 12% SDS/PAGE before transfer to polyvinylidene difluoride membranes. Rabbit polyclonal anti-TLR4 (Zymed Laboratories), mouse monoclonal anti-GAPDH (Santa Cruz Biotechnologies) and rabbit anti-beta tubulin (Sigma Aldrich) were used for antigen detection. HRP-conjugated secondary antibodies (Promega Corp.) were used at 1:10,000. The immunoreaction was visualized with ECL Pico (Amersham Pharmacia Biotech).
Clinical Outcome Parameters.
Acute rejection was proven by biopsy and graded according to the Banff classification. Only rejections within the first month were considered in this study. Patients with acute humoral rejection within the first week, vascular complications, or early urinary tract obstruction were excluded. IGF was defined as drop of serum creatinine within the first 24 h after transplantation of ≥25%, and non-IGF was defined as need for dialysis within the first 7 days or a drop of <25% of serum creatinine within the first 24 h after transplantation (2). To validate this definition, receiver operating characteristic curves were plotted for the occurrence of a TLR4-mutation in dependence to the drop of creatinine within the first 24 h, and the corresponding sensitivity and specificity rates for each drop value was computed. These values, as well as the mean of sensitivity and specificity, were plotted against the drop in creatinine. The intersection area of these 3 values displays the highest sensitivity-specificity ratio and therefore exhibits the best choice. Analyzing the whole population, the cut-off point of 28% drop in creatinine discriminated best between the occurrences of IGF and non-IGF. In patients receiving an organ from a deceased donor, the discrimination potential was stable between 17% and 28%. Therefore, the chosen definition of 25% discriminated between non-IGF and IGF has the highest specificity and sensitivity (living and deceased donors, 67% and 58%; deceased donors only, 41% and 80%).
Genotyping.
Donor DNA was extracted from a part of the preperfusion biopsies, from parts of additional material after fitting the kidney, or from donor blood using the salting-out method. The 2 TLR4 polymorphisms were analyzed either by using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems) using assays on demand (Asp299Gly, rs4986790, C__11722238_20; Thr399Ile, rs4986791, C__11722237_20) or by PCR with sequence-specific primers followed by restriction length fragment polymorphism analysis (16).
Statistical Analysis.
Results are expressed as mean ± SEM, unless stated otherwise. Non-parametric tests were used for comparisons of continuous variables, and two-sided χ2 or Fisher's Exact were performed for categorical variables. Tests were performed as two-sided. Potentially confounding covariates [recipient and donor age and gender, donor type (standard criteria donor vs. extended criteria donor/donation after cardiac death) cold-ischemia time, and center] were adjusted by using logistic regression analysis (uni- and multivariate). Differences in gene expression were calculated using the nonparametric Mann-Whitney U tests. P < 0.05 was considered as statistically significant. Statistical analysis was performed with the SPSS Version 16.0 software package (SPSS Inc.).
Supplementary Material
Acknowledgments.
For their assistance in enrollment and sample collection, the authors thank the surgical members of the Mount Sinai transplant institute: Benoit Blondeau, Ana Carolina del Pozo, Pablo Uva, and Hiroshi Sogawa. We thank Dr. Detlef Schlöndorff for the support in writing the manuscript. This work is supported by a Satellite Healthcare research grant (to B.S.) and a National Institutes of Health research grant to B.T.M. (UOI AI070107-01).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810169106/DCSupplemental.
References
- 1.Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814–1827. doi: 10.1016/S0140-6736(04)17406-0. [DOI] [PubMed] [Google Scholar]
- 2.Yarlagadda SG, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23:2995–3003. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoskes DA, Cecka JM. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66:1697–1701. doi: 10.1097/00007890-199812270-00022. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama I, et al. Effect of prolonged delayed graft function on long-term graft outcome in cadaveric kidney transplantation. Clin Transplant. 1994;8:101–106. [PubMed] [Google Scholar]
- 5.Barton GM. A calculated response: control of inflammation by the innate immune system. J Clin Invest. 2008;118:413–420. doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mollen KP, et al. Emerging paradigm: Toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 7.Alegre ML, et al. The multiple facets of Toll-like receptors in transplantation biology. Transplantation. 2008;86:1–9. doi: 10.1097/TP.0b013e31817c11e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leemans JC, et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groot K, et al. Toll-like receptor 2 and renal allograft function. Am J Nephrol. 2008;28(4):583–588. doi: 10.1159/000115974. [DOI] [PubMed] [Google Scholar]
- 11.Tsung A, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 13.Tsung A, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbour NC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 15.Rallabhandi P, et al. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol. 2006;177:322–332. doi: 10.4049/jimmunol.177.1.322. [DOI] [PubMed] [Google Scholar]
- 16.Kiechl S, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigo E, et al. Similar impact of slow and delayed graft function on renal allograft outcome and function. Transplant Proc. 2005;37:1431–1432. doi: 10.1016/j.transproceed.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 18.Pieringer H, Biesenbach G. Risk factors for delayed kidney function and impact of delayed function on patient and graft survival in adult graft recipients. Clin Transplant. 2005;19:391–398. doi: 10.1111/j.1399-0012.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 19.Remuzzi G, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006;354:343–352. doi: 10.1056/NEJMoa052891. [DOI] [PubMed] [Google Scholar]
- 20.Palmer SM, et al. Donor polymorphisms in Toll-like receptor-4 influence the development of rejection after renal transplantation. Clin Transplant. 2006;20:30–36. doi: 10.1111/j.1399-0012.2005.00436.x. [DOI] [PubMed] [Google Scholar]
- 21.Shen XD, et al. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplant. 2005;5:1793–1800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- 22.Dong X, et al. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007;71:619–628. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]
- 23.Furuichi K, et al. Gene therapy expressing amino-terminal truncated monocyte chemoattractant protein-1 prevents renal ischemia-reperfusion injury. J Am Soc Nephrol. 2003;14:1066–1071. doi: 10.1097/01.asn.0000059339.14780.e4. [DOI] [PubMed] [Google Scholar]
- 24.Avihingsanon Y, et al. On the intraoperative molecular status of renal allografts after vascular reperfusion and clinical outcomes. J Am Soc Nephrol. 2005;16:1542–1548. doi: 10.1681/ASN.2005020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melnikov VY, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaczorowski DJ, et al. Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation. 2007;84:1279–1287. doi: 10.1097/01.tp.0000287597.87571.17. [DOI] [PubMed] [Google Scholar]
- 27.Oyama J, et al. Reduced myocardial ischemia-reperfusion injury in Toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 28.Shen XD, et al. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13:1435–1443. doi: 10.1002/lt.21251. [DOI] [PubMed] [Google Scholar]
- 29.Braudeau C, et al. Contrasted blood and intragraft Toll-like receptor 4 mRNA profiles in operational tolerance versus chronic rejection in kidney transplant recipients. Transplantation. 2008;86:130–136. doi: 10.1097/TP.0b013e31817b8dc5. [DOI] [PubMed] [Google Scholar]
- 30.Wolfs TG, et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002;168:1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 31.Michel O, et al. Systemic responsiveness to lipopolysaccharide and polymorphisms in the toll-like receptor 4 gene in human beings. J Allergy Clin Immunol. 2003;112:923–929. doi: 10.1016/j.jaci.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Rabb H, Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007;248:4–11. doi: 10.1016/j.cellimm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–7897. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 34.Toronyi E, Lord R, Bowen ID, Perner F, Szende B. Renal tubular cell necrosis and apoptosis in transplanted kidneys. Cell Biol Int. 2001;25:267–270. doi: 10.1006/cbir.2000.0620. [DOI] [PubMed] [Google Scholar]
- 35.Park JS, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 36.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 37.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 38.Fekete A, et al. Association between heat shock protein 70s and Toll-like receptor polymorphisms with long-term renal allograft survival. Transpl Int. 2006;19:190–196. doi: 10.1111/j.1432-2277.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 39.Ducloux D, et al. Relevance of Toll-like receptor-4 polymorphisms in renal transplantation. Kidney Int. 2005;67:2454–2461. doi: 10.1111/j.1523-1755.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 40.Shigeoka AA, et al. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 41.Woehrle T, et al. Pathogen specific cytokine release reveals an effect of TLR2 Arg753Gln during Candida sepsis in humans. Cytokine. 2008;41:322–329. doi: 10.1016/j.cyto.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Johnsson C, Tufveson G, Wahlberg J, Hallgren R. Experimentally-induced warm renal ischemia induces cortical accumulation of hyaluronan in the kidney. Kidney Int. 1996;50:1224–1229. doi: 10.1038/ki.1996.431. [DOI] [PubMed] [Google Scholar]
- 43.Taylor KR, et al. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 44.Jiang D, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 45.El-Achkar TM, et al. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol. 2008;295:F534–544. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.