Abstract
Monoclonal antibodies (mAbs) to ErbB-2/HER2 or to its sibling, the epidermal growth factor receptor (EGFR), prolong survival of cancer patients, especially when combined with cytotoxic therapies. However, low effectiveness of therapeutic mAbs and the evolution of patient resistance call for improvements. Here we test in animals pairs of anti-ErbB-2 mAbs and report that pairs comprising an antibody reactive with the dimerization site of ErbB-2 and an antibody recognizing another distinct epitope better inhibit ErbB-2-overexpressing tumors than other pairs or the respective individual mAbs. Because the superiority of antibody combinations extends to tumor cell cultures, we assume that nonimmunological mechanisms contribute to mAb synergy. One potential mechanism, namely the ability of mAb combinations to instigate ErbB-2 endocytosis, is demonstrated. Translation of these lessons to clinical applications may enhance patient response and delay acquisition of resistance.
Keywords: cancer, growth factor, immunotherapy, signal transduction, tyrosine kinase
ErbB-2/HER2 is a member of the epidermal growth factor receptor (EGFR) family. When transactivated, ErbB-2/HER2 stimulates several downstream signaling cascades, including the mitogen-activated protein kinase cascade (1). This ligand-less receptor is moderately expressed in normal adult tissues, where it regulates cell growth and differentiation. By contrast, amplification of the corresponding gene and consequent overexpression of the HER2/ErbB-2 protein have been reported in 20–30% of tumors of the breast (2–4) and ovary (4). In general, erbB-2 gene amplification associates with enhanced metastatic potential and poor prognosis. Because ErbB-2 is expressed at relatively low levels in normal tissues, it makes an attractive target for immunotherapy. This was originally demonstrated in animals by Greene et al. (5), who targeted Neu, the rodent form of ErbB-2, and later developed this into a widely used clinical strategy (6). The molecular mechanisms underlying the growth-inhibitory effects of anti–ErbB-2 monoclonal antibodies (mAbs) may involve indirect tumor cell cytotoxicity through immunological mechanisms, including antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), increased cancer cell apoptosis, as well as direct interference with signaling cascades (6).
Clinical studies established that Trastuzumab (Herceptin), a humanized mAb directed against ErbB-2, is active against ErbB-2-overexpressing metastatic breast cancer, leading to its approval for clinical use (7). The objective response rates to Trastuzumab monotherapy is relatively low (≈15%) and short lived (median duration, 9 months) (8). On the other hand, mAbs seem to display a synergistic effect when combined with chemotherapy, probably because of interruption of ErbB-2-driven survival pathway (9). Yet another strategy, relevant to pancreatic cancer, combines antibodies to EGFR and to ErbB-2 (10). The present study explores an alternative strategy to enhance the therapeutic activity of anti-receptor antibodies, namely combining two or more epitope-distinct antibodies. This strategy was previously demonstrated by Drebin et al. (11) and by Kasprzyk et al. (12). It was later proposed that the superior activity of mAb combinations is attributable to a combination of various factors, including improved effector cell recruitment as ADCC and CDC (13, 14). We have previously demonstrated that combining anti-EGFR mAbs that engage distinct epitopes proved more effective in down-regulating the receptor in vitro than each antibody alone (15) because of the generation at the cell surface of very large receptor-antibody complexes or lattices, which collapse into the cytoplasm and eventually undergo degradation in lysosomes. Here we demonstrate that pairs of mAbs specific to distinct epitopes of ErbB-2, of which one epitope is involved in dimerization, are highly effective anti-tumor agents in vivo and are capable of inhibiting tumor cell growth in vitro. We also show that a noninhibitory mAb, which obviously does not induce a cellular response, contributes to the synergistic effect. This implies that direct effects of the mAbs occur in addition to effector mechanisms.
Results
Certain Combinations of Monoclonal Anti-ErbB-2 Antibodies Collaboratively Inhibit Tumor Growth In Vivo.
We have previously described a series of anti-ErbB-2 mAbs, which, when singly applied, variably inhibit the tumorigenic growth in vivo of N87 human gastric cells overexpresing ErbB-2 (16, 17). To examine the effect of combining two antibodies, N87 cells were injected s.c. into athymic mice, which elicited rapidly growing tumors. Thereafter, the four mAbs or their six combinations were intraperitoneally (i.p.) injected into groups of seven mice. Fig. 1 depicts the average tumor volume of each group as a function of postgrafting time. Although the antibodies differed in their therapeutic efficacy, with only one exception (a combination of mAbs L431 and L26), antibody combinations more effectively inhibited tumor growth than each antibody alone. Notably, tumors were completely eradicated in at least four of seven mice after treatment with the two most effective combinations, namely L26 plus N12 and L431 plus N12. Moreover, this effect persisted 6 weeks after the last injection. Interestingly, when singly applied, N29 was not effective. Nevertheless, this mAb enhanced the inhibitory effect of other mAbs, although tumors initially inhibited by N29-containing combinations displayed re-growth (Fig. 1). In conclusion, the majority of antibody combinations that we tested showed clear synergistic anti-tumor effects.
Fig. 1.
In vivo antitumor effects of antibodies to ErbB-2/HER2 and their combinations. Groups of seven CD-1/nude mice were injected s.c. with 5 × 106 N87 cells. mAbs (total, 720 μg per animal) were then injected i.p., either alone or in combinations, at days 7, 10, and 13 after grafting. Saline solution-injected mice were used for control (○). Combination treatments using the indicated antibodies are shown (●), along with mAb1 alone (▲) and mAb2 alone (△). Tumors were measured once a week by using calipers, and the mean tumor volume (cm3 ± SE) was plotted. Differences between the combined effects of N12+L431 or N12+L26 versus the individual mAbs are statistically significant (P < 0.05).
Antibody Combinations Inhibit Cell Growth In Vitro.
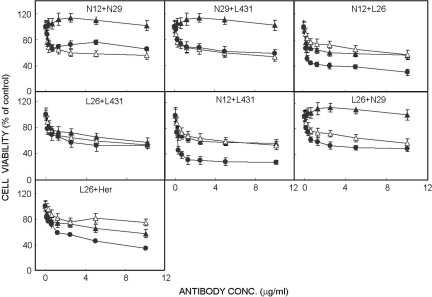
The synergistic in vivo effects that we observed may be caused by immunological mechanisms (e.g., ADCC). Hence, we expected that antibody synergy would not extend to an in vitro test, such as the MTT cell proliferation assay (18). Fig. 2 depicts the results of an assay performed with N87 cells incubated for 72 h with various antibodies and their combinations. Remarkably, a strong correlation was found with most antibody combinations between the results obtained in this assay and the in vivo experiments (Fig. 1): Four of the six combinations exhibited synergistic effects. Moreover, the relative potency of the various combinations was preserved in vitro. For instance, the superior antibody combination in animals, namely N12+L431, was also the most potent in vitro combination, yielding 54% reduction in cell proliferation already at 0.3 μg/ml. Likewise, our least potent in vivo combination, namely L26+L431, displayed no synergy in vitro. Notably, the N29 antibody elicited no inhibitory effects both in vitro and in vivo, and when tested in combinations it did not improve the effects of mAbs N12 and L431. Yet, this antibody reproducibly enhanced the in vitro antiproliferative effect of L26 (Fig. 2), which attributes a role for antigen crosslinking by a second antibody.
Fig. 2.
In vitro growth inhibitory effects of antibodies to ErbB-2/HER2 and their combinations. Cultured N87 cells were treated for 72 h with increasing concentrations of the indicated antibodies (mAb1, ▲; mAb2, △; HER indicates Herceptin/Trastuzumab) or their combinations (●). Thereafter, the MTT assay was performed and cell viability presented as percentage of control untreated cultures (±SD; n = 8). Differences between the combined effects of N12+L431 (> 0.1 μg/ml), N12+L26 (> 0.1 μg/ml) and L26+Her (> 0.6 μg/ml) versus the respective individual mAb are statistically significant (P < 0.05).
We previously reported that a combination of L26, an antibody capable of inhibiting heterodimerization of ErbB-2 (16), and Trastuzumab displays synergy in an in vivo antitumor test (15). Extension of this analysis is shown in Fig. 2: The combination is significantly more potent than each antibody alone. To exclude complement involvement, we repeated the MTT assay by using a serum replacement mixture containing heat-treated albumin. Because the synergistic effects of antibody combinations were observed also in the absence of serum (data not shown), we conclude that neither CDC nor ADCC contribute to the ability of anti-ErbB-2 antibodies to collaborate in vitro. Taken together, the overall similarity between the in vivo and in vitro effects of antibody combinations implies that the synergistic antigrowth effects are mediated by activities intrinsic to the antibody molecules.
Characterization of Epitope Sharing by Anti-ErbB-2 Antibodies.
Our previous analysis of a panel of antibodies to EGFR indicated that mAb synergy requires interactions with two mutually distinct antigenic determinants (15). Hence, we analyzed the ability of each anti-ErbB-2 mAb to displace radiolabeled versions of the other mAbs. The results shown in Fig. 3 confirmed that the nonsynergizing pair of antibodies, namely L26 and L431, is cross competitive. On the other hand, pairs of mAbs that displayed synergy both in vivo and in vitro, including L431 plus N12 and L26 plus N12, bind distinct epitopes of ErbB-2. In the case of N29, the unlabeled antibody did not interfere with the binding of any of the radiolabeled antibodies, whereas the binding of a radiolabeled derivative of N29 was reduced by L431, possibly through steric interference. Consistent with this possibility, an attempt to map the N29 epitope has failed, suggesting that N29 recognizes a carbohydrate-containing epitope (19).
Fig. 3.
Antibody displacement analyses. The ability of unlabeled mAbs to displace the indicated cell surface-bound 125I-mAb was used as a measure of the degree of antigenic overlap. N87 cells were treated for 1 h at 4 °C with mAbs L431 (○), L26 (▲), N29 (△), and N12 (●). The indicated radiolabeled mAbs (8 nmol/l) were then added, and the cells were incubated for additional 15 min before determination of cell-bound radioactivity. The experiment was repeated three times.
Specific Antibody Combinations Accelerate Removal of ErbB-2 From the Cell Surface.
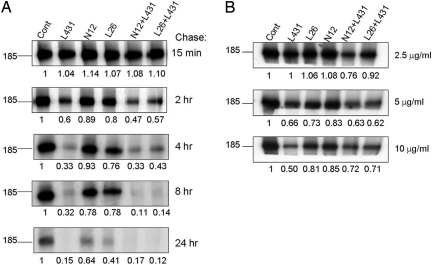
The interaction of receptor tyrosine kinases with their respective ligands is often coupled to rapid endocytosis and receptor degradation in lysosomes. It was shown that mAbs can induce an analogous, albeit slower, effect (20), which is associated with inhibition of tumorigenesis (12). Therefore, we tested the potential of our set of mAbs to alter the turnover rate of ErbB-2. To this end, N87 cells were biosynthetically labeled and then chased in fresh, mAb-containing medium. As shown in Fig. 4A, among the four mAbs that we tested, L431 remarkably accelerated degradation of ErbB-2, and this was slightly enhanced by the addition of N12. Because the nonsynergistic L26+L431 combination was less effective, we tested various concentrations of each antibody, as well as the two combinations (Fig. 4B; chase of 2 h). The results confirmed that the L431+N12 combination is superior at a relatively low antibody concentration, but this difference became less apparent at higher concentrations.
Fig. 4.
Effects of mAbs and their combinations on the turnover rate of ErbB-2. N87 cells were incubated for 16 h with a mixture of [35S]methionine and [35S]cysteine and then chased for the indicated periods of time with nonradioactive medium containing 20 μg/ml (A) or the indicated lower concentrations (B) of anti-ErbB-2 mAbs. Residual 35S-labeled ErbB-2 was subjected to immunoprecipitation with a rabbit polyclonal antibody directed to the carboxyl terminus of the protein and electrophoretically separated. Numbers below lanes represent densitometric quantification of ErbB-2 relative to control, untreated cells.
Because pulse–chase analysis examines the overall pool of ErbB-2, but mAbs interact with only the surface-exposed receptors, we applied surface biotinylation. Cells were surface-labeled with biotin after incubation for 8 h with single mAbs or their combinations. Thereafter, antibodies were acid-stripped and ErbB-2 immunoprecipitated. As depicted in Fig. 5A, the combination of mAbs L431 and N12 most potently down-regulated ErbB-2 from the cell surface. Interestingly, the other combination, L431+L26, was less effective than L431 alone, in line with their competitive interactions (Fig. 3). The superiority of the L431+N12 combination is evident also from the time course of ErbB-2 down-regulation (Fig. 5B): Almost all surface ErbB-2 molecules were cleared from the cell surface after a 24-h incubation with the L431+N12 combination, but each antibody exerted only a limited effect.
Fig. 5.
Cell surface biotinylation reveals enhanced effects of antibody combinations on endocytosis and ubiquitinylation of ErbB-2. N87 cells were treated for either 8 h with the indicated mAbs (5 μg/ml; A), or they were treated for increasing time intervals with the indicated antibodies (B). Thereafter, bound antibodies were acid-stripped and the cell surface was biotinylated. Cells were then lysed, and ErbB-2 immunoprecipitates (IP) or total cell lysates (TCL) were immunoblotted (IB) using the indicated antibodies or streptavidin horseradish peroxidase (HRP). Quantification of the signals is shown below the respective lanes. Ubiquitinylation of ErbB-2 (C) was determined by immunoblotting (with an anti-ubiquitin antibody from Babco/Covance) of ErbB-2 immunoprecipitates isolated from N87 cells treated with different mAbs for 1 h at 37 °C.
Sorting of receptor tyrosine kinases (e.g., EGFR) for intracellular degradation involves their ubiquitinylation, which recruits to the internalizing receptor ubiquitin-binding coat adaptors, such as Epsin (21). We previously proposed that this mechanism may underlie mAb-induced degradation of ErbB-2 (22). Hence, by using antiubiquitin antibodies we addressed the ability of mAbs and their combinations to enhance ErbB-2 ubiquitinylation. This assay revealed weak but reproducible mAb-induced ubiquitinylation of ErbB-2. Interestingly, L431 displayed higher effects than other mAbs, especially when combined with the noncompetitive N12 mAb. Once again, when combined with the competitive L26 antibody, the ubiquitinylation effect of L431 decreased. In conclusion, certain mAbs enhance ubiquitinylation of ErbB-2 and effectively target the surface-localized subpopulation of this receptor to intracellular degradation. Combining two noncompetitive mAbs enhances this activity, which correlates with the synergistic growth inhibitory effects of such combinations (Figs. 1 and 2). Below we discuss potential functional links between receptor internalization and inhibition of tumor growth in experimental and clinical settings.
Discussion
Understanding molecular mechanisms underlying cancer immunotherapy may guide optimal selection of mAbs for therapy and teach us how to overcome primary and acquired resistance (6). The present study addresses an emerging strategy, which enhances the therapeutic activity of antireceptor antibodies by combining two mAbs engaging distinct epitopes. In general, this superior activity may be attributable to a combination of factors that enhance immune effector cell-mediated cytotoxicity (14), including enhanced antiproliferative and antiapoptotic effects, improved Fc-mediated effector functions, and recruitment to tumors of natural killer cells (23). However, it can also include acceleration of receptor internalization (15), improved blockade of signaling pathways downstream to ErbB-2, as well as enhanced immune effector cell-mediated cytotoxicity (14). A reasonable assessment is that multiple mechanisms are involved in the antitumor efficacy by the anti-HER2 antibodies. These pathways, mAb induced or cellularly mediated, provide opportunities such as those proposed in our study to improve on the current single anti-HER2 therapeutic.
In favor of immune mechanisms, a mixture of anti-ErbB-2 mAbs requires Fc-mediated effector functions for optimal in vivo activity (24). On the other hand, other studies have shown that bivalent, Fc-lacking versions of antireceptor mAbs partially inhibit tumorigenic growth in animals (25–27), emphasizing the role of direct antibody binding to the receptor. This is supported in our present study: antibody combinations that displayed synergistic tumor inhibition in animals (Fig. 1) also collaborated in vitro in the absence of immune effector cells (Fig. 2). We previously proposed a mechanism attributing synergy to the ability of epitope-distinct antibodies to form large antigen–antibody lattices at the cell surface, which collapse into the cytoplasm and undergo degradation in lysosomes (15). Aggregation, in this case, is dramatically enhanced, as demonstrated by disappearance of the receptor from the cell surface (cotreatment after 3 h equals that by mono-treatment after 24 h), which correlates with attenuated ligand- or heterodimerization-induced signaling potency and duration.
It is worthwhile considering the identity of the most effective tumor-inhibitory antibody combinations (Fig. 1). It has been shown that ErbB-2 harbors multiple nonoverlapping antigenic sites (16, 27), including a site involved in receptor heterodimerization (16). The latter likely corresponds to the receptor's dimerization arm identified in crystal structures (28). Our most effective and durable antibody combinations, namely L431+N12 and L26+N12, each includes an antibody to the dimerization site: L26 is a bone fide dimerization-inhibitory mAb (16), and, according to our competition analysis (Fig. 3), mAb L431 binds with the same or with a closely localized site of ErbB-2. Interestingly, another dimerization blocker, mAb 2C4, and the humanized version, Pertuzumab/Omnitarg, effectively inhibits tumor xenografts (29). Moreover, when combined with Trastuzumab it improved the response rate of ErbB-2-positive metastatic breast cancer patients (30). These examples raise the intriguing possibility that a combination of a dimerization-blocking mAb and another mAb that binds with a distinct site of ErbB-2 would synergistically inhibit the tumorigenic growth of ErbB-2–overexpressing cancer cells, not only in vitro and in vivo in animals but also in cancer patients.
It is important noting that antibody combinations that do not interfere with heterodimerization are less effective in vivo in animals and in vitro, yet they display cooperative interactions in these assays. A remarkable example is provided by the N29 mAb, which displayed growth inhibitory activity neither in vivo in animals (Fig. 1) nor in vitro (Fig. 2), yet enhanced the inhibitory ability of other mAbs. Another illuminating combination has an antagonistic rather than a cooperative effect: When combined with the mutually competitive L26, antibody L431 lost part of its relatively strong activity as a tumor antagonist (Fig. 1) and as an accelerator of ErbB-2 endocytosis (Figs. 3B and 5A). Both the antagonistic and the cooperative interactions may be explained in terms of the “lattice model” that we previously proposed (15): Antireceptor antibodies collaborate as long as they do not interfere with each other, and their combination engages a large receptor aggregate, which is sorted for intracellular degradation.
Unlike the robust EGF-induced endocytosis and degradation of EGFR, ErbB-2 undergoes slow endocytosis, which is followed by recycling to the cell surface (31–33). To exit the recycling pathway and enter a route leading to degradation in lysosomes, internalizing receptors must recruit ubiquitin ligases, such as c-Cbl, and must undergo ubiquitinylation (21, 34). Unlike their natural ligands, antireceptor antibodies, such as anti-EGFR (15), anti-NCAM (35), and anti–ErbB-2 antibodies (22), induce relatively weak receptor ubiquitinylation. Consistent with immunotherapeutic relevance of antibody-induced receptor degradation (Fig. 4A), removal from the cell surface (Fig. 5 A and B), and ubiquitinylation (Fig. 5C), in these assays the synergistic combination L431+N12 is more active than the nonsynergistic pair (L431+L26).
In summary, by using combinations of anti-ErbB-2 antibodies, our study provides evidence supporting the possibility that mAb-induced internalization and degradation of surface receptors contribute to immunotherapy, at least in an animal model. We predict that certain combinations of mAbs directed to ErbB-2 or to other receptor tyrosine kinases will enhance therapeutic efficacy and synergy with chemotherapy. This may be especially important for breast cancer patients who eventually develop secondary resistance to antibodies like Trastuzumab (36). Future studies will address the molecular mechanisms underlying the endocytic superiority of antibody combinations.
Materials and Methods
Materials, Antibodies, and Cells.
Unless indicated, materials were purchased from Sigma (St. Louis), cells from the American Type Culture Collection (Manassas, VA), and antibodies from Santa Cruz Biotechnology (Santa Cruz, CA). Radiochemicals were purchased from Amersham (Buckinghamshire, United Kingdom). Trastuzumab was provided by Genentech (South San Francisco, CA). The previously described mAbs to ErbB-2 (16, 17) were purified on protein G plus agarose.
Surface Biotinylation Assay.
Cells were incubated at 37 °C with mAbs, transferred to ice, and the mAbs removed by using a low pH solution (0.15 mol/l acetic acid containing 0.15 mol/l NaCl; 4 min). The cells were washed and incubated for 60 min at 4 °C with N-hydroxysuccinimide (NHS)–biotin (0.5 mg/ml; Calbiochem, San Diego). Coupling of biotin was blocked with 15 mmol/l glycine (15). Thereafter, cells were solubilized by the addition of lysis buffer consisting of 50 mmol/l HEPES (pH 7.5), 150 mmol/l NaCl, 10% glycerol, 1% Triton X-100, 1 mmol/l EDTA, 1 mmol/l EGTA, 1 mmol/l phenylmethylsulfonyl fluoride, 50 mmol/l sodium fluoride, 0.5 mmol/l Na3VO4, 5 μg/ml leupeptin and 5 μg/ml aprotonin, and a rabbit antibody to ErbB-2 used for immunoprecipitation.
Radiolabeling of Antibodies.
MAbs (100 μg in 0.2 ml phosphate-buffered saline solution) were radiolabeled by using Na125I (5 μl; 0.5 mCi [18.5 MBq]) and chloramine-T (10 mg/10 μl). The reaction mixture was chromatographed on Sephadex G-25 yielding radiolabeled mAbs of 1–2 mCi/mg protein. MAb N29 was radiolabeled by using the [125I]-Bolton-Hunter reagent (PerkinElmer Sciences, Boston).
Antibody Competition Assay.
N87 cells (250,000 cells/well) grown in 24-well plates were treated for 1 h at 4 °C with increasing concentrations of unlabeled mAbs. Radiolabeled mAbs (8 nmol/l) were then added, and the cells incubated for an additional 15 min at 4 °C. After washing, the cells were solubilized in 0.5 N NaOH solution before determination of radioactivity.
Inhibition of N87 Tumor Cell Growth in Culture.
Antibodies were added to N87 cells (10,000 cells/well) grown in 96-well plates. Incubation at 37 °C proceeded for 72 h and cell viability determined by using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reagent (18).
Determination of the Effect of Antibodies on Receptor Turnover.
N87 cells were labeled by incubation (16 h at 37 °C) in methionine- and cysteine-free medium supplemented with 10% dialyzed fetal calf serum and Promix, a mixture of [35S]methionine and [35S]cysteine (50 μCi/ml). Thereafter, cells were chased for various periods of time by incubation in fresh medium in the absence or presence of the antibodies. The cells were then washed, and lysates were subjected to immunoprecipitation.
Tumorigenic Cell Growth in Animals.
Female CD/nude mice were injected s.c. with N87 cells (5 × 106 per mouse). The mAbs were injected i.p. at days 7, 10, and 13 after grafting. Groups of seven mice were used, with each mouse receiving 0.72 mg of purified mAb. Tumor parameters were measured once a week.
Statistical Analysis.
Student's t test (two-tailed) was used to test differences between the effects of antibody combinations and single treatment. Values of P < 0.05 were considered statistically significant.
Acknowledgments.
This work was supported by grants from the Mark Rich Foundation (the Linda de Picciotto Pancreas Cancer Research Program), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Evelyn and Harold Igdaloff Foundation, and the National Cancer Institute Grant CA72981.
Footnotes
The authors declare no conflict of interest.
References
- 1.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 2.King CR, et al. Heterogeneous expression of erbB-2 messenger RNA in human breast cancer. Cancer Res. 1989;49:4185–4191. [PubMed] [Google Scholar]
- 3.Slamon DJ, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 5.Drebin JA, Link VC, Weinberg RA, Greene MI. Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen. Proc Natl Acad Sci USA. 1986;83:9129–9133. doi: 10.1073/pnas.83.23.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Kasus T, Schechter B, Sela M, Yarden Y. Cancer therapeutic antibodies come of age: Targeting minimal residual disease. Mol Oncol. 2007;1:42–54. doi: 10.1016/j.molonc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter P, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobleigh MA, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 9.Arteaga CL, et al. p185c-erbB-2 signal enhances cisplatin-induced cytotoxicity in human breast carcinoma cells: Association between an oncogenic receptor tyrosine kinase and drug-induced DNA repair. Cancer Res. 1994;54:3758–3765. [PubMed] [Google Scholar]
- 10.Larbouret C, et al. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas. Clin Cancer Res. 2007;13:3356–3362. doi: 10.1158/1078-0432.CCR-06-2302. [DOI] [PubMed] [Google Scholar]
- 11.Drebin JA, Link VC, Greene MI. Monoclonal antibodies specific for the neu oncogene product directly mediate anti-tumor effects in vivo. Oncogene. 1988;2:387–394. [PubMed] [Google Scholar]
- 12.Kasprzyk PG, Song SU, Di Fiore PP, King CR. Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies. Cancer Res. 1992;52:2771–2776. [PubMed] [Google Scholar]
- 13.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 14.Spiridon CI, et al. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res. 2002;8:1720–1730. [PubMed] [Google Scholar]
- 15.Friedman LM, et al. Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: Implications for cancer immunotherapy. Proc Natl Acad Sci USA. 2005;102:1915–1920. doi: 10.1073/pnas.0409610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klapper LN, et al. A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER2 blocks crosstalk with growth factor receptors. Oncogene. 1997;14:2099–2109. doi: 10.1038/sj.onc.1201029. [DOI] [PubMed] [Google Scholar]
- 17.Stancovski I, et al. Mechanistic aspects of the opposing effects of monoclonal antibodies to the ERBB2 receptor on tumor growth. Proc Natl Acad Sci USA. 1991;88:8691–8695. doi: 10.1073/pnas.88.19.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Yip YL, Smith G, Koch J, Dubel S, Ward RL. Identification of epitope regions recognized by tumor inhibitory and stimulatory anti-ErbB-2 monoclonal antibodies: Implications for vaccine design. J Immunol. 2001;166:5271–5278. doi: 10.4049/jimmunol.166.8.5271. [DOI] [PubMed] [Google Scholar]
- 20.Yarden Y. Agonistic antibodies stimulate the kinase encoded by the neu protooncogene in living cells but the oncogenic mutant is constitutively active. Proc Natl Acad Sci USA. 1990;87:2569–2573. doi: 10.1073/pnas.87.7.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmor MD, Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–2070. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]
- 22.Klapper LN, Waterman H, Sela M, Yarden Y. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 2000;60:3384–3388. [PubMed] [Google Scholar]
- 23.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 24.Spiridon CI, Guinn S, Vitetta ES. A comparison of the in vitro and in vivo activities of IgG and F(ab′)2 fragments of a mixture of three monoclonal anti-Her-2 antibodies. Clin Cancer Res. 2004;10:3542–3551. doi: 10.1158/1078-0432.CCR-03-0549. [DOI] [PubMed] [Google Scholar]
- 25.Fan Z, Lu Y, Wu X, Mendelsohn J. Antibody-induced epidermal growth factor receptor dimerization mediates inhibition of autocrine proliferation of A431 squamous carcinoma cells. J Biol Chem. 1994;269:27595–27602. [PubMed] [Google Scholar]
- 26.Hurwitz E, Klapper LN, Wilchek M, Yarden Y, Sela M. Inhibition of tumor growth by poly(ethylene glycol) derivatives of anti-ErbB2 antibodies. Cancer Immunol Immunother. 2000;49:226–234. doi: 10.1007/s002620000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu F, et al. Antibody-induced growth inhibition is mediated through immunochemically and functionally distinct epitopes on the extracellular domain of the c-erbB-2 (HER-2/neu) gene product p185. Int J Cancer. 1993;53:401–408. doi: 10.1002/ijc.2910530310. [DOI] [PubMed] [Google Scholar]
- 28.Garrett TP, et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11:495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 29.Agus DB, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 30.Baselga J, et al. Objective response rate in a phase II multicenter trial of pertuzumab (P), a HER2 dimerization inhibiting monoclonal antibody, in combination with trastuzumab (T) in patients (pts) with HER2-positive metastatic breast cancer (MBC) which has progressed during treatment with T. J Clin Oncol (2007 ASCO Annual Meeting Proceedings Part I) 2007;25:1004. abstr. [Google Scholar]
- 31.Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 32.Pinkas-Kramarski R, et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 33.Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274:8865–8874. doi: 10.1074/jbc.274.13.8865. [DOI] [PubMed] [Google Scholar]
- 34.Katzmann DJ, Odorizzi G, Emr SD. Receptor down-regulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 35.Diestel S, Schaefer D, Cremer H, Schmitz B. NCAM is ubiquitylated, endocytosed and recycled in neurons. J Cell Sci. 2007;120:4035–4049. doi: 10.1242/jcs.019729. [DOI] [PubMed] [Google Scholar]
- 36.Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]