Abstract
Obesity is associated with increased risk for developing pancreatic cancer, and it is suggested that insulin resistance provides the missing link. Here we demonstrate that under the context of genetic susceptibility, a high fat diet (HFD) predisposes mice with oncogenic K-ras activation to accelerated pancreatic intraepithelial neoplasm (PanIN) development. Tumor promotion is closely associated with increased inflammation and abrogation of TNFR1 signaling significantly blocks this process underlining a central role for TNFα in obesity-mediated enhancement of PanIN lesions. Interestingly, however, despite increased TNFα levels, mice remain insulin sensitive. We show that, while aggravating tumor promotion, a HFD exerts dramatic changes in energy metabolism through enhancement of pancreatic exocrine insufficiency, metabolic rates, and expression of genes involved in mitochondrial fatty acid (FA) β-oxidation that collectively contribute to improved glucose tolerance in these mice. While on one hand these findings provide significant evidence that obesity is linked to tumor promotion in the pancreas, on the other it suggests alterations in inflammatory responses and bioenergetic pathways as the potential underlying cause.
Keywords: energy metabolism, cancer, TNFα, mitochondria, pancreas
Obesity increases the risk for gastrointestinal (GI) cancers (1). Over the past years, death rates have been significantly elevated among men and women with a body mass index (BMI) of ≥30 kg/m2 (1, 2). Increased risk in pancreatic cancer is found among individuals who had gained 12 kilos or more as adults, compared to those who only gained 2–5 kilos (3). Importantly, moderate exercise was associated with more than 50% reduction in risk for pancreatic cancer. Although epidemiological studies suggest that obesity through insulin resistance is associated with pancreatic cancer, the underlying cellular and molecular mechanisms remain largely unknown.
Obesity and cancer share inflammation as common pathogenesis. Recent studies have demonstrated that HFD-induced obesity is a low-grade inflammatory condition and through activation of the IKKβ-NF-κB pathway macrophages play an important role in the development of insulin resistance (4, 5). On the contrary, the connection between inflammation and cancer goes back to 19th century during which Virchow demonstrated that tumors arise from regions of chronic inflammation (6). It is now widely accepted that the tumor microenvironment contains inflammatory cells that secrete proinflammatory cytokines, chemokines, metalloproteases, growth factors, and reactive oxygen species, which can induce DNA damage and chromosomal instability and thereby drive carcinogenesis (7–9).
Among the animal models that recapitulate human pancreatic ductal adenocarcinoma (PDA), pancreas-specific activation of oncogenic K-ras displays the full spectrum of PanIN lesions thereby closely resembling the human malignancy from tumor promotion to progression even though in human conditions activation takes place most likely during adulthood (10). Recent studies suggest that in addition to somatic K-ras activation, PDA requires nongenetic events such as increased inflammation and tissue damage (11). It is suggested that during caerulein-induced chronic pancreatitis, infiltration of immune cells and increased production of inflammatory cytokines play a crucial role in the amplification of a certain pool of acinar progenitor cells that are susceptible to transformation by K-ras activation and/or in facilitating transdifferentiation of mature acinar cells implying that inflammation is essential for enhancement of PanIN development and PDA (11).
K-ras mice are characterized by a long phase of tumor promotion, and progression to full malignancy occurs rather late (10). Thus these mice represent a suitable model to investigate the role of a high caloric diet as a potential tumor promoter especially during very early tumor development. Here we demonstrate that a HFD predisposes mice with oncogenic K-ras activation to enhanced PanIN development and TNFα plays a causal role in linking obesity to tumor promotion interestingly in the absence of increased insulin resistance owing to dramatic changes in energy metabolism.
Results
Obesity Accelerates PanIN Development.
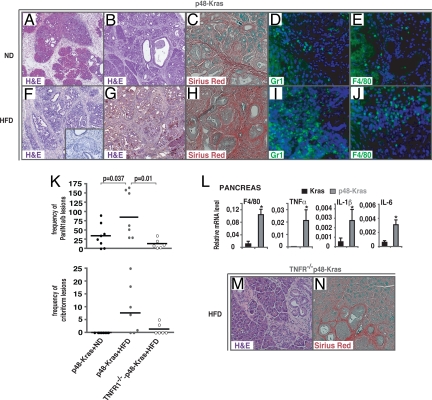
Obesity is a major risk factor for developing pancreatic cancer (1, 2). To elucidate the molecular mechanisms linking obesity to the pathogenesis of pancreatic cancer, we used a well-established mouse model, p48Cre-K-rasLSL-G12D/+, with K-ras expression, initially in the pancreatic progenitors, that retains oncogenic activity in all adult pancreatic cells (termed p48-Kras) (10). Development of PanINs in p48-Kras mice and littermate controls (K-rasLSL-G12D/+, termed Kras) was analyzed on histological sections fed on regular chow diet (ND) (Fig. 1 A and B, supporting information (SI) Fig. S1a) or HFD (Fig. 1 F and G, Fig. S1b) at 10 and 16 weeks, and lesion frequency and grade were scored at 14 weeks of age (Fig. 1K). p48-Kras mice displayed significantly increased PanIN 1a, 1b, and atypical cribriform lesions on a HFD compared to that of the age-matched ND group (Fig. 1K). Pancreata during a HFD were totally transformed with cystic dilation of ducts and they displayed complete atrophy of the acini, which was accompanied by a strong stromal reaction with increased fibrotic and inflammatory responses (Fig. 1 C–E and H–J, Fig. S1b). Further analysis of 30-week-old p48-Kras mice either fed on a ND (n = 9) or a HFD (n = 8) confirmed lesion extent in these animals (Fig. S2). Importantly, approximately 44% of p48-Kras mice showed cribriform lesions even when kept on a ND whereas 100% displayed cribriform lesions on a HFD. Furthermore, approximately 44% of p48-Kras mice on a ND showed PanIN 1b lesions in contrast to 75% of mice on a HFD. When approximately 22% of the ND group displayed PanIN2 lesions, 50% of pancreata had PanIN2 in the HFD group. Moreover, although none of the mice in the ND group showed PanIN3 lesions, 12.5% of mice fed on the HFD showed PanIN3 lesions, thereby suggesting a substantial effect of high caloric diet on enhanced PanIN development.
Fig. 1.
HFD accelerates tumor promotion and TNFR1 deletion ameliorates tumorigenesis. H&E staining of p48-Kras pancreata on a ND regimen at (A) 10 and (B) 16 weeks of age. (C) Fibrosis in p48-Kras mice kept on the ND was analyzed by Sirius Red staining. (D and E) Infiltration of inflammatory cells on the ND was checked by immunofluorescent staining using (D) α-Gr1 and (E) α-F4/80. (F and G) Representative H&E stained sections of p48-Kras pancreata on the HFD showing cribriform PanIN1 lesion at (F) 10 and (G) 16 weeks of age. (H) Fibrotic response in p48-Kras mice kept on the HFD was checked by Sirius Red staining. (I and J) Increased infiltration of (I) Gr-1+ and (J) F4/80+ cells on the HFD is shown by immunofluorescent staining. (K) PanIN frequency and grade were quantitated in all mouse groups at 14 weeks of age. (L) Expression analysis of genes involved in inflammatory responses in the pancreas of both p48-Kras mice and Kras control littermates during the HFD were measured by Real-Time PCR. (M and N) H&E staining of TNFR1−/−-p48-Kras mice pancreata on the HFD at 10 weeks of age (M). (N) Fibrosis was assessed by Sirius Red staining. Data are mean values ± SEM. N = 4–5 mice per genotype. *, P ≤ 0.05.
TNFR1 Deletion Significantly Ameliorates Tumor Promotion.
Because it has been suggested that chronic inflammation enhances tumor development (11), and because obesity is known to lead to low-grade inflammation (4), we checked myeloid cell marker and proinflammatory cytokine expression levels in p48-Kras mice and littermate controls following a HFD. Along with increased infiltration of inflammatory cells in the pancreas (Fig. 1 I and J), we found F4/80, TNFα, IL-1β, and IL-6 mRNA and circulating levels of TNFα and IL-6 to be significantly elevated in p48-Kras mice (Fig. 1l and Fig. S3a). To directly elucidate the role of TNFα, one of the most important modulators of inflammatory responses, on increased PanIN development, we crossed p48-Kras mice on a TNFR1-deficient background (termed TNFR1−/−-p48-Kras). Interestingly, TNFR1 deletion partially but significantly attenuated HFD-enhanced PanIN development in p48-Kras mice (Fig. 1K). Histological analysis in TNFR1−/−-p48-Kras mice revealed lesions mostly residing at the head of the pancreas (Fig. 1M and Fig. S4). The pancreata contained mostly intact acini and fibrosis was markedly reduced (Fig. 1N and Fig. S4). These results indicate that obesity enhanced tumor promotion in p48-Kras mice by increased inflammation and TNFα played a pivotal role in this process.
HFD-Induced Inflammation Enhances Tumor Promotion Independently of Insulin Resistance.
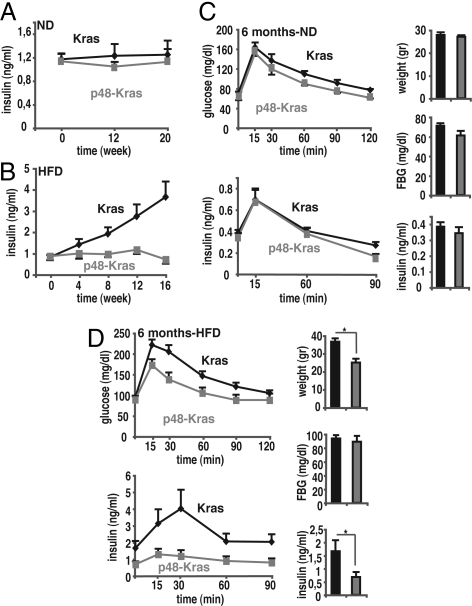
Inflammation is a major contributor to insulin resistance during HFD (4) and the role of TNFα is well established in this process (12). To elucidate whether insulin resistance was the molecular mechanism linking inflammation to increased PanIN development, we checked insulin levels in p48-Kras mice and controls under ND and HFD regimens. Nonfasting insulin levels in p48-Kras mice remained similar to Kras controls on the ND (Fig. 2A). Surprisingly, however, p48-Kras mice retained normoinsulinemia even on the HFD despite elevated TNFα levels and advanced PanIN formation (Fig. 2B). When we performed glucose tolerance tests (GTTs), regardless of gender, p48-Kras mice remained glucose tolerant as Kras controls on the ND (Fig. 2C and Fig. S5a). Postprandial insulin secretion achieved was similar in both p48-Kras mice and littermate controls indicating a state of enhanced insulin sensitivity (Fig. 2C and Fig. S5a). Importantly however, in contrast to Kras controls, p48-Kras mice on the HFD continued to display improved glucose tolerance although with considerably low postprandial insulin levels, suggesting that insulin resistance was not linked to advanced PanIN development in these animals (Fig. 2D and Fig. S5b).
Fig. 2.
HFD enhances tumorigenesis independent of insulin resistance. (A and B) Nonfasting plasma insulin values for p48-Kras mice and Kras control littermates kept on (A) a ND and (B) a HFD. (C) GTTs were performed in 6-month-old female p48-Kras mice and Kras control littermates on a ND. Insulin response curve during GTT together with corresponding weight, fasting glucose, and insulin values during the test are shown. (D) Glucose clearance and insulin response curve during GTT in 6-month-old female p48-Kras mice and Kras control littermates on the HFD are shown with corresponding weight, fasting glucose, and insulin values during the test. Data are mean values ± SEM. n = 5–7 mice per genotype. *, P ≤ 0.05.
Reduction in fat mass is certainly a major contributor to improved insulin sensitivity. To rule out that the significant weight difference observed at 6 months (Fig. 2D and Fig. S5b) was responsible for the enhanced glucose tolerance in p48-Kras mice, we performed GTTs on 3-month-old mice on a HFD regimen where the 2 genotypes showed no difference in body weight (Fig. S5c). Despite similar weights, p48-Kras mice at 3 months of age remained significantly refractory to obesity-induced insulin resistance (Fig. S5c), indicating a weight-independent cause for enhanced glucose tolerance in these mice.
Oncogenic K-ras expression takes place in all adult pancreatic cells in p48-Kras mice. To confirm that the exocrine compartment is responsible for the improved glucose tolerance observed in these animals, we crossed K-ras mice to the ElastaseCreER line to generate ElastaseCreER-K-rasLSL-G12D/+ mice (termed Ela-Kras), which have been shown to effectively recombine acinar cells, but not endocrine cells upon tamoxifen administration (13). Newly weaned mice received 5 consecutive injections of tamoxifen to induce deletion of the floxed cassette and were given the HFD at 4 weeks of age. Although Kras and Ela-Kras mice at 3 months of age did not show any difference in their glucose tolerance, at 5 months Ela-Kras mice displayed significantly low fasting glucose levels (Fig. S6 a and b). Moreover, during the GTTs, Ela-Kras mice required less insulin than Kras littermates to control their increased blood glucose levels and yet they still had lower glucose curves resembling p48-Kras mice, however, to a lesser extent (Fig. S6b). This was the case presumably because PanIN frequency and grade were significantly lower in Ela-Kras mice (14) when compared to p48-Kras animals at a similar age (Fig. S6c). These results demonstrate that exocrine pancreas played a more crucial role during improved glucose tolerance in p48-Kras animals.
HFD Alters Energy Metabolism Dramatically by Aggravating Pancreatic Exocrine Insufficiency.
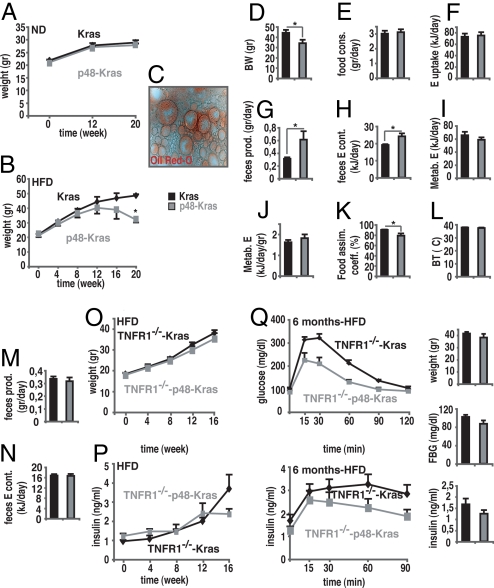
To define how mice retained insulin sensitivity, despite increased inflammation, we checked energy metabolism in p48-Kras mice and littermate controls. p48-Kras mice showed normal growth on the ND (Fig. 3A). Even though mice gained significant weight when kept on the HFD, p48-Kras mice remained leaner at around week 12 (Fig. 3B). Importantly, p48-Kras mice displayed pronounced weight loss thereafter, suggesting that this resulted from advanced disruption of the pancreas.
Fig. 3.
HFD alters energy metabolism by aggravating pancreatic exocrine insufficiency and TNFR1 deletion counteracts negative energy balance. (A and B) Weight curves of p48-Kras mice and Kras control littermates on (A) a ND and (B) a HFD. (C) Pancreatic exocrine insufficiency was determined by Oil Red O staining of fecal specimens in p48-Kras mice on the HFD. (D–L) p48-Kras mice and control littermates on the HFD were housed individually in cages over 5 days ad libitum and (D) body weight, (E) food consumption, (F) energy uptake, and (G) feces production were recorded. (H) Following dehydration, the energy content of feces was measured by using bomb calorimetry. (I) Absolute metabolized energy and (J) mass-specific metabolized energy were calculated on the basis of the difference between total energy uptake and energy content of the feces. (K) Food assimilation coefficient was calculated by % ratio of energy uptake to metabolized energy. (L) Rectal body temperature was measured. (M–N) TNFR1−/−-p48-Kras mice and TNFR1−/−-Kras control littermates on the HFD were housed individually in cages over 5 days ad libitum and (M) feces production was recorded. (N) Following dehydration, feces energy content was measured by using bomb calorimetry. (O) Weight curves of female TNFR1−/−-p48-Kras mice and TNFR1−/−-Kras control littermates on the HFD. (P) Nonfasting plasma insulin values for female TNFR1−/−-p48-Kras mice and TNFR1−/−-Kras control littermates during the HFD regimen. (Q) Glucose clearance and insulin response curve during GTT in 6-month-old female TNFR1−/−-p48-Kras mice and TNFR1−/−-Kras control littermates on the HFD are shown with corresponding weight, fasting glucose, and insulin values during the test. Data are mean values ± SEM. n = 5–7 mice per genotype. *, P ≤ 0.05.
Although substantial reduction in nutrient intake and increased energy expenditure have been shown to play a significant role in losing weight, malabsorption often seen in cancer patients contributes to negative energy balance (15). To check whether weight loss in p48-Kras mice was the result of malabsorption, mice on the HFD were housed individually in cages over 5 days ad libitum and alterations in energy uptake and loss were elucidated. Oil-Red-O staining of stool specimens showed increased lipid excretion in p48-Kras mice compared to littermate controls clearly indicating pancreatic exocrine insufficiency on the HFD (Fig. 3C). Accordingly, body mass remained significantly lower, although the daily food consumption and the energy uptake in p48-Kras mice were comparable to Kras controls (Fig. 3 D–F). On the contrary, the amount of stool produced and the energy loss through excretion in p48-Kras mice significantly exceeded that of controls (Fig. 3 G and H). Even though the mass-specific metabolized energy tended to be high, the food assimilation coefficient [% (metabolized energy/energy uptake)] remained significantly lower (79% in p48-Kras mice vs. 90% in controls) with no change in body temperature (Fig. 3 I–L). These results suggest that p48-Kras mice, similar to patients with chronic pancreatitis (16–18), experienced profound steatorrhea on the HFD.
In accordance with TNFα function in enhancing PanIN development, TNFR1 deficiency led to attenuation of metabolic alterations in p48-Kras mice (Fig. 3 M–P). Importantly, in contrast to age- and gender-matched counterparts on the HFD that showed significant weight loss (compare Fig. 3B) because of increased pancreatic exocrine insufficiency, TNFR1 deletion in p48-Kras mice corrected the negative energy balance (Fig. 3 M and N) and the weight loss (Fig. 3O), suggesting a role for TNFα in pancreatic exocrine insufficiency.
Moreover, although TNFR1 deficiency significantly restored nonfasting (compare Figs. 3P and 2B) and fasting insulin levels in p48-Kras mice (compare Figs. 3Q and 2D), TNFR1−/−-p48-Kras mice continued to display improved glucose tolerance with low postprandial insulin secretion on the HFD (Fig. 3Q), further demonstrating insulin sensitivity in p48-Kras mice occurred independent of weight changes.
HFD Enhances Metabolic Rates and Mitochondrial Fatty Acid β-Oxidation.
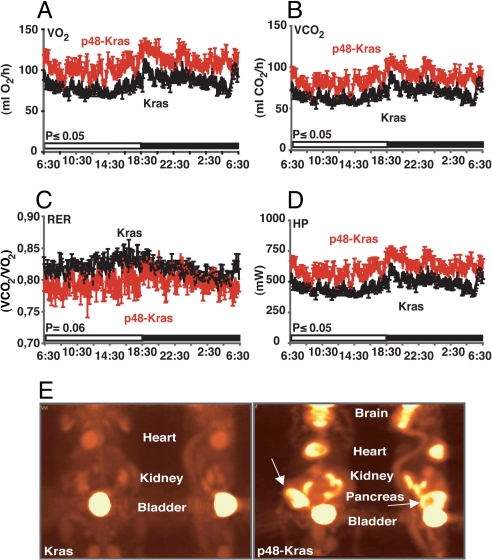
To elucidate changes in energy expenditure during enhanced PanIN development on the HFD, p48-Kras mice and littermate controls were housed individually in metabolic chambers for 24 h (12-h light cycle followed by 12-h dark cycle) and their metabolic rates were analyzed by indirect calorimetry. Interestingly, p48-Kras mice showed significantly increased O2 consumption and CO2 production (Fig. 4 A and B). Furthermore, they had decreased respiratory exchange rate (RER: VCO2/VO2) (Fig. 4C) suggestive of a shift toward fat utilization in these mice. Moreover, mice displayed elevated thermogenesis (Fig. 4D). Overall p48-Kras mice showed a 20% increase in their daily energy expenditure, suggesting significantly increased metabolic rates during HFD-enhanced tumor promotion.
Fig. 4.
p48-Kras mice are hypermetabolic and display increased energy uptake in the pancreas. (A–D) p48-Kras mice and Kras control littermates on a HFD were housed in metabolic chambers and (A) VO2 consumption, (B) VCO2 production, (C) respiratory exchange rate (RER), and (D) heat production (HP) were measured over 24 h (12-h light and 12-h dark cycle). (E) [18F]-FDG uptake in p48-Kras mice and control littermates was monitored by positron emission tomography (PET). Data are mean values ± SEM. n = 4–6 mice per genotype. *, P ≤ 0.05.
To determine whether increased metabolic rates (Fig. 4 A–D) were correlated with increased energy utilization in pancreas, we monitored uptake of glucose analog, fluorine-18-labeled 2-deoxy-2-fluoro-D-glucose ([18F]-FDG) with positron emission tomography (PET). PET scan analysis in mice showed significant [18F]-FDG uptake in tissues with increased energy demand and most importantly in the pancreas of p48-Kras mice (Fig. 4E). These results suggested that the HFD exerted significant alterations in host and tumor energy metabolism in p48-Kras mice.
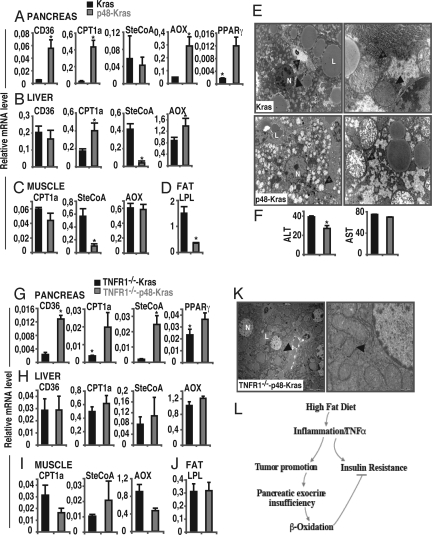
To provide insight into the changes in energy metabolism at the transcriptional level, gene expression analysis was used under a different diet regimen in p48-Kras mice and controls (Fig. 5 A–D). Among the mRNAs tested, genes encoding proteins involved in FA uptake and oxidation were significantly altered in the p48-Kras pancreata (Fig. 5A). FA transport across the cell membrane, determined by CD36 expression, was significantly increased in the pancreas. Meanwhile, expression of carnitine palmitoyltransferase 1a (CPT1a), acyl-CoA oxidase (AOX), along with peroxisome proliferator-activated receptor γ (PPARγ) were significantly elevated, mRNA levels for monounsaturated fatty acid stearoyl-CoA (SteCoA) was left unaffected (Fig. 5A). Moreover, analysis of circulating levels of postprandial FFA in p48-Kras mice showed a significant decrease (Fig. S7a), collectively suggesting increased FA oxidation in these animals during enhanced tumor promotion.
Fig. 5.
Pancreatic exocrine insufficiency induces mitochondrial β-oxidation. (A) Expression analysis of genes involved in fatty acid uptake and β-oxidation in the (A) pancreas, (B) liver, (C) muscle, and (D) fat tissue of p48-Kras mice and Kras control littermates on a HFD were measured by Real-Time PCR. (E) TEM pictures showing pronounced ultrastructural changes (arrowhead with a star) in hepatic mitochondria (arrowhead) in p48-Kras mice and Kras control littermates on the HFD. N, nucleus; L, lipid droplet. (F) Liver transaminases, ALT and AST, were analyzed in p48-Kras mice and Kras control littermates on the HFD. (G–J) Expression analysis of genes involved in lipid metabolism in (G) pancreas, (H) liver, (I) muscle, and (J) fat tissue of TNFR1−/−-p48-Kras mice and TNFR1−/−-Kras control littermates on the HFD were measured by Real-Time PCR. (K) TEM pictures showing ultrastructure of hepatic mitochondria (arrowhead) in TNFR1−/−-p48-Kras mice on HFD. N, nucleus; L, lipid droplet. (L) Schematic drawing suggesting how the HFD enhances pancreatic tumorigenesis. Data are mean values ± SEM. N = 4–7 mice per genotype. *, P ≤ 0.05.
Furthermore, PanIN development exerted secondary effects on the peripheral tissues. Although FA transport was equally achieved in both genotypes on the HFD, enzymes involved in FA oxidation, in particular CPT1a and AOX were significantly elevated in the liver only in the p48-Kras group (Fig. 5B). Moreover, SteCoA levels were significantly decreased in both the livers and the muscles of p48-Kras mice (Fig. 5 B and C). Importantly, expression of lipoprotein lipase (LPL) was reduced in the fat tissue of p48-Kras mice (Fig. 5D) thereby implicating decreased capacity for lipid mobilization in these animals.
To test whether altered mitochondria accompanied the metabolic switch observed in gene expression analysis, liver and muscle samples from p48-Kras mice were subjected to transmission electron microscopy (TEM). Electron micrographs revealed significant changes in the ultrastructure of particular mitochondria in p48-Kras mice, suggesting a secondary effect of pancreatic tumorigenesis in the liver (Fig. 5E). In contrast to control littermates that had mostly dense mitochondrial matrix and macrovesicular steatosis following the HFD, hepatocytes from p48-Kras animals displayed enlarged mitochondria with hypodense matrix and disrupted cristae. Interestingly, however, the changes were restricted only to the liver, while muscle mitochondria did not reveal any ultrastructural difference (Fig. S8). Moreover, these changes in the ultrastructure were not simply the result of increased damage because the liver transaminases, ALT and AST, remained normal and significantly lower in p48-Kras mice (Fig. 5F). These data suggest significant cross talk between the pancreas and liver during tumorigenesis.
Although abrogation of TNFα signaling did not abolish increased CD36 and CPT1a expression, it increased SteCoA levels in the pancreas (Fig. 5G). In contrast to the pancreas, TNFR1 deficiency counteracted the increased CPT1a and the decreased SteCoA expression in the liver and muscles and the reduced LPL mRNA levels observed in the fat tissue of p48-Kras mice (Fig. 5 H–J) and the decreased postprandial FFA levels (Fig. S7b), suggesting systemic effects for TNFα. Importantly, electron micrographs revealed that the enlarged mitochondria with hypodense matrix and disrupted cristae observed in p48-Kras mice were significantly changed on the TNFR1−/− background (Fig. 5K). These results clearly indicate a major role for TNFα in subsequent development of metabolic alterations in p48-Kras mice during HFD-associated early tumor promotion.
Discussion
Inflammation is a major feature of several chronic human diseases, including obesity and cancer (19). Importantly, HFD-induced proinflammatory cytokine expression is extensively elucidated, addressing macrophage activation as a crucial contributor to disease development during obesity in mouse models (4). Although inflammation has been independently linked to both disease moieties, the role of HFD-induced low-grade inflammation during tumor promotion has been underscored. Our findings raise the possibility that at least one of the mechanisms through which a HFD is associated to enhanced PanIN development is inflammation. Disease progression is significantly hampered when mice are rendered TNFR1 deficient, suggesting along with previous findings (20) that low concentrations of TNFα produced by inflammatory cells during a HFD could accelerate early tumor promotion.
One interesting finding in p48-Kras mice is that, in addition to its local paracrine/autocrine protumorigenic action, TNFα employs systemic effects on energy metabolism in various ways. First, TNFR1 deletion counteracts pancreatic exocrine insufficiency and restores the negative energy balance back to ND levels. This suggests a possible direct involvement of TNFα during development of pancreatic exocrine insufficiency, which needs further investigation using cell-type specific abrogation of TNFR1 signaling. Second, ablation of TNFR1 signaling normalizes adipose tissue mRNA levels of LPL, suggesting a further role for TNFα in lipid mobilization. This is in accordance with previous findings showing TNFα promotes fat wasting in cachexia by suppressing LPL gene expression (21). Finally TNFR1 deficiency abolishes the ultrastructural changes observed in hepatic mitochondria in p48-Kras mice implying a role for TNFα in cross talk between pancreas and peripheral tissue energy metabolism. Interestingly, there is a bulk of evidence suggesting pancreatic exocrine insufficiency (because of chronic pancreatitis or pancreatic cancer) exerts indirect effects on liver function via a rise in circulating cholecystokinin to pancreozymin levels (22). Because glucose and lipid metabolism are critical in supplying energy to extrahepatic tissues and all pancreatic secretions end up in hepatic blood flow, certain modifiers arising from the pancreas during a HFD could possibly modulate energy status in p48-Kras animals. Furthermore, malabsorption could lead to some specific nutrient deficiency, which could contribute to the overall metabolic effects.
Strikingly, p48-Kras mice on a HFD show accelerated tumor promotion in the absence of increased insulin resistance. Although it is well established that TNFα drives cachexia by inhibiting lipid storage and promoting FA release and it also leads to insulin resistance (12, 21, 23, 24), interestingly p48-Kras mice with PanIN lesions do not respond to elevated TNFα levels with insulin resistance. This discrepancy is explained by profound changes in energy metabolism. Concurrently aggravating tumor promotion, chronic inflammation and fibrosis destroys pancreatic exocrine tissue thereby augmenting inadequate delivery of digestive enzymes in the prandial and postprandial state. Interestingly, p48-Kras mice on a HFD, similar to patients with chronic pancreatitis, experience steatorrhea. This state of negative energy balance is counteracted by a profound increase in mitochondrial FA β-oxidation, which presumably coincides with increased need in processing fat caused by both a defect in storage and an increased demand for energy production in p48-Kras mice. It is important to note that increased energy consumption is characteristic of malignant cells and is closely associated with enhanced energy production from β-oxidation and glycolysis, the 2 major metabolic pathways in cancer (25, 26). It is possible that elevated metabolic rates and increased energy uptake correlate well with malignant transformation in p48-Kras mice. However, while the notion that increased energy metabolism possibly enhances tumor promotion in these animals is not only intriguing but conceivable because FA β-oxidation generates more ATP than carbohydrates and constitutes the dominant bioenergetic pathway in cancer (25, 27), on the other hand, elevated β-oxidation results in decreased FA levels in the circulation. It is well known that FFAs significantly impair insulin signaling and a decrease in FFA levels enhances glucose uptake (28, 29). Our results suggest that β-oxidation primarily induced to counteract increased energy demand because of negative energy balance accounts for the protection against HFD-induced insulin resistance and steatosis and for the enhanced glucose tolerance during GTTs in p48-Kras mice. Thus, these results provide significant evidence on how mice with enhanced PanIN lesions, despite chronic inflammation, do not develop insulin resistance. On the other hand, this does not rule out a causal role for insulin resistance in human pancreatic tumorigenesis as it has been suggested previously (2).
Collectively, our results define a context-specific function for TNFα, both its proinflammatory and lipolytic nature during obesity and suggest a model (Fig. 5L) in which TNFα plays a major role not only because of its local effect directly on tumor promotion and pancreatic exocrine insufficiency but also through its systemic effect on energy metabolism.
Materials and Methods
Mice.
LSL-K-rasG12D/+ mice (10) were crossed to p48-Cre (30) and Elastase-CreER (13) animals to generate mice with pancreas-specific (p48-Kras) and acinar-specific (Ela-Kras) activation of oncogenic K-ras. Mice were on mixed C57BL/6;129 background. p48-Kras mice were further crossed to TNFR1-deficient animals (initially obtained from the Jackson Laboratory and bred on mixed C57BL/6;129 background). Animals were housed under regular conditions (12-h light/dark cycle) and fed either on standard rodent chow or a high-fat diet (Research Diets). Mice underwent a HFD regimen at 6 weeks of age. Blood samples were collected for assessment of insulin (Crystal Chem), leptin (Crystal Chem), cytokines (eBioscience), FFA (WAKO), and TG (Sigma) levels according to manufacturer's instructions. Apoptotic rate was determined by TUNEL assay (BD Biosciences). All experimental procedures were approved by the Regierung von Oberbayern.
The methods used for measurement of glucose tolerance, direct and indirect calorimetry, fecal lipid content, and application of TEM, PET, various stainings, and RNA analysis are described in the SI Materials and Methods.
Statistical Analysis.
Data are expressed as mean values ± SEM. Differences were analyzed by Student's t test. P-values ≤ 0.05 were considered significant.
Supplementary Material
Acknowledgments.
We thank Luise Jennen for excellent technical assistance with TEM sessions. We also thank Kristin Retzlaff and Birgit Wittig for their help in maintaining the animal colony and Nicole Ehrhardt and Ann-Elisabeth Schwarz for performing indirect and bomb calorimetry. K-rasLSL-G12D/+, p48-Cre, and Elastase-CreER mice were kindly provided by David Tuveson, Hassan Nakhai, and Douglas Melton, respectively. Calorimetry studies were supported by grants from the German National Genome Research Network NGFNplus (01GS0822, 01GS0869, 01GS0850) to M.K. and M.H.d.A. This work was supported by grants from Wilhelm Sander Foundation (2005.146.1) and Deutsche Krebshilfe (107977) to M.C.A.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802864106/DCSupplemental.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Michaud DS, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. J Am Med Assoc. 2001;286:921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 3.Isaksson B, et al. Lifestyle factors and pancreatic cancer risk: a cohort study from the Swedish Twin Registry. Int J Cancer. 2002;98:480–482. doi: 10.1002/ijc.10256. [DOI] [PubMed] [Google Scholar]
- 4.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 5.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 10.Hingorani SR, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 11.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 13.Stanger BZ, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Hruban RH, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 15.Wigmore SJ, et al. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition. 1996;12:S27–30. doi: 10.1016/0899-9007(96)90014-3. [DOI] [PubMed] [Google Scholar]
- 16.Petersen JM, Forsmark CE. Chronic pancreatitis and maldigestion. Semin Gastrointest Dis. 2002;13:191–199. [PubMed] [Google Scholar]
- 17.Barber MD, Ross JA, Fearon KC. Cancer cachexia. Surg Oncol. 1999;8:133–141. doi: 10.1016/s0960-7404(99)00045-6. [DOI] [PubMed] [Google Scholar]
- 18.Dominguez-Munoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep. 2007;9:116–122. doi: 10.1007/s11894-007-0005-4. [DOI] [PubMed] [Google Scholar]
- 19.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002;13:135–141. doi: 10.1016/s1359-6101(01)00020-x. [DOI] [PubMed] [Google Scholar]
- 21.Fried SK, Zechner R. Cachectin/tumor necrosis factor decreases human adipose tissue lipoprotein lipase mRNA levels, synthesis, and activity. J Lipid Res. 1989;30:1917–1923. [PubMed] [Google Scholar]
- 22.Go VL, Gukovskaya A, Pandol SJ. Alcohol and pancreatic cancer. Alcohol. 2005;35:205–211. doi: 10.1016/j.alcohol.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Taishi P, et al. TNFalpha siRNA reduces brain TNF and EEG delta wave activity in rats. Brain Res. 2007;1156:125–132. doi: 10.1016/j.brainres.2007.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinberg GR, et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 26.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 27.Livesey G. The energy equivalents of ATP and the energy values of food proteins and fats. Br J Nutr. 1984;51:15–28. doi: 10.1079/bjn19840005. [DOI] [PubMed] [Google Scholar]
- 28.Roden M, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotamisligil GS, et al. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 30.Nakhai H, et al. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development. 2007;134:1151–1160. doi: 10.1242/dev.02781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.