Abstract
The IκB kinase (IKK)-NF-κB pathway plays a critical role in oncogenesis. Recently, we have shown that p53 regulates glucose metabolism through the IKK-NF-κB pathway and that, in the absence of p53, the positive feedback loop between IKK-NF-κB and glycolysis has an integral role in oncogene-induced cell transformation. Here, we demonstrate that IKKβ, a component of the IKK complex, was constitutively modified with O-linked β-N-acetyl glucosamine (O-GlcNAc) in both p53-deficient mouse embryonic fibroblasts (MEFs) and transformed human fibroblasts. In p53-deficient cells, the O-GlcNAcylated IKKβ and the activating phosphorylation of IKK were decreased by p65/NF-κB knockdown or glucose depletion. We also found that high glucose induced the O-GlcNAcylation of IKKβ and sustained the TNFα-dependent IKKβ activity. Moreover, the O-GlcNAcase inhibitor streptozotocin intensified O-GlcNAcylation and concomitant activating phosphorylation of IKKβ. Mutational analysis revealed that O-GlcNAcylation of IKKβ occurred at Ser 733 in the C-terminal domain, which was identified as an inactivating phosphorylation site, suggesting that IKKβ O-GlcNAcylation regulates its catalytic activity. Taken together, we propose a novel mechanism for the enhancement of NF-κB activity by loss of p53, which evokes positive feedback regulation from enhanced glucose metabolism to IKK in oncogenesis.
Keywords: glycolysis, NF-κB, O-GlcNAc, TNFα, high glucose
NF-κB is a critical regulator of innate and adaptive immune responses (1), and plays a key role in chronic inflammation-promoted tumor development (2, 3). Upon stimulation by pro-inflammatory cytokines such as TNFα, IκB, which sequesters NF-κB in cytoplasm, is phosphorylated by the IKK complex and is then degraded by the ubiquitin–proteasome system (4). NF-κB subsequently translocates into the nucleus and binds to the target DNA sequence (κB-site) to induce gene transcription. The IKK complex is composed of 2 homologous catalytic subunits, IKKα and IKKβ, and a regulatory scaffolding subunit, IKKγ/NEMO. IKKβ is essential for IκBα degradation induced by TNFα or IL-1 (5, 6) and has approximately 30-fold higher activity than IKKα for IκB phosphorylation (7). IKKβ is potentially phosphorylated on at least 16 serine residues, of which phosphorylation at 2 residues, Ser 178 and 181, in the T-loop is essential for activation. On the other hand, phosphorylation at the other 14 sites in the C terminus is critical for inactivation (8). Recently, Ser 733, 740, and 750 in/adjacent to the NEMO/γ-binding domain (NBD/γBD) were identified as inactivating phosphorylation sites (9, 10).
Many tumors preferentially use glycolysis and produce ATP through the conversion of glucose to lactate instead of oxidative phosphorylation in the presence of oxygen (11). This enhanced aerobic glycolysis is related to oncogenes such as ras, myc, or src through transcriptional up-regulation of the glycolysis-regulating genes (12). The tumor suppressor gene p53 is the most frequent target for genetic alterations in human cancer (13) and the loss of p53 function also changes the source of energy from cellular respiration to glycolysis (14). We have recently reported that IKKα/β kinase activity, transcriptional activity of NF-κB, and glycolysis are enhanced in p53-deficient cells, and that oncogenic Ras-induced cell transformation and enhanced aerobic glycolysis in p53-deficient cells were suppressed in the absence of p65/NF-κB expression (15). We also showed that a glycolytic inhibitor diminished the enhanced IKK activity in p53-deficient cells. These results suggest that the loss of p53 provokes a positive feedback loop, whereby glycolysis enhanced IKK–NF-κB activation.
The hexosamine biosynthetic pathway (HBP) shunts glycolysis to the production of uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), which is an activated substrate for modification of O-GlcNAc (16). Modification of O-GlcNAc of proteins is reversible, where the addition of the activated substrate is catalyzed by O-GlcNAc transferase (OGT) and removal of the sugar chain is catalyzed by O-GlcNAcase (17, 18). Some nuclear and cytoplasmic proteins are dynamically modified by the addition of O-GlcNAc to Ser or Thr, which regulates protein function as well as phosphorylation, and is related to some diseases such as diabetes and cancer (17, 18).
In this study, we revealed that IKKβ was specifically modified with O-GlcNAc in p53-deficient cells and in transformed human fibroblasts in which intracellular glucose metabolism is pathologically promoted. We propose an important role of IKKβ modification with O-GlcNAc in up-regulated aerobic glycolysis.
Results
Accelerated Aerobic Glycolysis by p53-deficiency Promotes IKKβ Modification with O-GlcNAc.
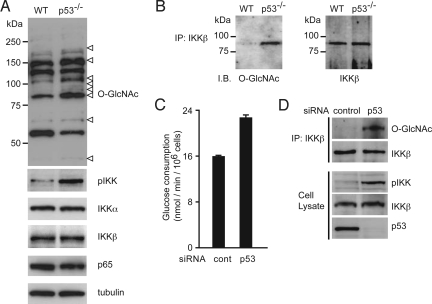
p53-deficient cells, as well as many cancers cells, obtain a large quantity of energy from glycolysis (14). The functional loss of p53 triggers the positive feedback loop for glycolysis-driven activation of the IKK–NF-κB pathway (15), suggesting that some factor in the IKK–NF-κB pathway acts as a glucose sensor. Approximately 2–5% of the glucose entering the cell is shunted to HBP, which generates UDP-GlcNAc, suggesting that proteins modified with O-GlcNAc are increased in p53-deficient cells. In fact, immunoblotting using the O-GlcNAc-specific antibody revealed a number of proteins with intensified O-GlcNAcylation in p53−/−MEFs (Fig. 1A). Because it was reported that high glucose augmented NF-κB transcriptional activity and the catalytic activity of IKKβ (19, 20), we tested whether IKKβ is modified with O-GlcNAc in p53-deficient cells. Fig. 1B clearly shows the O-GlcNAcylation of IKKβ. We also found that p53-knockdown MCF7 cells exhibited increased glucose consumption (Fig. 1C), enhanced O-GlcNAcylation of IKKβ and prominent activating phosphorylation of IKK (Fig. 1D).
Fig. 1.
O-GlcNAcylation of IKKβ in p53-deficient cells. (A) The cell extracts from wild-type and p53−/−MEFs were subjected to immunoblot analysis with an anti-O-GlcNAc antibody (Top) and the indicated antibodies. The open arrowheads indicate the intensified bands in p53−/−MEFs. (B) The cell extracts from wild-type and p53−/−MEFs were subjected to immunoprecipitation (IP) with antibodies specific for IKKβ followed by immunoblot analysis with an anti-O-GlcNAc antibody. (C and D) MCF7 cells were infected with control- or p53-siRNA-expressing retroviruses. Glucose consumption of the indicated cells was measured. Data are means ± SD from 3 independent experiments. *P < 0.01 for the indicated comparison (t test) (C). The levels of IKKβ O-GlcNAcylation and IKKα/β phosphorylation were examined by immunoblot analysis (D).
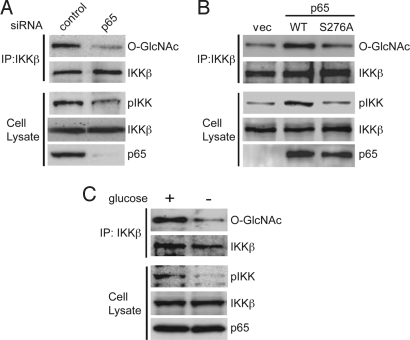
Because enhanced aerobic glycolysis in p53−/−MEFs is dependent on p65 (15), we next examined whether the intensified O-GlcNAcylation of IKKβ in p53−/−MEFs was influenced by NF-κB. In the p65-knockdown p53−/−MEFs, activating phosphorylation of IKK was suppressed and O-GlcNAcylation of IKKβ was markedly reduced (Fig. 2A). Reintroduction of the wild-type p65 to p65−/−p53−/−MEFs enhanced both IKKβ O-GlcNAcylation and activating phosphorylation of IKK. However, introduction of the p65 S276A mutant, which has lost the ability to activate transcription of multiple NF-κB-responsive genes (4), had almost no effect on IKKβ O-GlcNAcylation and activating phosphorylation of IKK (Fig. 2B). Exposure of p53−/−MEFs to the medium without glucose reduced IKKβ O-GlcNAcylation and activating phosphorylation of IKK (Fig. 2C).
Fig. 2.
p65-dependent IKKβ O-GlcNAcylation in p53−/−MEFs. (A) The cell extracts from p53−/−MEFs infected with control or p65 siRNA-expressing retroviruses were subjected to immunoprecipitation (IP) with an anti-IKKβ antibody, followed by immunoblot analysis with an anti-O-GlcNAc antibody. The cell extracts were subjected to immunoblot analysis with an anti-phospho-IKK (pIKK) antibody. (B) Levels of IKKβ O-GlcNAcylation and phosphorylation at the active site of IKKα/β were examined in p53−/−p65−/−MEFs infected with retroviruses encoding p65 (WT), p65 mutant (S276A) or empty vector (vec). (C) The cell extracts from p53−/−MEFs cultured in medium with or without glucose were subjected to immunoprecipitation (IP) with an anti-IKKβ antibody, followed by immunoblot analysis with an anti-O-GlcNAc antibody. The cell extracts were subjected to immunoblot analysis with an anti-phospho-IKK (pIKK) antibody.
O-GlcNAcylation of IKKβ is Induced by Exposure to High Glucose.
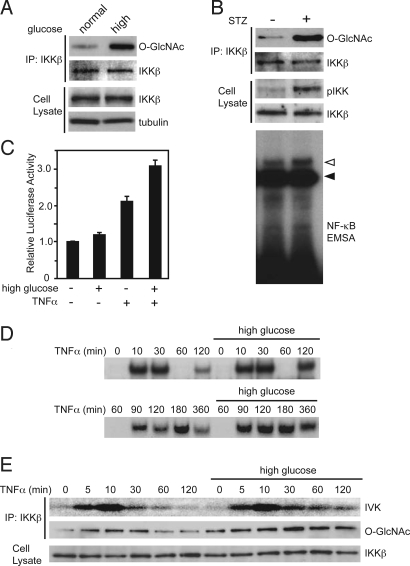
Because physiological flux through the HBP is implicated in the liver in vivo, we examined whether exposure to high glucose induces O-GlcNAcylation of IKKβ in the hepatic cancer cell line, HepG2. As expected, O-GlcNAcylation of IKKβ was induced by the high glucose culture condition (Fig. 3A). In db/db mice, which exhibit hyperglycemia, O-GlcNAcylation of IKKβ was detected in the liver [supporting information (SI) Fig. S1]. Because HepG2 cells have wild-type p53, we performed p53 knockdown and evaluated O-GlcNAcylation of IKKβ in HepG2 cells with or without high glucose. The IKKβ O-GlcNAcylation in HepG2 cells was increased by p53 knockdown, and the level of IKKβ O-GlcNAcylation in high glucose was also increased by p53 knockdown (Fig. S2). To assess whether O-GlcNAcylation of IKKβ promotes its kinase activity, we used the O-GlcNAcase inhibitor streptozotocin (STZ), which induces O-GlcNAcylation in conventional culture. STZ treatment of HepG2 cells markedly enhanced O-GlcNAcylation of IKKβ, activating phosphorylation of IKK (Fig. 3B Top) and DNA binding activity of NF-κB (Fig. 3B Bottom). The basal transcriptional activity of NF-κB was slightly induced under the high glucose condition and the TNFα-stimulated NF-κB transcriptional activity was synergistically enhanced (Fig. 3C). The peak of NF-κB DNA binding activity was observed at 30 min after TNFα stimulation in both the high and normal glucose conditions. At 60 min, the DNA binding activity of NF-κB was significantly decreased (Fig. 3D), and resynthesis of IκBα was strongly induced (Fig. S3). Under the high glucose condition, the TNFα-dependent DNA binding activity of NF-κB was pronounced in the late phase (Fig. 3D), and constitutively O-GlcNAcylated IKKβ and sustained kinase activity of IKKβ were detected after TNFα treatment (Fig. 3E). It was reported that the oscillation of NF-κB activity upon stimulation by proinflammatory cytokines is regulated by the amount of IκB and is dependent on p65 phosphorylation at serine 536 by IKK (21–23). Therefore, the sustained IKK activity in high glucose may contribute to enhanced oscillated NF-κB activation through IκBα degradation and p65 phosphorylation. Furthermore, TNFα-induced activating phosphorylation of IKK was prolonged in STZ-treated cells (Fig. S4). These results suggest that the induction of O-GlcNAcylated IKKβ is associated with sustained TNFα-stimulated activity of IKKβ and concomitant NF-κB activity.
Fig. 3.
TNFα-induced NF-κB activation is prolonged by exposure to high glucose. (A) HepG2 cells were incubated in normal medium (5.6 mM) or high glucose medium (30 mM) for 3 h. The cell extracts were subjected to immunoprecipitation with an anti-IKKβ antibody, followed by immunoblot analysis with an anti-O-GlcNAc antibody. (B) HepG2 cells were treated with STZ (5 mM) for 3 h. Levels of IKKβ O-GlcNAcylation and the phosphorylation at the active-site of IKKα/β (pIKK) were examined (Top). The nuclear extracts from the cells were subjected to EMSA with a radiolabeled probe containing the NF-κB consensus sequence (Bottom). The open arrowhead indicates the band for NF-κB with the p65 component. The solid arrowhead indicates a nonspecific band. (C) HepG2 cells were transfected with a NF-κB Luc reporter plasmid and phRL-TK as an internal control. After 24 h, the transfected cells were preincubated in normal medium (5.6 mM) or high glucose medium (30 mM) for 3 h and then stimulated with TNFα (100 ng/ml) for 12 h before harvesting. The luciferase activity is shown normalized to Renilla luciferase activity. Data are means ± SD from 3 independent experiments. (D and E) HepG2 cells were incubated in normal medium (5.6 mM) or high glucose medium (30 mM) for 3 h and stimulated with TNFα (100 ng/ml) for the indicated periods. The nuclear extracts were subjected to EMSA using a radiolabeled-κB oligonucleotide probe. The upper and lower panels are results from same experiment at different time points (D). The catalytic activity of IKKβ was estimated by an in vitro kinase assay (IVK) (E; Top). The cell extracts from the treated cells were subjected to immunoprecipitation with an anti-IKKβ antibody, followed by immunoblot analysis with an anti-O-GlcNAc antibody (E; Middle).
O-GlcNAcylated IKKβ on Ser 733 at the C-terminal Regulates its Catalytic Activity.
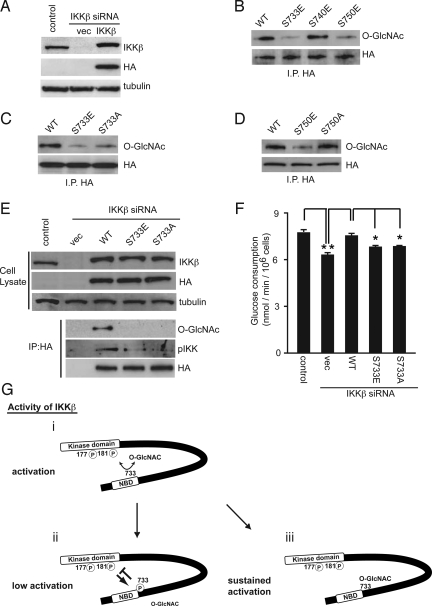
Because both O-GlcNAcylation and phosphorylation occur on Ser/Thr residues, we hypothesized that O-GlcNAcylation competes with and prevents the residue from inactivating phosphorylation of IKKβ. The kinase activity of the IKKβ mutant in which Ser 733, Ser 740, and Ser 750 residues at the C terminus are changed to Ala was intensified and that of the mutant in which both Ser 740 and Ser 750 residues are changed to Glu, mimicking phosphorylation, was diminished (10). Then, we examined whether the potential O-GlcNAcylation impairs known inactivating phosphorylation at Ser 733, Ser 740, or Ser 750. To exclude the involvement of endogenous IKKβ in the IKK complex, HA-tagged human IKKβ was reintroduced into National Institutes of Health 3T3 cells, where the mouse IKKβ-specific siRNA suppressed endogenous IKKβ expression (Fig. 4A). The amount of the reintroduced human IKKβ was almost comparable to that of reconstituted endogenous mouse IKKβ. As shown in Fig. 4B, O-GlcNAcylation of HA-tagged wild-type IKKβ was observed, whereas O-GlcNAcylation of the HA-tagged IKKβ S733E and S750E mutants was hardly detected. The O-GlcNAcylation level of the IKKβ S733A mutant as well as the S733E mutant was lower than that of the wild-type IKKβ (Fig. 4C), while the O-GlcNAcylation level of the IKKβ S750A mutant was comparable to that of the wild-type IKKβ (Fig. 4D). These findings suggest that IKKβ phosphorylation at the Ser 750 residue reduced O-GlcNAcylation in other site(s), including Ser 733, to inactivate the catalytic activity of IKK. The in vitro OGT assay also showed that wild-type IKKβ was O-GlcNAcylated in the presence of UDP-GlcNAc, while O-GlcNAcylated levels of the IKKβ 733E and 733A mutants were lower than that of wild-type IKKβ (Fig. S5). Furthermore, it was shown that STZ treatment induces the O-GlcNAcylation of the IKKβ S733A and S733E mutants as well as of the wild type but failed to promote phosphorylation of the activation site in the T-loop of the IKKβ S733A and S733E mutants (Fig. S6). When the human HA-IKKβ were reintroduced into the IKKβ knockdown p53−/−MEFs, O-GlcNAcylation and activating phosphorylation of the reconstituted human IKKβ were remarkable in the wild-type but not in the phosphorylation-mimicking S733E and nonphosphorylatable S733A mutants (Fig. 4E), indicating that O-GlcNAcylation at S733 positively regulates the catalytic activity of IKK even without competition from inactivating phosphorylation. In the same experiment, the glucose consumption in p53−/−MEFs was attenuated by mouse IKKβ-specific siRNA, which was restored by reconstitution with wild-type human IKKβ but not with human IKKβ S733E and S733A mutants (Fig. 4F). These results suggest that IKKβ is modified with O-GlcNAc not only at Ser 733 but also other site(s). However, Ser 733 is the crucial site that regulates IKK complex activity through O-GlcNAcylation not only by preventing inactivating phosphorylation at the site but also by O-GlcNAcylation-mediated positive regulation such as conformational changes in the kinase domain (Fig. 4G).
Fig. 4.
O-GlcNAcylation of IKKβ at Ser 733 regulates its catalytic activity. (A) NIH 3T3 cells infected with mouse IKKβ siRNA-expressing retrovirus were superinfected with HA-tagged human IKKβ wild type (WT). The cell extracts from the infected cells were subjected to immunoblot analysis with the indicated antibodies. Tubulin was used as a loading control. (B) NIH 3T3 cells infected with mouse IKKβ siRNA-expressing retrovirus were superinfected with retroviruses encoding HA-tagged human IKKβ wild type (WT), S733E, S740E, or S750E mutants. The cell extracts were subjected to immunoprecipitation with an anti-HA antibody followed by immunoblot analysis with an anti-O-GlcNAc antibody. (C) NIH 3T3 cells infected with mouse IKKβ siRNA-expressing retrovirus were superinfected with retroviruses encoding HA-tagged human IKKβ WT, S733E, or S733A mutants. The level of IKKβ O-GlcNAcylation was examined in cells infected with each retrovirus. (D) National Institutes of Health 3T3 cells infected with mouse IKKβ siRNA-expressing retrovirus were superinfected with retroviruses encoding HA-tagged human IKKβ WT, S750E, or S750A mutants. The level of IKKβ O-GlcNAcylation was examined in the cells infected with each retrovirus. (E and F) p53−/−MEFs infected with mouse IKKβ siRNA-expressing retrovirus were superinfected with HA-tagged human IKKβ wild-type (WT), S733E, or S733A mutants. The cell extracts from the infected cells were subjected to immunoprecipitation with an anti-HA antibody followed by immunoblot analysis with the indicated antibodies to examine the levels of IKKβ O-GlcNAcylation and phosphorylation at the active site of IKKα/β (E). Glucose consumption was estimated from the concentration of glucose in the culture media. Data are means ± SD. from 3 independent experiments *P < 0.01 for the indicated comparison (t test) (F). (G) A model of IKKβ activation regulated by phosphorylation and O-GlcNAcylation at the C-terminal domain. In the basal state, IKKβ is reciprocally modified with O-GlcNAc at Ser 733. Stimulation, such as by TNFα, induces activating phosphorylation in its kinase domain of IKKβ (i) and subsequently autophosphorylates the inactivating phosphorylation sites at its C-terminal domain, including the Ser 733 residue (ii). For IKKβ modified with O-GlcNAc, the TNFα-induced kinase activity is sustained by blockade of inactivating phosphorylation (iii).
IKKβ is Constitutively O-GlcNAcylated in Transformed Cells.
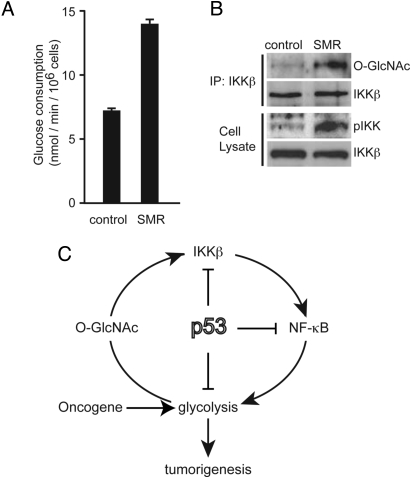
We next examined whether IKKβ O-GlcNAcylation was induced by transformation with oncogenes. TIG-3 human primary fibroblasts that were transformed by the introduction of SV40 T antigen, c-Myc, and Ha-RasV12 (SMR cells) (24) exhibited increased glucose consumption (Fig. 5A), and O-GlcNAcylation of IKKβ and activating phosphorylation of IKK were enhanced (Fig. 5B). We previously showed that Ha-RasV12 induces cell transformation and further promotes glycolysis in p53−/−MEFs (15). Accordingly, Ha-RasV12 promoted both O-GlcNAcylation of IKKβ and activating phosphorylation of IKK in p53−/−MEFs, which were reduced by p65 knockdown (Fig. S7 A and B). Because Ha-RasV12 increased IKKβ O-GlcNAcylation even in p53 deficient cells, we examined whether OGT expression was induced by Ha-RasV12. Although the amount of OGT was not affected by p53-deficiency (data not shown), Ha-RasV12 increased OGT expression (Fig. S7 C and D), suggesting that the further increase of O-GlcNAcylation in Ha-RasV12 expressing p53−/−MEFs is due to the induced OGT expression by Ha-RasV12.
Fig. 5.
IKKβ O-GlcNAcylation is enhanced in transformed human fibroblasts. (A and B) Tig3 cells were transformed by serial infection with retroviruses encoding the SV40 T antigen, Myc, and Ha-Ras (SMR cells). The glucose consumption of control Tig3 cells and transformed Tig3 cells (SMR) was estimated from the concentration of glucose in the culture media. Data are means ± SD. from 3 independent experiments. *P < 0.01 for the indicated comparison (t test) (A). Levels of IKKβ O-GlcNAcylation and the active-site of IKKα/β phosphorylation were examined in control Tig3 cells and transformed Tig3 cells (SMR) (B). (C) A model for the proposed link between p53, IKK, NF-κB, and glycolysis. p53 regulates an activation loop between IKK, NF-κB, and glucose metabolism.
Discussion
Accumulating evidence has shown that modification with O-GlcNAc plays an important role in the regulation of transcription, translation, protein trafficking, stability, stress survival, and cell cycle (18). In this study, we have shown that the IKK–NF-κB signaling pathway and enhanced glycolysis induce O-linked glycosylation of IKKβ in p53-deficient cells (Fig. 1B and 2 A and C). The same modification was also observed in hepatic cancer cells, HepG2, that were exposed to high glucose medium (Fig. 3A), which indicates that IKKβ is O-GlcNAcylated in response to enhanced glycolysis. As shown in Fig. 3E, constitutively O-GlcNAcylated IKKβ and sustained kinase activity of IKKβ in the high glucose condition were detected after TNFα treatment. These findings may be closely correlated with enhanced transcriptional activity and prolonged DNA-binding activity of NF-κB (Fig. 3 C and D). Furthermore, this notion is supported by the result that O-GlcNAcylation occurs at the inactivating phosphorylation site of IKKβ at Ser 733 (Fig. 4C) and that mutation of this site inhibits enhanced glycolysis in p53-deficient cells (Fig. 4F). Because O-GlcNAcylation of IKKβ constitutively occurred in p53−/−MEFs, we hypothesized that the IKK–NF-κB pathway is susceptible to TNFα. Indeed, the activation of IKK and NF-κB by TNFα in p53−/−MEFs was enhanced compared with wild-type MEFs (Fig. S8 A–C). It was reported that the IKK–NF-κB signaling pathway plays a key role in inflammation-associated tumor development (25, 26). Then, increasing O-GlcNAcylation of IKKβ that sensitizes it to activation by inflammatory cytokines such as TNFα may affect the positive feedback loop regulation of NF-κB activation in tumors. We recently showed that activated p53 inhibits the kinase activity of IKKβ through direct binding (27). Moreover, it has been shown that O-GlcNAcylation stabilizes p53 (28). Therefore, it is possible that, in normal cells, p53 directly restricts IKKβ activity in response to enhanced glycolysis; however, the precise mechanism remains to be elucidated.
Numerous studies have demonstrated that constitutive NF-κB activation is frequently observed in many types of cancer (2, 3), and we found that the positive feedback loop between the IKK–NF-κB pathway and glycolysis plays an important role in oncogenic Ras-induced transformation of p53-deficient cells (15). O-GlcNAcylation of IKKβ and activating phosphorylation of IKK were enhanced by the expression of Ha-RasV12 in p53−/−MEFs (Fig. S7A). Using a human tumor cell creating system established by Weinberg's group (29), we also showed that, in normal human diploid fibroblast Tig3 cells, oncogene-induced transformation enhances glycolysis, O-GlcNAcylation of IKKβ, and activating phosphorylation of IKK (Fig. 5 A and B). These results suggest that O-GlcNAcylation of IKKβ is responsible for constitutive NF-κB activation in cancers. A recent report has demonstrated that p65 is modified with O-GlcNAc, which regulates its transcriptional activity (30, 31). It seems likely that NF-κB activation through O-glycosylated p65 is involved in the positive feedback loop between the IKK–NF-κB pathway and glycolysis; however, we could not detect O-GlcNAcylation of immunoprecipitated p65 (data not shown) and our previous results showed that inhibition of IKKβ by RNAi suppressed NF-κB activity and glycolysis in p53-deficient cells (15), indicating that activation of IKKβ is important for maintaining this positive feedback loop. In conclusion, we have found a novel mechanism by which glucose metabolism enhances the IKK–NF-κB signaling pathway through O-linked glycosylation of IKKβ. Because p53 suppresses IKK activity, NF-κB transcriptional activity (15, 27) and decreases glycolysis (14), restriction of the positive feedback loop by p53 may be an important role in the tumor surveillance system (Fig. 5C).
Materials and Methods
Cell Culture and Retrovirus Infection.
p53−/−, p65−/−, and p53−/−p65−/−MEFs were prepared as previously described (15). Cells were cultured in DMEM (Nissui Pharmaceutical Co.) supplemented with 10% FBS and 50 μg/ml kanamycin. For the medium without glucose, cells were cultured in DMEM (Sigma) supplemented with 1% FBS. Retroviral infection was performed as previously described (32). The infected cells were selected using either 1 μg/ml puromycin (Sigma) for 3 days, 200 μg/ml hygromycin (Wako) for 3 days, or 2.5 μg/ml blasticidin (Invitrogen) for 7 days.
Plasmids and RNAi.
pNF-κB Luc was obtained from Clontech Laboratories Inc., and the internal control plasmid phRL-TK (Renilla luciferase reporter) was obtained from Toyobo. The retrovirus vectors pBabe HA-IKKβ wild type, pBabe Ha-RasV12, pBabe SV40 T antigen, and pBabe c-Myc with puromycin, hygromycin, blasticidin, and neomycin selectable markers, respectively, were used. pBabe HA-IKKβ S733E, S733A, S740E, S750E, and S750A were constructed by site-directed mutagenesis. The retrovirus vectors, pSuper shIKKβ puro, pSuper shp65 puro, pBabe p65 wild type, and pBabe p65 S276A were used as previously described (15).
Antibodies and Materials.
Anti-O-GlcNAc (Covance), monoclonal anti-IKKα (Oncogene), monoclonal anti-IKKβ (Cell Signaling Technology), anti-p65 (sc-372; Santa Cruz Biotechnology), anti-p53 (FL393; Santa Cruz Biotechnology and Ab-5; Neo Markers Inc.), anti-phospho-IKK (IKKα [Ser-180]/IKKβ [Ser-181]); (Cell Signaling Technology) and anti-α tubulin (DM1A; Sigma) antibodies were used for immunoblot analysis. STZ was obtained from Sigma.
Immunoprecipitation and Immunoblot Analysis.
Cells were solubilized in ice-cold buffer [25 mM Hepes pH 7.2, 150 mM CH3COOK, 2 mM EDTA, 0.1% Nonidet P-40, protease inhibitor mixture (Nacalai Tesque) and 1 mM DTT], and then centrifuged at 20,000 × g for 20 min. The supernatants were used as the total cell extracts. Immunoprecipitations were performed using IKKβ (Cell Signaling Technology), or HA (Covance) antibodies with protein G Sepharose (Amersham Bioscience). The immune complexes were then subjected to SDS/PAGE.
Measurement of Glucose Consumption.
Measurement of glucose consumption was performed as previously described (15). The glucose level was determined using a glucose assay kit (Sigma).
Electrophoretic Mobility Shift Assay (EMSA).
Nuclear extracts were prepared and EMSA was performed as previously described (15).
in Vitro Kinase Assay.
The kinase activity of IKKβ was analyzed by an immune complex kinase assay as previously described (33). Briefly, the cells were solubilized in ice-cold buffer [20 mM Tris pH 7.4, 10 mM EGTA, 10 mM MgCl2, 1 mM benzamidine, 60 mM β-glycerophosphate, 1 mM Na3VO4, 20 mM NaF, 1 mM DTT, protease inhibitor mixture (Nacalai Tesque) and 1% Triton X-100] and then centrifuged at 20,000 × g for 20 min. The supernatant was used as the cell extract. HA-tagged IKKβ was recovered from the cell extract by immunoprecipitation, and the precipitated complexes were incubated in 10 μl of a reaction mixture (20 mM Hepes pH 7.4, 10 mM MgCl2, 50 mM NaCl, 100 mM Na3VO4, 20 mM β-glycerophosphate, 1 mM DTT, 100 μM ATP, 0.05 μCi [γ-32P]-ATP, and 5 μg of GST-IκBα [1–55] as a substrate) at 30 °C for 20 min. After SDS-polyacrylamide gel electrophoresis (PAGE), the level of GST-IκBα phosphorylation was measured by autoradiography.
Additional materials and methods are provided in the SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Drs. Y. Akimoto, S. Minami, I. Ohsawa, Y. Shibukawa, K. Sada, E. Oda-Sato, Y. Abe, I. Uehara, W. Nakajima, and M. Ando for discussions, and Mr. M. Ishikawa, Dr T. Doi, M. Kawagoe, Y. Asano, and H. Hiroike for technical support. We appreciate the gift of IKKβ from Dr. D.V. Goeddel (Tularic Inc., San Francisco, CA) and the retroviral vectors pSuper-sh human p53 puro from Dr. R. Agami (Netherlands Cancer Institute, Amsterdam, The Netherlands). This work was supported by Grants-in-aid from the Mitsubishi Foundation and the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813210106/DCSupplemental.
References
- 1.Barnes PJ, Karin M. Nuclear factor-κB: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 2.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-κB is the lynchpin. Trends Immunol. 2005;26:318–325. doi: 10.1016/j.it.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 7.Huynh QK, et al. Characterization of the recombinant IKK1/IKK2 heterodimer. Mechanisms regulating kinase activity. J Biol Chem. 2000;275:25883–25891. doi: 10.1074/jbc.M000296200. [DOI] [PubMed] [Google Scholar]
- 8.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 9.May MJ, Marienfeld RB, Ghosh S. Characterization of the IκB-kinase NEMO binding domain. J Biol Chem. 2002;277:45992–46000. doi: 10.1074/jbc.M206494200. [DOI] [PubMed] [Google Scholar]
- 10.Schomer-Miller B, Higashimoto T, Lee YK, Zandi E. Regulation of IκB kinase (IKK) complex by IKKγ-dependent phosphorylation of the T-loop and C terminus of IKKβ. J Biol Chem. 2006;281:15268–15276. doi: 10.1074/jbc.M513793200. [DOI] [PubMed] [Google Scholar]
- 11.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 12.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 13.Hamroun D, et al. The UMD TP53 database and website: Update and revisions. Hum Mutat. 2006;27:14–20. doi: 10.1002/humu.20269. [DOI] [PubMed] [Google Scholar]
- 14.Bensaad K, Vousden KH. p53: New roles in metabolism. Trends Cell Biol. 2007;17:286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Kawauchi K, Araki K, Tobiume K, Nobuyuki T. p53 regulates glucose metabolism through IKK and NF-κB: An integral role in cell transformation. Nat Cell Biol. 2008;10:611–618. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
- 16.McClain DA, Crook ED. Hexosamines and insulin resistance. Diabetes. 1996;45:1003–1009. doi: 10.2337/diab.45.8.1003. [DOI] [PubMed] [Google Scholar]
- 17.Comer FI, Hart GW. O-Glycosylation of nuclear and cytosolic proteins. Dynamic interplay between O-GlcNAc and O-phosphate. J Biol Chem. 2000;275:29179–29182. doi: 10.1074/jbc.R000010200. [DOI] [PubMed] [Google Scholar]
- 18.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 19.Mohan S, Konopinski R, Bo Y, Centonze VE, Natarajan M. High glucose induced-IKKβ-Hsp-90 interaction contributes to endothelial dysfunction. Am J Physiol Cell Physiol. 2009;296:C182–C192. doi: 10.1152/ajpcell.00575.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X, et al. Adiponectin suppresses IκB kinase activation induced by tumor necrosis factor-α or high glucose in endothelial cells: Role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab. 2007;293:E1836–E1844. doi: 10.1152/ajpendo.00115.2007. [DOI] [PubMed] [Google Scholar]
- 21.Nelson DE, et al. Oscillations in NF-κB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 22.Park SG, et al. The influence of the signal dynamics of activated form of IKK on NF-κB and anti-apoptotic gene expressions: A systems biology approach. FEBS Lett. 2006;580:822–830. doi: 10.1016/j.febslet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Sillitoe K, Horton C, Spiller DG, White MR. Single-cell time-lapse imaging of the dynamic control of NF-kappaB signalling. Biochem Soc Trans. 2007;35:263–266. doi: 10.1042/BST0350263. [DOI] [PubMed] [Google Scholar]
- 24.Araki K, Kawauchi K, Tanaka N. IKK/NF-κB signaling pathway inhibits cell-cycle progression by a novel Rb-independent suppression system for E2F transcription factors. Oncogene. 2008;27:5696–5705. doi: 10.1038/onc.2008.184. [DOI] [PubMed] [Google Scholar]
- 25.Greten FR, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Pikarsky E, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 27.Kawauchi K, Araki K, Tobiume K, Tanaka N. Activated p53 induces NF-κB DNA binding but suppresses its transcriptional activation. Biochem Biophys Res Commun. 2008;372:137–141. doi: 10.1016/j.bbrc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Yang WH, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8:1074–1083. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 29.Hahn WC, et al. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 30.James LR, et al. Flux through the hexosamine pathway is a determinant of nuclear factor κB- dependent promoter activation. Diabetes. 2002;51:1146–1156. doi: 10.2337/diabetes.51.4.1146. [DOI] [PubMed] [Google Scholar]
- 31.Yang WH, et al. NFκB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA. 2008;105:17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato M, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 33.Kamata K, et al. Cycloprodigiosin hydrochloride suppresses tumor necrosis factor (TNF) α-induced transcriptional activation by NF-κB. FEBS Lett. 2001;507:74–80. doi: 10.1016/s0014-5793(01)02946-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.