Abstract
All-trans retinoic acid (ATRA)/arsenic trioxide (ATO) combination-based therapy has benefitted newly diagnosed acute promyelocytic leukemia (APL) in short-term studies, but the long-term efficacy and safety remained unclear. From April 2001, we have followed 85 patients administrated ATRA/ATO with a median follow-up of 70 months. Eighty patients (94.1%) entered complete remission (CR). Kaplan–Meier estimates of the 5-year event-free survival (EFS) and overall survival (OS) for all patients were 89.2% ± 3.4% and 91.7% ± 3.0%, respectively, and the 5-year relapse-free survival (RFS) and OS for patients who achieved CR (n = 80) were 94.8% ± 2.5% and 97.4% ± 1.8%, respectively. Upon ATRA/ATO, prognosis was not influenced by initial white blood cell count, distinct PML-RARα types, or FLT3 mutations. The toxicity profile was mild and reversible. No secondary carcinoma was observed, and 24 months after the last dose of ATRA/ATO, patients had urine arsenic concentrations well below the safety limit. These results demonstrate the high efficacy and minimal toxicity of ATRA/ATO treatment for newly diagnosed APL in long-term follow-up, suggesting a potential frontline therapy for de novo APL.
Keywords: combination therapy, 5-year EFS, 5-year OS, residual disease, arsenic retention
Acute promyelocytic leukemia (APL) is the M3 subtype of acute myeloid leukemia (AML) and is characterized by an accumulation of abnormal promyelocytes in the bone marrow and a severe bleeding tendency. Additionally, in the great majority of cases, APL is associated with the t(15,17) chromosomal translocation and resultant PML-RARα transcripts that encode the leukemogenic PML-RARα fusion protein (1). Five decades ago, APL was the most fatal type of acute leukemia and was considered essentially untreatable (2). The first breakthrough came with the use of anthracyclines, which improved the complete remission (CR) rate but provided a low 5-year overall survival (OS) (3). The introduction of all-trans retinoic acid (ATRA) therapy resulted in terminal differentiation of APL cells and a 90–95% CR rate in patients (1, 4), and subsequent combination of ATRA with chemotherapy raised the 5-year disease-free survival (DFS) rate up to 74% (5). In the 1990s, significant benefits of arsenic trioxide (ATO) were reported, which further improved the outcome of patients with APL (6, 7). ATO exerts dose-dependent dual effects on APL cells, with low concentrations inducing partial differentiation and relatively high concentrations triggering apoptosis. Of note, both ATRA and ATO induce catabolism of the PML-RARα fusion protein, demonstrating a paradigm for rationally targeted therapy in leukemia. However, between 20% and 30% of newly diagnosed APL patients treated with regimens based on the use of ATRA or ATO as single agents will develop disease recurrence or drug resistance.
To improve further the clinical outcome of APL, therapeutic strategies should be designed to include combinatorial use of drugs with distinct but convergent mechanisms that may amplify treatment efficacies and diminish adverse effects. A striking convergence in the effects of ATRA and ATO is the degradation of PML-RARα through distinct pathways, with ATRA targeting the RARα and ATO targeting the PML moieties of the fusion protein (8–10). Interestingly, ATRA has been shown to up-regulate the gene encoding transmembrane protein aquaglyceroporin 9 (AQP9), leading to a significantly increased arsenic uptake by leukemic cells (11), and combined use of ATRA and ATO synergizes to induce differentiation, apoptosis, and transcriptome/proteome pattern in vitro (12, 13) and accelerate tumor regression through enhanced differentiation and apoptosis in vivo (14, 15). These were the impetus for a clinical trial to test the efficacy of ATRA plus ATO in newly diagnosed APL. In a previous study with a median follow-up of 18 months, we randomized patients to receive either ATRA or ATO singly, or ATRA/ATO-based regimens incorporating chemotherapy treatment as a frontline therapy for new APL cases. We showed that the ATRA/ATO combination significantly shortened the time to achieve CR, reduced disease burden, and enhanced DFS compared with approaches based on the use of either ATRA or ATO as single agents (16). These benefits of the ATRA/ATO-based combination were subsequently confirmed by several groups (17–19). Being aware of these advances, APL patients entering into our clinical trials preferred ATRA/ATO-based treatment to gain maximal therapeutic efficacy. Therefore, for obvious ethical reasons, since early 2004 all newly diagnosed patients have been treated with the ATRA/ATO combination regimen.
The sustainability of the effects of the ATRA/ATO combination as a basis for frontline therapy of APL patients, however, remained unclear. Furthermore, the potential eventual long-term sequelae due to the incorporation of ATO into the therapeutic strategy for new APL cases represented major concerns. To characterize the long-term efficacy, safety, tolerability, and pharmacological features of ATO in our regimen, we followed 85 APL patients who received ATRA/ATO combination therapy for 7 years. Additionally, we present the results of an analysis of prognostic factors in APL.
Results
Accrual of Patients.
From April 2001 to December 2005, 85 newly diagnosed APL patients without prior exposure to any antileukemic therapy were recruited with informed consent to the study. The diagnosis of APL was established according to the clinical presentation and morphological criteria of the French-American-British classification and was confirmed by cytogenetic assay for t(15;17)(q22;q21) and RT-PCR analysis for PML-RARα transcripts (16). The characteristics of the patients are shown in supporting information (SI) Table S1.
Clinical Outcome.
Treatment regimens were designed in 2000, and the clinical trial was started in 2001 with 3 arms: ATRA alone, ATO alone, and ATRA/ATO combination, as selective differentiation/apoptosis induction therapies together with chemotherapy during remission induction and postremission therapy. The detailed information with regard to the dose, time course, interval, and overall regimen design of ATRA, ATO, and chemotherapy during remission induction, consolidation and maintenance therapy has been described previously (16). The benefits of combining ATRA with ATO for newly diagnosed APL, which were statistically significant over a median follow-up of 18 months in our trial, were published in 2004 (16) and got support from some recent reports (18, 19), and patients who thereafter entered the trial requested this treatment regimen. As a result of a meticulous evaluation of our trial in terms of both clinical outcome and ethics, we terminated the randomized grouping and prescribed the ATRA/ATO-based regimen for induction and postremission therapy for the maximal benefit of patients. With ATRA/ATO treatment, 80 (94.1%) of 85 patients achieved CR with a median of 27 days (range, 15–38 days). Five patients suffered from early deaths within 15 days during the induction therapy due to intracranial hemorrhage (3 cases), retinoic acid syndrome (1 case), or disseminated intravascular coagulation (1 case). In 71 evaluable responders, a rapid normalization of coagulopathy with a median of 6 days (range, 1–24 days) was observed.
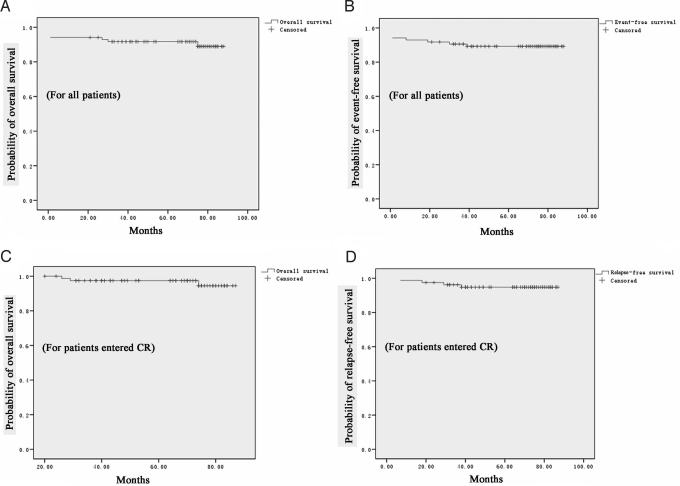
For the 80 patients who entered CR, the median follow-up was 70 months (range, 21–88 months), and 76 remained in good clinical remission. Only 4 patients relapsed, and all presented with central nervous system (CNS) leukemia as the first sign of relapse: 2 patients experienced severe headache and died subsequently at 30 and 27 months, respectively, after CR with documented abnormal CNS test, whereas the other 2 patients had documented CNS leukemia 8 and 39 months, respectively, after CR and then developed full bone marrow relapse 29 and 23 months later, respectively. As shown in Fig. 1, the 5-year OS rates for all 85 patients and for those who achieved CR (n = 80) were 91.7% ± 3.0% (Fig. 1A) and 97.4% ± 1.8% (Fig. 1C), respectively. The 5-year event-free survival (EFS) rates for all patients were 89.2% ± 3.4% (Fig. 1B), and 5-year relapse-free survival (RFS) rates for those who entered CR were 94.8% ± 2.5% (Fig. 1D).
Fig. 1.
Survival analysis. The 5-year OS (A) and EFS (B) for all 85 patients treated with the ATRA/ATO combination. The 5-year OS (C) and RFS (D) for the 80 patients who obtained CR after ATRA/ATO treatment.
Prognostic Factors.
The response to the ATRA/ATO-based regimen was not influenced by sex, age, and type of PML-RARα transcript (Table S1). Previous reports suggested a white blood cell (WBC) count of 10 × 109/L (10,000/μL) and higher as an adverse prognostic factor in APL (20, 21), but analyses carried out here showed that patients with WBC counts above or below 10 × 109/L had equivalent CR rates (Table S1). Previous studies have shown that the presence of FLT3 mutation was an adverse prognostic factor for AML (22–24), including APL (25), whereas others reported that mutant FLT3 did not correlate with prognosis of APL (22) and was not an indicator for stem cell transplantation in AML (excluding APL) (24). Also shown in Table S1, our results indicate that status of FLT3 did not, in the context of our therapeutic approach, correlate with low CR rates in patients receiving ATRA/ATO. We further analyzed the influence of these factors on long-term survival and found that either traditional parameters for unfavorable prognosis, such as age over 55 years and WBC count above 10 × 109/L before the treatment or molecular markers including distinct isoforms of PML-RARα and FLT3, were not significantly associated with worse OS or RFS (Table S1).
Molecular Response.
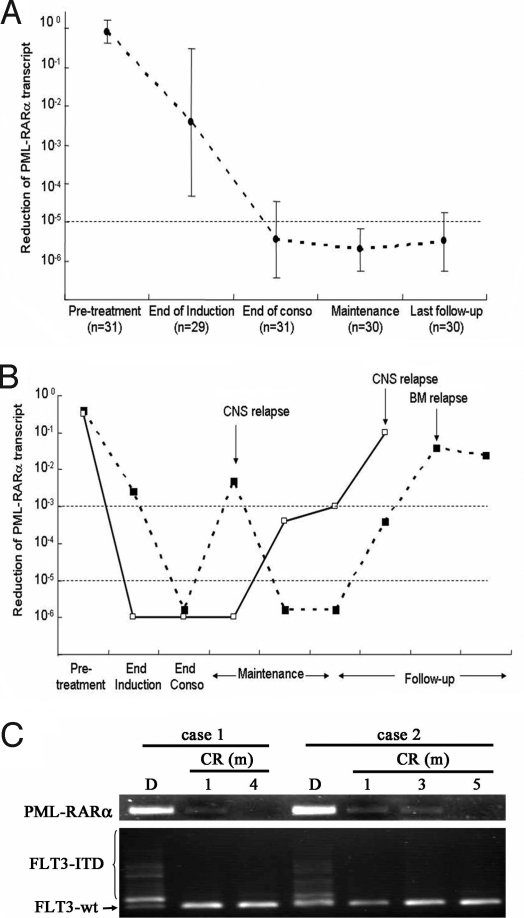
To monitor residual disease, real-time quantitative RT-PCR was performed for PML-RARα transcript levels in serial bone marrow samples of 31 eligible patients as described previously (16). We found that the expression of PML-RARα in these patient samples was high at diagnosis, significantly decreased (more than 2-log reduction on average) after induction therapy, and further reduced at the end of consolidation therapy using chemotherapy, with an average 5-log reduction (Fig. 2A). Importantly, during maintenance therapy and throughout follow-up, those patients in continuous remission displayed consistently low or undetectable levels of PML-RARα (>5-log reduction; Fig. 2A). In 2 cases, the expression of PML-RARα was significantly reduced at the end of consolidation but elevated markedly when disease relapsed in the CNS and/or bone marrow (Fig. 2B).
Fig. 2.
Dynamics of PML-RARα and FLT3 ITD in patients treated with ATRA/ATO. (A) In patients who maintained continuous clinical remission, the PML-RARα transcript level was significantly decreased during the treatment and throughout the follow-up. (B) In 2 patients who experienced relapse, the PML-RARα transcript became increased when disease relapse was documented either in CNS or bone marrow. (C) Monitoring of PML-RARα and FLT3 ITD in patients treated with ATRA/ATO at different time courses. End of conso indicates end of consolidation chemotherapy; D, diagnosis..
FLT3 mutations, including internal tandem duplications (ITDs) and D835 point mutations (PMs), also were monitored among evaluable patients. For the results to be comparable, the sensitivity of the method for assaying the transcripts of both PML-RARα and mutant FLT3 was carefully adjusted to similar levels (10−4). At diagnosis, 10 (20%) of 50 investigated patients harbored FLT3 abnormalities (8 ITDs and 2 PMs). Interestingly, the FLT3 mutation could no longer be detected at an early stage of CR (1 month), whereas PML-RARα became undetectable after 4 to 5 months, a relatively late stage of CR (Fig. 2C), suggesting that mutant FLT3 was not an adverse indicator for poor prognosis for APL with ATRA/ATO-based therapy, which was further confirmed by the outcome in patients with long-term follow-up (Table S1).
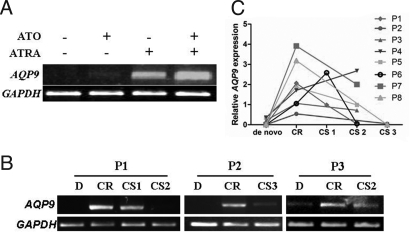
Consistent with a previous in vitro study that suggested that ATRA could facilitate arsenic uptake by HL60 leukemic cells through up-regulation of AQP9 (11), we found that treatment with ATRA up-regulated AQP9 expression in NB4 cells (Fig. 3A). Semiquantitative RT-PCR was performed to analyze the dynamics of AQP9 expression in 8 patients, and expression of AQP9 was found to be low at diagnosis, was markedly increased when patients received ATRA/ATO, and was maintained at a relatively high level or decreased when patients underwent consolidation chemotherapy (Fig. 3 B and C).
Fig. 3.
Effects of ATRA/ATO combination on expression of AQP9. (A) In NB4 cells, AQP9 expression was significantly up-regulated after treatment with ATRA/ATO. (B) Expression of AQP9 in bone marrow mononuclear cells isolated from patients on ATRA/ATO treatment in 3 patients (P1–P3). (C) Dynamic changes of AQP9 expression in 8 patients (P1–P8) treated with ATRA/ATO-based regimen. D indicates diagnosis; CS, consolidation therapy.
Toxicity Profile in Long-Term Survivors.
Apart from the 5 early deaths recorded during remission induction and 3 deaths due to disease relapse in CNS or bone marrow, there were no further deaths in this study. During remission induction, 55 of 73 evaluable patients developed tolerable and reversible grade I-II liver dysfunction, whereas no grade III-IV liver toxicity was observed. Moreover, hepatic function returned to normal in all of these patients after termination of induction therapy. One of the potential safety issues associated with our treatment regimen was long-term toxicity linked to exposure to multiple cycles of ATO. To evaluate this, we performed systemic physical examination and laboratory studies to screen for possible toxic effects in 33 eligible patients when they were being treated with ATRA/ATO and at least 24 months after administration of the last dose of ATO. The tests performed were as follows: blood counts with peripheral smears, electrolyte panels with blood urea nitrogen and creatinine, urinalysis, liver function test, evaluation of serum tumor markers (CEA, α-FP, CA199, CA125), electrocardiograms (ECGs) and echocardiograms, chest X-ray, dermatologic consultations, neurologic consultations, and nerve conduction velocity to test peripheral neurologic symptoms as necessary. Careful evaluation at final follow-up showed that all of these patients were in generally good condition and presented no skin lesions (hyperpigmentation and hyperkeratosis). Only 1 case of mild anemia with a hemoglobin level of 98 g/L and another case with a decreased WBC count at 3.2 × 109/L were documented. There were no abnormal ECGs and echocardiograms that might be associated with the long-term toxicity of ATO, and chest X-rays were negative in all cases. Serum tumor marker screening revealed mildly increased levels of CEA in one male and CA125 in a female patient, but no tumors were detected in either of the 2 cases after thorough screening. In further follow-up, CEA and CA125 levels returned to normal. In general, the results of laboratory study and examination were comparable between patients and healthy donors, as shown in Table S2.
Evaluation of Arsenic Retention After Cessation of ATRA/ATO Treatment.
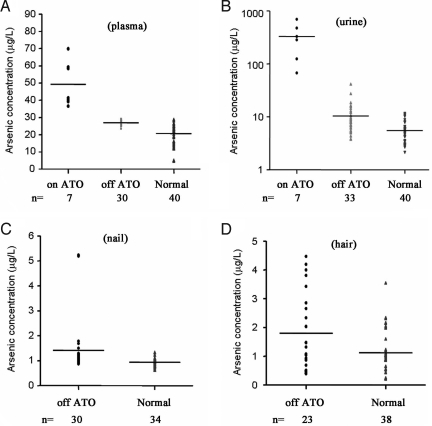
Another concern surrounding the use of ATO was arsenic retention. We collected plasma and urine samples to assess the concentration of residual arsenic in 33 eligible long-term survivors who had ceased arsenic treatment more than 24 months prior. Samples from 40 healthy controls (including family members of patients) and 7 patients with APL undergoing ATO treatment were used as controls. When patients were receiving ATO, the plasma arsenic content was high (49.3 ± 13.0 ng/mL), but when treatment was terminated, the plasma arsenic concentration in APL patients drastically decreased to 26.4 ± 2.1 ng/mL. This was, however, still somewhat higher than the levels found in healthy controls (18.4 ± 6.0 ng/mL; Fig. 4A). The urine arsenic concentration also was high (330.2 ± 232.1 ng/mL) when patients were receiving ATRA/ATO, but it was significantly decreased when treatment was ceased (10.4 ± 7.4 ng/mL). Again, this concentration remained higher than that found in healthy controls (4.2 ± 1.6 ng/mL; Fig. 4B). Of note, because of a short half-life of arsenic in plasma, its level generally is not used as a marker to monitor arsenic exposure and chronic toxicity. The urine arsenic levels in long-term survivors were below the Standard of Diagnosis for Endemic Arsenism (90 μg/L) set by the Ministry of Health of China and below the safety limit of 200 μg/L recommended by the U.S. Agency for Toxic Substances and Disease Registry (26, 27). These levels also were within the normal range of 36–541 nmol/L (range, 2.7–40.6 μg/L) set by the Trace Element Reference Values in Human Tissues project (28). Finally, the nail (Fig. 4C) and hair (Fig. 4D) arsenic contents in these patients (reflecting the long-term exposure and in vivo accumulation of arsenic) were 0.8–5.2 μg/g and 0.4–4.5 μg/g, respectively, which were only slightly higher than the levels in healthy controls (range 0.6–1.3 μg/g and 0.2–3.5 μg/g, respectively; Fig. 4 C and D).
Fig. 4.
Study on pharmacological features: arsenic concentration in plasma (A), urine (B), nails (C), and hair (D) in different patient groups. (A) The plasma arsenic concentrations of the off-ATO group were considerably lower than those of the on-ATO treatment group (P < 0.001), albeit statistically significantly higher than normal (P < 0.001). (B) Urine arsenic concentrations of the off-ATO group were also lower than those of the ATO treatment group (P = 0.0002) but higher than normal (P = 0.0001). (C and D) Arsenic concentrations in nails and hair of the off-ATO group compared with normal (P = 0.011 and 0.014, respectively). On ATO indicates patients with ongoing ATO treatment; Off ATO, patients treated with ATRA/ATO-based regimen who were at least 24 months off the ATO treatment; Normal, control samples from healthy family members of patients or unrelated healthy blood donors.
Discussion
A synergistic therapeutic effect with ATRA/ATO-based combination treatment for newly diagnosed APL (16, 18, 19, 29, 30) and for those cases in first recurrence (17) has been documented in short-term studies. Here, we report that in 85 de novo patients with a median follow-up of 70 months, CR was achieved in 80 cases (94.1%), whereas the 5-year EFS and OS rates were 89.2% ± 3.4% and 91.7 ± 3%, respectively, for all patients. For patients who achieved CR (n = 80), the 5-year RFS and OS rates were 94.8% ± 2.5% and 97.4% ± 1.8%, respectively. These are consistent with recent studies (31–33) and demonstrate a better long-term outcome with the ATRA/ATO-based regimen in induction or consolidation than with that obtained using the ATRA-based regimen in our own group or reported by many others (1). More interestingly, Estey et al. (18) reported a major benefit for the combination of ATO and ATRA with minimal or even without chemotherapy in the treatment of low-risk APL patients. Although trials with larger number of cases still need to be conducted, ATRA/ATO combination approach could be considered as a potential frontline therapy for de novo APL.
The chimeric PML-RARα protein has been shown to play a key role in the pathogenesis of APL through interaction with nuclear receptor corepressors, with abnormally high affinity leading to deacetylation of histones and transcription repression of RARα target genes (34). In addition, we previously (16, 35, 36) showed that quantitative assay of PML-RARα fusion transcripts represents a specific and sensitive marker for disease burden. In this current study, we demonstrate a rapid reduction in the level of PML-RARα fusion transcripts when patients received ATRA/ATO treatment, as well as recurrence of PML-RARα transcripts when disease relapsed in 2 cases (Fig. 2 A and B). It is obvious that FLT3 mutation is not an adverse indicator in the context of our therapeutic approach, because the status of FLT3 did not affect the response and long-term outcome (Table S1), and mutant FLT3 transcripts become negative before the PML-RARα marker (Fig. 2C). Studies show that AQP9 controls arsenic transportation and may determine ATO sensitivity (11, 37). In HL-60 and K562 cells, ATRA up-regulated AQP9 expression, facilitated arsenic uptake, and sensitized the cells to ATO. In NB4 cells, we found that ATRA treatment led to significant up-regulation of AQP9 (Fig. 3A). In de novo APL patients, the expression of AQP9 in bone marrow mononuclear cells was relatively low at diagnosis (Fig. 3 B and C). Interestingly, AQP9 was markedly up-regulated when patients received ATRA/ATO treatment (Fig. 3 B and C). In a previous study testing the efficacy of ATRA/ATO in relapsed APL, Raffoux et al. (38) showed that patients receiving ATRA/ATO had a lower serum arsenic concentration compared with those who received ATO alone. These results suggested that ATRA-triggered AQP9 up-regulation might contribute to enhanced arsenic uptake by leukemic cells, which could be related to the mild toxicity and high therapeutic efficacy, and induced expression of AQP9 might play an important role in effects of ATRA/ATO on APL cells. Of course, the precise mechanisms of action of ATRA/ATO on AQP9 warrant investigation.
The therapeutic benefit of ATRA/ATO use in relapsed APL has been described previously in some case reports (39–41), but in a randomized study in which only 10 cases were treated with ATRA/ATO, the combination regimen failed to induce any synergistic effect (38). Hence, additional trials with a larger sample size are warranted before any conclusion can be drawn. Furthermore, it should be clarified whether the loss of synergistic effect in relapsed APL could be due to the development of ATRA resistance.
Although the ATRA/ATO combination therapy yielded a remarkable improvement in the survival rate, there were 5 patients who died early because of intracranial hemorrhage and disseminated intravascular coagulation in the induction therapy, necessitating the development of effective measures to prevent or control this lethal complication. Additionally, although the combination regimen significantly decreased the incidence of bone marrow relapse, the incidence of CNS relapse remains a challenge, probably because of the relatively poor entry of the drugs through the blood–brain barrier (42–44). Because CNS leukemia may be accompanied or preceded by resurgence of the PML-RARα transcript level, intrathecal prophylactics should be considered in the future, with closer monitoring of this specific disease marker.
The long-term therapeutic benefit of ATO as a single agent for the treatment of APL has been reported previously (45, 46), and we show here that incorporation of ATRA drastically enhances its efficacy. On the other hand, ATO use as a single agent causes only minimal toxicity, including gastrointestinal reactions, grade I-II hepatotoxicity, neurotoxicity, and mildly abnormal ECG. Reversible grade III-IV hepatotoxicity was seen in a small proportion of patients (45, 46). Interestingly, Wang et al. (19) reported that the ATO/ATRA combination is less toxic when compared with the use of ATO or ATRA alone. Our results are consistent with this observation, because no severe side effects, including grade III-IV hepatotoxicity or cardiac dysfunction, were documented. Carcinogenesis is a major concern associated with long-term exposure to arsenic, but we found no cases that developed secondary tumors, such as skin cancer, although transiently 1 male tested positive for CEA, and a mild, unsustained increase in CA125 in a female patient was recorded. Moreover, arsenic concentrations in the urine of patients who had ceased ATRA/ATO treatment for 24 months were below the safety limits recommended by government agencies in several countries or regions (Fig. 4B), whereas arsenic levels in plasma, nails, and hair were only slightly higher than those found in healthy controls (Fig. 4 A, C, and D).
In summary, we report here that the ATRA/ATO-based treatment regimen incorporating chemotherapy for both remission induction and postremission therapy for newly diagnosed APL yields an encouraging long-term survival rate greater than 90% with alleviated side effects, and thus reinforces its potential use as frontline therapy for de novo APL.
Methods
Patients and Treatment Protocols.
Between April 2001 and December 2005, the dates when the first and final patients were included in the study, 85 patients with newly diagnosed APL were seen, with the diagnosis requiring cytogenetic assay for t(15;17)(q22;q21) and/or RT-PCR evidence of the PML-RARα rearrangement (6). The clinical features of patients are described in Table S1. Informed consent was obtained from all patients entering into this study. All patients received ATRA/ATO combination and previously described chemotherapy protocols primarily for postremission consolidation and maintenance therapy (16). Briefly, for induction therapy, patients received ATRA and ATO daily until documentation of CR, whereas patients who presented with WBC counts higher than 10 × 109/L before or during the ATRA/ATO treatment also received additional chemotherapy to control the hyperleukocytosis. After achieving CR, all patients were given 3 courses of consolidation therapy, including daunorubicin plus Ara-C, Ara-C “pulse” regimen, and homoharringtonine plus Ara-C regimen. The maintenance treatments consisted of 5 cycles of sequential use of ATRA, ATO, and low-dose chemotherapy. In the instances of relapse, ATRA/ATO or chemotherapy was given (16). CR, relapse, RFS, and EFS were defined according to criteria recommended by the International Working Group (47).
Detection of PML-RARα Fusion Transcripts and Analysis of FLT3 Mutation.
Bone marrow samples were collected at diagnosis, after remission induction, at the end of consolidation, and throughout the maintenance therapy. Total RNA from mononuclear cells was extracted by using TRIzol LS reagent (Invitrogen). Regular RT-PCR of 3 distinct [long (L), short (S), and variant (V)] types of PML-RARα transcripts and quantitative real-time RT-PCR were analyzed as described previously (16) by using GAPDH as a control.
The screening of FLT3 mutations was performed based on amplification of the full-length FLT3 gene by RT-PCR, and then the PCR products were purified and sequenced on an ABI Prism 3700 DNA Analyzer using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) (48).
Analysis of Arsenic Concentration and Quantification of AQP9 Gene Expression.
Plasma, urine, hair (from the nape of the head near the scalp), and tips of fingernail were collected in patients who had terminated the treatment for at least 24 months. All samples were sealed separately in labeled polyethylene Ziploc bags (hair and nails) or frozen (urine and blood) at −20 °C until further processing. The arsenic concentrations were measured as described previously (9, 37). AQP9 expression was quantified by semiquantitative RT-PCR as previously described (39).
Statistical Analysis.
OS, EFS, and RFS were estimated according to the Kaplan–Meier method. The comparison of arsenic concentration between groups of patients off ATO and on ATO or healthy controls was performed by the Student's t test, and all statistical analyses were performed by using SPSS 12.0 for Windows.
Supplementary Material
Acknowledgments.
We are indebted to all members of the Shanghai Institute of Hematology for their long-term support and to Dr. Jian-Xiang Liu for his constructive input. This work was supported in part by the National High Tech Program for Biotechnology (863:2006AA02A405), the Chinese National Key Basic Research Project (973, 2004 CB 518606), the National Natural Science Foundation of China (30672772, 30623010, 30521003), the Key Discipline Program of Shanghai Municipal Education Commission (Y0201), the Shanghai Municipal Commission for Science and Technology (06DZ22021), and the Samuel Waxman Cancer Research Foundation Co-PI Program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813280106/DCSupplemental.
References
- 1.Wang ZY, Chen Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 2.Hillestad LK. Acute promyelocytic leukemia. Acta Med Scand. 1957;159:189–194. [PubMed] [Google Scholar]
- 3.Bernard J, et al. Acute promyelocytic leukemia: Results of treatment by daunorubicin. Blood. 1973;41:489–496. [PubMed] [Google Scholar]
- 4.Huang ME, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- 5.Tallman MS, et al. All-trans retinoic acid in acute promyelocytic leukemia: Long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100:4298–4302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- 6.Shen ZX, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 7.Sun HD, Ma L, Hu XC, Zhang TD. Ai-Lin I treated 32 cases of acute promyelocytic leukemia. Chin J Integrat Chin West Med. 1992;12:170–171. [Google Scholar]
- 8.Raelson JV, et al. The PML/RAR alpha oncoprotein is a direct molecular target of retinoic acid in acute promyelocytic leukemia cells. Blood. 1996;88:2826–2832. [PubMed] [Google Scholar]
- 9.Chen GQ, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 10.Lallemand-Breitenbach V, et al. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008;10:547–555. doi: 10.1038/ncb1717. [DOI] [PubMed] [Google Scholar]
- 11.Leung J, Pang A, Yuen WH, Kwong YL, Tse EWC. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109:740–746. doi: 10.1182/blood-2006-04-019588. [DOI] [PubMed] [Google Scholar]
- 12.Zheng PZ, et al. Systems analysis of transcriptome and proteome in retinoic acid/arsenic trioxide-induced cell differentiation/apoptosis of promyelocytic leukemia. Proc Natl Acad Sci USA. 2005;102:7653–7658. doi: 10.1073/pnas.0502825102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianni M, et al. Combined arsenic and retinoic acid treatment enhances differentiation and apoptosis in arsenic-resistant NB4 cells. Blood. 1998;91:4300–4310. [PubMed] [Google Scholar]
- 14.Jing Y, et al. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia cells in vitro and in vivo. Blood. 2001;97:264–269. doi: 10.1182/blood.v97.1.264. [DOI] [PubMed] [Google Scholar]
- 15.Lallemand-Breitenbach V, et al. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J Exp Med. 1999;189:1043–1052. doi: 10.1084/jem.189.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen ZX, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2004;101:5328–5335. doi: 10.1073/pnas.0400053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aribi A, et al. Combination therapy with arsenic trioxide, all-trans retinoic acid, and gemtuzumab ozogamicin in recurrent acute promyelocytic leukemia. Cancer. 2007;109:1355–1359. doi: 10.1002/cncr.22524. [DOI] [PubMed] [Google Scholar]
- 18.Estey E, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–3473. doi: 10.1182/blood-2005-10-4006. [DOI] [PubMed] [Google Scholar]
- 19.Wang G, et al. An efficient therapeutic approach to patients with acute promyelocytic leukemia using a combination of arsenic trioxide with low-dose all-trans retinoic acid. Hematol Oncol. 2004;22:63–71. doi: 10.1002/hon.728. [DOI] [PubMed] [Google Scholar]
- 20.Fenaux P, et al. Long-term follow-up confirms the benefit of all-trans retinoic acid in acute promyelocytic leukemia. European APL group. Leukemia. 2000;14:1371–1377. doi: 10.1038/sj.leu.2401859. [DOI] [PubMed] [Google Scholar]
- 21.Fenaux P, et al. Effect of all trans retinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood. 1993;82:3241–3249. [PubMed] [Google Scholar]
- 22.Kiyoi H, et al. Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia Study Group of the Ministry of Health and Welfare (Kohseisho) Leukemia. 1997;11:1447–1452. doi: 10.1038/sj.leu.2400756. [DOI] [PubMed] [Google Scholar]
- 23.Schnittger S, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: Correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 24.Gale RE, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): An analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005:1063658–3665. doi: 10.1182/blood-2005-03-1323. [DOI] [PubMed] [Google Scholar]
- 25.Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: The combination matters—an analysis of 3082 patients. Blood. 2008;111:2527–2537. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 26.Sun GF, Liu JY, Sun DJ, Luong TV, Wang LY. Endemic Arsenicosis: A Clinical Diagnostic Manual with Photo Illustrations. Bangkok, Thailand: UNICEF East Asia and Pacific Regional Office; 2004. [Google Scholar]
- 27.Agency for Toxic Substances and Disease Registry; U.S. Department of Health and Human Services. Toxicological profile for arsenic. [Accessed January 2008 and December 2008];2000 Available at www.atsdr.cdc.gov/csem/arsenic/clinical_evaluation.html.
- 28.Kristiansen J, Christensen JM, Iversen BS, Sabbioni E. Toxic trace element reference levels in blood and urine: Influence of gender and lifestyle factors. Sci Total Environ. 1997;204:147–160. doi: 10.1016/s0048-9697(97)00155-1. [DOI] [PubMed] [Google Scholar]
- 29.Quezada G, Kopp L, Estey E, Wells RJ. All-trans-retinoic acid and arsenic trioxide as initial therapy for acute promyelocytic leukemia. Pediatr Blood Cancer. 2008;51:133–135. doi: 10.1002/pbc.21529. [DOI] [PubMed] [Google Scholar]
- 30.Zhang GC, et al. Effects of combination therapy with all-trans retinoic acid and arsenic trioxide on acute promyelocytic leukemia. Chin J Cancer. 2008;23:430–434. [PubMed] [Google Scholar]
- 31.Tsimberidou AM, et al. All-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) combination therapy induces high rates of durable molecular remission in newly diagnosed acute promyelocytic leukemia (APL) ASH Annual Meeting Abstracts. 2007;110:1834. [Google Scholar]
- 32.Li A, Zhang L, Liu G. Clinical study on induction therapy of arsenic trioxide in combination with all-trans retinoic acid for childhood acute promyelocytic leukemia. Chin J Pract Pediatr. 2006;21:93–95. [Google Scholar]
- 33.Powell BL, et al. Effect of consolidation with arsenic trioxide (As2O3) on event-free survival (EFS) and overall survival (OS) among patients with newly diagnosed acute promyelocytic leukemia (APL): North American Intergroup Protocol C9710. J Clin Oncol. 2007;25(suppl):2. [Google Scholar]
- 34.Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- 35.Liu YF, et al. Molecular response in acute promyelocytic leukemia: A direct comparison of regular and real-time RT-PCR. Leukemia. 2006;20:1393–1399. doi: 10.1038/sj.leu.2404262. [DOI] [PubMed] [Google Scholar]
- 36.Gu BW, et al. Feasibility and clinical significance of real-time quantitative RT-PCR assay of PML-RARalpha fusion transcript in patients with acute promyelocytic leukemia. Hematol J. 2001;2:330–340. doi: 10.1038/sj.thj.6200128. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raffoux E, et al. Combined treatment with arsenic trioxide and all-trans-retinoic acid in patients with relapsed acute promyelocytic leukemia. J Clin Oncol. 2003;21:2326–2334. doi: 10.1200/JCO.2003.01.149. [DOI] [PubMed] [Google Scholar]
- 39.Au WY, Chim CS, Lie AK, Liang R, Kwong YL. Combined arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia recurring from previous relapses successfully treated using arsenic trioxide. Br J Haematol. 2002;117:130–132. doi: 10.1046/j.1365-2141.2002.03409.x. [DOI] [PubMed] [Google Scholar]
- 40.Grigg A, Kimber R, Szer J. Prolonged molecular remission after arsenic trioxide and all-trans retinoic acid for acute promyelocytic leukemia relapsed after allogeneic stem cell transplantation. Leukemia. 2003;17:1916–1917. doi: 10.1038/sj.leu.2403050. [DOI] [PubMed] [Google Scholar]
- 41.Galimberti S, et al. Arsenic and all-trans retinoic acid as induction therapy before autograft in a case of relapsed resistant secondary acute promyelocytic leukemia. Bone Marrow Transplant. 1999;24:345–348. doi: 10.1038/sj.bmt.1701875. [DOI] [PubMed] [Google Scholar]
- 42.Patriarca F, et al. Activity of all-trans-retinoic acid in a case of central nervous system extramedullary relapse of acute promyelocytic leukemia. Eur J Haematol. 2002;68:310–313. doi: 10.1034/j.1600-0609.2002.01660.x. [DOI] [PubMed] [Google Scholar]
- 43.Knipp S, Gattermann N, Schapira M, Kaferstein H, Germing U. Arsenic in the cerebrospinal fluid of a patient receiving arsenic trioxide for relapsed acute promyelocytic leukemia with CNS involvement. Leuk Res. 2007;31:1585–1587. doi: 10.1016/j.leukres.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Au WY, Tam S, Fong BM, Kwong YL. Determinants of cerebrospinal fluid arsenic concentration in patients with acute promyelocytic leukemia on oral arsenic trioxide therapy. Blood. 2008;112:3587–3590. doi: 10.1182/blood-2008-06-161000. [DOI] [PubMed] [Google Scholar]
- 45.Mathews V, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: Durable remissions with minimal toxicity. Blood. 2006;107:2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 46.Ghavamzadeh A, et al. Treatment of acute promyelocytic leukemia with arsenic trioxide without ATRA and/or chemotherapy. Ann Oncol. 2006;17:131–134. doi: 10.1093/annonc/mdj019. [DOI] [PubMed] [Google Scholar]
- 47.Cheson BD, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 48.Nakao M, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.