Abstract
The Fv1 virus resistance gene is a coopted endogenous retrovirus (ERV) sequence related to the gag gene of the MuERV-L ERV family. Three major Fv1 resistance alleles have been identified in laboratory mice, and they target virus capsid genes to produce characteristic patterns of resistance to mouse leukemia viruses (MLVs). We identified Fv1 in 3 of the 4 Mus subgenera; its absence from Coelomys and 1 of 3 species of Pyromys indicate Fv1 was acquired shortly after the origin of the Mus genus. We sequenced Fv1 genes from 21 mice representative of the major taxonomic groups of Mus. Two lines of evidence indicate that Fv1 has had antiviral function for 7 million years of evolution. First, 2 species of African pygmy mice (subgenus Nannomys) show an Fv1-like MLV resistance, and transduced cells expressing the Nannomys Fv1 gene reproduce this resistance pattern. Second, sequence comparisons suggest that Fv1 has been involved in genetic conflicts throughout Mus evolution. We found evidence for strong positive selection of Fv1 and identified 6 codons that show evidence of positive selection: 3 codons in the C-terminal region including 2 previously shown to contribute to Fv1 restriction in laboratory mice, and 3 codons in a 10-codon segment overlapping the major homology region of Fv1; this segment is known to be involved in capsid multimerization. This analysis suggests that Fv1 has had an antiviral role throughout Mus evolution predating exposure of mice to the MLVs restricted by laboratory mouse Fv1, and suggests a mechanism for Fv1 restriction.
Keywords: Fv1 gammaretrovirus restriction gene, mouse endogenous retrovirus, Mus evolution
Wild mouse species and inbred laboratory strains vary in their susceptibility to gammaretrovirus infection, and such resistance can be due to constitutively expressed antiviral factors that target various stages of the retroviral life cycle. The prototype for such virus resistance factors is the Fv1 gene, discovered 40 years ago in studies on resistance to Friend murine leukemia virus (MLV) (1).
There are 4 well characterized functional variants of Fv1 and additional Fv1-like restrictions found in inbred strains and wild mouse species (2). Three of these alleles, termed Fv1n, Fv1b, and Fv1nr, produce characteristic patterns of resistance to subgroups of mouse-tropic viruses that are designated N-, B-, or NR-tropic. Cells with the Fv10 (null) allele restrict none of these virus subgroups, and NB-tropic viruses are not restricted by any of these Fv1 alleles.
Fv1 was cloned and identified as a coopted ERV sequence that is related to the gag gene of MuERV-L (3, 4), a Class III (spumavirus-related) ERV transposit family that is transpositionally active in mice but has no known infectious virus counterparts. The major resistance variants of Fv1 differ from one another at 3 amino acid sites in its C-terminal region, and Fv1b additionally differs from Fv1n and Fv1nr at its C terminus due to a 1.3-kb indel (3). Substitutions at the 3 sites and variation at the C terminus all contribute to resistance (5, 6). The mechanism of resistance is unknown, but Fv1 typically blocks replication after reverse transcription and before integration. Fv1 is known to target the virus capsid gene; a single amino acid substitution at position 110 distinguishes N- and B-tropic viruses (7), and substitutions at additional residues in the capsid N-terminal domain are responsible for NR- and NB-tropism (5, 8).
Until recently, Fv1-type restriction had only been identified in laboratory mice and wild mouse species closely related to laboratory mice (2). Our examination of an African pygmy mouse, subgenus Nannomys, identified an unusual postentry resistance to ecotropic MuLVs (9). Although this resistance targets some of the same amino acid residues as the mouse Fv1 gene, the pattern of virus resistance in the pygmy mouse cells does not resemble that attributed to any of the laboratory mouse Fv1 alleles.
We have now screened additional Mus species distantly related to laboratory strains for Fv1-like resistance phenotypes, and analyzed the Fv1 sequence in wild mouse species of 3 Mus subgenera. We show here that the pygmy mouse Fv1 has antiviral activity and demonstrate that Fv1 has been under strong positive selection throughout 7 MY of Mus evolution. We identified 6 codons under strong positive selection including 2 residues implicated in Fv1-mediated virus restriction, and 3 codons in a segment overlapping the Fv1 major homology region (MHR) region, a region that in retroviruses produces the interface for capsid binding and dimerization.
Results
Analysis of the 4 Subgenera of Mus for Fv1.
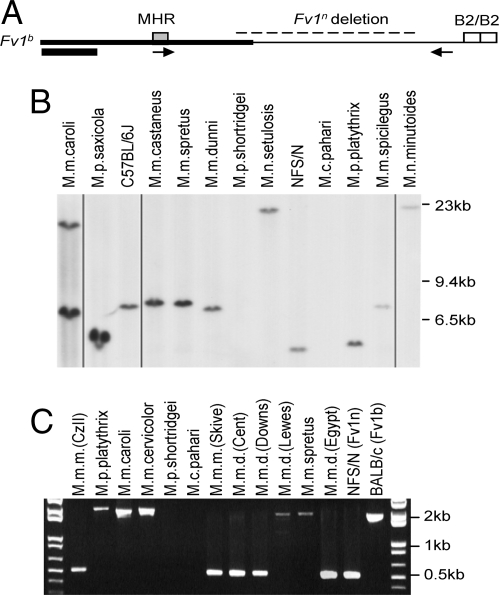
We examined representative species of all 4 Mus subgenera (Mus, Pyromys, Nannomys and Coelomys) for Fv1 sequences by Southern blot analysis using a probe from the 5′ end of Fv1 (Fig. 1A). One Fv1-reactive band was detected in most species; Mus mus caroli has 2 BglI-generated Fv1-related copies (Fig. 1B). Fv1 was identified in 3 of the 4 Mus subgenera; it was missing in Mus coelomys pahari and in Mus pyromys shortridgei, 1 of 3 Pyromys species tested.
Fig. 1.
Detection of Fv1 in DNAs of Mus species. (A) The structure of Fv1b is shown with a gray box marking the MHR, open boxes representing B2 repeats and a dashed line representing the 1.3-kb segment deleted in Fv1n. The arrows represent the PCR primers Fb3003 and Rb4831, and the black box represents the segment used for blot hybridization. Fv1 is encoded by a single exon. (B) Southern blot analysis of BglI-digested Mus DNAs. All lanes taken from the same exposure of a single blot; deleted lanes are indicated by vertical lines. (C) PCR products of Mus DNAs.
PCR using primers designed to amplify the 3′ end of Fv1 along with flanking sequences confirmed the absence of Fv1 from these 2 species (Fig. 1C). These primers also distinguish the 2 major Fv1 variants found in laboratory mouse strains; Fv1n has a 1.3-kb deletion at its 3′ end relative to Fv1b. Both Fv1 variants were identified in Mus species. Most mice carry the 1.3-kb segment characteristic of Fv1b; the Fv1n deletion was found only in house mouse species, specifically all 4 Mus mus musculus samples tested and some Mus mus domesticus and Mus mus spretus mice.
Mus originated 7–8 MYA and quickly radiated into 4 subgenera. The radiations leading to these subgenera are difficult to order, but Coelomys is generally regarded as the most basal group in Mus (10). Our results indicate that Fv1 is absent from species in 2 of the non-Mus subgenera, including Coelomys, and that when Fv1 entered the Mus germ line it contained the 1.3-kb segment found in the laboratory mouse Fv1b allele.
Restriction of Ecotropic MLVs in Cells of 2 Species of Nannomys.
To determine if Fv1 sequences serve an antiviral function in species from non-Mus subgenera, we infected cells from these mice with various viruses known to be subject to restriction by laboratory mouse Fv1, and susceptibility was compared with NIH 3T3 (Fv1n), 129/J (Fv1nr) and M. dunni (Fv10) (Table 1). M. pyromys platythrix was fully susceptible to all viruses tested indicating that its Fv1 gene has no antiviral activity against this particular panel of MLVs. Cells of a second species, Mus nannomys minutoides, were completely resistant to all AKV MLVs, but susceptible to MoMLV and Friend MLVs, as reported (9). In contrast, cells of another Nannomys species, Mus nannomys setulosus, show low, but detectable susceptibility to AKV-B and AKV-NR, reduced susceptibility to MoMLV, and differential susceptibility to the 2 Friend MLVs. M. n. setulosus cells, like M. n. minutoides, are completely resistant to infection with AKV-N and to an MoMLV chimera, Mo-CA3, in which part of the Fv1 target region (codon positions 99–129) of the capsid gene was replaced with that of AKV-N (9). Thus, these 2 pygmy mice have similar but distinctive patterns of susceptibility to MLVs, and their resistance to AKV-N is mediated by the region of capsid that is targeted by Fv1.
Table 1.
Virus titers of MLVs on cells of various Mus species and cells with known Fv1 restriction
| Cells | Log10 Virus Titer*/Fv1 Tropism |
|||||
|---|---|---|---|---|---|---|
| AKV MLV |
MoMLV |
FBLV |
F-S MLV |
|||
| N | B | NR | NB | NB | NR | |
| NIH 3T3 (Fv1n) | 4.4 | 0.5 | 4.4 | 6.5 | 5.1 | 5.6 |
| 129/J EF (Fv1nr) | 1.8 | 0.9 | 4.2 | 5.8 | ND | 4.7 |
| M. dunni (Fv10) | 4.1 | 4.5 | 4.4 | 2.5† | 4.1 | 4.7 |
| M. n. minutoides | <0 | <0 | <0 | 4.5 | 4.3 | 4.0 |
| M. n. setulosus | <0 | 1.4 | 0.7 | 3.6 | 2.8 | 1.1 |
| M. p. platythrix | 3.7 | 4.4 | 4.4 | 5.8 | 4.1 | 4.4 |
*Virus titers were determined by the XC overlay test in which the indicated cells were infected with virus dilutions, irradiated 4 days later and overlaid with XC cells to identify clusters of virus infected cells (9, 11). Titers represent the number of XC PFU in 0.2 ml in representative experiments. ND, not done.
†Restriction is mediated by receptor polymorphism (12), and is unrelated to Fv1.
Fv1 gene of M. n. minutoides and M. n. setulosis.
The 5′ and 3′ ends of the Fv1 gene of M. n. minutoides, Fv1m, were amplified from genomic DNA and sequenced (Fig. 2); this sequence included a complete 1.3-kb Fv1-related ORF and flanking sequences on both sides of the gene. The 24-bp segment flanking the 5′ end is identical to that flanking Fv1b. The 803-bp flanking the 3′ end of the ORF are homologous to the Fv1b flanking segment, but also contain a 170-bp B1 repeat inserted near the end of the coding region. The 437-aa Fv1m ORF is highly homologous to the 440-aa Fv1 gene of the laboratory mouse. The Fv1m predicted protein sequence is 87% identical to Fv1n. The differences include a 9-base in-frame deletion near the N terminus, and 9-aa differences at the C terminus due to the B1 insertion. The rest of the differences are scattered and notably include differences at the 3 amino acid positions (352, 358, and 399) that distinguish Fv1n, Fv1b, and Fv1nr and have been shown to modulate resistance (3, 5, 6). Residues at these positions for the known restrictive Fv1 alleles are: SKV (Fv1n), SER (Fv1b), and FKV (Fv1nr). The Fv1m sequence has a different combination at these sites, FKS.
Fig. 2.
Comparison of predicted amino acid sequence of the M. n. minutoides Fv1 gene and Fv1b. The position of MHR is indicated by a solid bar and shading indicates the 3 amino acids identified as critical for restriction based on analysis of laboratory mouse Fv1 alleles (3, 5, 6).
The predicted amino acid sequence of the Fv1 gene cloned from M. n. setulosus cells, Fv1s, is 98% identical to Fv1m, and has a similar C terminus and the same 9-bp deletion in the N terminus relative to Fv1n. For Fv1s, the amino acids at the 3 sites that distinguish the 3 known restrictive alleles are FKE, a unique pattern.
Functional Analysis of Fv1m.
Full length Fv1 ORFs from M. n. minutoides cells and from NIH 3T3 were expressed in MDTF cells (Fv10) (13). Cells were tested for susceptibility to viruses restricted by NIH 3T3 and by M. n. minutoides cells (Table 2). The transduced cells were fully susceptible to NB-tropic Friend MLV (FBLV), but MDTF cells expressing Fv1n showed reduced susceptibility to AKV-B, and cells expressing Fv1m showed reduced susceptibility to both AKV-B and AKV-N. The fact that the transduced cells did not reproduce the level of restriction seen in M. n. minutoides or NIH 3T3 cells is consistent with previous observations that Fv1 is highly sensitive to concentration and that not all transfected cells show restriction (3, 14). In 3 independent experiments, however, the transduced MDTF cells reproduced the distinctive restriction patterns of M. n. minutoides and NIH 3T3.
Table 2.
Virus titers of MLVs on MDTF cells transduced with Fv1n or Fv1m
| Cells | Log10 Virus Titer* |
||
|---|---|---|---|
| AKV-N | AKV-B | FBLV | |
| MDTF | 4.4 | 3.4 | 5.8 |
| MDTF-Fv1n | 4.7 | 0.8 | 5.4 |
| MDTF-Fv1m | 2.7 | 1.5 | 5.3 |
*Virus titers determined as in Table 1.
Fv1 Evolution.
Host genes involved in antagonistic interactions with pathogens can be identified by sequence comparisons that reveal evidence of positive selection. To determine if Fv1 has had an antiviral role throughout Mus evolution, and to identify possible sites of virus interaction, we analyzed 24 sequenced Fv1 genes from various Mus species.
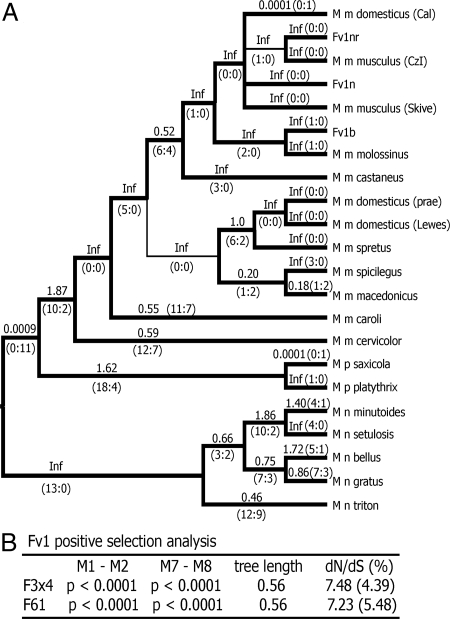
Two of the Fv1 genes, in M. mus dunni and M. mus cookii, had stop codons in the 5′ half of the gene as found in a previous analysis of this segment of the Fv1 sequence (15); these were excluded from further analysis. The near full-length Fv1 sequences used for selection analysis did not include gaps in the sequence, and codons after the stop codon in M. nannomys triton (Dataset S1). All of the sequenced Fv1 genes were amplified using one or more primers designed from the sequences flanking the Fv1b ORF, indicating that these genes represent the same integration site. Twenty-two Fv1 genes were used to construct a maximum parsimony tree (Fig. 3A). Values of dN/dS along each branch were calculated using the free-ratio model of PAML (16). A dN/dS value >1 suggests that positive selection has acted along that lineage. Many branches of the tree show evidence of positive selection with dN/dS >1, or, when dS = 0, show 4 or more replacement substitutions, which is also suggestive of selection.
Fig. 3.
Positive selection of Fv1 in the genus Mus. (A) Cladogram showing branch values of dN/dS calculated using the free-ratio model of PAML, with the number of replacement and synonymous changes in parentheses. When dS = 0, dN/dS is infinite (Inf). dN/dS > 1 suggests positive selection along that lineage. Bootstrap support for this tree topology was generally good, with most bootstrap percentages >90%. The thin lines represent branches with bootstrap values <70%. (B) Likelihood ratio tests were used to test for positive selection. Neutral models (M0, M1, M7) were compared with selection models (M2, M8) using 2 different models of codon frequency (F3 × 4 or F61). P values <0.0001 provide strong evidence of selection. Tree length is the average number of substitutions per codon along all branches. For codons under positive selection, the dN/dS ratio is given along with the % of codons with this ratio.
Likelihood ratio tests indicate that Fv1 has a significant probability of having experienced positive selection (Fig. 3B and SI Appendix); this is consistent with a previous analysis of a smaller segment of Fv1 (15). The Bayes empirical Bayes calculation of posterior probabilities in PAML (17) identified specific Fv1 codon positions as having significant probability of positive selection. Results identified 6 codons as being under positive selection with posterior probability >0.95 (Fig. 4), and for all 6 codons P ≥ 0.99. A second analysis done with an equally parsimonious PAUP-generated tree identified the same 6 codons with nearly identical statistics. The 6 codons identified as being under positive selection included 3 at positions 261, 265, and 270, clustered at the 5′ end of the MHR, a highly conserved region known to be involved in capsid interactions (18–22). The other 3 codons identified as being under positive selection were at positions 352, 399, and 401; 2 of these codons, 352 and 399, distinguish the laboratory mouse Fv1 restriction genes (3, 5), and substitutions at both of these codons have been shown to contribute to virus restriction (5, 6). A third codon, 358, previously implicated in Fv1 restriction in laboratory mice was not identified as being under positive selection; all Fv1 genes except for Fv1b have K at this site suggesting that the laboratory mouse Fv1b variant K358E arose fairly recently in Mus. The demonstration of positive selection at these 6 codons suggests that the Fv1 gene has been subjected to positive selection over nearly 7 MY of Mus evolution, and indicates that residues known to be responsible for restriction of MLVs in laboratory mice also have antiviral activity in more evolutionarily divergent species and subgenera.
Fig. 4.
Fv1 sites that have been subject to positive selection. At the top are sequence alignments for 22 Mus genes in the regions containing the 6 positively selected codons; all changes relative to Fv1b are shaded. MHR is boxed. At the bottom is a diagram of the Fv1 coding region showing the locations of the MHR (black box), the 6 positively selected codons and the 1 additional codon (358) implicated in restriction (3, 6).
Discussion
These results indicate that the mouse Fv1 gene has had antiviral activity since shortly after its introduction into the Mus genome, an event that occurred during a period of rapid diversification that gave rise to the 4 Mus subgenera ≈7 MY ago (10, 23). That this sequence has long had antiviral function is supported by the observation that the Fv1 genes of 2 species of Nannomys have novel Fv1-mediated antiviral phenotypes, and by the demonstration that Fv1 has been under positive Darwinian selection. We identified 2 segments of Fv1 potentially involved in restriction: an MHR segment known to function in capsid binding, and 3 residues in the C-terminal domain (CTD) of which 2 are known to contribute to the specificity of Fv1 restriction in laboratory mice.
The mechanism of Fv1 restriction has not been elucidated after 40 years of investigation, but the determination that Fv1 encodes a retroviral capsid-like protein suggests that Fv1 may bind capsids of exogenous virus and interfere with capsid disassembly and reverse transcription. Three of the positively selected codons identified in this study are in a 10-codon segment associated with the Fv1 capsid-like MHR region. Mutations of codons in and around the MHR regions of various retroviruses disrupt virus assembly, maturation and infectivity (18–20). Mutational analysis in and around the MHR of MoMLV and HIV-1 has determined that the segment described here is critical for formation of the high-affinity capsid interface, and that this segment determines the specificity of heterodimeric interactions (20, 21). These interactions are important in the early stages of replication as the majority of replication defective MoMLV CA mutants are blocked before reverse transcription (22). Mutations within this segment of the Fv1 MHR demonstrate this region is critical for Fv1 restriction (6), but while studies on Fv1 have not produced direct evidence for Fv1-capsid binding, our results support an antiviral model based on Fv1-capsid interactions.
Three of the 6 positively selected residues include 2 in the CTD that have already been identified as critical for the specificity of Fv1 restriction. F/S352 distinguishes Fv1n and Fv1nr restriction types, and substitutions at codon 399 alter restriction specificity (5, 6). Our analysis also identified positive selection at a position in this region not previously known to affect restriction, 401; its role in restriction may be defined by further mutational analysis. A 4th codon in this region of the CTD, 358, is known to affect MLV-N/B/NB restriction specificity (6), but we found this site to be highly invariant in Mus species. Lack of sequence variation at this site suggests that the only known substitution at the 358 codon identified to date, in the Fv1b laboratory mouse strains, likely emerged very recently in Mus evolution, perhaps in response to the emergence of the N-tropic MLV it restricts.
Fv1 is not found in all mice. Fv1 entered the mouse germ line shortly after the origination of Mus during a 1 MY period of diversification ≈7 MYA; this is supported by the observation that 2 species from 2 of the 4 subgenera that originated at that time lack Fv1. Evidence of positive selection of the Fv1 gene, the identification of 2 different but related Fv1-like restriction genes in Nannomys and the demonstration that one of these cloned Fv1 genes, Fv1m, reproduces this resistance phenotype in transduced cells suggests Fv1 has had an antiviral role throughout Mus evolution.
The exposure of mice to MLVs is marked by the appearance of related ERVs, but the appearance of Fv1 in Mus significantly predates the acquisition of ERVs of MLVs, the viruses restricted by Fv1. We demonstrated that MLV ERVs are largely found only in house mouse species that appeared only ≈1 MYA, that is, M. m. domesticus, castaneus and musculus (24). Our evolutionary and functional analysis of Fv1 in other species and subgenera uncovered evidence of antiviral activity in MLV-free species, which suggest that Fv1 may have broader antiretroviral activity than previously appreciated.
Materials and Methods
Cells and Viruses.
Ecotropic (mouse-tropic) MLV isolates were obtained from J. W. Hartley (National Institute of Allergy and Infectious Diseases, Bethesda, MD) and included AKV MLV (AKV-N), WN1802B (AKV-B), AKR-L1 (AKV-NR), Moloney MLV (MoMLV), and 2 Friend MLV (FrMLV) isolates, NR-tropic F-S MLV and NB-tropic FBLV (9). Mo-CA3 is a chimera of MoMLV with a segment of AKV MLV capsid containing the Fv1 target region between residues 99 and 129 (9). Virus stocks were made by collecting culture fluids from virus infected cells.
The XC overlay test (9, 11) was used to test for susceptibility to virus infection in various cell lines including 2 lines derived from the Asian species Mus m. dunni, MDTF and M. dunni (13), NIH 3T3 and cell lines derived from tail fibroblasts of the wild mouse species M. n. minutoides, M. n. setulosus, and M. p. platythrix obtained from J. Rodgers (Baylor College of Medicine, Houston, TX) (25). Embryo fibroblasts were prepared from strain 129/J from The Jackson Laboratory.
Wild Mouse Genomic DNA.
DNA was isolated from the wild mouse-derived cell lines listed above, from individual NFS/N mice from our colony and from wild mice from various sources (Table S1). Most mice were originally obtained from M. Potter (National Cancer Institute, Bethesda, MD). A set of African pygmy mouse DNA samples was obtained from Y. Cole and P. D'Eustachio (New York University, New York); these mice had been classed into 4 species of Nannomys mice on the basis of skeletal features by J. T. Marshall (Smithsonian Natural History Museum, Washington, DC). A sample of M. m. macedonicus DNA was provided by R. Elliott (Roswell Park, Buffalo, NY), and M. m. domesticus mice trapped in California were provided by S. Rasheed (University of Southern California, Los Angeles). M. m. spretus (SPRET/EiJ), M. m. castaneus (CAST/EiJ), M. m. molossinus, BALB/cJ, and C57BL/6J were obtained from The Jackson Laboratory. All animal protocols were reviewed and approved (Proposal no. LMM1) by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
All wild-derived mice are named using genus, subgenus, and species.
Cloning and Functional Analysis of M. n. minutoides Fv1.
The 5′ end of the Fv1 gene of M. n. minutoides (Fv1m) was PCR amplified and sequenced using primers based on Fv1b and numbered according to GenBank accession no. X97719: Fb2136-AGCCGAGTTCTAGGGAAACAA and Rb3133-TCAGCCAACCAATCAAACAAACT. The 3′ end of Fv1m was then amplified from NcoI digested DNA using inverse PCR (26) with Fv1m-based primers from GenBank accession no. FJ603554: Fm568-ATAGATTCTGATGGGACTGAG and Rm184-TACACAGAGTCATTAAGTTCCTTACC. The PCR product was sequenced.
The Fv1m coding region was then amplified from total RNA of M. n. minutoides cells using primers with introduced HindIII and ClaI sites (Fm22-TCAAGCTTAGGATGAATTTCCTACGTGCGCTTGCTG and Rm1313-TTATCGATTTAAAGGCCACCACGCCCGGCTTTTG) and cloned into the retroviral vector pLNCX2 (Clontech). The full length Fv1n gene was similarly amplified from total RNA extracted from NIH 3T3 cells with primers based on GenBank accession no. X97720: Fn35-TCAAGCTTAGGATGAATTTCCCACGTGCGCTTGCTG and Rn1332-TTATCGATTCAGAGTTTTGTAGCTGCTGTTGGCT and cloned into pLNCX2. The Fv1 genes were packaged by cotransfecting GP2–293 cells (Clontech) with the Fv1 clones or empty vector and pVSV-G (Clontech). Culture fluids were collected and used to infect MDTF cells, and stably transduced cells were selected for virus infection.
Fv1 Sequences of Other Wild Mouse Species.
BglI digested DNAs were screened for the presence of Fv1 by Southern blot analysis using as probe a 358-bp segment of the 5′ end of Fv1b amplified from C57BL/6 DNA using primers Fb2165-AGATGAATTTCCCACGTGC and Rb2497-AGGACACACTTAGAAGCCTTTAGATC. This segment shows 27% identity to the MuERV-L family of which Fv1 is a member (4).
Fv1 was amplified from various mouse genomic DNAs using primers designed from coding and flanking sequences of Fv1m and Fv1b. Flanking sequence primers included Fb2136-AGCCGAGTTCTAGGGAAACAA, Rb3703-TTTGCAACCAACCAGTGGCA, Rb4179-TCATAGCATATGTGAACAATCA, and Rb4831-CATCTATACTATCTTGGTGAG. From Fv1 coding sequences, we used primers Fb2165, F/Rb3003-TTTAAGGGTGTGGGATAATGGT and F/Rb3133-AGTTTGTTTGATTGGTTGGCTGA. Most Fv1 genes were sequenced as 2 overlapping PCR products, some were first cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, CA) before sequencing (Dataset S1).
Selection Analysis of Lineages and Codons.
DNA sequences were aligned using MUSCLE (27) and improved manually. The phylogeny used was 1 of 4 equally parsimonious phylogenies returned by PAUP* (version 4.0b10) (28), and was chosen by correspondence to consensus Mus phylogeny (10, 23). Two of the 4 equally parsimonious trees differed only in the arrangement of zero-length branches, leaving only 2 different tree topologies. The codeml program of the PAML4 package (16) was used for maximum likelihood analysis of codon evolution (29). The free ratio model (codon model = 1) was used to calculate branch-specific rates of dN/dS. In this model each branch is assumed to have a specific dN/dS ratio. The likelihood of the phylogeny under this model was tested against the likelihood of the phylogeny under the model of one uniform dN/dS ratio across all branches (codon model 0) using a likelihood ratio test (LRT). The significance of the LRT value was assessed using a χ2 distribution with 36° of freedom.
Selection acting on Fv1 codons was analyzed using 2 models of equilibrium codon frequencies and 4 models of codon selection. The 2 codon frequency models used were the F3 × 4 model (codon frequencies estimated from the nucleotide frequencies in the data at each codon site) and the F61 codon table model (frequencies of each of the 61 non-stop codons estimated from the data). The codon selection models were 2 neutral/negative selection models (M1 and M7), which were compared against corresponding models including a category for dN/dS >1 (M2 and M8, respectively). The significance of this additional codon selection category was assessed using LRTs of the phylogeny likelihoods under the neutral and positive selection models. Significance of the test statistics was calculated using a χ2 distribution with 2 degrees of freedom. The Bayes empirical Bayes algorithm (17) was used to calculate the posterior probability of individual codons experiencing dN/dS >1.
Supplementary Material
Acknowledgments.
We thank Esther Shaffer and Qingping Liu for expert technical assistance. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. X97719, FJ603554, and X97720).
This article contains supporting information online at www.pnas.org/cgi/content/full/0900181106/DCSupplemental.
References
- 1.Lilly F. Susceptibility to two strains of Friend leukemia virus in mice. Science. 1967;155:461–462. doi: 10.1126/science.155.3761.461. [DOI] [PubMed] [Google Scholar]
- 2.Kozak CA. Analysis of wild-derived mice for the Fv-1 and Fv-2 murine leukemia virus restriction loci: A novel wild mouse Fv-1 allele responsible for lack of host range restriction. J Virol. 1985;55:281–285. doi: 10.1128/jvi.55.2.281-285.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Best S, LeTossier P, Towers G, Stoye JP. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature. 1996;382:826–829. doi: 10.1038/382826a0. [DOI] [PubMed] [Google Scholar]
- 4.Benit L, et al. Cloning of a new murine endogenous retrovirus, MuERV-L, with strong similarity to the human HERV-L element and with a gag coding sequence closely related to the Fv1 restriction gene. J Virol. 1997;71:5652–5657. doi: 10.1128/jvi.71.7.5652-5657.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens A, et al. Retroviral capsid determinants of Fv1 NB and NR tropism. J Virol. 2004;78:9592–9598. doi: 10.1128/JVI.78.18.9592-9598.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop KN, Bock M, Towers G, Stoye JP. Identification of the regions of Fv1 necessary for murine leukemia virus restriction. J Virol. 2001;75:5182–5188. doi: 10.1128/JVI.75.11.5182-5188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozak CA, Chakraborti A. Single amino acid changes in the murine leukemia virus capsid protein gene define the target of Fv1 resistance. Virology. 1996;225:300–305. doi: 10.1006/viro.1996.0604. [DOI] [PubMed] [Google Scholar]
- 8.Jung YT, Kozak CA. A single amino acid change in the murine leukemia virus capsid gene responsible for the Fv1nr phenotype. J Virol. 2000;74:5385–5387. doi: 10.1128/jvi.74.11.5385-5387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Y, Kozak CA. Novel post-entry resistance to AKV ecotropic mouse gammaretroviruses in the African pygmy mouse, Mus minutoides. J Virol. 2008;82:6120–6129. doi: 10.1128/JVI.00202-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veyrunes F, et al. Phylogenomics of the genus Mus (Rodentia: Muridae): Extensive genome repatterning is not restricted to the house mouse. Proc R Soc B. 2006;273:2925–2934. doi: 10.1098/rspb.2006.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe WP, Pugh WE, Hartley JW. Plaque assay techniques for murine leukemia viruses. Virology. 1970;42:1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- 12.Eiden MV, Farrell K, Warsowe J, Mahan LC, Wilson CA. Characterization of a naturally occurring ecotropic receptor that does not facilitate entry of all ecotropic murine retroviruses. J Virol. 1993;67:4056–4061. doi: 10.1128/jvi.67.7.4056-4061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lander MR, Chattopadhyay SK. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984;52:695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bock M, Bishop KN, towers G, Stoye JP. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J Virol. 2000;74:7422–7430. doi: 10.1128/jvi.74.16.7422-7430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi C-F, et al. Molecular phylogeny of Fv1. Mamm Genome. 1998;9:1049–1055. doi: 10.1007/s003359900923. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 17.Yang Z, et al. Bayes empirical Bayes inference of amino acid sites under positive selection. Mo Bio Evol. 2005;22:1107–1118. doi: 10.1093/molbev/msi097. [DOI] [PubMed] [Google Scholar]
- 18.Strambio-de-Castilla C, Hunter E. Mutational analysis of the major homology region of Mason-Pfizer monkey virus by use of saturation mutagenesis. J Virol. 1992;66:7021–7032. doi: 10.1128/jvi.66.12.7021-7032.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlinsky KJ, Gu J, Hoyt M, Sandmeyer S, Menees TM. Mutations in the Ty3 major homology region affect multiple steps in Ty3 retrotransposition. J Virol. 1996;70:3440–3448. doi: 10.1128/jvi.70.6.3440-3448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamble TR, et al. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science. 1997;278:849–853. doi: 10.1126/science.278.5339.849. [DOI] [PubMed] [Google Scholar]
- 21.Alin K, Goff SP. Mutational analysis of interactions between the Gag precursor proteins of murine leukemia viruses. Virology. 1996;216:418–424. doi: 10.1006/viro.1996.0078. [DOI] [PubMed] [Google Scholar]
- 22.Alin K, Goff SP. Amino acid substitutions in the CA protein of Moloney murine leukemia virus that block early events in infection. Virology. 1996;222:339–351. doi: 10.1006/viro.1996.0431. [DOI] [PubMed] [Google Scholar]
- 23.Lundrigan BL, Jansa SA, Tucker PK. Phylogenetic relationships in the genus Mus, based on paternally, maternally, and biparentally inherited characters. Syst Biol. 2002;51:410–431. doi: 10.1080/10635150290069878. [DOI] [PubMed] [Google Scholar]
- 24.Kozak CA, O'Neill RR. Diverse wild mouse origins of xenotropic, mink cell focus-forming, and two types of ecotropic proviral genes. J Virol. 1987;61:3082–3088. doi: 10.1128/jvi.61.10.3082-3088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis BK, Cook RG, Rich RR, Rodgers JR. Hyperconservation of the putative antigen recognition site of the MHC Class I-b molecule TL in the subfamily Murinae: Evidence that thymus leukemia antigen is an ancient mammalian gene. J Immunol. 2002;169:6890–6899. doi: 10.4049/jimmunol.169.12.6890. [DOI] [PubMed] [Google Scholar]
- 26.Silver J, Keerikatte V. Novel use of polymerase chain reaction to amplify cellular DNA adjacent to an integrated provirus. J Virol. 1989;63:1924–1928. doi: 10.1128/jvi.63.5.1924-1928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swofford DL. Sunderland, Massachusetts: Sinauer Associates; 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods) Version 4. [Google Scholar]
- 29.Bielawski JP, Yang Z. In: Statistical Methods in Molecular Evolution. Nielsen R, editor. New York: Springer; 2005. pp. 103–124. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.