Abstract
Mesothelin is a cell-surface molecule over-expressed on a large fraction of carcinomas, and thus is an attractive target of immunotherapy. A molecularly targeted therapy for these cancers was created by engineering T cells to express a chimeric receptor with high affinity for human mesothelin. Lentiviral vectors were used to express a single-chain variable fragment that binds mesothelin and that is fused to signaling domains derived from T-cell receptor zeta, CD28, and CD137 (4–1BB). When stimulated by mesothelin, lentivirally transduced T cells were induced to proliferate, express the antiapoptotic gene Bcl-XL, and secrete multiple cytokines, all features characteristic of central memory T cells. When transferred intratumorally or intravenously into NOD/scid/IL2rγ−/− mice engrafted with large pre-established tumors, the engineered T cells reduced the tumor burden, and in some cases resulted in complete eradication of the tumors at low effector-to-target ratios. Incorporation of the CD137 signaling domain specifically reprogrammed cells for multifunctional cytokine secretion and enhanced persistence of T cells. These findings have important implications for adoptive immunotherapy of cancer, especially in the context of poorly immunogenic tumors. Genetically redirected T cells have promise of targeting T lymphocytes to tumor antigens, confer resistance to the tumor microenvironment, and providing immunosurveillance.
Keywords: adoptive immunotherapy, chimeric receptor, mesothelin
A principal limitation of cancer immunotherapy is that most solid tumors are poorly antigenic, expression of MHC Class I molecules is very low, no T cells are available that have high avidity for tumor specific antigens, or no T cells that have the desired specificities remain in the patient after chemotherapy. To address these problems, tumor antigen-specific T cells have been engineered by introduction of chimeric immunoreceptors (CIR)—or “T bodies”—that have antibody-based external receptor structures and cytosolic domains that encode signal transduction modules of the T-cell receptor (1). These constructs can function to retarget T cells in vitro in an MHC-unrestricted manner. Several pilot clinical trials to test this concept have recently been reported (2–4). In all of the trials, safety has been documented. However, the principal issue revealed in the initial trials has been poor in vivo persistence and expression of the transgene. Approaches to remedy these shortcomings currently involve improved receptor design, the incorporation of costimulatory signaling domains into the signaling module, and reducing the immunogenicity of the T-body construct (5).
Mesothelin is a glycosyl-phosphatidyl inositol-linked membrane glycoprotein expressed in a variety of cancers (6). Immunohistochemistry studies have shown that mesothelin is over-expressed in virtually all pancreatic ductal adenocarcinomas and mesotheliomas (7), and in a high percentage of epithelial ovarian cancers, as well as non-small cell carcinomas of the lung (8, 9). Because mesothelin has recently been reported to bind to CA125/MUC16, one possible biological role of this membrane protein may be linked to heterotypic cell adhesion, which may facilitate the metastatic spread of mesothelin-bearing cancer cells (10). In earlier studies, tumor cells expressing mesothelin have been shown to be killed by MHC Class I-restricted T cells (11). In addition, mesothelin-specific antibodies and antibody-based immunotoxins have been shown to cause tumor regression in preclinical and clinical studies (12, 13). Here we report that lentiviral vectors can efficiently generate T cells that specifically target mesothelin. The mesothelin-retargeted T cells eradicate large pre-established mesothelioma xenografts in NOD/scid/IL2rγ–/– mice at low effector-to-target (E:T) ratios, and the incorporation of costimulatory domains enables the prolonged persistence of the engineered T cells in tumor-bearing mice following intratumoral or i.v. administration.
Results
Chimeric receptors were designed that contain the SS1 scFv that recognizes human mesothelin (Fig. 1A). SS1 was chosen because it has a high binding affinity to mesothelin (Kd = 0.7 nM) (14), and because it has been found to be safe in patients when administered as a recombinant immunotoxin (13). We created a series of SS1 scFv-based chimeric receptors that contain the TCR-ζ signal-transduction domain with the CD28 and CD137 (4–1BB) intracellular domains in tandem. Chimeric receptors that contained a truncated form of the TCR-ζ intracellular domain (SS1-Δz) or an anti-CD19 scFv (anti-CD19-z) were designed as controls for signal transduction and binding specificity, respectively.
Fig. 1.
Generation and cytolytic activity of antimesothelin lentiviral vector-engineered T cells. (A) Schematic representation of the mesothelin-binding chimeric receptors. A binding-control chimeric receptor with a truncated TCRζ domain and a specificity control receptor with an anti-CD19 scFv were also constructed. (B) Expression of the SS1 scFv fusion proteins was examined on human primary CD4 T cells. Transduction efficiencies are indicated with mean fluorescence intensity of the transduced populations in parentheses. (C) Cell surface expression of mesothelin on K562, K562.meso, OvCa61.4, OvCa68.4, and M108 was determined by flow cytometry (Top). Cells were incubated with either the mouse anti-human mesothelin antibody CAK1 (solid black line) or an isotype control (dotted line) followed by staining with a PE-conjugated goat anti-mouse Ig. The cytolytic activity of the chimeric receptors on primary human CD8+ T cells targeting cell lines expressing mesothelin was determined using a 4 h 51Cr release assay (Bottom). Results are expressed as a mean and SD of triplicate wells from 1 of at least 3 separate experiments.
Because of the increased efficiency of transduction and expression, and the possible decreased potential for adverse effects, such as insertional mutagenesis compared to retroviruses, we used lentiviral-vector technology to express the fusion constructs in primary human T cells using clinically validated techniques (15). The cDNA sequences containing the various fusion constructions were cloned into a third-generation lentiviral vector in which the CMV promoter was replaced with the EF-1α promoter (16). Lentiviral vector supernatants transduced primary T cells with high efficiency (Fig. 1B). In preliminary experiments, the SS1-transduced T cells were found to have sustained proliferation in the presence of various cell lines that express mesothelin, while culture in the absence of mesothelin-expressing feeder cells failed to sustain T-cell proliferation (data not shown). All constructs were brightly expressed on the surface of CD4 and CD8 T cells, and expression was stable for at least 2 months in culture (not shown). T-cell expansion during culture with mesothelin-expressing tumor cells was higher for T cells that contained SS1 scFv fused with either costimulatory domain than for T cells expressing the SS1 TCR-ζ signaling domain only (data not shown), consistent with previous reports that have tested T cells with other chimeric receptors in vitro (17–22). Furthermore, the SS1 scFv fused with costimulatory domains enriched the population of T cells to near purity during culture on mesothelin-expressing tumors (data not shown).
To investigate the antitumor potential of the transduced T cells, effector function was measured in standard 51Cr release assays using mesothelin-negative K562 cells, K562.meso (a derivative engineered to express mesothelin), and primary tumor lines isolated from patients with ovarian cancer and malignant mesothelioma (Fig. 1C). T cells transduced with SS1 scFv efficiently lysed K562.meso but did not kill parental K562 cells. Importantly, the SS1-transduced T cells were also highly cytotoxic for carcinoma cells that express mesothelin, killing OvCa68.4 and M108 cell lines derived from patients with ovarian cancer and mesothelioma, while failing to kill ovarian tumor cells that did not express mesothelin (OvCa61.4). Transduction of OvCa61.4 cells with a mesothelin-expressing lentiviral vector rendered them susceptible to SS1 scFv lysis (data not shown). The inclusion of CD28 and CD137 costimulatory domains in tandem or in triplicate with TCR-ζ generally failed to increase in vitro cytotoxicity above that of T cells expressing SS1-TCR-ζ only. The killing was efficient, with plateau lysis occurring at a 10:1 E:T ratio during a 4-h assay, and at <1:1 E:T ratio during a 48-h culture [supporting information (SI) Fig. S1], suggesting that the redirected T cells were capable of serial killing. Moreover, the lysis was specific because T cells transduced with GFP or an irrelevant anti-CD19 scFv chimeric receptor showed no cytotoxic activity against the same target cells, excluding alloreactivity or nonspecific lysis. Furthermore, T cells expressing a truncated TCR-ζ intracellular domain (SS1-Δz) also failed to kill mesothelin-expressing targets, demonstrating the requirement for an intact TCR-ζ signaling domain.
Studies in mouse tumor models indicate that antitumor effects of redirected CD8 T cells will likely be sustained by CD4 cells (23). Therefore, we transduced primary human CD4 and CD8 T cells and measured cytokine secretion after exposure to mesothelioma cells (Fig. S2A). The release of inflammatory cytokines was triggered by mesothelin, and both CD4 and CD8 T cells secreted Th1 cytokines. In contrast, the T cells secreted low or undetectable amounts of IL-4, IL-5, IL-10, and IL-17 (data not shown), consistent with a predominant Th1 cytokine bias under these culture conditions. The pattern of cytokine release observed was consistent with the known signal transduction properties of the various signaling domains, confirming the modular nature of the domains and that they can function in cis on the fusion proteins. For example, IL-2 and TNF-α were only secreted by CD4 T cells, and this was dependent on the CD28 domain. Both CD4 and CD8 T cells released IFN-γ and IL-6, and either the CD28 or the CD137 signaling domain was sufficient. Therefore, in contrast to cytotoxicity, where TCR-ζ was sufficient, cytokine secretion was more pronounced in primary T cells expressing a costimulatory signaling domain. Mesothelin-independent cytokine secretion was not observed.
An emerging concept in the vaccine field indicates that the presence of multifunctional T cells is associated with improved control of chronic viral infections, and that this may be important for T-cell-based cancer therapies (24). Transduced T cells were stimulated with mesothelin-expressing tumor cells and the CD4 and CD8 T-cell subsets stained for intracellular IFN-γ, TNF-α, IL-2, and GM-CSF (Fig. S2B). Cytokine production was restricted to the fraction of T cells expressing chimeric receptors (data not shown). The incorporation of the CD28 signaling domain increased the fraction of cytokine secreting CD4 and CD8 T cells early in culture; however, after 20 h of culture, the fraction of responding cytokine-secreting cells was similar for all signaling constructs. However, the fraction of polyfunctional T cells was highly dependent on the presence of costimulatory domains, as nearly all cytokine-secreting cells expressing only the TCR-ζ domain were monofunctional. In contrast, highly multifunctional T cells secreting all 4 cytokines were primarily observed in the cells expressing the BB:TCR-ζ or 28:BB:TCR-ζ signaling domains. Importantly, the CD137 signaling domain was notable for supporting multifunctional CD4 and CD8 T cells, a feature that might prolong the function of the T cells in the tumor microenvironment.
The expression of antiapoptotic genes can confer resistance to the toxic effects of the tumor microenvironment (25). Most of the T cells expressing CD28, and to a lesser extent the CD28:BB-signaling domains, expressed Bcl-XL after culture with the M108 mesothelioma cell line (Fig. S2C). In contrast, T cells expressing BB:TCR-ζ, or a truncated TCR-ζ had lower or undetectable levels of Bcl-XL. The expression of Bcl-XL in T cells expressing CD28 and CD137 signaling domains was not constitutive, as T cells cultured in media (see Fig. S2C) or with mesothelin-negative tumor cells (data not shown) did not express detectable levels of Bcl-XL.
As an initial test of in vivo specificity of the SS1 constructs, a modified Winn assay was done using A431 cells transduced with mesothelin (26). Rag2γ–/– mice were injected s.c. on opposite flanks with either A431.meso or A431 parental tumor cells. T cells transduced with SS1 chimeric receptors were coinjected with the tumor cells at a 1:2 E:T ratio (Fig. S3). SS1 receptors containing the CD28 and CD137 domains were able to inhibit tumor growth in a mesothelin-specific manner. However, the TCR-ζ group was only able to delay A431.meso tumor growth. In contrast, T cells transduced with the truncated TCRζ or with GFP had no effect on tumor growth. Even at a high E:T ratio (8:1), the control T cells had no effect on tumor growth, confirming an allogeneic effect was not contributing to the antitumor effects (data not shown).
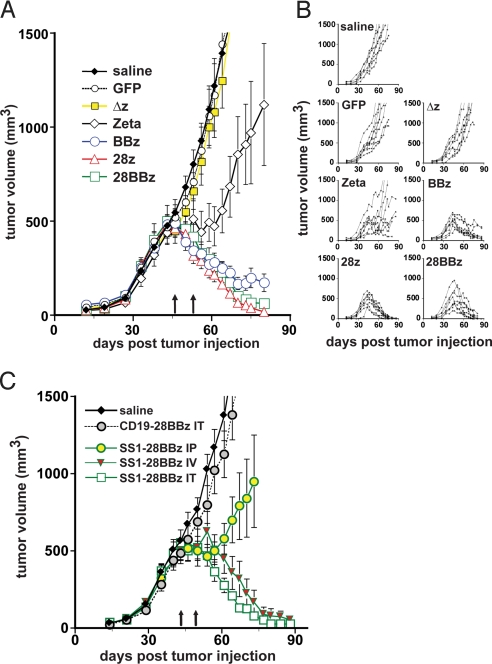
To further explore the potential antitumor efficacy of the SS1 scFv constructs, we developed a rigorous xenograft model using M108 tumor cells. When implanted into the flanks of NOD/scid/IL2rγ–/– (NOG) mice, M108 cells proliferated and continued to express mesothelin (Fig. S4). Groups of NOG mice were injected in the flanks with serially passaged primary M108 tumor cells (Fig. 2A). On weeks 6 and 7 after establishing the tumor, when the tumor burden was ≈500 mm3, the mice were treated with intratumoral injections of 15 × 106 T cells (≈70%–80% transgene-positive). A potent antitumor effect was observed (see Fig. 2A). Statistical modeling of the tumor growth curves revealed significant differences among the treatment groups (P < 0.0001 by a Wald test that all groups gave equal log-tumor volume curves). The 7 curves separated themselves into 4 groups: the 3 control treatments (saline, GFP, and Δz) were not significantly different from each other and showed continued growth beyond the time of T cell transfer; the TCR-ζ treatment showed a mixed pattern of continued growth in some animals but slowed growth or tumor decline in others (Fig. 2B); the BBz treatment showed relatively slow tumor decline; and the 28z and 28BBz treatments showed rapid tumor decline and were statistically indistinguishable from each other.
Fig. 2.
Mesothelin retargeted T cells eradicate large pre-established tumors in vivo: effect of costimulatory signaling domains and route of administration. (A) Human primary M108 tumors were established in the flanks of NOG mice. After 6 weeks, when the tumors reached a volume of ≈500 mm3, the mice were treated with 2 intratumoral injections of 15 × 106 T cells (≈70%–80% chimeric receptor-positive) on days 46 and 53 (arrows). Results are expressed as a mean tumor volume (mm3 ± SEM) with n = 8 for all groups, and are representative of 2 experiments. The plots for BBz, CD28z, and CD28BBz are significantly different from the control treatment groups (P < 0.0001 by a Wald test that all groups gave equal log tumor volume curves). (B) The tumor-growth curves of the individual mice in each group are shown. (C) M108-bearing NOG mice were treated with T lymphocytes expressing the 28BBz chimeric receptors (against mesothelin or CD19) via intratumoral (IT), i.p. (IP), and i.v. (IV) routes and the effect on tumor growth was assessed. After tumors reached a mean volume of ≈500 mm3, 2 injections of 10 × 106 T cells (>90% CIR-positive) on days 43 and 49 (arrows) were performed. The results are expressed as a mean tumor volume (mm3 ± SEM) with n = 8 for the saline and intratumoral injection groups, and n = 7 for the i.v. and i.p. groups.
The plots of tumor burden for the individual mice (see Fig. 2B) indicate the magnitude and reproducibility of the antitumor effects of the T cells endowed with chimeric receptors containing costimulatory domains, with 1 mouse reaching a tumor volume of 1,000 mm3 before tumor regression to subpalpable levels. The mice were killed when tumors reached volumes >2,000 mm3 and tumors were photographed (Fig. S5). Tumors from mice treated with control T cells were highly vascularized, while many of the small tissue masses harvested from the 28z or 28BBz mice and several of the BBz mice were fibrotic or necrotic masses. However, small regions of tumor with abnormal morphology were still evident, as determined by histology. Thus, T cells expressing mesothelin-specific chimeric receptors containing CD28 and 4–1BB domains were able to control or eradicate large established tumors after intratumoral injections. There was no significant contribution of alloreactivity to the antitumor effects because mice treated with T cells expressing GFP or a truncated TCR-ζ receptor had tumor growth that was equivalent to mice injected with saline.
In the previous experiment, vigorous and specific antitumor effects were noted in mice treated with intratumoral injections of the engineered T cells, directly assessing their potential antitumor effects in the vascularized tumor microenvironment. We next tested whether the T cells would be effective using several routes of administration (Fig. 2C). Tumors were established with M108 cells, and after the tumors reached a mean volume of ≈500 mm3, injections of 10 × 106 T cells transduced with SS1–28:BB:TCR-ζ (>90% positive) on weeks 6 and 7 were given by i.v., i.p., or intratumoral routes. For these experiments, the CD28:BB:TCR-ζ construct was chosen because it has displayed the most potent and reproducible antitumor effects, as well as enhanced engraftment properties (see below). Following i.v. and intratumoral injections, a potent antitumor effect was again observed, with a more rapid reduction in tumor mass following the intratumoral route of administration. We analyzed tumor volumes on the natural log scale. For log-tumor volume, the ranking (lowest to highest) was intratumoral SS1–28BBz, i.v. SS1–28BBz, i.p. SS1–28BBz, intratumoral anti-CD19–28BBz, and saline. The overall F test for comparison of the means was significant at P < 0.0001, and in pairwise comparisons each treatment was not significantly different (at P = 0.05) from the adjacent treatments, but was significantly different from the others. Thus, we conclude that SS1–28BBz eradication of M108 tumor is antigen-specific because the anti-CD19–28BBz group displayed no antitumor activity, and that intratumoral appears to be the superior route of administration, marginally faster than i.v. (≈7 day delay in response) but significantly better than i.p.
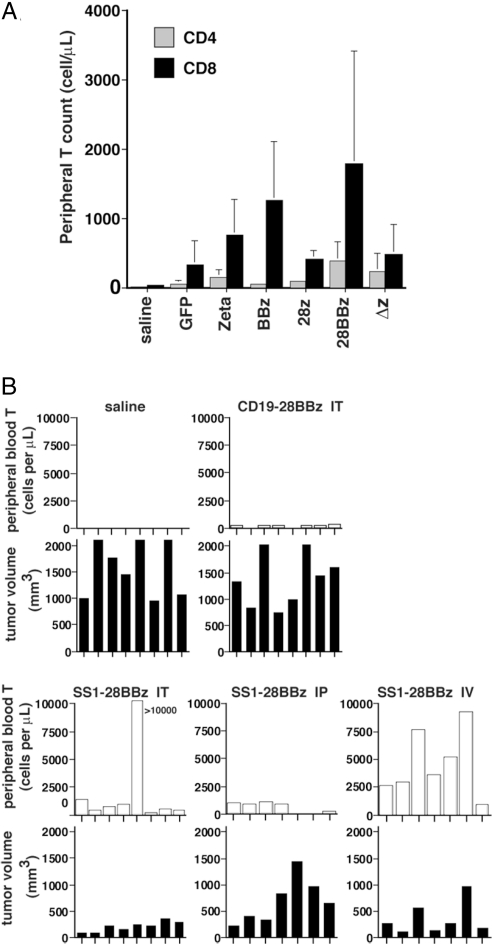
Because surprisingly short-term engraftment has been observed in the pilot clinical trials testing chimeric receptors (2–4), and because long-term engraftment of adoptively transferred T cells correlates with antitumor effects (27, 28), we next determined the persistence of the lentiviral vector-engineered T cells in tumor-bearing mice. We first examined the mice from the experiment shown in Fig. 2A. Peripheral blood from M108-bearing NOG mice treated with intratumoral injections of SS1 scFv-transduced T cells on days 46 and 53 after tumor injection was obtained on day 73: that is, 20 days after the last adoptive T cell transfer, and quantified for the presence of CD4 and CD8 T cells (see Fig. 3A). For all conditions tested, the CD8 T cell counts were higher than the CD4 T cell counts. Analysis of variance of the CD4 data indicates significant differences among the treatment arms (P < 0.0001). In pairwise comparisons of the treatment groups, adjusted for multiplicity by the Tukey method, cell counts in mice endowed with 28BBz were significantly higher than BBz and GFP, while the BBz, 28z, Zeta, and Δz groups were indistinguishable. Analysis of the CD8 data also revealed significant differences among the treatment arms (P < 0.0001). In Tukey-adjusted pairwise comparisons, the cell counts in mice engrafted with cells expressing 28BBz and BBz were significantly higher (P < 0.05) than GFP, and the 28BBz, BBz, Zeta, 28z, and Δz groups were indistinguishable. It was unexpected that the T-cell counts in the mice given T cells transduced with the 28z were not higher than T cells expressing TCR-ζ only, given their superior IL-2 production and increased Bcl-XL expression shown in Fig. S2). Furthermore, the persistence of T cells in peripheral blood did not have a direct correlation with tumor burden (compare Fig. 2 A and B with Fig. 3A).
Fig. 3.
CD28 and 4–1BB signals enhance the persistence of human T lymphocytes after treatment of M108 tumor. (A) Peripheral blood from M108-bearing NOG mice treated with intratumoral injections of SS1 chimeric receptor-transduced T cells was obtained on day 73 and quantified for the presence of CD4 and CD8 T cells by a FACS Trucount assay. Analysis of variance of the CD4 and CD8 data indicates significant differences among the treatment arms (P < 0.0001). Results are expressed as a mean absolute count per μL of peripheral blood ± SD with n = 8 for all groups. (B) Persistence of T cells in vivo was assessed after i.v. (IV), intratumoral (IT), or i.p. (IP) route of administration. Mice from the experiment in Fig. 2C were bled and analyzed for peripheral T cell persistence on day 65 by a FACS Trucount assay. Absolute T-cell count per μL of peripheral blood is shown for individual mice in each group with corresponding tumor volumes directly below at day 65. The overall F test comparing the mean T-cell counts across groups was significant (P < 0.0001). In Tukey-adjusted pairwise comparisons of the means (at P = 0.05), i.v. and intratumoral SS1 administration gave a significantly higher T-cell count than i.p. SS1 administration.
Finally, we measured the persistence of T cells in vivo in the mice given 28BBz as a function of the route of administration. In the experiment shown in Fig. 2C, mice were injected with T cells on days 43 and 49, and were bled on day 65 to measure T-cell engraftment. Tumor volumes and peripheral blood persistence on day 65 are plotted for each individual mouse in each group (see Fig. 3B). By inspection of the plots, the presence of SS1–28:BB:z T cells generally correlates inversely with tumor burden in that the groups of mice given T-cell injections by intratumoral or i.v. routes all had substantial levels of T cells in peripheral blood and the lowest tumor burdens, while mice given SS1–28:BB:z by i.p., or anti-CD19–28:BB:z by intratumoral administration, had high-tumor burdens and low levels of T-cell engraftment. The overall F test comparing the mean T-cell counts across groups was significant (P < 0.0001). The group with the highest mean T-cell count was i.v. SS1–28BBZ. In Tukey-adjusted pairwise comparisons of the means, i.v. SS1 administration gave a significantly higher (P < 0.05) count than i.p. SS1 administration and intratumoral anti-CD19 scFv administration. Mice treated with intratumoral SS1 also had significantly higher (P < 0.05) T-cell counts than the intratumoral anti-CD19 group, suggesting that tumor antigen drives the expansion of the adoptively transferred T cells in vivo. Furthermore, in addition to a low-level T-cell engraftment, mice injected with T cells expressing a nonbinding anti-CD19 scFv 28:BB:z receptor had tumor burdens equivalent to mice injected with saline, indicating that the specific triggering of the T cell by the M108 cells rather than allogeneic or xenogeneic effects are required for high-level engraftment and antitumor effects observed for the T cells expressing the SS1 scFv 28:BB:z receptor. Thus, long-term systemic persistence of SS1 scFv 28:BB:z engineered T cells is driven by tumor antigen and occurs in tumor-bearing mice following either intratumoral or i.v. administration, with i.v. statistically equivalent to intratumoral but significantly better than i.p.
Discussion
Retargeted T cells are particularly attractive for the therapy of carcinomas, where the available endogenous T-cell receptor repertoire may be limiting because of tolerance and previous cytotoxic chemotherapy, and MHC Class I expression may be decreased. However, while initial clinical studies demonstrate feasibility with the retargeted T cells, poor in vivo persistence and expression of the transgene have been documented, and the therapy has had less clinical activity than expected (2–4). Our studies have addressed all of these issues. Mesothelin proved to be an attractive target as the retargeted T cells efficiently and specifically killed a variety of tumors that express mesothelin. The engineered T cells could kill tumor cells at ≈1:10 E:T ratio in vitro, and at an unprecedented 1:40 E:T ratio in vivo (see below). It was unexpected that expression of the CD137 signaling domain was most correlated with persistence of the T cells in vivo, as the CD28 domain was predicted to be most efficient by the preponderance of in vitro measures of cytokine function and Bcl-XL expression.
The eradication of large, long-term pre-established tumors by immunotherapy has rarely been reported. Most preclinical models in a therapeutic setting have tested tumors that have been implanted for less than 2 weeks before initiation of therapy (29). We have presented evidence that T cells expressing SS1 fused to CD28 and CD137 costimulatory domains were able to reject very large, well-established tumors of 500 to 1,000 mm3. Given that the experiments in Fig. 2A used 2 injections of T cells containing a total of 20 to 24 × 106 T cells transduced with the SS1 construct, and assuming that a 1,000-mm3 tumor mass contains at least 1 × 109 cells, we estimate that the T cells are able to eradicate tumors at an initial E:T ratio of ≈1:40 in vivo. Therefore, our preclinical data would support treating patients with tumor burdens of at least 1 × 1012 cells, because our current large-scale manufacturing can routinely produce 1 × 1011 transduced T cells during a 10-day culture (15). Tumor cells of 1 × 1012 is a clinically relevant tumor burden, representing 1 kg of tumor bulk. Further studies will be required to determine to what extent this tumor eradication can be attributed to serial killing of tumor cells by the infused T cells and to the tumor-induced proliferation of the retargeted T cells and the cytotoxic effects of the daughter cells.
It is likely that several mechanisms account for the enhanced efficiency of the redirected T cells observed in the present study. First, previous studies have generally used T cells transduced with retroviruses. The high efficiencies associated with lentiviral-mediated transduction, combined with robust in vitro cell-expansion methods, make the rapid expansion of large numbers of therapeutically relevant number feasible. In the present study we have used lentiviral vectors, which have a higher transduction efficiency, thus shortening the in vitro culture to 7 to 10 days, and permitting the use of the T cells early at a time when we have shown previously that the average telomere length of the cultured T cells is actually longer than at the start of culture (30). We attribute this to the previous demonstration that the anti-CD28-driven culture system induces telomerase activity (31) and preserves central memory cells (32). The use of T cells with longer telomeres may be important, as the cells have a more extensive replicative capacity and in adoptive transfer studies with tumor-infiltrating lymphocytes, there is enhanced antitumor efficacy in patients infused with “younger” tumor-infiltrating lymphocytes (33). Second, in comparison to previous reports, our transgenes are brightly expressed on the surface of the T cells, which may be the result of the lentiviral vector design, the use of EF-1α as an internal promoter (34), and the use of CD28 costimulation, which increases the expression level of transgenes (35). Although a number of studies have shown that lentiviral vectors are superior in hematopoietic stem cells (36), only one has directly compared the expression of transgenes in human T cells following retroviral or lentiviral transduction, and the lentiviral approach was superior (37). Third, we demonstrated that incorporation of the costimulatory domains in cis enhanced the persistence of T cells, confirming and extending recent work indicating that costimulatory domains expressed in trans in T cells can enhance the effects of retargeted T cells (38).
Our study suggests that in vitro experiments can be misleading in predicting the efficacy of the engineered T cells in vivo. Furthermore, engineered cells expressing the CD137 signaling domain were more likely to be multifunctional and persist in tumor-bearing mice. See the SI Text for a discussion of these issues.
Previous studies have shown that T cells in the tumor microenvironment have a number of signaling perturbations (39). One study has shown that chimeric receptors with a CD28 signaling domain enhances the resistance of T cells to regulatory T cells (40). While we have not yet directly tested the effects of regulatory T cells on T-effector cells expressing our chimeric receptors, our data would suggest that the combination of CD137 and CD28 are more effective than either CD137 or CD28 alone, based on the antitumor effects and persistence in tumor-bearing mice. Although CD28 is sufficient for in vivo antitumor activity and the addition of CD137 is required for engraftment, the combination of the 2 signals may provide the most effective therapy and long-term protection.
We tested several routes of administration, and found that the intratumoral and i.v. routes were nearly equivalent and distinctly superior to the i.p. route. The delay in tumor regression with the i.v. route compared to the intratumoral route may be at least partially explained by soluble mesothelin in the plasma of the mice (data not shown), reflecting that patients with mesothelin-expressing tumors have been documented to have circulating mesothelin (41). We expect to primarily test the i.v. route in phase I clinical studies; however, the intratumoral route may have certain advantages. First, trafficking of T cells into solid tumors can be rate-limiting (42) and direct injection eliminates trafficking as a variable. Second, the intratumoral route may increase the therapeutic index in situations where there is a potential for on-target, off-organ toxicity. In specific, the mesothelin-redirected T cells may cause peritonitis or pleuropericarditis, because of patterns of tissue-specific expression (6). We do not expect that this will be a major problem because previous clinical studies with mesothelin antibodies and immunoconjugates have not yet revealed unmanageable toxicity (12, 13). Finally, recent studies have shown the safety and feasibility of intratumoral injection of T cells (43). In summary, chimeric T-cells targeted to mesothelin and possessing intracytoplasmic-signaling domains from TCRζ, CD28, and CD137 appear highly effective at treating large, well-established tumors in mice. Clinical translation of this approach to tumors, such as mesothelioma and ovarian cancer, is currently underway.
Methods
Generation of Antimesothelin T-Body Molecules.
Chimeric antimesothelin scFv-fusion proteins were generated as described in the SI Text.
Animal Experiments.
Xenograft tumors were established by serial passage in mice of primary pleural effusion cells from mesothelioma patient M108. All injections were s.c., and performed in the presence of a 50% solution of Matrigel (BD Biosciences) in PBS. For T-cell experiments, s.c. M108 tumors were allowed to grow in NOG mice for 6 to 7 weeks. The route, dose, and timing of T-cell injections is indicated in the individual figure legends. Tumor dimensions were measured with calipers, and tumor volumes calculated using the formula V = ½ × L × W × W, where L is length (longest dimension) and W is width (shortest dimension). Peripheral blood was obtained from retro-orbital bleeding and stained for the presence of human CD45, CD4, and CD8 T cells. After gating on the human CD45+ population, the CD4+ and CD8+ subsets were quantified using TruCount tubes (BD Biosciences).
Statistical Analysis.
All results were expressed as means ± SD or SEM, as indicated. Tumor-volume data were transformed to the log scale before analysis. Additional information for methods used is provided in the SI Text.
Supplementary Material
Acknowledgments.
We thank Gwendolyn Binder, Chrystal Paulos, and Gwenn-aël Danet-Desnoyers for helpful discussions, Cynthia June for support, Elizabeth Jaffee for the generous gift of mesothelin vector, and Ronghua Liu and Anthony Secreto for expert technical assistance. We also thank the Center for AIDS Research Immunology Core for providing primary human T cells. This work was supported by National Institutes of Health Grants 1R01CA120409, 5P50CA083638, and 2P01CA066726, the Alliance for Cancer Gene Therapy (the Joan Miller and Linda Bernstein Gene Therapy for Ovarian Cancer Award), and in part of by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813101106/DCSupplemental.
References
- 1.Eshhar Z, et al. The T-body approach: Potential for cancer immunotherapy. Springer Semin in Immunopathol. 1996;18:199–209. doi: 10.1007/BF00820666. [DOI] [PubMed] [Google Scholar]
- 2.Kershaw MH, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JR, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 4.Till BG, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 6.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 7.Argani P, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 8.Chang K, et al. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res. 1992;52:181–186. [PubMed] [Google Scholar]
- 9.Ho M, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–1575. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 10.Rump A, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 11.Thomas AM, et al. Mesothelin specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan R, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20–29. [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan R, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Verschraegen CF, Mendoza J, Hassan R. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1(dsFv)PE38, towards tumor cell lines established from ascites of patients with peritoneal mesotheliomas. Anticancer Res. 2004;24:1327–1335. [PubMed] [Google Scholar]
- 15.Levine BL, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parry RV, et al. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 17.Haynes NM, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood. 2002;100:3155–3163. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]
- 18.Maher J, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCR zeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 19.Finney HM, Akbar AN, Lawson ADG. Activation of resting human primary T cells with chimeric receptors: Costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 20.Friedmann-Morvinski D, et al. Redirected primary T cells harboring a chimeric receptor require costimulation for their antigen-specific activation. Blood. 2005;105:3087–3093. doi: 10.1182/blood-2004-09-3737. [DOI] [PubMed] [Google Scholar]
- 21.Pule MA, et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Hombach A, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167:6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 23.Moeller M, et al. Sustained antigen-specific antitumor recall response mediated by gene-modified CD4+ T helper-1 and CD8+ T cells. Cancer Res. 2007;67:11428–11437. doi: 10.1158/0008-5472.CAN-07-1141. [DOI] [PubMed] [Google Scholar]
- 24.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 25.Gastman BR, Johnson DE, Whiteside TL, Rabinowich H. Tumor-induced apoptosis of T lymphocytes: elucidation of intracellular apoptotic events. Blood. 2000;95:2015–2023. [PubMed] [Google Scholar]
- 26.Winn HJ. Immune mechanisms in homotransplantation. II. Quantitative assay of the immunologic activity of lymphoid cells stimulated by tumor homografts. J Immunol. 1961;86:228–239. [PubMed] [Google Scholar]
- 27.Greenberg PD, Cheever MA. Treatment of disseminated leukemia with cyclophosphamide and immune cells: tumor immunity reflects long-term persistence of tumor-specific donor T cells. J Immunol. 1984;133:3401–3407. [PubMed] [Google Scholar]
- 28.Zhou J, et al. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol. 2006;18:226–231. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Weng N-P, et al. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol Rev. 1997;160:43–54. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 31.Weng N-P, Levine BL, June CH, Hodes RJ. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183:2471–2480. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bondanza A, et al. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood. 2006;107:1828–1836. doi: 10.1182/blood-2005-09-3716. [DOI] [PubMed] [Google Scholar]
- 33.Shen X, et al. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother. 2007;30:123–129. doi: 10.1097/01.cji.0000211321.07654.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serafini M, Bonamino M, Golay J, Introna M. Elongation factor 1 (EF1alpha) promoter in a lentiviral backbone improves expression of the CD20 suicide gene in primary T lymphocytes allowing efficient rituximab-mediated lysis. Haematologica. 2004;89:86–95. [PubMed] [Google Scholar]
- 35.Costello E, et al. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 2000;7:596–604. doi: 10.1038/sj.gt.3301135. [DOI] [PubMed] [Google Scholar]
- 36.Miyoshi H, et al. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 37.Zhou X, et al. Lentivirus-mediated gene transfer and expression in established human tumor antigen-specific cytotoxic T cells and primary unstimulated T cells. Hum Gene Ther. 2003;14:1089–1105. doi: 10.1089/104303403322124800. [DOI] [PubMed] [Google Scholar]
- 38.Stephan MT, et al. T cell-encoded CD80 and 4–1BBL induce auto-and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 39.Mizoguchi H, et al. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 40.Loskog A, et al. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–1828. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 41.Robinson BW, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 42.Palmer DC, et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol. 2004;173:7209–7216. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duval L, et al. Adoptive transfer of allogeneic cytotoxic T lymphocytes equipped with a HLA-A2 restricted MART-1 T-cell receptor: A phase I trial in metastatic melanoma. Clin Cancer Res. 2006;12:1229–1236. doi: 10.1158/1078-0432.CCR-05-1485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.