Abstract
The “master clock” in the suprachiasmatic nucleus (SCN) of the hypothalamus controls most behavioral, physiological, and molecular circadian rhythms in mammals. However, there are other, still unidentified, circadian oscillators that are able to carry out some SCN functions. Here we show that one of these, the methamphetamine-sensitive circadian oscillator (MASCO), which generates behavioral rhythms in the absence of the SCN, is based on an entirely different molecular mechanism. We tested mice lacking, or with mutations of, genes that form the canonical circadian machinery. In all cases, animals that were arrhythmic as a consequence of genetic defect expressed circadian locomotor rhythms when treated with methamphetamine. These results strongly support the hypothesis that the mechanism generating MASCO does not involve the molecular feedback loops that underlie canonical circadian rhythmicity. The properties of MASCO may provide insight into the evolution of circadian mechanisms. Importantly, MASCO may play a role in addiction to psychostimulants.
Keywords: mouse, suprachiasmatic nucleus, psychostimulant
The dominant circadian pacemaker in the mammalian brain is contained within the suprachiasmatic nucleus (SCN) of the hypothalamus (1, 2). However, if the SCN is ablated by lesion or suppressed by constant bright light, resulting in arrhythmic locomotor behavior, circadian rhythmicity can be reconstituted with methamphetamine (3, 4). Arrhythmic, SCN-lesioned rats (SCNX) (3) and mice (4) given ad libitum access to drinking water containing a low dose of methamphetamine produce clear, robust locomotor rhythms with circadian periods that are considerably longer than those of intact animals drinking pure water. Such methamphetamine-induced rhythms continue under constant conditions as long as the drug is present in the drinking water and often for several cycles after its removal (4). These experiments, together with the appropriate controls (3, 4), demonstrate that these rhythms are not the result of a non-oscillating “hourglass” but rather constitute clear evidence of the existence of an effective circadian pacemaker outside the SCN. We have called this pacemaker the methamphetamine-sensitive circadian oscillator (MASCO) and are studying its properties.
As yet very little is known about MASCO despite overwhelming evidence of its existence. Its location is unknown, although it would be surprising if it were not somewhere in the brain, perhaps associated with dopaminergic and/or serotonergic pathways. Parsimony would suggest that it might be generated by the same molecular feedback loops that produce circadian rhythmicity in the SCN itself and in many peripheral cells, tissues, and organs. However there is reason to believe that this may not be the case. Mice carrying the ClockΔ19 gene mutation and others in which the 2 cryptochromes have been knocked out have arrhythmic phenotypes in constant conditions but become rhythmic when given methamphetamine (5, 6). These data suggest that the molecular events that underlie MASCO may differ substantially from those that generate canonical circadian oscillations. The extent of this potential difference is important. Is MASCO a modified canonical circadian oscillator, or is it based on a radically different molecular mechanism? The available data are not sufficient to decide between these alternatives. We know that in CLOCK-deficient mice, NPAS2 can substitute for this core protein (7); indeed, even in wild-type mice, NPAS2 replaces CLOCK functionally in the forebrain (8). A similar situation might exist for 1 or both of the CRY proteins (only 1 of which is necessary for rhythmicity). Therefore, the molecular mechanism of MASCO might be similar to the canonical mechanism with 2 substituted proteins, 1 of which could be NPAS2. We cannot test these possibilities directly because we do not yet know where in the brain to look for such substitute proteins. However, it is possible to assess the involvement of other known circadian genes in MASCO. We have done so by using both circadian mutants and knockout animals. In all cases, the affected animals continue to respond to methamphetamine, suggesting that MASCO is indeed radically different from known circadian oscillators.
Results
We assessed the effects of methamphetamine in mice with defects in the well-known canonical circadian clock genes (Per1, Per2, Cry1, Cry2, Bmal1, Npas2, Clock, and Ck1ε), some with lesions of their SCN. All circadian mutants and knockouts tested exhibited methamphetamine-induced changes in their free-running rhythms. Rhythmic mutants had lengthened circadian free-running periods (τ) in the presence of methamphetamine, and arrhythmic mutants and SCNX animals became rhythmic on methamphetamine. The lengths of free-running periods (as measured by running-wheel activity or, in the case of Bmal1 knockouts, general locomotor activity) before and during methamphetamine treatment are presented in Table 1.
Table 1.
Free-running periods of mice with circadian defects
| Genotype | N | Baseline (h) | 0.005% MA (h)* | Change in τ (h) |
|---|---|---|---|---|
| ClockΔ19 heterozygous | 5 | 24.20 (± 0.08) | 26.50 (± 1.29) | 2.30 |
| ClockΔ19 homozygous | 6 | 27.83 (± 0.23) | 28.50 (± 0.22) | 0.67 |
| Tau heterozygous | 15 | 21.92 (± 0.05) | 26.93 (± 1.36) | 5.01 |
| Tau homozygous | 6 | 20.11 (± 0.04) | 25.86 (± 2.13) | 5.75 |
| SCNX Tau homozygous | 2 | Arrhythmic | 34.09 (± 3.09) | |
| Npas2−/− | 5 | 23.54 (± 0.10) | 24.13 (± 0.08) | 0.59 |
| SCNX Npas2−/− | 3 | Arrhythmic | 28.17 (± 1.23) | |
| Per1/Per2−/− | 6 | Arrhythmic/ultradian | 27.14 (± 1.76) | |
| Bmal1−/− | 5 | Arrhythmic | 32.77 (± 2.30) | |
| Cry1/Cry2−/− | 6 | Arrhythmic/ultradian | Range: 24.50–35.33 | |
| SCNX Cry1/Cry2−/− | 4 | Arrhythmic | 26.67 (± 3.06) |
Mean (± SEM) or range of the dominant free-running periods observed on pure water (baseline) and on 0.005% methamphetamine. Mean τ on MA for Bmal1-knockout (Bmal1−/−) mice represents the average of the longest free-running periods observed for a given mouse. The range for Cry1/Cry2−/− mice (intact) represents free-running periods throughout the duration of methamphetamine treatment (free-running period was measured following 1 week, 2 weeks, 4 weeks, 6 weeks, and ≈ 7 weeks on 0.005% methamphetamine). All other means on methamphetamine represent the average free-running period during the last 2 weeks of methamphetamine treatment. Note that not all genotypes were on methamphetamine for the same length of time but instead were treated until a stable free-running period was achieved.
*, For all mutations that free-ran on pure water, the free-running period was significantly longer on 0.005% methamphetamine (P < 0.05). For comparison, the free-running period of untreated, wild-type C57BL/6 mice is ≈ 23.6 h; when mice were treated with 0.005% methamphetamine, the period lengthened to ≈ 24.0 h. (Data from: Tataroglu O, Davidson AJ, Benvenuto LJ, Menaker M (2006) The methamphetamine-sensitive circadian oscillator (MASCO) in mice. Journal of Biological Rhythms 21:185–194.)
Clock-mutant mice (both heterozygotes and homozygotes) exhibited the anticipated phenotypes before methamphetamine administration (9): long free-running rhythms of wheel-running activity. The τ of these mice became longer yet following 0.005% methamphetamine treatment (administered chronically in the drinking water (Fig. 1). Additionally, none of the Clock homozygous mutant mice became arrhythmic while treated with methamphetamine, as would be expected had they been held on pure water in constant darkness (DD) for that long (≈ 7 weeks). In fact, rhythmicity in these animals persisted for at least 10 (and in 1 case for as many as 29) days following methamphetamine removal.
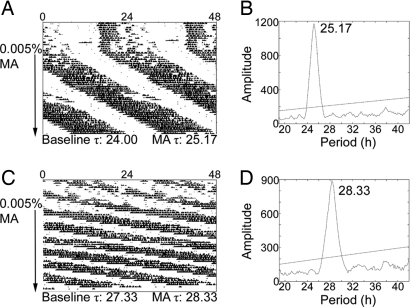
Fig. 1.
Clock Δ19 mice on methamphetamine (MA). Sample actograms (A and C) and periodograms (B and D) from a ClockΔ19 heterozygous (A and B) and a ClockΔ19 homozygous mouse (C and D). Actograms are double plotted. The arrow indicates the period of drug administration. τ in h is listed along the bottom of each actogram. Baseline τ was calculated in DD before drug administration. The methamphetamine τs were calculated using the last 2 weeks of drug treatment and are represented in the periodograms (B and D). Methamphetamine use significantly lengthened τ in Clock-mutant mice (F (1, 9) = 5.863, P < 0.05). The effects of methamphetamine administration did not differ by genotype (heterozygote or homozygous Clock mutation).
Tau-mutant mice exhibited the expected free-running periods (10). As expected, the circadian periods of Tau heterozygotes were 2 h shorter, and those of Tau homozygotes were ≈ 4 h shorter than those of wild-type animals. While on 0.005% methamphetamine, however, the free-running periods of Tau-mutant mice lengthened significantly (Fig. 2). Some of the mice exhibited multiple free-running components that seemed to influence each other but did not share the same period length (i.e., the free-running components were relatively coordinated). Relative coordination between these components (1 of which may be driven by the SCN and 1 by MASCO) may explain the shifts in phase that occurred, seemingly spontaneously, in some animals (Fig. 2, A and C). SCNX Tau homozygotes were arrhythmic before methamphetamine administration. Methamphetamine induced rhythmicity in these mice: 1 animal free-ran with a period of 31.00 h, the other with a period of 37.17 h (Fig. 3).
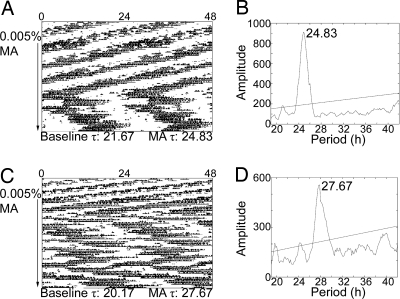
Fig. 2.
Tau-mutant mice on methamphetamine (MA). Sample actograms (A and C) and periodograms (B and D) from a Tau heterozygous mutant (A and B) and a Tau homozygous mutant (C and D). Actograms are double plotted. The arrow indicates the period of drug administration (0.005% methamphetamine). τ in h is listed along the bottom of each actogram. Baseline τ was calculated in DD before drug administration. The methamphetamine τs were calculated using the last 2 weeks of drug treatment and are represented in the periodograms (B and D). Methamphetamine use significantly lengthened τ in Tau-mutant mice (F (1, 19) = 18.104, P < 0.05). The effects of methamphetamine administration did not differ by genotype (heterozygote or homozygous Tau mutation). Note that the heterozygote animal (A) was on methamphetamine for many days before a substantial lengthening of the free-running period was achieved.
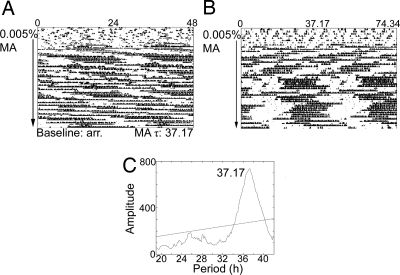
Fig. 3.
SCNX Tau-mutant mice on methamphetamine (MA). (A) Sample actogram from an SCN-lesioned Tau homozygous mutant mouse. The data from (A) are replotted in (B); the x-axis has been changed to 37.17 h to aid in visualizing rhythmicity. Actograms are double plotted. The arrow indicates the period of drug administration (0.005% methamphetamine). Baseline τ was calculated in DD before drug administration. The methamphetamine τ was calculated using the last 2 weeks of drug treatment and is represented in the periodogram (C). A significant peak is observed at 37.17 h.
Npas2−/− mice lengthened their free-running periods during 0.005% methamphetamine treatment (Fig. 4). Arrhythmic SCNX Npas2−/− mice became rhythmic following the application of methamphetamine, sometimes demonstrating multiple free-running components (Fig. 4).
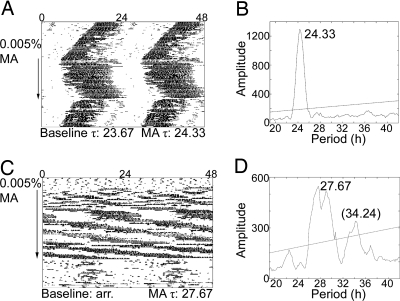
Fig. 4.
Npas2−/− mice on methamphetamine (MA). Sample actograms (A and C) and periodograms (B and D) from an intact Npas2−/− mouse (A and B) and an SCNX Npas2−/− mouse (C and D). Actograms are double plotted. The arrow indicates the period of drug administration (0.005% methamphetamine). τ in h is listed along the bottom of each actogram. Baseline τ was calculated in DD before drug administration. Methamphetamine τs were calculated using the last 2 weeks of drug treatment and are represented in the periodograms (B and D). A significant peak is observed at 24.33 h for the intact mouse (B). Methamphetamine use significantly lengthened τ in intact Npas2−/− mice (t = −4.384, P < 0.05). The SCNX animal had no significant free-running period in DD before drug administration; however, note the weak 24-h rhythm following methamphetamine removal. During methamphetamine treatment a significant peak is observed at 27.67 h with a smaller peak at 34.24 h (D).
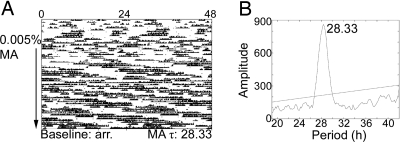
Per1−/−/Per2−/−, Bmal1−/−, and Cry1−/−Cry2−/−mice were all arrhythmic before treatment with methamphetamine, consistent with previous reports (11–13). All these animals became rhythmic on methamphetamine. Per1−/−/Per2−/− mice exhibited varied free-running periods on 0.005% methamphetamine; during the last 2 weeks of drug treatment, the range of free-running periods was 21.0–33.5 h (Fig. 5).
Fig. 5.
Per1−/−/Per2−/− mice on methamphetamine (MA). Sample actogram (A) and periodogram (B) from a Per1−/−/Per2−/− mouse. The actogram is double plotted. The arrow indicates the period of drug administration (0.005% methamphetamine). τ in h is listed along the bottom of the actogram. Methamphetamine τ was calculated using the last 2 weeks of drug treatment and is represented in the periodogram (B). A significant peak is observed at 28.33 h.
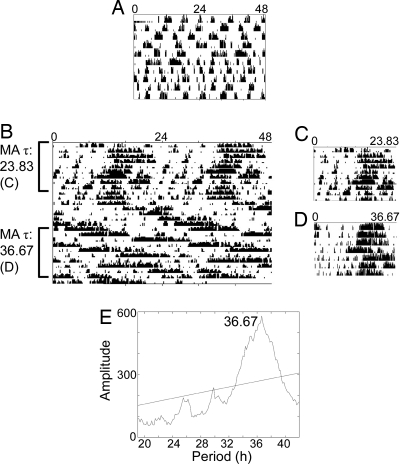
During pilot studies, beginning methamphetamine treatment at the 0.005% concentration proved lethal for a number of the Bmal1-knockout mice; these animals therefore were started on a lower dose of methamphetamine (0.0035% methamphetamine), and the dose was increased to 0.005% methamphetamine after 1 month (any animals that did not survive at least 2 weeks into the 0.005% methamphetamine treatment were excluded from analyses). Methamphetamine induced locomotor rhythmicity in Bmal1−/− animals. One animal displayed a free-running period close to 24 h while on 0.0035% methamphetamine and during the first 10 days of 0.005% methamphetamine administration. This animal's period lengthened to 36.67 h after ≈ 2.5 weeks of 0.005% methamphetamine (Fig. 6). Another animal exhibited a period of 24.50 h after ≈ 3 weeks of 0.005% methamphetamine. All other animals displayed transient, extremely long periods (> 30 h) of locomotor activity while on 0.005% methamphetamine but did not display robust rhythms at the lower concentration of methamphetamine. Wild-type animals (n = 5) were rhythmic with lengthened periods on 0.0035% and 0.005% methamphetamine.
Fig. 6.
Bmal1−/− mouse on methamphetamine (MA). Double-plotted actogram from a Bmal1-knockout mouse depicting infrared activity data before methamphetamine treatment (A) and in the initial period of 0.005% methamphetamine water administration (B). The first day of the actogram in (B) is the first day of 0.005% methamphetamine availability; before that point the animal was consuming 0.0035% methamphetamine water. The τ in h is listed along the side of the actogram. The abrupt change in period that occurred toward the middle of the record was spontaneous. (C) A single-plotted actogram of the initial period of 0.005% methamphetamine treatment (when the observed τ was 23.83 h). The x-axis has been changed to 23.83 h to aid in visualizing rhythmicity. (D) A single-plotted actogram of the latter portion of (B) with the x-axis changed to 36.67 h to aid in visualizing rhythmicity. (E) Periodogram analysis from this mouse after ≈2.5 weeks of 0.005% methamphetamine treatment. This periodogram covers the last 10 days of the record shown in (B); a significant peak is observed at 36.67 h.
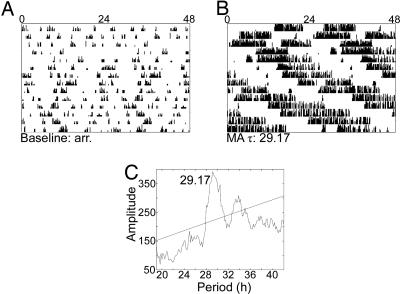
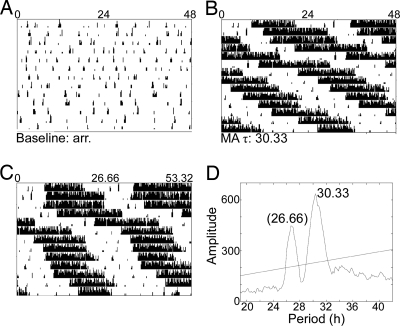
Cry1−/−/Cry2−/− mice had long periods of circadian locomotor activity while on 0.005% methamphetamine (Fig. 7). For some animals rhythmicity was not achieved for several weeks, and in some cases the observed rhythms were transient. After ≈ 12 weeks on 0.005% methamphetamine, the dose was increased to 0.010% to determine if a further increase in drug would lengthen the period further. The increased dose resulted in a dramatic increase in period length for 1 animal, but all other animals became arrhythmic at the higher dose. SCNX Cry1−/−/Cry2−/− mice were arrhythmic on pure water and became rhythmic on 0.005% methamphetamine (Fig. 8). Observed free-running periods after 1 month of 0.005% methamphetamine treatment were in the range of 23.17–35.83 h.
Fig. 7.
Cry1−/−/Cry2−/− mouse on methamphetamine (MA). Double-plotted actograms from an intact Cry1−/−/Cry2−/− mouse before methamphetamine treatment (A) and during the last 2 weeks of drug treatment (B). This animal had no significant free-running period in DD before drug administration but takes on a long free-running period in the presence of 0.005% methamphetamine, as shown in the periodogram (C). A significant peak is observed at 29.17 h.
Fig. 8.
SCNX Cry1−/−/Cry2−/− mouse on methamphetamine (MA). Actograms from an SCNX Cry1−/−/Cry2−/− mouse before methamphetamine treatment (A) and during the last 2 weeks of drug treatment (B). This animal had no significant free-running period in DD before drug administration but takes on a long free-running period in the presence of 0.005% methamphetamine. The data from (B) are replotted in (C) with the x-axis changed to 26.66 h to aid in visualizing the 2 periods present. (D) Periodogram analysis of these data shows a significant peak at 30.33 h and a smaller peak at 26.66 h.
Discussion
Methamphetamine has several robust effects on overt circadian locomotor rhythms in rats and mice. In intact animals it alters locomotor activity patterns, sometimes causing the rhythm to separate into long and short components and often yielding long free-running periods of locomotor activity (4, 14, 15). In arrhythmic animals, whether the arrhythmicity is caused by SCN lesion (3, 4), constant light (4), or, as in the current study, by mutation or knockout of critical canonical clock genes (5, 6), methamphetamine administration produces rhythms of locomotor activity with circadian properties. This remarkable result was first observed in SCNX rats and led to the suggestion that the overt rhythms were driven by a circadian oscillator in an unidentified location outside the SCN (3). The work reported here was undertaken to test the hypothesis that this oscillator uses the well-known canonical circadian clock genes (Per1, Per2, Cry1, Cry2, Bmal1, Npas2, Clock, and Ck1ε) to generate its oscillation. We tested the effects of methamphetamine on mice carrying mutations in Clock and Ck1ε, Bmal1 and Npas2 knockouts and double Per1/Per2 and Cry1/Cry2 knockouts. In no case did the genetic alteration abolish the effect of methamphetamine; mice that were rhythmic on pure water (Npas2−/−, Tau- mutants, and Clock mutants) lengthened their free-running periods in the presence of methamphetamine, whereas those that were arrhythmic (Per1−/−/Per2−/−, Cry1−/−/Cry2−/−, and Bmal1−/− mice) became rhythmic under the influence of methamphetamine. Thus we can conclude that the molecular mechanism that underlies MASCO differs substantially from that which produces canonical circadian oscillations in the SCN and many peripheral cells and tissues.
The response to the removal of methamphetamine (MA) varied. Clock homozygous mutants remained rhythmic for several days, and sometimes for weeks, following the removal of the drug. Likewise, several of the Clock heterozygotes maintained somewhat longer periods even after several weeks on pure drinking water. Most other intact animals reverted to their previous free-running periods very quickly. In some cases SCNX animals displayed weak rhythmicity for a few cycles after methamphetamine removal. This persistence of rhythmicity in the absence of drug has been reported previously (4) and is further evidence that MASCO is an endogenous oscillator.
The ability of methamphetamine to induce circadian rhythms in the absence of the SCN and known critical components of the molecular circadian clockwork demonstrates the existence of 2 separate oscillators: the canonical (SCN-driven) circadian oscillator and the methamphetamine-sensitive circadian oscillator. These oscillators seem to be both molecularly and anatomically distinct. They are, however, coupled under some conditions. This distinction can be observed best in intact mice, which sometimes demonstrate 2 relatively coordinated free-running components, 1 of which seems to be driven by the SCN, whereas the other has a long period characteristic of MASCO (see figure 1A in ref. 4). The free-running period on methamphetamine is longer for SCNX animals than for intact animals (4), further demonstrating that in the presence of the SCN, MASCO interacts with the circadian clock in the SCN.
The coupling between MASCO and the SCN may be at least partially dependent on canonical clock genes. This dependence might account for the observed variation in the period-lengthening effect of methamphetamine among genotypes (see Table 1). Similarly, the free-running periods of many of the circadian mutants on methamphetamine were longer than would be expected for an intact wild-type mouse (4). Npas2-null mice, which have the weakest circadian phenotype of the mutants we tested (i.e., their free-running periods are closest to those of wild-type animals), experienced the smallest change in τ, whereas mice carrying mutations causing more severe circadian phenotypes had longer free-running periods on methamphetamine. Some Cry1−/−/Cry2−/− mice had periods as long as 35 h, and the average free-running period for Bmal1-null mice was longer than 32 h. These long free-running periods could be the result of mutations in canonical clock genes altering interactions between the SCN and MASCO. If so, one would expect a different free-running period in SCNX Cry1−/−/Cry2−/− mice than in intact mice of the same genotype. Our limited sample suggests that this might be the case.
The relationship between the free-running periods of the canonical and methamphetamine-sensitive circadian oscillators is not linear, as illustrated by the results obtained in Tau homozygous mutant mice. These mice free-ran at 20.11 h before drug treatment, and the period lengthened to 25.86 h on methamphetamine, even longer than the free-running period of wild-type mice on methamphetamine (24.04–24.17 h; ref. 4). Thus, although the mutation in Ck1ε shortens the period of the canonical circadian oscillator, it does not shorten the period of MASCO in the same animal, supporting the conclusion that MASCO and the canonical circadian clock are mechanistically distinct.
Importantly, although methamphetamine induced rhythmicity in Cry1−/−/Cry2−/− mice, the free-running periods observed were not always consistent and sometimes alternated with periods of arrhythmicity. A similar finding has been reported previously by Honma et al. (5). However, these investigators found that some Cry1−/−/Cry2−/− mice did not become rhythmic in the presence of methamphetamine, whereas all the mice we tested exhibited at least transient periods of rhythmicity. The concentration of methamphetamine we used was twice that used by Honma et al. (5), and the difference in dose probably explains the different result. The observed free-running rhythms in Cry1−/−/Cry2−/− mice make it clear that cryptochromes are not necessary for the expression of MASCO, although rhythmicity is less consistent in Cry1−/−/Cry2−/− animals than in wild-type mice on methamphetamine. This inconsistency might result from an effect of cryptochromes on general locomotor activity, or it could point to a role for cryptochromes in the stability of MASCO itself.
A possible interpretation of the effects of methamphetamine on mutant mice is that methamphetamine amplifies low-level signals still produced by the mutant SCN and thus “restores” locomotor rhythmicity. To test this interpretation, we made SCN lesions in Tau-mutant, Npas2−/−, and Cry1−/−/Cry2−/− mice. In no case did SCN lesion eliminate the response to methamphetamine. In fact, the rhythms of SCNX Cry1−/−/Cry2−/− mice on methamphetamine are more robust than those of intact mice of the same genotype. The same effect was observed in Npas2−/− animals, which had free-running periods on methamphetamine that were, on average, several hours longer after SCN lesion. These results suggest that the SCN may partially inhibit methamphetamine-induced expression of circadian locomotor rhythms and underscore the importance of understanding the interaction between the canonical circadian oscillator and MASCO.
Bmal1−/− mice often are small and weak and do not do well on running wheels. It therefore was necessary to measure their circadian behavior using infrared beam breaks. Methamphetamine-induced lengthening of τ (for wild-type mice) and induced rhythmicity (for Bmal1−/− mice) was observed easily using this measure of locomotor activity. It has been suggested that the presence of a running wheel may be necessary to observe a methamphetamine-induced effect on circadian locomotor behavior (16). Our data do not support this suggestion.
Our data demonstrate that the canonical molecular circadian feedback loop is not necessary for the generation of circadian locomotor rhythmicity by methamphetamine. We tested mice with mutations or deletions in both the positive (Clock, Npas2, Bmal1) and negative (Per, Cry) limbs of the canonical circadian molecular loop and failed to abolish methamphetamine-sensitive circadian locomotor activity. Although negative results would be difficult to interpret, the observation of MASCO in animals with a variety of circadian defects suggests that this oscillator is able to operate without input from known circadian machinery. We conclude that MASCO is an independent oscillator capable of inducing locomotor rhythms in the circadian range.
Because methamphetamine never has been part of the natural environment of rodents, MASCO probably has a normal function unrelated to drugs of abuse. We do not know what that function is. The ability of MASCO to interact with the SCN suggests that it normally might work with the canonical circadian system to fine-tune rhythmic outputs, particularly those that respond to rewarding stimuli such as food. Indeed, it is reasonable to suggest that MASCO is identical to the food-entrainable oscillator, which also does not require (at least some) canonical clock genes (17). Although in some cases the free-running periods elicited by methamphetamine were very long, most animals displayed a free-run between 25 and 29 h. This period is longer than the SCN-driven oscillations produced in most mammals, but it is not outside the biological range for circadian rhythms. Some fungi, for example, exhibit rhythms with periods longer than 30 h (18). Further research on MASCO is likely to provide insight into the function and, perhaps, the evolution of biological oscillators. Moreover, the existence of MASCO reveals a strong, and unexpected, effect of psychostimulants: their ability to alter dramatically and even to induce circadian rhythms. These findings may change our understanding of the development and symptomology of substance abuse and potentially could influence its treatment. MASCO provides a new window into the interaction of circadian rhythms and drugs of abuse (19–21). Determining its location in the brain and its mechanistic basis may provide insight into mechanisms of addiction.
Materials and Methods
Subjects.
All mice were obtained from breeding colonies housed at the University of Virginia. Npas2-knockout mice and Per1−/−/Per2−/− mice were maintained by null/null crosses. All other animals were obtained from crosses with at least 1 wild-type allele for the gene of interest. The offspring then were phenotyped and/or genotyped by PCR before entering the experiments. Per1−/−/Per2−/− animals were maintained on a mixed genetic background; all other strains were on a C57BL/6 background (although the number of backcross generations was not consistent among all genotypes). Colony founders were generously donated by Joseph Takahashi of Northwestern University (Clock Δ19-mutant mice and Per1−/−/Per2−/− mice), Andrew Loudon of the University of Manchester (Tau-mutant mice), Steven McKnight of the University of Texas Southwestern Medical Center (Npas2−/− mice), Charles Weitz of Harvard University (Bmal1−/− mice, and Marina Antoch of Roswell Park Cancer Institute (Cry1 −/−/Cry2−/− mice).
The Animal Care and Use Committee at the University of Virginia approved all experimental procedures.
Animal Housing.
Animals were housed individually in Nalgene cages with food and water available ad libitum. With the exception of the Bmal1−/− mice, all animals' cages were equipped with running wheels. Because the presence of a running wheel increased mortality in Bmal1−/− mice, these animals and a cohort of wild-type controls were housed in cages equipped with Quorum passive infrared devices (Electronic Goldmine) to monitor general cage activity. All activity data were recorded using ClockLab software (Actimetrics). Animals were kept on a 12 h:12 h light:dark cycle before experimental manipulation. During the light phase, light intensity at the level of the animals' cages was ≈ 350 lux.
SCN Lesion Procedure.
Animals were anesthetized with isoflurane. Bilateral electrolytic lesions of the SCN were made by passing 1.1-mA DC current through a Teflon-coated (0.5-mm exposed tip) tungsten wire electrode for 20 s. For an animal with a bregma−lambda distance of 3.8 mm, the electrode was placed 0.30 mm rostral, 0.25 mm lateral, and 6.00 mm ventral to the bregma. The rostral and ventral coordinates were adjusted based on the bregma–lambda distance of a given animal. Animals recovered for at least 1 week before the start of the experimental paradigm. Lesions were confirmed by observation of arrhythmic behavior or, for arrhythmic mutant animals, by histology. For histological verification, tissue was fixed with 4% paraformaldehyde, brains were sliced, and tissue was stained with thionin to determine the structural extent of the lesion. Lesions were considered successful if cell damage extended across the bulk of the SCN.
Methamphetamine Treatment.
Animals were released into DD for ≈ 2 weeks to assess arrhythmicity or to obtain a baseline free-running period. Animals' drinking water then was replaced with a solution containing 0.005% methamphetamine (Sigma). Bmal1−/− mice and wild-type controls were started on a lower dose of methamphetamine (0.0035%) for the first 4 weeks; then the dose was increased to 0.005%. The lower dose was administered first to reduce the potential for methamphetamine-induced mortality in the Bmal1-null mice, which generally are weak and are particularly sensitive to the toxic effects of methamphetamine. All groups of mice were maintained on the methamphetamine water until they developed a stable pattern of locomotor activity (for most genotypes this pattern took approximately 40–60 days). Unless otherwise noted, animals then were given pure drinking water and monitored until they again achieved a stable circadian behavioral pattern or arrhythmicity.
Analyses.
Activity data were double plotted in actograms using a 10-min block size. The circadian period was evaluated using ClockLab (Actimetrics). χ2 periodogram analyses were performed on at least 1 week of continuous data over a range of 16–42 h, with alpha set at 0.01. Because in most cases a week or more of drug treatment was needed before a change in the free-running period was observed, the reported period lengths on methamphetamine reflect the dominant free-running period during the last 2 weeks of drug treatment, unless otherwise noted. When multiple significant period lengths were observed, the reported τ reflects the most robust peak (i.e., the period length with the largest amplitude above significance). Comparisons between period lengths before and after drug treatment were done by t test; comparisons involving multiple mutant alleles (i.e., both heterozygous and homozygous mice) were made using a repeated measures analysis of variance. In all cases P < 0.05 was considered significant.
Acknowledgments.
We thank Naomi Ihara, Denise Holmes, Ozgur Tataroglu, Andrew Doering, Cara Iorianni, and James Pilachowski for their technical support and expertise. This research was supported by Conte Center grant 1P50MH074924 and National Space Biomedical Research Institute grant NCC 9–58-HPF 00406 (M.M.). J.M. was supported by National Institutes of Health grants T32DK007646 and F32DA024542.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 2.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honma K, Honma S, Hiroshige T. Activity rhythms in the circadian domain appear in suprachiasmatic nuclei lesioned rats given methamphetamine. Physiol Behav. 1987;40:767–774. doi: 10.1016/0031-9384(87)90281-2. [DOI] [PubMed] [Google Scholar]
- 4.Tataroglu O, Davidson AJ, Benvenuto LJ, Menaker M. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. Journal of Biological Rhythms. 2006;21:185–194. doi: 10.1177/0748730406287529. [DOI] [PubMed] [Google Scholar]
- 5.Honma S, Yasuda T, Yasui A, van der Horst GT, Honma K. Circadian behavioral rhythms in Cry1/Cry2 double-deficient mice induced by methamphetamine. Journal of Biological Rhythms. 2008;23:91–94. doi: 10.1177/0748730407311124. [DOI] [PubMed] [Google Scholar]
- 6.Masubuchi S, Honma S, Abe H, Nakamura W, Honma K. Circadian activity rhythm in methamphetamine-treated Clock mutant mice. European Journal of Neuroscience. 2001;14:1177–1180. doi: 10.1046/j.0953-816x.2001.01749.x. [DOI] [PubMed] [Google Scholar]
- 7.DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nature Neuroscience. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: An analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- 9.Vitaterna MH, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng QJ, et al. Setting clock speed in mammals: The CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae K, et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 12.Bunger MK, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Horst GT, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 14.Honma K, Honma S, Hiroshige T. Disorganization of the rat activity rhythm by chronic treatment with methamphetamine. Physiol Behav. 1986;38:687–695. doi: 10.1016/0031-9384(86)90265-9. [DOI] [PubMed] [Google Scholar]
- 15.Rietveld WJ, Korving J, Boon ME, Wirz-Justice A. The circadian control of behavior in the rat affected by the chronic application of methamphetamine. Prog Clin Biol Res. 1987;227B:513–517. [PubMed] [Google Scholar]
- 16.Honma S, Honma K, Hiroshige T. Methamphetamine effects on rat circadian clock depend on actograph. Physiol Behav. 1991;49:787–795. doi: 10.1016/0031-9384(91)90319-j. [DOI] [PubMed] [Google Scholar]
- 17.Pitts S, Perone E, Silver R. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am J Physiol Regulatory Integrative, and Comparative Physiology. 2003;285:R57–67. doi: 10.1152/ajpregu.00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene AV, Keller N, Haas H, Bell-Pedersen D. A circadian oscillator in Aspergillus spp. regulates daily development and gene expression. Eukaryotic Cell. 2003;2:231–237. doi: 10.1128/EC.2.2.231-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci USA. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- 21.Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]