Abstract
Current influenza A virus vaccines do not generate significant immunity against serologically distinct influenza A virus subtypes and would thus be ineffective in the face of a pandemic caused by a novel variant emerging from, say, a wildlife reservoir. One possible solution would be to modify these vaccines so that they prime cross-reactive CD8+ cytotoxic T lymphocytes (CTL) cell-mediated immunity directed at conserved viral epitopes. A further strategy is to use novel adjuvants, such as the immunomodulatory glycolipid α-galactosylceramide (α-GalCer). We show here that giving α-GalCer with an inactivated influenza A virus has the paradoxical effect of diminishing acute CTL immunity via natural killer T (NKT) cell-dependent expression of indoleamine 2,3-dioxygenase (IDO), an important mediator of immune suppression, while at the same time promoting the survival of long-lived memory CTL populations capable of boosting protection against heterologous influenza A virus challenge. This enhancement of memory was likely due to the α-GalCer-induced upregulation of prosurvival genes, such as bcl-2, and points to the potential of α-GalCer as an adjuvant for promoting optimal, vaccine-induced CD8+ T cell memory.
Keywords: T cell memory, viral immunity, vaccine, adjuvant
The commonly used influenza A virus (IAV) vaccines generate strain-specific antibody responses against the surface viral hemagglutinin (H, HA) and neuraminidase (N, NA) proteins. Neutralizing antibodies specific for the viral HA in particular provide a reasonable measure of protection if there is sufficient similarity between the vaccine (1) and current circulating IAV strains. However, in the event of a pandemic where a novel IAV subtype is introduced into human circulation, it is unlikely that the present inactivated whole or disrupted (split) virus vaccines will be adequate as there will be no preexisting humoral immunity (2). Recent outbreaks of highly pathogenic H5N1 IAVs in poultry and humans throughout Southeast Asia have raised the possibility that a new IAV pandemic could be imminent (2). The development of novel vaccines that elicit immunity to heterologous (HA-different) IAV infection is thus of considerable interest.
At least in mice, virus-specific CD8+ cytotoxic T lymphocytes (CTL) play a critical role in terminating IAV infections (3, 4). The role of CD8+ T cell-mediated immunity in humans is much less clear, although it is receiving renewed attention (5, 6). These CD8+ CTLs are specific for peptide epitopes derived from internal virus proteins that are not subject to antibody-mediated selection pressure and are relatively conserved between different IAVs (7). Infection with HA and NA-different viruses thus primes for a measure of broad, heterosubtype-specific CTL-mediated immunity that is not induced by the current inactivated vaccines (8). An ideal solution would be to find an adjuvant that could, when given with inactivated (i) IAV, promote both influenza-specific antibody and CTL-based immunity. Unfortunately, the adjuvants approved for human use (alum and MF59) are poor inducers of CD8+ T cell responses (9).
The potent immune modulator α-galactosylceramide (α-GalCer) is presented to natural killer T (NKT) cells by the MHC class I-like molecule, CD1d (10, 11). The NKTs are specialized T lymphocytes expressing markers of the NK cell lineage and an invariant TCR (12). Following α-GalCer/CD1d recognition, activated NKT cells produce a range of cytokines and cell-surface stimulatory molecules that contribute to the activation of NK cells, T and B lymphocytes, and dendritic cells (DC) (13). Therefore, NKT cells could be important regulators of immune responsiveness in a broad variety of diseases. α-GalCer has, and continues to be, tested in human cancer immunotherapy trials.
Recent mouse studies have shown that α-GalCer is an effective adjuvant in vaccination against a range of immune challenges (13, 14). Such experiments have shown that combining iIAV vaccine with α-GalCer can significantly enhance protective efficacy (15–18). However, though CTL cytotoxicity could be demonstrated after in vitro restimulation, no attempt was made to enumerate IAV-specific CD8+ T cell numbers directly ex vivo (16). Furthermore, as these studies used homologous virus challenge, preexisting neutralizing antibody rather than the recall of virus-specific CTL memory was the likely mechanism of immune protection (16, 17). Thus, though NKT cell activation appears to enhance immunity to influenza, the question remains: do α-GalCer-adjuvanted influenza vaccines augment heterologous CTL-mediated immunity? Utilizing a well-characterized model of IAV infection of C57BL/6J mice, we ask whether α-GalCer serves as an effective adjuvant for enhancing protective, iIAV vaccine-induced CTL-mediated immunity.
Results
α-GalCer Inhibits CTL Responses After Primary Vaccination.
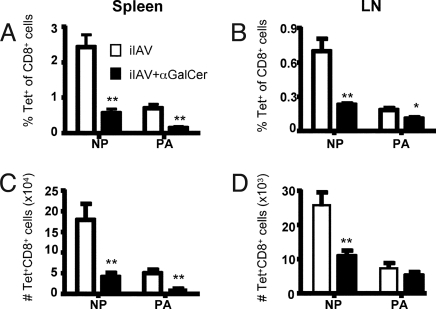
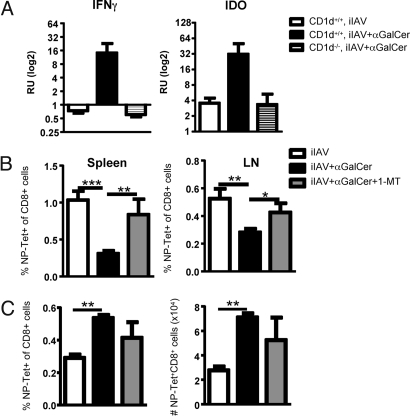
Prior in vitro expansion experiments indicated that α-GalCer may augment IAV-specific CTL responses after high-dose vaccination (16, 17). To determine whether virus-specific CTL responses could be measured directly ex vivo after iIAV priming (with and without α-GalCer administration), mice were injected s.c. with 7.5 μg of iIAV together with 1 μg of α-GalCer or PBS and sampled 7 days later. The primary response to DbNP366 is clearly dominant (compared to DbPA224) in both spleen (Fig. 1 A and C) and LN (Fig. 1 B and D). This is presumably due to the greater amount of nucleoprotein (NP) (compared to acidic polymerase [PA]) within the inactivated virus (19). Surprisingly, mice that were vaccinated with iIAV and α-GalCer together displayed significantly reduced percentages (Fig. 1 A and B, black bars) and absolute numbers (Fig. 1 C and D, black bars) of both DbNP366 and DbPA224 CD8+ T cells compared with those given iIAV alone. This was observed in both the spleen (Fig. 1 A and C) and draining lymph node (Fig. 1 B and D). Repeated immunization (iIAV ± α-GalCer 1−3 times at 2-week intervals and analyzed 7 days after the last vaccination) did not boost DbNP366-specific CD8+ T cell numbers at any time point [supporting information (SI) Fig. S1], and in fact, we again found this CTL response to be diminished by the α-GalCer. Interestingly, there was no increase in the number of DbNP366-specific T cells upon boosting with iIAV alone. A likely explanation is that influenza-specific antibodies induced after 2 vaccinations limits available antigen for subsequent CTL expansion upon a third vaccination (Fig. S1 C and D). The extent of NKT cell activation following α-GalCer administration was also evaluated using α-GalCer-loaded CD1d tetramer. A week after the α-GalCer treatment, both the percentage and absolute numbers of NKT cells were significantly increased (Fig. S1 E and F). It is thus reasonable to think that α-GalCer-induced NKT cell activation impairs the development of acute, iIAV-induced CTL responses.
Fig. 1.
Quantitative analysis of DbNP366+ and DbPA224+ CD8+ T cells following influenza vaccination with an adjuvant. The B6 mice were immunized with iIAV ± α-GalCer, and lymphocytes from the spleen and brachial lymph node (BLN) were sampled 7 days later. The percentage (A and B) and absolute number (C and D) of tetramer-positive populations was evaluated among the CD8+ T cells in the spleen (A and C) and BLN (B and D). Results are expressed as mean ± SD for groups of 5 mice. *P < 0.05; **P < 0.01.
NKT Cell Activation Enhances Vaccine-Induced Memory T Cell Generation.
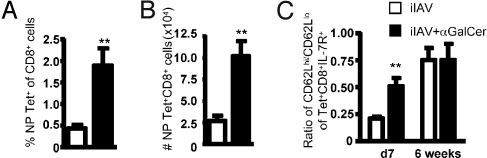
Given the diminished CTL counts found at the peak of the primary response after iIAV + α-GalCer priming, it was important to determine if the magnitude of CTL memory was also affected. Mice were vaccinated with iIAV ± α-GalCer, and DbNP366-specific CTLs were measured 6 weeks later. In contrast to the early (day 7) time point, a significant (P < 0.01) increase (≈4-fold) in the proportion (Fig. 2A) and number (Fig. 2B) of DbNP366-specific memory CTLs was observed in mice given iIAV + α-GalCer. Memory CTL populations can be divided into 2 major subsets identified by their phenotype: “effector memory” cells (TEM) are CD62Llow IL-7R+, whereas “central memory” cells (TCM) are CD62Lhigh IL-7R+ (20). Interestingly, the diminished effector magnitude at the acute time point correlated with an increased ratio of CD62Lhi to CD62Llo DbNP366-specific T cells (Fig. 2C). There was, however, no difference in this CD62Lhi to CD62Llo ratio at the memory time point. As such, α-GalCer + iIAV apparently favors the generation of the TCM set early after vaccination, contributing to an increased pool of memory T cells.
Fig. 2.
α-GalCer promotes the generation of long-term CD8+ T cell memory. The B6 mice were immunized with iIAV ± α-GalCer and killed 6 weeks later. Splenocytes were harvested and analyzed for the percentage (A) and absolute number (B) of tetramer-positive CD8+ CTLs. (C) The proportion of TCM vs. TEM cells was evaluated at early and late time points after immunization. Results are expressed as mean ± SD for groups of 5 mice. *P ≤ 0.05; **P < 0.01
NKT Cells Play a Physiological Role in Regulating CTL Response.
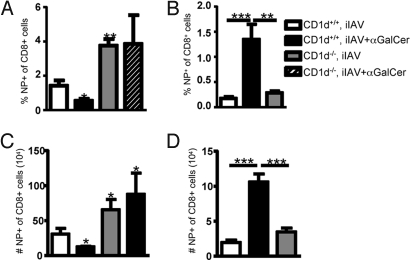
To determine if the α-GalCer effect was mediated via NKT cell activation, NKT-deficient CD1d−/− or wild-type CD1d+/+ mice were vaccinated with iIAV ± α-GalCer. The DbNP366-specific CTL response was then analyzed at acute (d7) and memory (d42) time points, and, as expected, there was a diminished CTL response in wild-type mice after iIAV + α-GalCer administration compared with iIAV alone (Fig. 3 A and C, white vs. black bars). Interestingly, irrespective of α-GalCer treatment, effector DbNP366-specific CTL responses were significantly greater in the CD1d−/− mice (Fig. 3 A and C). This suggests that NKT cell activation following iIAV vaccination plays a key role in suppressing the effector CTL response in normal mice, with the effect being magnified by the concurrent administration of α-GalCer. However, iIAV vaccination of CD1d−/− and CD1d+/+ mice gave equivalent numbers of DbNP366-specific CTLs on day 35 (Fig. 3 B and D), indicating that NKT cell activation is also key for the improved memory CTL generation after vaccination.
Fig. 3.
NKT cells limit the acute CTL response but are essential for memory. Groups of CD1d+/+ and CD1d−/− mice were immunized with iIAV ± α-GalCer and analyzed 7 days (A and C) or 6 weeks later (B and D) for the percentage (A and B) and absolute number (C and D) of DbNP366+CD8+ T cells in the spleen. Results are expressed as mean ± SD. *P ≤ 0.05; **P < 0.01.
α-GalCer Promotes Efficient Secondary Responses and Faster Viral Clearance.
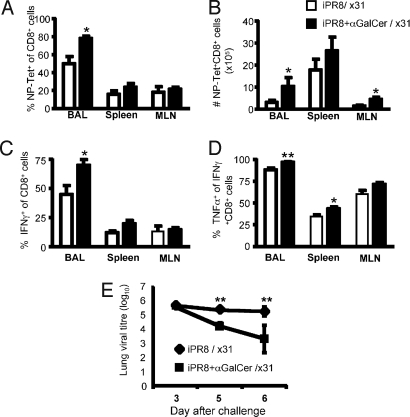
Does this α-GalCer augmentation of CTL memory after iIAV vaccination (Fig. 2) enhance recovery following heterologous IAV infection? Mice were primed with 7.5 μg of iIAV PR8 (H1N1) ± α-GalCer and challenged intranasally (i.n.) with 104 pfu of the HKx31 (H3N2) IAV at least 6 weeks later. The percentage (Fig. 4A) and number (Fig. 4B) of DbNP366-specific CTLs in lymphoid and nonlymphoid organs was then assessed by tetramer and intracellular cytokine staining (ICS) (Fig. 4 C and D) 8 days after infection. Secondary i.n. challenge with A/HKx31 of iPR8 IAV-primed mice resulted in a large recall response, equivalent to that found routinely in those primed with live PR8 IAV (data not shown and ref. 21). Importantly, the DbNP366-specific recall response was significantly greater in both the mediastinal lymph node (MLN) and respiratory tract airways (isolated by bronchoalveolar lavage [BAL]) of mice vaccinated with iPR8 + α-GalCer compared with mice primed with iPR8 alone. Furthermore, the quality of the response was enhanced, as shown by the increased prevalence of IFNg+ CD8+ T cells (Fig. 4C; P < 0.05) and by the greater TNF expression in the IFNγ+DbNP366-specific sets from BAL and the spleen (Fig. 4D; P < 0.01 and P < 0.05, respectively). Despite differences in the proportion of CTL making cytokine, analysis of the mean fluorescence intensity showed there was no difference in the amount of cytokine produced on a per-cell basis (data not shown). It thus seems that α-GalCer coadministration with iIAV augments the recall CTL response to heterologous IAV challenge, presumably as a consequence of the increased numbers of CTL memory precursors (Fig. 2).
Fig. 4.
Increased protection of mice vaccinated with α-GalCer after infection. Mice were challenged i.n. with 104 pfu of live HKx31 IAV at 6 weeks after priming with iIAV + (black bars) or − (white bars) α-GalCer. The percentage (A) and absolute numbers (B) of influenza-specific CTLs were evaluated for the bronchoalveolar lavage (BAL), spleen, and mediastinal lymph node (MLN). Cells were stained for IFNγ and TNFα after stimulation with NP366 peptide. Shown is the percentage of IFNγ+ CD8+ T cells (C) and the proportion of IFNγ+ T cells that are also TNFα+ (D). *P ≤ 0.05 and **P < 0.01 comparing iIAV ± α-GalCer. (E) Virus clearance from the lung of mice that had been primed with iIAV ± α-GalCer. The graphs show the mean pfu per lung (n = 5) ± SD. **P < 0.01.
Given there was an increase in both the magnitude and quality of the secondary DbNP366-specific CTL response, it was important to determine if this correlated with more rapid viral clearance. Mice were vaccinated with iPR8 IAV ± α-GalCer, then challenged i.n. with the HKx31 IAV at 6 weeks. Though lung virus titres were equivalent on day 3 (Fig. 4E), those that had received iPR8 + α-GalCer showed evidence of significantly enhanced virus clearance on day 5 and day 6 (Fig. 4E). There were no differences in the levels of influenza A virus-specific IgG1 and IgG2a antibodies in the sera of mice primed with iPR8 ± α-GalCer following the HKx31 challenge (Fig. S2). This supports the notion that improved CTL memory, rather than improved antibody responses, was the major contributor to the enhanced viral clearance.
α-GalCer Does Not Impair Dendritic Cell Antigen Presentation.
Administration of α-GalCer and the subsequent activation of NKT cells induce rapid maturation of CD8+ and CD8− dendritic cell (DC) subsets (22). The diminished CTL response observed on day 7 after vaccination with iIAV + α-GalCer might thus be thought to reflect rapid DC maturation and subsequent inefficient DC processing and presentation of viral antigens (23). In contrast, we found evidence that α-GalCer induced upregulation of costimulatory molecules and this correlated with no impact on DC priming of naïve T cells (Fig. S3 and data not shown). These findings suggest that impaired DC function does not explain the decrease in the acute IAV-specific CTL responses found for iIAV + α-GalCer-immunized mice.
α-GalCer-Dependent Indoleamine 2,3-Dioxygenase Expression Inhibits T Cell Expansion.
Indoleamine 2,3-dioxygenase (IDO) plays a role in tryptophan metabolism and has been shown to inhibit T cell proliferation (24). The expression of IDO is stimulated by IFNγ, an effect shown clearly for α-GalCer-treated human peripheral blood mononuclear cells (25). We thus asked whether the diminished CTL effector responses observed after iIAV + α-GalCer reflected increased IDO expression and the consequent suppression of CTL expansion. Quantitative RT-PCR analysis showed a significant upregulation of IFN-γ and IDO (Fig. 5A) mRNA in the lymph nodes of mice vaccinated with iIAV + α-GalCer compared with those given iIAV alone. Increased IDO expression was not observed in CD1d-deficient mice (NKT cell deficient), suggesting a requirement for NKT cell activation.
Fig. 5.
Increased expression of IFNγ and IDO inhibit T cell proliferation. (A) Quantitative analysis of IFNγ and IDO transcript accumulation was performed for the LNs of CD1d+/+ and CD1d−/− mice given iIAV ± α-GalCer. Results are expressed in arbitrary units (log2) of molecules normalized to hypoxanthine phosphoribosyltransferase ± SD for groups of 9 to 3 mice. (B) Mice were vaccinated with iIAV ± α-GalCer, and some were given 1-MT for 7 days. The proportion of CTLs was evaluated in the spleen (Left) and the LN (Right). Results are expressed as mean ± SD for groups of 10 mice. (C) Mice were analyzed 6 weeks after treatment for the percentage and number of CTLs in the spleen. Results are expressed in mean ± SD for groups of 5 mice. **P ≤ 0.001.
We further evaluated the role of IDO at early and late time points by blocking its activity with 1-methyl tryptophan (1-MT). Administration of 1-MT from the day of immunization together with α-GalCer treatment largely restored the influenza-specific primary effector response in both the spleen and lymph node (Fig. 5B). However, IDO inhibition had little effect on IAV-specific memory CTL generation, because mice treated with 1-MT in addition to iIAV + α-GalCer displayed the same increased percentage and number (Fig. 5C) of DbNP366-specific CD8+ memory T cells found at 6 weeks in those given only iIAV + α-GalCer (Fig. 5C). These findings thus suggest that NKT cell-derived IFNγ induces increased IDO expression, which in turn inhibits the full expansion and maturation of the acute CTL effector response after iIAV + α-GalCer priming. Furthermore, it seems that the α-GalCer-induced inhibition of acute CTL effector generation and the increase in memory CTL cells may reflect independent mechanisms.
α-GalCer Induces the Upregulation of Prosurvival Genes in T Cells.
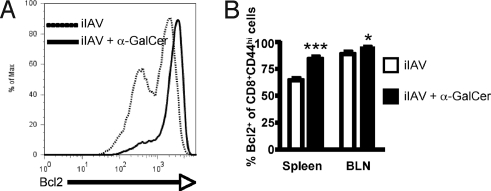
Administration of α-GalCer inhibits the expansion of CTL effectors at the peak of the response while enhancing T cell memory. Prosurvival gene expression has been found to correlate with the establishment of long-lived memory T cells (26). We thus measured bcl-2 expression CD8+ T cells 7 days after vaccinating mice with iIAV ± α-GalCer (Fig. 6). The α-GalCer treatment was associated with increased bcl-2 expression (Fig. 6) in CD44+CD8+ CTLs (memory phenotype) compared with the same population from mice that received iIAV alone. This α-GalCer-dependent increase in bcl-2 levels was observed in both the spleen (P < 0.001) and the draining LN (P < 0.05), supporting the notion that α-GalCer promotes CTL survival and development into long-lived memory by inducing the expression of survival genes.
Fig. 6.
Enhanced memory-cell generation correlates with increased survival signals. (A) Representative histograms of intracellular Bcl-2 expression by splenic CD8+CD44+ T cells from mice given iIAV + (Right) or − (Left) a-GalCer. (B) Quantitation of the proportion of CD8+CD44+ CTLs expressing intracellular Bcl2 at 7 days after iIAV ± α-GalCer. Results are expressed as mean ± SD for groups of 5 mice. *P ≤ 0.05; ***P < 0.001.
Discussion
In this study we show that α-GalCer given at the time of vaccination with a high dose of an inactivated, nonreplicating virus has a powerful adjuvant effect that augments the later recall of CD8+ T cell memory. This enhanced memory is associated with a diminution in the primary effector CTL response after vaccination, an effect dependent on NKT cell activation and subsequent IDO expression, and likely reflects both a greater prevalence of TCM CTL precursors and increased expression of prosurvival genes like bcl-2.
Previous studies in a variety of immune models have shown that α-GalCer can improve antibody and T cell responses (13, 15–17, 22, 27, 28). For example, α-GalCer has been shown to have potent adjuvant activity when combined with iIAV vaccines (15–17). However, though α-GalCer promotes the IAV-specific antibody response, the protective effect was probed only with homologous virus challenge, a situation where the neutralization of the input virus would mask any possible CTL-mediated effect (16, 17). The present study minimizes the antibody component by using the well-characterized model of heterologous IAV challenge (21). The findings show clearly that giving α-GalCer with iIAV (H1N1) virus results in enhanced protection following respiratory challenge with a serologically distinct HKx31 (H3N2) IAV.
We found that α-GalCer diminished iIAV-induced effector CTL generation at the peak of the primary response while at the same time augmenting CD8+ memory T cell numbers. This finding was counterintuitive, as the size of the memory pool has been generally thought of as a direct reflection of the extent of clonal expansion during the initial primary response (29). It is known that α-GalCer increases IL-12 production (30) via NKT cell-dependent CD40 ligation (31) and induces the rapid maturation of CD8+ and CD8− DC subsets (22). Furthermore, early DC maturation can diminish acute CTL responses in virus infections (23). However, though some effect on DC phenotype was observed after α-GalCer + iIAV, it was only marginally different from that induced by iIAV alone. Furthermore, αGalCer resulted in efficient antigen presentation after iIAV vaccination. It thus seems unlikely that α-GalCer-induced activation of early DC maturation is the primary explanation for the diminished effector CTL response.
It is known that α-GalCer-dependent activation of NKT cells results in the release of a large number of cytokines, including IFN-γ. Interestingly, a recent report showed that α-GalCer promotes the IFN-γ-dependent expression of IDO, an enzyme that is important in tryptophan metabolism. Tryptophan is essential for optimal T cell proliferation, and IDO-induced tryptophan degradation results in the suppression of T cell expansion and more rapid apoptosis (32). Following iIAV, the concurrent administration of α-GalCer is associated with increased IFN-γ and IDO expression, indicating that the acute suppression of vaccine-induced CTL responses is dependent on NKT cell activation and IDO activity. This points to a natural role for NKT cell regulation in IAV-induced CTL responses that can be overcome by the inhibition of IDO catalytic activity. Given that lung resident NKT cells can be activated to produce a range of cytokines (33), it would be intriguing to determine what cytokines are produced during influenza A virus infection, and which, if any, are important in modulating responsiveness.
Both the level of inflammation and the antigen load during the initial priming phase are thought to be crucial for determining the size of the effector CTL response and the rate of memory T cell recall after virus challenge (34). This more rapid generation of CTL memory has been attributed to a relative lack of inflammatory signals (such as IFN-γ) in the absence of infection (35). Though α-GalCer does induce some production of proinflammatory cytokines, such as IFN-γ, this is transient, and the NKT cells are refractory to subsequent α-GalCer treatment (36). Perhaps α-GalCer causes just enough inflammation to optimize DC activation for efficient memory CTL generation. The fact that CD62Lhi IL7R+ DbNP366-specific CTLs are found in greater proportions at early time points in α-GalCer-treated mice supports this notion. Furthermore, bcl-2 expression within CTLs is enhanced after α-GalCer treatment, and the capacity of αGalCer to promote the TCM set while diminishing the effector CTL response reflects the known role of prosurvival genes such as bcl-2 in facilitating memory (37).
Given the influence of α-GalCer on both the acute and memory CTL responses to influenza, we speculate that NKT cells may be important regulators of CD8+ T cell-mediated immunity. In support of this, antigen-specific CTL responses were increased in magnitude after iIAV vaccination in NKT cell-deficient mice compared with wild-type mice. Interestingly, this did not extend to improved cytokine production on a per cell basis. We are currently examining the capacity of NKT cell activation to modulate cytotoxic capacity of virus-specific CTL. Thus, though the participation of NKT cells in host defense against infection is still controversial, it is now clear that the NKT population can function to promote (at least) vaccine responses that enhance protective CTL memory.
Overall, the present analysis indicates that adding α-GalCer to inactivated virus vaccines enhances protective T cell-mediated immunity. The current influenza immunization strategy depends totally on inducing a neutralizing antibody response, with the risk that any protective effect is lost if the virus mutates or there is the emergence of a new strain from wildlife reservoirs. Though vaccines have been produced against some of the H5N1 viruses that cause a low level of human infection (but with very high mortality), there is always the possibility that the virus will change or that the pandemic threat may be a novel H7N7 or H9N2 variant. Priming a cross-reactive CTL response directed at peptides from conserved internal proteins could never prevent such infections, but mouse experiments suggest that, if the level of memory is sufficiently high, the CTL response does function to moderate the severity of the disease process. Applications using α-GalCer as an adjuvant are already in clinical trial, so there is some justification for exploring this possibility with influenza.
Materials and Methods
Mice, Viruses, and Treatments.
Female C57BL/6J (B6, H-2b) and C57BL/6.CD1d−/− were bred in the animal facility of the Department of Microbiology and Immunology at the University of Melbourne (Parkville, Australia). The CD1d−/− mice were originally provided by L. Van Kaer (Vanderbilt University School of Medicine, Nashville, TN) and backcrossed to B6 for 10 generations. Naïve mice were anesthetized at 6 weeks of age by inhalation of (methoxyfluorane) penthrane and injected s.c. (100 μl) in the scruff of the neck with 7.5 μg of inactivated PR8 IAV vaccine (iIAV) with PBS alone or with 1 μg of α-GalCer adjuvant. For challenge experiments, mice were infected i.n with 104 pfu of the HKx31 (H3N2) IAV in 30 μl of PBS at least 6 weeks after the primary immunization. Virus stocks were grown in eggs. The PR8 virus was inactivated via formalin fixation and purified on a sucrose gradient by ultracentrifugation.
Adjuvants and Inhibitors.
1-Methyltryptophan (1-MT; Sigma-Aldrich) was dissolved in 1 M hydrochloric acid at a ratio of 1 g 1-MT in 10 ml of 1 M HCl, then diluted in drinking water at 5 mg/ml 1-MT in water. The pH was adjusted with 5 M sodium hydroxide solution. When administered to mice in drinking water at 5 mg/ml during 7 days, this gives a 2 × 10−3 mM 1-MT concentration in serum (38). α-GalCer, α-C-GalCer, and OCH were dissolved in 0.5% Tween 20 in PBS, which is used as a vehicle in all experiments.
Tissue Sampling and Cell Preparation.
Spleens, BAL, and lymph nodes were recovered from the vaccinated and virus-challenged mice. Spleens were enriched for CD8+ T cells by using anti-mouse IgG and IgM antibodies (Jackson ImmunoResearch).
Tetramer and Cytokine (ICS Assay) Staining.
Virus-specific CD8+ T cells were stained with APC-conjugated DbPA224 or DbNP366 tetramers, then with anti-mouse CD8α-allophycocyanin-Cy7 (57–6.7), anti-CD62L-fluorescein isothiocyanate (MEL-14), and anti-CD127-phycoerythrin (SB/199) mAbs (BD Biosciences Pharmingen).
Cells were stimulated with NP366 peptides in cRPMI medium containing 1 μg/ml GolgiPlug (BD Biosciences Pharmingen), washed, and stained with a PerCP-Cy5.5 conjugated mAb to CD8. Cells were fixed, permeabilized with BD Cytofix/Cytoperm Kit, stained with mAbs to IFN-γ (FITC), TNF-α (APC) and IL-2 (PE) (BD Biosciences Pharmingen), washed, and analyzed by flow cytometry.
For analysis of intracellular bcl-2 staining, splenic CD8+ T cells were isolated from mice 8 days after iIAV vaccination ± αGalcer, fixed, and permeabilized as above and stained with mAbs to murine bcl-2, CD8, and CD44. Cells were analyzed by flow cytometry.
Quantitative RT-PCR.
Total RNA was isolated using TRIzol (Invitrogen), and 10 μg of RNA was treated with DNase and reverse transcribed using an MMLV Reverse Transcriptase Kit (Invitrogen). Quantitative RT-PCR was performed using the Roche LightCycler 480 System. All qRT-PCR reactions were prepared in 10 μl with final concentrations of 1× LightCycler 480 Probes Master, 200 nM forward and reverse primers, and 100 nM Universal ProbeLibrary probe, using the following cycling conditions: 95 °C for 10 min; 45 cycles of 95 °C (10 sec) and 60 °C (30 sec); and 40 °C 1 min to cool. Direct detection of PCR products was monitored by measuring increase in fluorescence. Relative expression (AU, arbitrary units) was calculated using the 2−ΔΔCt method.
Lung Virus Titration.
Mice were killed by cervical dislocation and the lungs were removed and homogenized for virus titration by plaque assay on MDCK cells. Near-confluent 25 cm2 monolayers were infected with serial dilutions of lung homogenate for 1 h at 37 °C, then washed with PBS and 3 ml of MEM containing 1 mg/ml L-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin (Worthington). Agarose (0.8%) was added and the cultures were incubated at 37 °C under 5% CO2 atmosphere for 72 h. Plaques were then enumerated.
Statistical Analysis.
Results are expressed as mean ± SD, statistical significance was evaluated using the Mann-Whitney rank sum U test, and P values ≤0.05 were considered significant.
Supplementary Material
Acknowledgments.
This work was supported by a National Health and Medical Research Council (NHMRC) Project Grant 499455 (to S.J.T, D.I.G, and I.G.B.). C.G. is supported by a fellowship from the Sixth Framework Programme of the European Union, Marie Curie (040840), and by the Fondation pour la Recherche Médicale. P.C.D was supported by NHMRC Project Grant 454595 and National Institutes of Health Grant AI70251; D.I.G. by an NHMRC Principal Research Fellowship, S.J.T. by a Pfizer Senior Research Fellowship; J.D.M. by a CJ Martin Fellowship; and F.-X.H. by a Marie Curie Fellowship (040998). G.S.B. acknowledges the Medical Research Council (G9901077 and G0500590) and The Wellcome Trust (081569/2/06/2). The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Aging.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813309106/DCSupplemental.
References
- 1.de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. 2000;61:94–99. [PubMed] [Google Scholar]
- 2.Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 3.Bender BS, Croghan T, Zhang L, Small PA., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty PC, Riberdy JM, Belz GT. Quantitative analysis of the CD8+ T-cell response to readily eliminated and persistent viruses. Philos Trans R Soc Lond B Biol Sci. 2000;355:1093–1101. doi: 10.1098/rstb.2000.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty PC, Kelso A. Toward a broadly protective influenza vaccine. J Clin Invest. 2008;118:3273–3275. doi: 10.1172/JCI37232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee LY, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118:3478–3490. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty PC, et al. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 8.Deliyannis G, et al. Immunopotentiation of humoral and cellular responses to inactivated influenza vaccines by two different adjuvants with potential for human use. Vaccine. 1998;16:2058–2068. doi: 10.1016/s0264-410x(98)00080-2. [DOI] [PubMed] [Google Scholar]
- 9.Singh M, O'Hagan D. Advances in vaccine adjuvants. Nat Biotechnol. 1999;17:1075–1081. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 10.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 11.Hong S, et al. Lipid antigen presentation in the immune system: Lessons learned from CD1d knockout mice. Immunol Rev. 1999;169:31–44. doi: 10.1111/j.1600-065x.1999.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 12.Kronenberg M. Toward an understanding of NKT cell biology: Progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey DI, Kronenberg M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 15.Galli G, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko SY, et al. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175:3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 17.Youn HJ, et al. A single intranasal immunization with inactivated influenza virus and alpha-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine. 2007;25:5189–5198. doi: 10.1016/j.vaccine.2007.04.081. [DOI] [PubMed] [Google Scholar]
- 18.Ho LP, et al. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur J Immunol. 2008;38:1913–1922. doi: 10.1002/eji.200738017. [DOI] [PubMed] [Google Scholar]
- 19.Renfrey S, Watts A. Morphological and biochemical characterization of influenza vaccines commercially available in the United Kingdom. Vaccine. 1994;12:747–752. doi: 10.1016/0264-410x(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Ann Rev Immun. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 21.Flynn KJ, et al. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 22.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson NS, et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 24.Mellor AL, Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nature Rev. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 25.Fallarini S, Paoletti T, Panza L, Lombardi G. Alpha-galactosylceramide modulates the induction of indoleamine 2,3-dioxygenase in antigen presenting cells. Biochem Pharmacol. 2008;76:738–750. doi: 10.1016/j.bcp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Aseguinolaza G, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung Y, Chang WS, Kim S, Kang CY. NKT cell ligand alpha-galactosylceramide blocks the induction of oral tolerance by triggering dendritic cell maturation. Eur J Immunol. 2004;34:2471–2479. doi: 10.1002/eji.200425027. [DOI] [PubMed] [Google Scholar]
- 29.Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura H, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallarino F, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 33.Akbari O, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 34.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 35.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 36.Uldrich AP, et al. NKT cell stimulation with glycolipid antigen in vivo: Costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 38.Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.