Abstract
It is widely appreciated that neurotransmission systems interact in their effects on human cognition, but those interactions have been little studied. We used genetics to investigate pharmacological evidence of synergisms in nicotinic/muscarinic interactions on cognition. We hypothesized that joint influences of nicotinic and muscarinic systems would be reflected in cognitive effects of normal variation in known SNPs in nicotinic (CHRNA4 rs1044396) and muscarinic (CHRM2 rs8191992) receptor genes. Exp. 1 used a task of cued visual search. The slope of the cue size/reaction time function showed a trend level effect of the muscarinic CHRM2 SNP, no effect of the nicotinic CHRNA4 SNP, but a significant interaction between the 2 SNPs. Slopes were steepest in individuals who were both CHRNA4 C/C and CHRM2 T/T homozygotes. To determine the specificity of this synergism, Exp. 2 assessed working memory for 1–3 locations over 3 s and found no significant effects on either SNP. Interpreting these results in light of Sarter's [Briand LA, et al. (2007) Modulators in concert for cognition: Modulator interactions in the prefrontal cortex. Prog Neurobiol 83:69–91] claims of tonic and phasic modes of cholinergic activity, we argue that reorienting attention to the target after invalid cues requires a phasic response, dependent on the nicotinic system, whereas orienting attention to valid cues requires a tonic response, dependent on the muscarinic system. Consistent with that, shifting and scaling after valid cues (tonic) were strongest in CHRNA4 C/C homozygotes who were also CHRM2 T/T homozygotes. This shows synergistic effects within the human cholinergic system.
Keywords: cholinergic, genetics, synergism, cognition
The role played by neurotransmitters in human cognitive functioning has been much investigated in recent years, most notably in selective attention (1, 2), working memory (3), and vigilance (4). It is widely appreciated that neurotransmitters do not always act independently of one another, yet interactive effects on cognitive functions, either between or within neurotransmission systems, are not well understood. Specifically, ACh has both synergistic and antagonistic interactions with other neurotransmitters. Synergistic interactions between cholinergic and NMDA receptors are claimed to play a role in cortical plasticity (5), and the cholinergic and noradrenergic systems appear to have both synergistic and antagonistic effects associated with cue-related uncertainty (6). There are also interactions within the cholinergic system. Both fast-acting nicotinic receptors and slow-acting muscarinic receptors influence the efficiency of shifting visuospatial attention. Nicotine weakens the effect of cue validity in the Posner cued-detection task (7) by speeding responses to invalidly-cued targets in both rats (8) and humans (2, 9). In contrast, the muscarinic antagonist scopolamine selectively slows responses to invalidly-cued targets in rats (8) and monkeys (10).
Cholinergic drugs modulate cognition-related brain activation patterns. Furey et al. (11) found that cholinergic potentiation by physostigmine eliminated the increase in prefrontal blood flow seen with greater memory load. They argued that the cholinergic agonist did not influence memory per se, but rather reduced the need to recruit prefrontal regions with greater task demands. They also found that cholinergic enhancement or inhibition influenced ability to maintain selective attention on the cued category of target, but did not alter the ability to shift category (12). A different group (13) observed that muscarinic effects on blood flow were strongest in primary and association visual cortices, whereas nicotinic effects were strongest in inferior parietal regions. Interactive effects of muscarinic and nicotinic drugs on cognition have also been reported for visual discrimination (14) and working memory tasks (15, 16). Performance was poorer after muscarinic antagonism with scopolamine, but was unaffected after nicotinic antagonism with mecamylamine. However, simultaneous antagonism using both drugs produced an interaction, with deficits greater on several tasks after combined administration compared with either drug alone (16).
Pharmacological methods have some limitations for investigating the role of nicotinic-muscarinic interactions in attention and working memory. Cholinergic antagonists have known sedative and peripheral autonomic effects, e.g., increases in pupil size and changes in blood pressure and pulse (17). Although these changes could contribute to cognitive effects, Ellis et al. (16) used the critical flicker fusion test to control for sedative effects.
As an alternative to investigating interactive influences of neurotransmission systems on cognition using pharmacology, endogenous effects related to normal variation in genes controlling receptors can be measured. SNPs in a gene can alter the gene's protein production. Each receptor type is controlled by at least 1 gene, and the heteromeric nicotinic receptors are composed of subunits, each controlled by a different gene (18). The genetic association approach investigates influences of normal genetic variation on cognition (19, 20). Most studies of cognitive genetics, however, have examined the association between variation in 1 gene and (typically) 1 cognitive test (e.g., refs. 21 and 22). A single gene approach may be too limiting when investigating cognitive functions that almost certainly are influenced by >1 neuromodulator. Accordingly, our study investigated the separate and combined effects of nicotinic and muscarinic SNPs on attention and working memory.
Nicotinic receptors are composed of both α and β subunits assembled together to form the most common cortical cholinergic nicotinic receptor (23). Some SNPs in both α4 and β2 subunit genes have been shown to reduce receptor response to extracellular Ca2+ (24), resulting in increased affinity of the receptor for ACh. One SNP in the CHRNA4 gene encoding the α4 subunit of the nicotinic receptor rs1044396 T/C [previously termed 1545 T/C (25)] modulates vulnerability to nicotine addiction (26) and visuospatial attention (27, 28), but not working memory (28). Specifically, in a cued-attention shifting task (29), we found increasing dose of the C allele was associated with increased benefits of valid cues and reduced costs of invalid cues. We observed no effects on working memory (28). In a cued-attention scaling task (30), we found increasing dose of the C allele associated with heightened dependence on cues that strengthened scaling effects (27).
The behavioral effects of this CHRNA4 nicotinic SNP are reflected in neuroimaging findings. During a visual “oddball” task in which a rare stimulus is detected, differential fMRI blood-oxygen-level-dependent (BOLD) responses were observed over left parietal cortex, with the strongest response in T/T homogygotes and the weakest response in C/C homogygotes (31). Also in oddball tasks, T allele carriers had higher amplitude auditory N1 and visual P1 components compared with noncarriers (32). Thus, the C allele of this CHRNA4 SNP is associated with both greater effects of valid, precise cues and reduced brain activity. The combination of greater cue effects and reduced brain activity could be interpreted as a sign of heightened neural efficiency (33) underlying cue-directed shifts of attention.
There are also cognitive consequences of normal variation in muscarinic genes. The M2 muscarinic receptor is of particular interest as genomewide scans have revealed an association between IQ and the CHRM2 gene (34–36). A known SNP in the CHRM2 gene controlling the M2 receptor (rs8191992 A/T, previously termed 1890 A/T) has been linked to IQ (37, 38) and to EEG oscillations evoked in a visual oddball task (39).
We hypothesized that synergistic effects of nicotinic and muscarinic systems would be reflected in effects of normal variation in nicotinic and muscarinic receptor genes on attentional scaling. This hypothesis was based on evidence of interactions between muscarinic and nicotinic systems in drug studies (16, 17) and evidence (reviewed above) that variation in a muscarinic gene modulates IQ and variation in a nicotinic gene modulates ability to adjust the scale of the attentional focus. To test this hypothesis we selected the SNP in the muscarinic gene CHRM2 (rs8191992 A/T) linked to IQ and the SNP in the nicotinic gene CHRNA4 (rs1044396 C/T) linked to attentional scaling (40, 41) and used them to investigate effects of nicotinic–muscarinic interactions on attentional scaling in a sample of healthy adults.
Exp. 1
Results.
Neuropsychological performance.
Demographic and neuropsychological data are presented in Table S1 for the combinations of SNPs. The only statistically significant difference was that the CHRNA4 C/T heterozyotes were marginally younger on average than the other groups (P = 0.08). The genotype groups did not differ on Wechsler Adult Intelligence Scale (WAIS) vocabulary or Wechsler Memory Scale (WMS)-delayed. The WMS-immediate score was significantly higher in the CHRM2 A/T heterozyotes [F(2,397) = 3.55].
Search performance.
Test-retest analysis.
To assess reliability of performance on this cued visual search task, 74 individuals underwent retesting within 1 week of the initial test. The test–retest correlation of cue size/reaction time (RT) slope was 0.51 for conjunction search.
Interaction of CHRNA4 and CHRM2.
Performance on the cued visual search task can be quantified as the slope of RT as a function of cue size. Slopes of RT/cue size functions were calculated for each participant from the RT data under feature and conjunction search. Slopes were analyzed in a repeated measures ANOVA with between-subjects factors of CHRNA4 genotype (C/C, C/T, T/T) and CHRM2 genotype (A/A, A/T, T/T). The within-subjects factor was task type (feature, conjunction search).
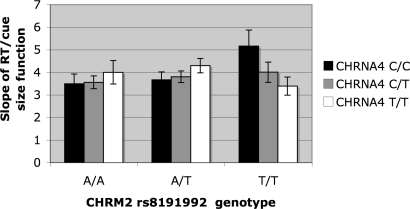
Search slopes did not vary significantly with either SNP, although the main effect of CHRM2 was a nonsignificant trend (P = 0.07). There was an interaction between the 2 SNPs [F(4,397) = 2.52, η2 = 0.03] that can been seen in Fig. 1, which shows that slopes increased slightly with copy number of CHRNA4 T alleles (0, 1, 2) in both the CHRM2 AA and A/T genotypes. However, in CHRM2 T/T homozygotes, slopes decreased with number of CHRNA4 T alleles. Overall slopes were highest in individuals who were both CHRNA4 C/C and CHRM2 T/T homozygotes. With regard to within-subjects effects, slopes were steeper on conjunction compared with feature search [F(1,397) = 705.61]. The interaction between CHRNA4 and task type was also significant [F(2,397) = 3.06]. This result is attributable to shallower slopes for heterozygotes on conjunction search but steeper slopes for heterozygotes on feature search. The 3-way interaction was not significant.
Fig. 1.
Exp. 1. Interaction of effect of CHRM2 rs8191992 and CHRNA4 1044396 genotypes on cued visual search RT/cue size slope.
Discussion.
As hypothesized, SNPs in CHRNA4 and CHRM2 genes exerted synergistic effects on attentional scaling. We found a trend level effect of the muscarinic SNP (CHRM2) on performance, no effect of the nicotinic SNP (CHRNA4), but a significant interaction when effects of both SNPs were considered together.
These findings are consistent with previous pharmacological evidence that nicotinic and muscarinic drugs had greater effects when administered together than separately on declarative memory (17) and working memory tasks (15). In Green et al. (15) a nicotinic antagonist (mecamylamine) did not affect performance on an N-back working memory task, whereas a muscarinic antagonist (scopolamine) did. When the 2 antagonists were administered together, there was a greater effect on performance than when scopolamine was administered alone (15). Effects of genetic variation are more specific than drug effects. In contrast to the nonselective effects of scopolamine [attributed to blockade of postsynaptic M1 and presynaptic M2 and M4 receptors (42)], we assessed only the effect of variation in M2. The present results thus provide more specific evidence for the existence of synergistic nicotinic–muscarinic interactions in attention and working memory.
The present results extend our previous findings of steeper slopes in CHRNA4 rs1044396 SNP C allele carriers on the same task (27). We here report similar results, but only in C allele carriers who were also CHRM2 rs8191992 T allele carriers. We recently reported similar results in a flanker task. Posner's Attention Network Task (ANT) flanker task (43) requires discrimination of the direction of a central arrow flanked on each side by arrows that are either congruent (same direction) or incongruent (opposite direction). The difference in RT between congruent and incongruent trials is considered a measure of executive attention. We found that people with both the CHRM2 T allele and the CHRNA4 C allele had greater differences between congruent and incongruent RTs than other combinations of genotypes that did not show an orderly pattern. That similar nicotinic–muscarinic interactions were seen in both the ANT and the scaling task of the present study suggests overlapping task demands. On incongruent trials of the ANT, successful performance requires that processing of the incongruous flankers be inhibited. That inhibition may be achieved by flexible adjustment of the scale of attention around the target, similar to adjustment of the scale of attention around the target in the cued search task of the present study. The combination of CHRM2 T allele and CHRNA4 C allele appears to heighten effects of stimuli in controlling attention, greater effect of cues in the cued search task of the present study and greater effect of flankers in the ANT of our recent report.*
The present results suggest synergy normally exists between muscarinic and nicotinic systems. Although we found synergistic nicotinic and muscarinic gene effects on attention, synergistic drugs effects have been reported for declarative memory, working memory, and vigilance (16), suggesting fairly broad effects on cognition. To determine whether the synergistic effects of nicotinic and muscarinic genes is similarly broad, we assessed their separate and combined effects on a working memory task.
Exp. 2
Exp. 1 observed synergistic effects of nicotinic and muscarinic SNPs on cued visual search. As discussed above, synergistic effects of nicotinic and muscarinic antagonists have been seen on several tasks, iconic memory, vigilance, and working memory (15, 16). Effects of receptor variation are generally more specific than effects of drug variation. If the synergistic effect of the 2 SNPs on cued visual search is specific to attention, it should not be evident on a task of working memory.
Results.
Neuropsychological data.
Demographic and neuropsychological data are presented in Table S2 for the combinations of SNPs. The only statistically significant difference was that the CHRNA4 T/T homozygotes were slightly older on average than the other genotype groups [F(2,429) = 4.47]. No other effects were significant and the genotype groups did not differ on WAIS vocabulary, WMS-immediate, or WMS-delayed. The MiniMental State Examination was compared across genotype groups for the 29% of the participants who were older. The means were essentially the same, ranging from 28 to 29 of 30 across genotype groups.
Accuracy data: Interaction of CHRNA4 and CHRM2.
Accuracy of memory for location after a 3-s delay was analyzed in a repeated measures ANOVA. The between-subjects factors were rs1044396 CHRNA4 genotype (C/C, C/T, T/T) and rs819199 CHRM2 genotype (A/A, A/T, T/T). The within-subjects factor was location number (nos. 1, 2, and 3). Consistent with our previous reports on this task (27, 28), accuracy decreased with location number [F(2,858) = 311.12]. There were no effects of genotype or other significant effects.
Discussion.
In contrast to the interaction we observed in the attention task in Exp. 1, there were no main effects of either the nicotinic or the muscarinic SNP on working memory performance, which is consistent with our previous findings with the nicotinic SNP and working memory (28).† Based on primate work showing the basal forebrain cholinergic system mediates attention rather than memory (44, 45), Robbins et al. (46) have argued that the cholinergic system is only important for working memory under conditions of attentional load. Nevertheless, effects of cholinergic agents on memory have been seen in rodents (47), even when attention is carefully controlled (48). Consistent with the literature on primates, our human work has revealed that when there is no attentional manipulation variation in cholinergic SNPs does not modulate working memory performance (28), but when there is an attentional manipulation of working memory (27) variation in cholinergic SNPs does modulate working memory performance (28, 49). The present findings indicate that nicotinic and muscarinic systems interact in influencing attention but not working memory, which suggests a degree of specificity in the effects of nicotinic and muscarinic receptor function on cognition.
General Discussion
We observed synergistic effects of normal variation in nicotinic and muscarinic cholinergic receptor genes on attention but not on working memory. The present findings are consistent with previous pharmacological work (15, 16) in that we observed synergistic effects on cognition, but they are also inconsistent in that we observed effects on attention but not working memory. The present study adds to what is known about the effect of normal genetic variation on cognition (19) by showing selective synergistic effects of nicotinic–muscarinic receptor efficiency on attention but not working memory.
The present results extend our previous reports that cognition is modulated by the nicotinic CHRNA4 (rs1044396) SNP. We previously observed that the C allele of this SNP was associated with greater benefits of valid cue location on discrimination speed (28) and greater effects of precue size on search speed (27, 28). Using this much larger sample, we have again observed the CHRNA4 C allele to be associated with the strongest effects of cue size, but only in CHRM2 rs8191992 T/T homozygotes. In the other genotypes, there was a slight benefit for the T allele (Fig. 1). We have observed a similar pattern with the same 2 SNPs on a flanker paradigm in Posner's ANT task (43) where the difference between congruent and incongruent conditions was greatest in CHRM2 T/T individuals with the CHRNA4 C allele (44). It appears that nicotinic and muscarinic receptor systems normally interact in modulating visuospatial attention, with at least somewhat selective synergism.
What is the basis at the receptor level for the observed effect of cholinergic SNPs on cognition? The CHRNA4 gene controls the α4 subunit of the common α4/β2 neuronal nicotinic cholinergic receptor. In a heritable form of epilepsy, CHRNA4 mutations produce nicotinic receptors with heightened sensitivity to ACh (50). Hoda et al.‡ assessed the electrophysiology of human CHRNA4 haplotypes, including the CHRNA4 rs1044396 SNP. They concluded that the haplotype containing the C allele differed from the haplotype containing the T allele by the numbers of receptors in the high-affinity state. Therefore, altered receptor responsiveness could underlie the behavioral differences we observed associated with that SNP. Although all of the SNPs in the CHRNA4 haplotype identified by Hoda et al.‡ appear to be synonymous (do not alter the protein product of the gene), such SNPs can nevertheless exert effects by altering protein folding and enzyme activity (51). It is also possible that the CHRNA4 rs1044396 SNP is inherited together with another, as yet unidentified, SNP.
What are the implications of cholinergic receptor variation for cortical function? Compared with the C allele, the T allele of CHRNA4 rs1044396 SNP has been found to be protective against nicotine addiction (26) and linked to reduced effects of cues on attention (27). Two recent studies indicate that these behavioral traits are associated with differences in cortical function. Winterer et al. (31) observed greater fMRI BOLD responses during an oddball task in CHRNA4 rs1044396 T/T homozygotes over anterior cingulate and parietal cortex compared with C/C homozygotes. Moreover, a gene dose effect was seen over left parietal cortex, with the heterozygote activation at intermediate levels compared with greater activation in T/T and lower in C/C homozygotes (31). What is the meaning of differential activation levels? Rypma et al. (33) reported that faster-performing people show less prefrontal but greater parietal activation, arguing that such individuals require less top-down executive control (33, 52). Measuring event-related potentials (ERPs) in an oddball task, Espeseth et al. (32) found CHRNA4 rs1044396 T allele carriers had higher amplitude auditory N1 and visual P1 components than noncarriers. That CHRNA4 rs1044396 T allele carriers exhibit heightened cortical responsiveness by using both blood flow and electrophysiological measures are consistent with our behavioral findings that the T allele was associated with greater costs of invalid cues (28, 53) and weaker effects of valid cue size (27). The present study extends that work by showing weaker effects of cue size on search in CHRNA4 T allele carriers and stronger effects in C allele carriers, but only in CHRM2 T/T homozygotes.
In terms of brain measures, the T allele of that SNP has been associated with an increase in regional brain activity to an unexpected target (oddball task), in both fMRI (31) and early ERP components (32). In terms of cognitive measures, the T allele is associated with greater slowing of responses when cues are invalid (28) and weaker effects of predictable cues (27). These cognitive and brain effects suggest that T allele carriers experience greater reactivity to unexpected events, possibly because of greater sensitivity of the nicotinic receptors to ACh.
However, the present study shows that the nicotinic system should not be considered in isolation. What underlies the apparent interaction? Based on evidence that cue validity effects vary inversely with levels of nicotine or ACh in attentional cuing tasks (8, 9, 54), Hasselmo (55) recently considered the interaction between nicotinic and muscarinic effects in the context of Yu and Dayan's model of the neuromodulation of uncertainty (6). In that model, ACh signals “expected uncertainty” about an information source, e.g., the known unreliability of unpredictable cues in the Posner directed attention task. The Yu and Dayan model was designed to accommodate evidence that the cue validity effect varies inversely with ACh level in rodents and primates (8, 9) and, as we have shown (56), in patients with Alzheimer's disease who have depleted cortical ACh levels. Hasselmo's physiological data reveal the role of muscarinic and nicotinic receptors in cuing effects. Through muscarinic receptors, ACh induced presynaptic suppression of excitatory glutamatergic potentials in feedback circuits evoked by cortical layer I stimulation without affecting afferent and feedforward synapses in layer IV (57). Through nicotinic receptors, ACh heightened thalamocortical transmission of new sensory input (58). In Hasselmo's view, top-down cue effects would be dampened by muscarinic inhibition, whereas bottom-up sensory input target effects would be heightened by nicotinic receptor enhancement of thalamocortical transmission (55). This view is consistent with the above-summarized findings that the CHRNA4 T allele heightens bottom-up sensory effects of unexpected stimuli (28, 31, 32), but not of expected stimuli (27). Although our explanation is speculative, it seems likely that a well-coordinated interaction exists between nicotinic and muscarinic receptors, given previous pharmacological, and now genetic, evidence of synergism.
What synaptic mechanism underlies the effect of variation in M2 receptors seen in the present results? M2 is the primary muscarinic autoreceptor presynaptically regulating ACh release in the forebrain of rodents and primates (59, 60). Therefore, varying efficiency in the M2 receptor caused by variation in the CHRM2 gene could selectively influence muscarinic presynaptic inhibition. Other muscarinic receptors could also play a role. M4 receptors are also largely presynaptic (61). However, because M1 receptors act as postsynaptic heteroreceptors onto glutamatergic synapses (62), any role for M1 receptors controlling ACh release would likely be indirect. The specificity with which CHRM2 modulates attention should be examined in future genetic work.
The present findings of nicotinic/muscarinic synergism can also be considered in the context of Parikh et al.'s (63) recent argument for the existence of 2 modes of cholinergic activity, each exerting unique effects on cognition. Based on microelectrode recordings of the real time course of extracellular ACh release, Briand et al. (68) propose that a slow-acting tonic mode influences background arousal, whereas a fast-acting phasic mode mediates cue detection and attentional performance. Although M2 autoreceptors are known to modulate ACh release, the method developed by Parikh et al. does not allow assignment of the modulation or influence of the ACh release to presynaptic or postsynaptic effects. We can extend these arguments to speculate that the nicotinic system could control the phasic mode through its fast-acting receptors in response to thalamocortical sensory input, whereas the muscarinic system could control the tonic mode through its slower-acting receptors. Consistent with Briand et al. (64), invalid cuing would demand a phasic response, dependent on the excitatory (5) fast-acting nicotinic receptors (65), by requiring a rapid shift to target onset (bottom-up) from the invalidly cued location.
That CHRNA4 T allele carriers show selectively slowed RT after invalid cues (28, 53), and greater cortical responsiveness (31, 32) suggests the T allele of this CHRNA4 SNP produces a more sensitive receptor. However, shifting and scaling after valid cues, as in the present study, would require a more tonic response and therefore depend more on the muscarinic system. Consistent with that idea, we found that scaling after valid cues was strongest in CHRNA4 C allele carriers who were also CHRM2 T/T homozygotes. We speculate that the CHRNA4 C allele receptor is less responsive to phasic sensory input, whereas the CHRM2 T allele receptor is more responsive to tonic effects of precuing. If this explanation were correct, then the strongest effect of unpredictable stimuli would be seen in individuals with the CHRNA4 T/T and CHRM2 C/C genotype.
We acknowledge the limited nature of our data on nicotine use and on menstrual cycle phase in young women. Both of these factors could alter the results in a subset of our sample.
Materials and Methods
Exp. 1.
Participants.
Healthy individuals ranged in age from 18 to 90 years and were recruited from the greater Washington, DC, area (n = 406). All procedures were approved by institutional review boards and performed in accordance with the 1964 Declaration of Helsinki. All persons gave informed consent before their inclusion in the study. Vision was tested to ensure at least 20/30 acuity (after correction) on a Rosenbaum pocket screener. All participants were screened by questionnaire for neurologic and psychiatric disorders and screened cognitively with the WAIS vocabulary subtest (66) and the WMS subtest (67). This study was originally designed to assess gene–gene interactions in the context of healthy aging. Although there were effects of age on the standardized neuropsychological tests, there were no interactions with genotype whether individuals were grouped by CHRNA4 rs1044396 C/T or CHRM2 rs8191992A/T genotype. To investigate this further, we analyzed the experimental data by using between-subjects factors of CHRM2, CHRNA4, and age group. The 2-way interaction of CHRM2 × CHRNA4 remained significant [F(4,388) = 3.2] but the 3-way interaction of CHRM2 × CHRNA4 x age was not significant. This analysis must be considered preliminary, because 2 of the cells had only 7 people. Based on these analyses, age effects were not analyzed further. To eliminate individuals with a dementing illness, participants aged 65 and older (29% of participants) were screened with the MiniMental State Examination. Means ranged from 28 to 29 and did not vary across genotype groups.
Reanalysis of Exp. 1 data without the 5% of individuals reporting nicotine use did not change the results. Regarding the effect of endogenous hormones, we did not attempt to control the phase of the menstrual cycle in the subset of participants who were young women. We reanalyzed Exp. 1 data by using only the 192 young women. We observed the same pattern of steeper slopes in the combination of CHRM2 T/T, CHRNA4 C carriers that we observed in our full sample. With the smaller sample (1 of the cells was <10) the CHRM2 × CHRNA4 interaction was not significant.
Genotyping.
Both SNPs were in Hardy-Weinberg equilibrium (P > 0.05 in χ2 tests). See SI Text.
Stimuli and Procedures.
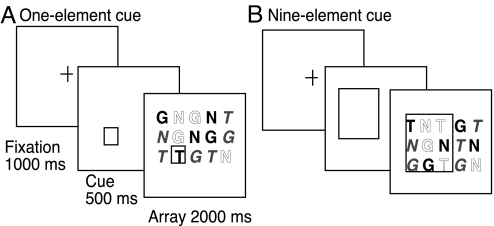
A spatially cued visual search task was used (Fig. 2). This task required speeded search for a pink T target whose location was cued in advance. The target was embedded in a 6.3 ×× 4.2° array of 15 uppercase letters (3 rows, 5 letters each). The 1.5° letters were pink, blue, and green (T, N, G), and their location was randomly determined. After a 1,000-ms fixation cross, target location was precued (500-ms stimulus onset asynchrony) with a rectangle enclosing 1, 3, 9, or 15 letters. On 83% of trials the target appeared inside the randomly-placed cue, although on 17% (catch trials) the target was absent. Both cue and target remained visible until the speeded button press response indicating target presence/absence occurred or 2.5 s elapsed. There were 2 search conditions: (i) easy (feature) search in which was target letter “popped out” from the distractors as the only pink letter, and (ii) hard (conjunction) search in which the target properties of color and form appeared together only in the target but separately with equal frequency among the distractors.
Fig. 2.
Exp. 1. Cued visual search task. Cued visual search required for a pink T target embedded randomly in an array of pink, blue, green T, G, N letters (pink represented by bolding, blue by outline, green by italics). (A) Cue precisely indicates the pink (bold) T target of search. (B) Cue imprecisely indicates the pink (bold) T target of search by including distractors inside the cue.
Exp. 2.
Participants.
Participants (n = 438) were drawn from the same pool of individuals described in Exp. 1. The neuropsychological and demographic characteristics of this group are shown in Table S2.
Stimuli and procedures.
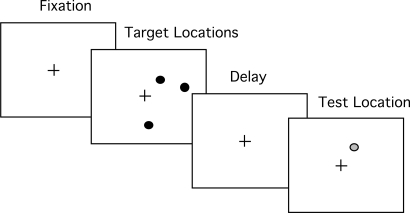
A spatial working memory task (68) was used, illustrated in Fig. 3. After a fixation cross for 1 s, 1, 2, or 3 (0.67°) black dots, each indicating a target location appeared at randomly chosen screen locations for 500 ms. At dot offset, the fixation cross reappeared for 3 s. At the end of the delay, a single red test dot appeared alone. This test dot appeared either at the same location as 1 of the target dots (match condition) or at a different location (nonmatch condition). On nonmatch trials, the distance between the correct location and the test dot varied over 3 levels, being ≈2°, 4°, or 8° of visual angle. Participants had 2 s to decide whether the test dot location matched 1 of the target dots. Accuracy was the measure of interest.
Fig. 3.
Exp. 2. Working memory task. One, 2, or 3 target locations had to be retained over a 3-s delay. Remembered target location was compared with test location.
Genotyping.
Procedures were as described in Exp. 1.
Supplementary Material
Acknowledgments.
This work was supported by Virginia Center on Aging Grant Alzheimer's and Related Diseases Research Award Fund 06-2 (to P.M.G.) and National Institute on Aging Grant AG19653 (to R.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807891106/DCSupplemental.
Squire PN, et al., Society for Neuroscience Meeting, November 3–7, 2007, San Diego, CA.
Greenwood P, et al., Society for Neuroscience Meeting, October 14–18, 2006, Atlanta, GA.
Hoda J-C, et al., Society for Neuroscience Meeting, November 3–7, 2007, San Diego, CA.
References
- 1.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 2.Shirtcliff EA, Marrocco RT. Salivary cotinine levels in human tobacco smokers predict the attentional validity effect size during smoking abstinence. Psychopharmacology. 2003;166:11–18. doi: 10.1007/s00213-002-1293-x. [DOI] [PubMed] [Google Scholar]
- 3.Abi-Dargham A, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarter M, Bruno JP. Developmental origins of the age-related decline in cortical cholinergic function and associated cognitive abilities. Neurobiol Aging. 2004;25:1127–1139. doi: 10.1016/j.neurobiolaging.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 6.Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 8.Phillips JM, McAlonan K, Robb WG, Brown VJ. Cholinergic neurotransmission influences covert orientation of visuospatial attention in the rat. Psychopharmacology. 2000;150:112–116. doi: 10.1007/s002130000437. [DOI] [PubMed] [Google Scholar]
- 9.Witte EA, Davidson MC, Marrocco RT. Effects of altering brain cholinergic activity on covert orienting of attention: Comparison of monkey and human performance. Psychopharmacology. 1997;132:324–334. doi: 10.1007/s002130050352. [DOI] [PubMed] [Google Scholar]
- 10.Davidson MC, Marrocco RT. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. J Neurophysiol. 2000;83:1536–1549. doi: 10.1152/jn.2000.83.3.1536. [DOI] [PubMed] [Google Scholar]
- 11.Furey ML, Ricciardi E, Schapiro MB, Rapoport SI, Pietrini P. Cholinergic enhancement eliminates modulation of neural activity by task difficulty in the prefrontal cortex during working memory. J Cognit Neurosci. 2008;20:1342–1353. doi: 10.1162/jocn.2008.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mentis MJ, et al. Muscarinic versus nicotinic modulation of a visual task: A pet study using drug probes. Neuropsychopharmacology. 2001;25:555–564. doi: 10.1016/S0893-133X(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 14.Erskine FF, et al. Evidence for synergistic modulation of early information processing by nicotinic and muscarinic receptors in humans. Hum Psychopharmacol. 2004;19:503–509. doi: 10.1002/hup.613. [DOI] [PubMed] [Google Scholar]
- 15.Green A, et al. Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Biochem Behav. 2005;81:575–584. doi: 10.1016/j.pbb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Ellis JR, et al. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175–189. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- 17.Little JT, Johnson DN, Minichiello M, Weingartner H, Sunderland T. Combined nicotinic and muscarinic blockade in elderly normal volunteers: Cognitive, behavioral, and physiologic responses. Neuropsychopharmacology. 1998;19:60–69. doi: 10.1016/S0893-133X(98)00002-5. [DOI] [PubMed] [Google Scholar]
- 18.Flores CM, et al. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- 19.Greenwood PM, Parasuraman R. Normal genetic variation, cognition, and aging. Behav Cognit Neurosci Rev. 2003;2:278–306. doi: 10.1177/1534582303260641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cognit Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Hariri AR, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattay VS, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. Neuronal nicotinic receptor expression in sensory neurons of the rat trigeminal ganglion: Demonstration of α3β4, a novel subtype in the mammalian nervous system. J Neurosci. 1996;16:7892–7901. doi: 10.1523/JNEUROSCI.16-24-07892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues-Pinguet N, et al. Five ADNFLE mutations reduce the Ca2+ dependence of the α4β2 acetylcholine response. J Physiol (London) 2003;550:11–24. doi: 10.1113/jphysiol.2003.036681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinlein OK, et al. Neuronal nicotinic acetylcholine receptor α4 subunit (CHRNA4) and panic disorder: An association study. Am J Med Genet. 1997;74:199–201. doi: 10.1002/(sici)1096-8628(19970418)74:2<199::aid-ajmg17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 26.Feng Y, et al. A common haplotype of the nicotine acetylcholine receptor α4 subunit gene is associated with vulnerability to nicotine addiction in men. Am J Hum Genet. 2004;75:112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwood PM, Fossella JA, Parasuraman R. Specificity of the effect of a nicotinic receptor polymorphism on individual differences in visuospatial attention. J Cognit Neurosci. 2005;17:1611–1620. doi: 10.1162/089892905774597281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parasuraman R, Greenwood PM, Kumar R, Fossella J. Beyond heritability: Neurotransmitter genes differentially modulate visuospatial attention and working memory. Psychol Sci. 2005;16:200–207. doi: 10.1111/j.0956-7976.2005.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- 30.Eriksen CW, St James JD. Visual attention within and around the field of focal attention: A zoom lens model. Percept Psychophys. 1986;40:225–240. doi: 10.3758/bf03211502. [DOI] [PubMed] [Google Scholar]
- 31.Winterer G, et al. Association of attentional network function with exon 5 variations of the CHRNA4 gene. Hum Mol Genet. 2007;16:2165–2174. doi: 10.1093/hmg/ddm168. [DOI] [PubMed] [Google Scholar]
- 32.Espeseth T, Endestad T, Rootwelt H, Reinvang I. Nicotine receptor gene CHRNA4 modulates early event-related potentials in auditory and visual oddball target detection tasks. Neuroscience. 2007;147:974–985. doi: 10.1016/j.neuroscience.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Rypma B, et al. Neural correlates of cognitive efficiency. NeuroImage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 34.Dick DM, et al. Linkage analyses of IQ in the collaborative study on the genetics of alcoholism (COGA) sample. Behav Genet. 2006;36:77–86. doi: 10.1007/s10519-005-9009-8. [DOI] [PubMed] [Google Scholar]
- 35.Dick DM, et al. Association of CHRM2 with IQ: Converging evidence for a gene influencing intelligence. Behav Genet. 2007;37:265–272. doi: 10.1007/s10519-006-9131-2. [DOI] [PubMed] [Google Scholar]
- 36.Luciano M, et al. Genomewide scan of IQ finds significant linkage to a quantitative trait locus on 2q. Behav Genet. 2006;36:45–55. doi: 10.1007/s10519-005-9003-1. [DOI] [PubMed] [Google Scholar]
- 37.Comings DE, et al. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. Am J Med Genet. 2002;114:527–529. doi: 10.1002/ajmg.10406. [DOI] [PubMed] [Google Scholar]
- 38.Comings DE, et al. Role of the cholinergic muscarinic 2 receptor (CHRM2) gene in cognition. Mol Psychiatry. 2003;8:10–11. doi: 10.1038/sj.mp.4001095. [DOI] [PubMed] [Google Scholar]
- 39.Jones KA, et al. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: Implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Greenwood PM, Parasuraman R. Scale of attentional focus in visual search. Percept Psychophys. 1999;61:837–859. doi: 10.3758/bf03206901. [DOI] [PubMed] [Google Scholar]
- 41.Greenwood PM, Parasuraman R. The scaling of spatial attention in visual search and its modification in healthy aging. Percept Psychophys. 2004;66:3–22. doi: 10.3758/bf03194857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brazhnik E, Borgnis R, Muller RU, Fox SE. The effects on place cells of local scopolamine dialysis are mimicked by a mixture of two specific muscarinic antagonists. J Neurosci. 2004;24:9313–9323. doi: 10.1523/JNEUROSCI.1618-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan J, et al. Testing the efficiency and independence of attentional networks. J Cognit Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 44.Voytko ML. Cognitive functions of the basal forebrain cholinergic system in monkeys: Memory or attention? Behav Brain Res. 1996;75:13–25. doi: 10.1016/0166-4328(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 45.Dunnett SB, Rogers DC, Jones GH. Effects of nucleus basalis magnocellularis lesions in rats on delayed matching and nonmatching to position tasks. Eur J Neurosci. 1989;1:395–406. doi: 10.1111/j.1460-9568.1989.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 46.Robbins TW, et al. Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: Further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behav Brain Res. 1989;35:221–240. doi: 10.1016/s0166-4328(89)80143-3. [DOI] [PubMed] [Google Scholar]
- 47.Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- 48.Chudasama Y, et al. Cholinergic modulation of visual attention and working memory: Dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learn Mem. 2004;11:78–86. doi: 10.1101/lm.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenwood PM, et al. Both a nicotinic SNP and a noradrenergic SNP modulate working memory performance when attention is manipulated. J Cognit Neurosci. 2009 doi: 10.1162/jocn.2008.21164. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertrand D. Neuronal nicotinic acetylcholine receptors and epilepsy. Epilepsy Curr. 2002;2:191–193. doi: 10.1046/j.1535-7597.2002.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimchi-Sarfaty C, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 52.Haier RJ, et al. Regional glucose metabolic changes after learning a complex visuospatial/motor task: A positron emission tomographic study. Brain Res. 1992;570:134–143. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- 53.Espeseth T, et al. Interactive effects of APOE and CHRNA4 on attention and white matter volume in healthy middle-aged and older adults. Cognit Affect Behav Neurosci. 2006;6:31–43. doi: 10.3758/cabn.6.1.31. [DOI] [PubMed] [Google Scholar]
- 54.Voytko ML, et al. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasselmo M. Expecting the unexpected: Modeling of neuromodulation. Neuron. 2005;46:526–528. doi: 10.1016/j.neuron.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Parasuraman R, Greenwood PM, Haxby JV, Grady CL. Visuospatial attention in dementia of the Alzheimer type. Brain. 1992;115:711–733. doi: 10.1093/brain/115.3.711. [DOI] [PubMed] [Google Scholar]
- 57.Hasselmo ME, Cekic M. Suppression of synaptic transmission may allow combination of associative feedback and self-organizing feed-forward connections in the neocortex. Behav Brain Res. 1996;79:153–161. doi: 10.1016/0166-4328(96)00010-1. [DOI] [PubMed] [Google Scholar]
- 58.Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 59.Zhang W, et al. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knockout mice. J Neurosci. 2002;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mrzljak L, Levey AI, Belcher S, Goldman-Rakic PS. Localization of the m2 muscarinic acetylcholine receptor protein and mRNA in cortical neurons of the normal and cholinergically deafferented rhesus monkey. J Comp Neurol. 1998;390:112–132. [PubMed] [Google Scholar]
- 61.Rouse ST, Gilmor ML, Levey AI. Differential presynaptic and postsynaptic expression of m1–m4 muscarinic acetylcholine receptors at the perforant pathway/granule cell synapse. Neuroscience. 1998;86:221–232. doi: 10.1016/s0306-4522(97)00681-7. [DOI] [PubMed] [Google Scholar]
- 62.Alcantara AA, et al. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: Anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 2001;434:445–460. doi: 10.1002/cne.1186. [DOI] [PubMed] [Google Scholar]
- 63.Parikh V, et al. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 2004;20:1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- 64.Briand LA, et al. Modulators in concert for cognition: Modulator interactions in the prefrontal cortex. Prog Neurobiol. 2007;83:69–91. doi: 10.1016/j.pneurobio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: A mechanism for inhibition and disinhibition of neuronal networks. J Neurosci. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- 67.Wechsler D. Wechsler Memory Scale- Revised Manual. New York: Psychological Corporation; 1987. [Google Scholar]
- 68.Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the National Institute of Mental Health's BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.