Abstract
BACKGROUND:
Refractory congestive heart failure (CHF) with diuretic resistance is life-threatening and predicts a short life expectancy. Glucocorticoids have been proven to have potent diuretic effects in animal studies; however, their efficacy in CHF patients with diuretic resistance is not known.
METHODS:
Thirteen CHF patients with significant volume overload and diuretic resistance who failed to respond to a conventional sequential nephron blockade therapeutic strategy; that is, the coad-ministration of a thiazide (hydrochlorothiazide) and spironolactone, in combination with loop diuretics, were studied. Prednisone (1 mg/kg daily) was then added to standard care, with other medications unchanged, to determine diuretic efficacy in these CHF patients. Variables included body weight, urine volume, serum electrolytes and renal function.
RESULTS:
Adding prednisone resulted in striking diuresis with a mean (± SD) body weight reduction of 9.39±3.09 kg. Prednisone significantly decreased serum creatinine by 52.21±48.68 μmol/L and increased glomerular filtration rate by 33.63±15.87 mL/min/1.73 m2 compared with baseline. All patients were discharged from hospital with improved clinical status and renal function, and 11 patients remained alive in the long term. The main side effect of prednisone appeared to be hyperglycemia in diabetic patients.
CONCLUSIONS:
The present study demonstrated that prednisone can rapidly eliminate volume overload and improve clinical status and renal function in CHF patients with diuretic resistance. Further prospective randomized clinical studies are warranted to confirm its clinical efficacy.
Keywords: Diuretic resistance, Furosemide, Heart failure, Prednisone, Renal function, Spironolactone
Abstract
HISTORIQUE :
L’insuffisance cardiaque congestive (ICC) réfractaire accompagnée d’une résistance diurétique met en jeu le pronostic vital et est prédictive d’une courte espérance de vie. Dans les études sur des animaux, il est démontré que les glucocorticoïdes ont de puissants effets diurétiques. On ne connaît toutefois pas leur efficacité chez des patients atteints d’ICC accompagnée d’une résistance diurétique.
MÉTHODOLOGIE :
Treize patients atteints d’ICC réfractaire présentant une importante surcharge de volume et une résistance diurétique et qui n’avaient pas réagi à la stratégie thérapeutique classique de blocage des néphrons (c’est-à-dire la coadministration d’hydrochlorothiazide de thiazide) et de spironolactone en association avec des diurétiques en boucle. On a ensuite ajouté la prednisone (1 mg/kg tous les jours) aux soins classiques, sans modifier le reste de la médication, afin d’en déterminer l’efficacité diurétique chez les patients atteints d’ICC ayant une résistance diurétique réfractaire. Les variables incluaient le poids corporel, le volume urinaire, les électrolytes sériques et la fonction rénale.
RÉSULTATS :
L’ajout de prednisone a entraîné une diurèse énergique associée à une réduction de poids moyenne (±ÉT) de 9,39±3,09 kg. La prednisone a suscité une réduction considérable de créatinine sérique de 52,21±48,68 μmol/L et une augmentation du taux de filtration glomérulaire de 33,63±15,87 mL/min/1.73 m2 par rapport aux données de départ. L’état clinique et la fonction rénale de tous les patients s’étaient améliorés au moment du congé, et 11 patients ont survécu à long terme. L’hyperglycémie chez les patients diabétiques semblait être le principal effet secondaire de la prednisone.
CONCLUSIONS:
La présente étude a révélé que la prednisone peut rapidement éliminer la surcharge de volume ainsi qu’améliorer l’état clinique et la fonction rénale des patients atteints d’ICC accompagnée d’une résistance diurétique réfractaire. Il faudrait mener d’autres études cliniques prospectives aléatoires pour en confirmer l’efficacité clinique dans une telle situation.
Congestive heart failure (CHF) is a complex clinical hemodynamic disorder characterized by progressive pump failure and fluid accumulation. Diuretics are a vital component of symptomatic CHF management. However, over time, sodium and water excretion are equalized before adequate fluid elimination occurs. This phenomenon is thought to occur in one in three CHF patients on diuretic therapy and is termed diuretic resistance. In clinical settings, diuretic resistance in edematous patients has been defined as a clinical state in which sodium intake and excretion are equalized before adequate elimination of fluid occurs (1). One therapeutic strategy to overcome diuretic resistance is by sequential nephron blockade. Concurrent use of diuretics acting on different segments of the nephron, and loop diuretics with either proximal or distal tubule diuretics may produce an additive or synergistic diuretic response. However, there remain some cases refractory to this therapeutic strategy.
Animal studies (2–4) have shown that glucocorticoids can increase renal plasma flow and glomerular filtration rate (GFR) without changes in the glomerular filtration fraction. Also, glucocorticoids may have important effects on atrial natriuretic peptide (ANP), a potent endogenous diuretic peptide. It has been reported that glucocorticoids can regulate the synthesis and release of ANP (5), upregulate ANP receptors on vascular endothelial cells (6), and thus have an important effect on diuresis and natriuresis in patients with volume overload. Therefore, glucocorticoids may be a promising class of drugs for CHF patients with diuretic resistance refractory to a sequential nephron blockade therapeutic strategy.
The present study was designed to determine the clinical efficacy of chronic administration of prednisone in the management of patients with diuretic resistance.
METHODS
Study design and prednisone treatment
This was an open-label, noncontrolled prospective study approved by the institutional ethics committee and conducted in adherence with local guidelines for good clinical practice. After written consent was obtained, prednisone (1 mg/kg/day, with a maximum dose of 60 mg/day) was administered for four weeks and then gradually decreased.
Patient selection
Inclusion criteria included severe CHF with significant volume overload, New York Heart Association (NYHA) functional class IV and diuretic resistance refractory to sequential nephron blockade. The exclusion criteria included patient refusal, signs of infection, chronic renal failure, pericardial diseases, prerenal azotemia, hypovolemia, renal disease (eg, inflammatory renal disease), shock as the eventual cause of the acute renal failure and cardiogenic shock.
The diagnosis of CHF was established using echocardiographic criteria, signs of volume overload by clinical or radiological evidence of pulmonary edema and internal jugular venous distention (JVD). Significant volume overload was defined as at least two of the following: elevated JVD of more than 10 cm, pulmonary edema or pleural effusion on chest x-ray, ascites or enlarged liver, or significant peripheral or sacral edema (moderate, indentation remaining 10 s to 15 s after pressure). Sequential nephron blockade was defined as coadministration of a thiazide (hydrochlorothiazide) and spironolactone, in combination with loop diuretics. The definition of diuretic resistance was the failure to attain a negative fluid balance, despite bed rest, salt and water restriction, and standard medical care, ie, digoxin (when appropriate), furosemide (more than 200 mg/day administered orally or by continuous intravenous infusion for at least three days), hydrochlorothiazide (more than 50 mg/day with a creatinine clearance of more than 0.417 mL/s), spironolactone (more than 50 mg/day with serum potassium of less than 5.0 mmol/L) and positive inotropic drugs for at least three days.
Clinical assessment and laboratory values
Body weight, supine and seated blood pressure, pulse rate, JVD and edematous status were assessed every day during the study period. The total daily output of urine was collected. Blood electrolytes were monitored at least twice per week.
Statistical analysis
Continuous variables were expressed as mean ± SD unless otherwise stated. Wilcoxon’s signed rank test was used to analyze paired differences in variables before and after treatment. All statistical tests were performed using SPSS software (version 11.0, SPSS Inc, USA) with two-sided alternatives; a type I error of 0.05 was considered significant.
RESULTS
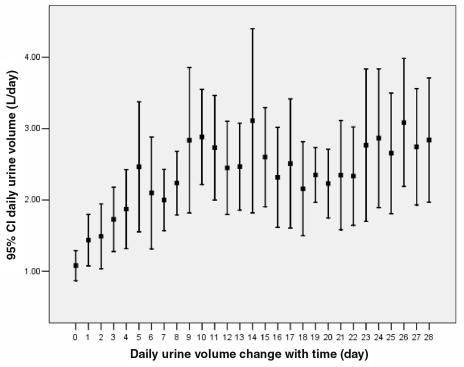
From April 2003 to September 2005, 13 consecutive patients with diuretic resistance were enrolled from the coronary care unit at the First Hospital of Hebei Medical University (Hebei, China). Patient clinical characteristics are shown in Table 1. After prednisone was added, urine volume began to increase; diuresis established approximately three to four days after initiating therapy, and the effect persisted during the treatment (Figure 1). Adding prednisone resulted in a significant body weight reduction (9.39±3.09 kg). On average, dry weight (defined as body weight maintained the same for at least three days without signs of fluid retention) was achieved on day 20.4 (range from 11 to 40 days). More important was that prednisone significantly decreased serum creatinine (52.21±48.68 μmol/L, P<0.01) and increased the GFR (33.63±15.87 mL/min/1.73m2, calculated by modified Cockroft-Gault formula, P<0.01) (Table 2). Of note, the maximum daily urine volume was more than 5 L in four patients, 6 L in three patients and 7 L in one patient.
TABLE 1.
Patient clinical characteristics at baseline (n=13)
| Characteristic | |
|---|---|
| Age, years | 50.2±12.9 |
| Sex (male/female) | 10/3 |
| Etiology | |
| Idiopathic dilated cardiomyopathy, n | 6 |
| Rheumatic heart disease, n | 4 |
| Hypertrophic cardiomyopathy, n | 1 |
| Coronary artery disease, n | 2 |
| Time with heart failure, years | 3.7±2.7 |
| Left ventricular ejection fraction, % | 35.8±15.9 |
| Jugular venous distension >10 cm, n | 13 |
| Peripheral or sacral edema ≥2, n | 13 |
| Ascites, n | |
| Mild | 1 |
| Moderate | 3 |
| Gross | 9 |
| Enlarged liver, n | 10 |
| Pleural effusion, n | |
| Moderate | 4 |
| Gross | 1 |
| Medication | |
| Furosemide, mg | 212.3±25.2 |
| Thiazide, mg | 65.4±28.0 |
| Spironolactone, mg | 101.5±45.0 |
| Angiotensin-converting enzyme inhibitor, n | 6 |
| Beta-blocker, n | 5 |
| Digoxin, n | 12 |
| Nitrate, n | 13 |
| Inotropic drug, n | 13 |
Figure 1).
Changes in daily urine volume. Day 0 was the day before administration of prednisone, day 1 was the day of initiation of prednisone treatment, etc. Daily urine volume is expressed as mean and 95% CI
TABLE 2.
Effects of prednisone combined with conventional therapy
| Before prednisone | After prednisone | Change from baseline | |
|---|---|---|---|
| Body weight, kg | 75.35±17.9 | 65.96±16.22** | –9.39±3.09 |
| SPotassium, mmol/L | 4.27±0.7 | 4.23±0.45 | –0.03±0.87 |
| SSodium, mmol/L | 134.23±6.4 | 136.08±3.81 | 1.85±6.30 |
| SChloride, mmol/L | 97.93±6.6 | 99.81±4.93 | 1.88±6.37 |
| SCreatinine, μmol/L | 128.37±58.8 | 76.16±28.30** | –52.21±48.68 |
| SUrea, mmol/L | 14.42±10.6 | 8.62±3.80* | –5.80±10.56 |
| SUric acid, μmol/L | 630.38±214.5 | 393.92±179.62** | –236.46±215.67 |
| Glomerular filtration rate, mL/min/1.73 m2 | 71.06±41.4 | 104.69±48.27** | 33.63±15.87 |
Results are presented as mean ± SD. P values are results compared with before prednisone treatment using the Wilcoxon’s signed rank test.
P<0.05;
P<0.01. S Serum concentration
At the end of study, all but one patient had improved in terms of NYHA class. Two patients were in NYHA class I, five patients were in NYHA class II and five patients were in NYHA class III at the end of study.
All patients in the study were taking higher doses of spironolactone, ranging from 60 mg/day to 180 mg/day (mean 100 mg/day). However, there were still five patients suffering from transient hypokalemia (serum potassium 2.75 mmol/L to 3.3 mmol/L) who needed potassium supplementation.
Notably, the dosage of furosemide could have been reduced four days after adding prednisone, once diuresis was established. It was decreased gradually (range from 20 mg/day to 60 mg/day) with time, with little impact on diuresis.
The data showed that adding prednisone led to an increase in blood pressure – systolic pressure increased from 98.2±10.8 mmHg at baseline versus 103.9±9.4 mmHg at the end of study (P=0.04). Diastolic pressure was insignificantly increased. There was also a reduction in heart rate – 81±12 beats/min at baseline versus 75±9 beats/min at the end of study (P=0.007).
Follow-up
On average, patients were followed up for 396 days (range 50 to 933 days). Two patients died. One patient died of blood dyscrasia three months after discharge, and the other died of renal failure eight months later. The other 11 patients remained clinically stable on a low dose of furosemide.
During the period of prednisone administration, one patient with diabetes mellitus developed poorly controlled hyperglycemia and one patient developed a skin infection.
DISCUSSION
To our knowledge, the present study is the first to explore the diuretic effects of prednisone in CHF patients with diuretic resistance. Adding prednisone resulted in a rapid, striking and persistent diuretic effect, as well as a significant improvement in NYHA class and renal function.
Conventional teaching dictates that glucocorticoids increase tubule secretion of potassium and hydrogen ions, as well as renal tubule sodium reabsorption, resulting in fluid and sodium retention, and should therefore be used with caution in CHF patients. Surprisingly, despite the widespread prevalence of this belief within the medical community, there are few supporting clinical data. In fact, several animal studies have shown that glucocorticoids given acutely or chronically at physiological or pharmacological doses can increase GFR and have profound effects on diuresis and natriuresis (2–4). Suggested mechanisms include that glucocorticoids can enhance nitric oxide production (7), and activate the endogenous renal dopaminergic mechanism and, thus, specifically dilate renal vasculature (8), leading to a significant increase in glomerular plasma flow (4,8). More important is that glucocorticoids may have important effects on ANP. It has been reported that glucocorticoids can regulate the synthesis and release of ANP (5), upregulate ANP receptors on vascular endothelial cells (6) and potentiate the response of cyclic GMP to ANP (9). A human study indicated that glucocorticoids may have a permissive effect on human ANP-mediated natriuresis and diuresis (10). An observational study designed to detect the relationship between ANP and Cushing’s syndrome also revealed that ANP levels in patients with Cushing’s syndrome were three times higher than in the normal population, which is in accordance with our postulation above (11).
Patients with severe heart failure and diuretic resistance remain a therapeutic challenge. Alternative approaches in these critically ill patients include hemofiltration and hemodialysis. However, the effects only persist for the short term (usually less than four months), and long-term results have been quite discouraging, with annual survival rates of approximately 30% to 40% in most reports (12–15). Therefore, the use of these treatments is of limited value in end-stage CHF patients with edema refractory to maximal conventional treatment. Prednisone may be an alternative to hemofiltration and hemodialysis.
Concerns and study limitations
Several concerns arose from the present study. First, clinical and experimental evidence suggests that chronic immune myocardial injury may play a key part in the development of heart failure in patients with cardiomyopathy due to ischemic heart disease or in nonischemic cases (16). Whether prednisone has an important effect on the immune system in CHF patients with refractory diuretic resistance is still unknown, for our study was not designed to determine its immune effects. However, Parrillo et al (17) reported that treatment with prednisone did not improve cardiac function in 102 patients with idiopathic dilated cardiomyopathy who were enrolled in a randomized, double-blinded, placebo-controlled study over the long term. A meta-analysis of immunosuppressive therapy in inflammatory cardiomyopathy also did not verify its clinical efficacy (18). Another concern is the furosemide dose. Renal responsiveness to loop diuretics may be decreased in patients with severe heart failure. However, there is a ceiling effect, and the response is not increased by larger doses (19). Thus, we thought that 200 mg was enough to elicit diuresis and natriuresis, especially in combination with high doses of hydrochlorothiazide and spironolactone. However, this may not be the case, and higher doses might have been effective. The third concern is the spironolactone dose. The mean dose of spironolactone used in the present study was 100 mg, which was much higher than the mean dose of 26 mg in the Randomized Aldactone Evaluation Study (RALES) (20), which can predispose patients to hyperkalemia. However, studies have shown that a high dose of spironolactone in combination with loop diuretics may be more effective in producing diuresis when circulating aldosterone concentrations are increased (which is usually the case in more advanced CHF) (21). At the same time, glucocorticoids can increase tubule secretion of potassium. It is important to note that all patients in the present study were closely monitored. Overall, they tolerated the high dose of spironolactone very well. The last concern is function of the adrenal cortex. We did not test adrenal cortex function in all patients; however, a recent randomized, placebo-controlled clinical study (22) in which all patients had normal adrenal cortex function showed that prednisone had potent diuretic effects in stable CHF patients.
Limitations
Limitations of this study include the lack of a control group, the relatively small sample size, and the heterogeneity of age and etiology of CHF in the study population. Other limitations include the potential for underdosing of diuretics and not using metolazone or etacrynic acid. However, it is highly unlikely that patients with diuretic resistance who were refractory to the sequential nephron blockade strategy would spontaneously restore their diuretic function after a three-day waiting period. In our study, prednisone restored diuresis in these patients without an increased dose of diuretics, or the addition of metolazone or etacrynic acid.
CONCLUSIONS
Our preliminary data show that prednisone may have a potent diuretic effect in patients with severe CHF and diuretic resistance, and that it can improve renal function at the same time. Additional studies are needed to clarify its mechanisms of action and verify its clinical efficacy.
REFERENCES
- 1.Krämer BK, Schweda F, Kammerl M, Riegger GA. Diuretic therapy and diuretic resistance in cardiac failure. Nephrol Dial Transplant. 1999;14(Suppl 4):39–42. doi: 10.1093/ndt/14.suppl_4.39. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre JA, Ibarra FR, Barontini M, Arrizurieta EE, Armando I. Effect of glucocorticoids on renal dopamine production. Eur J Pharmacol. 1999;370:271–8. doi: 10.1016/s0014-2999(99)00121-1. [DOI] [PubMed] [Google Scholar]
- 3.Nevskaia TL, Shcherbinina AV, IuI Ivanov. [Effect of glucocorticoids on electrolyte excretion after expansion of the extracellular space.] Probl Endokrinol (Mosk) 1977;23:48–51. [PubMed] [Google Scholar]
- 4.Baylis C, Brenner BM. Mechanism of the glucocorticoid-induced increase in glomerular filtration rate. Am J Physiol. 1978;234:F166–70. doi: 10.1152/ajprenal.1978.234.2.F166. [DOI] [PubMed] [Google Scholar]
- 5.Garcia R, Debinski W, Gutkowska J, et al. Gluco- and mineralocorticoids may regulate the natriuretic effect and the synthesis and release of atrial natriuretic factor by the rat atria in vivo. Biochem Biophys Res Commun. 1985;131:806–14. doi: 10.1016/0006-291x(85)91311-7. [DOI] [PubMed] [Google Scholar]
- 6.Lanier-Smith KL, Currie MG. Effect of glucocorticoids on the binding of atrial natriuretic peptide to endothelial cells. Eur J Pharmacol. 1990;178:105–9. doi: 10.1016/0014-2999(90)94800-d. [DOI] [PubMed] [Google Scholar]
- 7.De Matteo R, May CN. Glucocorticoid-induced renal vasodilatation is mediated by a direct renal action involving nitric oxide. Am J Physiol. 1997;273:R1972–9. doi: 10.1152/ajpregu.1997.273.6.R1972. [DOI] [PubMed] [Google Scholar]
- 8.May CN, Bednarik JA. Regional hemodynamic and endocrine effects of aldosterone and cortisol in conscious sheep. Comparison with the effects of corticotropin. Hypertension. 1995;26:294–300. doi: 10.1161/01.hyp.26.2.294. [DOI] [PubMed] [Google Scholar]
- 9.Kanda K, Ogawa K, Miyamoto N, Hatano T, Seo H, Matsui N. Potentiation of atrial natriuretic peptide-stimulated cyclic guanosine monophosphate formation by glucocorticoids in cultured rat renal cells. Br J Pharmacol. 1989;96:795–800. doi: 10.1111/j.1476-5381.1989.tb11886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damjancic P, Vierhapper H. Permissive action of glucocorticoid substitution therapy on the effects of atrial natriuretic peptide (hANP) in patients with adrenocortical insufficiency. Exp Clin Endocrinol. 1990;95:315–21. doi: 10.1055/s-0029-1210971. [DOI] [PubMed] [Google Scholar]
- 11.Yamaji T, Ishibashi M, Yamada A, et al. Plasma levels of atrial natriuretic hormone in Cushing’s syndrome. J Clin Endocrinol Metab. 1988;67:348–52. doi: 10.1210/jcem-67-2-348. [DOI] [PubMed] [Google Scholar]
- 12.Grapsa E, Alexopoulos GP, Margari Z, Terrovitis JV, Kontoyannis DA, Nanas JN. Ultrafiltration in the treatment of severe congestive heart failure. Int Urol Nephrol. 2004;36:269–72. doi: 10.1023/b:urol.0000034633.95171.64. [DOI] [PubMed] [Google Scholar]
- 13.Iorio L, De Santo LS, Violi F. Hemodialitic treatment of cardiac failure. Semin Nephrol. 2001;21:278–81. doi: 10.1053/snep.2001.21657. [DOI] [PubMed] [Google Scholar]
- 14.Ragazzoni E, Sacco A, Cusinato S, et al. [Heart failure resistant to drug therapy. Nephrologic approach.] Minerva Urol Nefrol. 1998;50:133–8. [PubMed] [Google Scholar]
- 15.Iorio L, Simonelli R, Nacca RG, DeSanto LS. Daily hemofiltration in severe heart failure. Kidney Int Suppl. 1997;59:S62–5. [PubMed] [Google Scholar]
- 16.Torre-Amione G. Immune activation in chronic heart failure. Am J Cardiol. 2005;95:3C–8C. 38C–40C. doi: 10.1016/j.amjcard.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Parrillo JE, Cunnion RE, Epstein SE, et al. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. N Engl J Med. 1989;321:1061–8. doi: 10.1056/NEJM198910193211601. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Chen J, Liu K. Immunosuppressive treatment for inflammatory cardiomyopathy: Meta-analysis of randomized controlled trials. Int Heart J. 2005;46:113–22. doi: 10.1536/ihj.46.113. [DOI] [PubMed] [Google Scholar]
- 19.Brater DC. Diuretic therapy. N Engl J Med. 1998;339:387–95. doi: 10.1056/NEJM199808063390607. [DOI] [PubMed] [Google Scholar]
- 20.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–51. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 21.van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol. 1993;71:21A–8A. doi: 10.1016/0002-9149(93)90241-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Chen H, Zhou C, et al. Potent potentiating diuretic effects of prednisone in congestive heart failure. J Cardiovasc Pharmacol. 2006;48:173–6. doi: 10.1097/01.fjc.0000245242.57088.5b. [DOI] [PubMed] [Google Scholar]