Abstract
BACKGROUND:
Dobutamine stress echocardiography (DSE) is an established method of detecting myocardial ischemia. Its diagnostic accuracy solely depends on wall motion assessment. Clear visibility of the left ventricular endocardium is essential for reliable assessment of a wall motion abnormality. However, incremental benefits of contrast DSE for the detection of coronary artery disease (CAD) have not been demonstrated in overweight or obese patients.
OBJECTIVES:
The purpose of the present study was to test the incremental benefits of contrast DSE in detecting CAD in overweight or obese patients.
METHODS:
Sixty-two overweight or obese patients (body mass index 26 kg/m2 to 33 kg/m2) underwent DSE with or without contrast and coronary angiography. Contrast-enhanced images were achieved at rest and during peak DSE after administration of SonoVue (Bracco Diagnostics Inc, Italy) or Optison (Mallinckrodt, USA). The endocardial border resolution for each myocardial segment was graded as 0, 1 or 2. A total of 992 segments from 62 subjects were analyzed. The results of DSE with or without contrast were compared with the findings on angiography.
RESULTS:
The differences in the score grading between the two groups with or without contrast, at rest and during peak DSE were statistically significant (P<0.001). The sensitivity, specificity and accuracy of contrast DSE in detecting CAD, compared with the studies without contrast, were improved (82% versus 70%, 78% versus 67% and 81% versus 69%, respectively).
CONCLUSIONS:
SonoVue and Optison can enhance left ventricular endocardial border delineation in overweight or obese patients, optimizing the evaluation of wall motion both at rest and during peak stress. This increases the diagnostic value of DSE in detecting CAD.
Keywords: Angiography, Coronary artery disease, Echocardiographic contrast, Stress
Abstract
HISTORIQUE :
L’échocardiographie d’effort à la dobutamine (ÉED) est une méthode établie pour déceler l’ischémie myocardique. Sa précision diagnostique dépend entièrement de l’évaluation du mouvement des parois. Il est essentiel de bien voir l’endocarde ventriculaire gauche pour bien évaluer l’anomalie du mouvement des parois. Cependant, les avantages incrémentiels de l’ÉED de contraste n’ont pas été démontrés pour les patients obèses ou qui font de l’embonpoint.
OBJECTIFS :
La présente étude visait à vérifier les avantages incrémentiels de l’ÉED de contraste pour déceler une coronaropathie chez les patients obèses ou qui font de l’embonpoint.
MÉTHODOLOGIE :
Soixante-deux patients obèses ou qui font de l’embonpoint (indice de masse corporelle de 26 kg/m2 à 33 kg/m2) ont subi une éED avec ou sans contraste et une coronarographie. Les images de contraste ont été obtenues au repos et pendant l’ÉED de pointe, après l’administration de SonoVue (Bracco Diagnostics Inc., Italie) ou d’Optison (Mallinckrodt, États-Unis). On a attribué une cotation de 0, 1 ou 2 à la résolution du bord endocardiaque de chaque segment myocardique. On analysé un total de 992 segments provenant de 62 sujets. On a comparé les résultats de l’ÉED avec ou sans contraste et angiographie.
RÉSULTATS :
Les différences de cotation entre les deux groupes, avec ou sans contraste, au repos et pendant l’ÉED de pointe, étaient statistiquement significatives (P<0,001). La sensibilité, la spécificité et la précision de l’ÉED de contraste pour déceler la coronaropathie étaient accrues (70 % par rapport à 82 %, 67 % par rapport à 78 % et 69 % par rapport à 81 %, respectivement).
CONCLUSIONS:
SonoVue et Optison peuvent améliorer la délimitation du bord endocardiaque chez les patients obèses ou qui font de l’embonpoint et optimiser l’évaluation du mouvement des parois tant au repos que pendant un stress de pointe. Cette constatation accroît la valeur diagnostique de l’ÉED pour dépister la coronaropathie.
Dobutamine stress echocardiography (DSE) is well established for detecting inducible myocardial ischemia. With DSE, ischemia is defined by a regional reduction or deterioration of myocardial thickening or inward motion of the endocardial border (1,2). Clear visibility of the left ventricular endocardium is essential for reliable assessment of wall motion abnormalities (WMA). Various factors, such as lung disease, obesity or chest deformities, may impair image quality at rest in about 15% of patients, and during stress echocardiography, in up to 30% of patients (3,4), thus limiting reader confidence and decreasing diagnostic accuracy for detecting coronary artery disease (CAD).
Several studies (5–7) have demonstrated the usefulness of administering contrast agents in enhancing the endocardial border, thereby optimizing wall motion analysis in patients with CAD for both at-rest echocardiography and exercise or pharmacological stress echocardiography. However, few studies assessed this approach only in overweight or obese patients. The present study was performed to evaluate the feasibility and usefulness of contrast-enhanced DSE for the assessment of left ventricular wall motion and its diagnostic accuracy for detecting CAD in overweight or obese patients.
METHODS
Patient selection
A total of 62 overweight or obese patients (body mass index [BMI] 26 kg/m2 to 33 kg/m2) who were known to have, or suspected of having, CAD were prospectively studied. Both contrast DSE and diagnostic coronary angiography were performed within one month of each other at the University of Heidelberg (Heidelberg, Germany). All patients gave informed consent, and the study protocol was approved by the local ethics committee. Exclusion criteria included recent myocardial infarction (less than eight weeks previously), unstable angina, previous surgical or percutaneous revascularization, severe hypertension (blood pressure 180/110 mmHg or higher), congenital or valvular heart disease, cardiomyopathy, left bundle branch block, supraventricular tachycardia and ventricular tachycardia. Patients were asked to abstain from using nitrates and beta-blockers at least 12 h before the test.
Clinical evaluation
All patients were assessed clinically, including complete history, physical examination, cardiac risk factors, medication use and BMI.
Contrast DSE
A Sonos 7500 system (Phillips Medical Systems, USA) with second harmonic imaging was used in the study. There is an S3 probe in this system, which transmits ultrasound at a mean frequency of 1.6 MHz while receiving at 3.2 MHz. The electrocardiogram was monitored continuously and blood pressure was measured every 3 min. A quad-screen format display was created by digitizing four standard imaging planes at baseline, during a low dose and the highest dose (peak stress) of dobutamine, as well as during recovery. These images were also recorded on videotape at the end of each stage. Side-by-side comparison of wall motion in the digitized images was performed by two experienced investigators who were blinded to patient clinical and coronary angiography data. If a discrepancy appeared, a third observer assessed the images and complete agreement was reached. After baseline images (without and with contrast) of four-, three- and two-chamber views were recorded, dobutamine was administered in 3 min stages at an infusion rate of 5 μg/kg/min, 10 μg/kg/min, 20 μg/kg/min, 30 μg/kg/min and 40 μg/kg/min. Patients who did not achieve 85% of their age-predicted maximal heart rate were given intravenous atropine (1 mg or less), and dobutamine infusion was continued until the target heart rate was obtained. The end points for termination of the test included attainment of the target heart rate, completion of the study protocol, development of severe ischemia (increasing angina, extensive WMA or ST segment shift of more than 3 mm on electrocardiogram). Other reasons for terminating the test included severe palpitation, headache, significant arrhythmia or blood pressure of 220/120 mmHg or higher. Regional wall motion was given a score (wall motion score) of 0 (hyperkinesis), 1 (normal), 2 (hypokinesis), 3 (akinesis) and 4 (dyskinesis). A WMA consistent with ischemia was considered present when dobutamine stress induced an increase in the wall motion score, with the exception of the change from akinesis to dyskinesis (8).
The contrast agents in the present study included SonoVue (Bracco Diagnostics Inc, Italy) and Optison (Mallinckrodt, USA), the second-generation ultrasound contrast agents commercially available, which have outstanding stability, resistance to pressure, and biological safety (9,10). A 20-gauge intravenous catheter was inserted into a right antecubital vein, and 1.25 mL of SonoVue or 0.25 mL of Optison was injected as a bolus at rest after baseline images without contrast were recorded. The same method was used at peak stress after images without contrast were recorded. Each subject received 2.5 mL of SonoVue or 0.5 mL of Optison.
Endocardial border analysis
The left ventricle was divided into six segments in both the apical four-chamber view (basal, mid- and apical interventricular septum and lateral wall) and the two-chamber view (basal, mid- and apical anterior and inferior walls), and four segments in the three-chamber view (basal, mid-anteroseptal and posterior walls). There were a total of 16 segments for each subject. Endocardial border visualization was scored for each wall segment using a three-level scale of 0 (border invisible), 1 (barely visible – border visualized partially throughout a heart cycle and/or incomplete wall length) or 2 (complete visualization of the endocardial border).
Coronary angiography
All patients underwent selective coronary angiography within one month of DSE. CAD was defined as 50% or more of luminal diameter narrowing in at least one major epicardial artery or a major branch. If an artery had several stenoses, the most severe was used to define the presence or absence of CAD. Multivessel CAD was determined to be present when either both the left anterior descending artery and the left circumflex artery or the left anterior descending artery and the right coronary artery had 50% or greater luminal diameter stenosis. Coronary angiography analysis was performed by an experienced interventionalist who was blinded to the echocardiographic data. The degree of angiographic stenosis was quantified through the use of quantitative coronary analysis.
Statistical analysis
All categorical variables are expressed as percentages and continuous variables as mean ± SD. The paired t test was used to compare continuous variables between rest and stress. The scores of rest and peak stress of endocardial border delineation with standard and contrast echocardiography were calculated with the χ2 test. Sensitivity, specificity, and positive and negative predictive values for detecting significant CAD were also calculated. Accuracy was derived by adding the true positive and negative results, and dividing the sum by the total number considered. P<0.05 was considered significant. All analyses were performed with standard software (SPSS version 10, SPSS Inc, USA).
RESULTS
The demographic and clinical characteristics of the study population are summarized in Table 1.
TABLE 1.
Demographic and clinical characteristics (n=62)
| Characteristic | |
|---|---|
| Age, years (mean ± SD) | 68.8±7.7 |
| Male sex, n (%) | 44 (71) |
| History of myocardial infarction, n (%) | 15 (24) |
| Hypertension, n (%) | 54 (87) |
| Diabetes, n (%) | 28 (45) |
| Hypercholesterolemia, n (%) | 31 (50) |
| Family history of coronary artery disease, n (%) | 16 (26) |
| Smoking, n (%) | 18 (29) |
| Typical chest pain, n (%) | 34 (55) |
| Atypical chest pain, n (%) | 28 (45) |
| Body mass index, kg/m2 (mean ± SD) | 29.1±1.2 |
| No significant coronary artery disease, n (%) | 18 (29) |
| Single-vessel coronary artery disease, n (%) | 24 (39) |
| Multivessel coronary artery disease, n (%) | 20 (32) |
| Beta-blocker use, n (%) | 32 (52) |
| Calcium channel blocker use, n (%) | 22 (35) |
| Angiotensin-converting enzyme inhibitor use, n (%) | 51 (82) |
| Statin use, n (%) | 56 (90) |
All 62 patients achieved 85% of their maximal heart rate. Heart rate increased from 68±8 beats/min at baseline to 139±14 beats/min at peak stress. Systolic blood pressure increased from 138±18 mmHg to 163±21 mmHg and diastolic blood pressure increased from 70±10 mmHg to 76±12 mmHg (P<0.001 for both). The highest dose of dobutamine (40 μg/kg/min) was applied to 52 (84%) patients. Atropine was added for 13 (21%) patients. As minor side effects of dobutamine, five (8%) patients had headaches, five (8%) had palpitation and two (3%) had flush. No patient experienced a myocardial infarction or other life-threatening side effects.
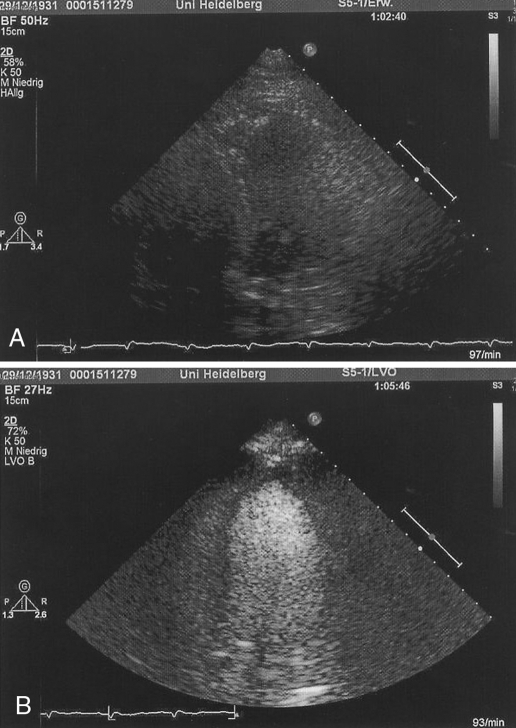
Endocardial border delineation of the LV was enhanced after the administration of the contrast agent (Figure 1). A total of 992 segments from 62 subjects were analyzed (Table 2). The differences in grading of the score between the two groups with and without contrast, at rest and at peak stress were statistically significant (P<0.001). The percentage increments in the identification of the endocardial border in the at-rest echocardiography and peak DSE for a score of 2 were 81% and 116%, respectively.
Figure 1).
A An apical four-chamber view without contrast. The endocardial border of the left ventricle is barely visible. B A four-chamber view with contrast. The endocardial border of the left ventricle is completely visualized
TABLE 2.
Scores for 992 segments analyzed using at-rest echocardiography (echo) and during peak dobutamine stress echo (DSE) with and without contrast
| Score | At-rest echo, n (%) | Peak DSE, n (%) | At-rest contrast echo, n (%) | Peak contrast DSE, n (%) |
|---|---|---|---|---|
| 0 | 128 (13) | 179 (18) | 0 | 0 |
| 1 | 328 (33) | 380 (38) | 18 (2) | 50 (5) |
| 2 | 536 (54) | 433 (44) | 974 (98)* | 942 (95)** |
| Total | 992 | 992 | 992 | 992 |
P<0.001 compared with at-rest echo;
P<0.001 compared with peak DSE
In the 62 patients who underwent coronary angiography, a severity of 50% diameter stenosis or greater of at least one major vessel was present in 44 patients; multivessel CAD was documented in 20 of these patients. There were 18 patients without obstructive CAD. During DSE, it was found that 31 patients had a WMA consistent with angiographically significant CAD and 12 had no WMAs consistent with the absence of angiographically significant CAD. During contrast DSE, 36 patients had a WMA consistent with angiographically significant CAD and 14 had no WMA consistent with absence of angiographically significant CAD. The diagnostic values of DSE and contrast DSE for the detection of CAD in overweight or obese patients are shown in Table 3. The sensitivity, specificity and accuracy of contrast DSE for single-vessel versus multivessel disease are shown in Table 4. The interobserver variability of contrast DSE for wall motion analysis was 6.3% in 20 patients, including 10 patients with CAD and 10 patients without CAD.
TABLE 3.
Comparison of the accuracy of dobutamine stress echocardiography (DSE) with contrast DSE in detecting coronary artery disease
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % | |
|---|---|---|---|---|---|
| DSE | 70 | 67 | 84 | 46 | 68 |
| Contrast DSE | 82 | 78 | 90 | 64 | 81 |
| Increment (relative change) | 17 | 16 | 7 | 39 | 17 |
NPV Negative predictive value; PPV Positive predictive value
TABLE 4.
Comparison of the accuracy of contrast dobutamine stress echocardiography for single-vessel versus multivessel disease
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % | |
|---|---|---|---|---|---|
| Single-vessel | 71 | 78 | 81 | 66 | 74 |
| Multivessel | 90 | 83 | 86 | 88 | 87 |
| P | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
NPV Negative predictive value, PPV Positive predictive value
DISCUSSION
Although tissue harmonic imaging modalities improve signal-to-noise ratio and overall image quality, unsatisfactory left ventricular border definition precludes a meaningful interpretation of regional left ventricular function in some patients with a poor acoustic window. Secondary generation transpulmonary contrast agents increase backscatter in an ultrasound field and opacify the left ventricular cavity. Some studies demonstrated an improvement of endocardial border delineation at rest and during stress testing after the administration of a transpulmonary contrast agent (11–14). In the present study, at-rest echochardiography graded 536 of 992 (54%) myocardial segments as a score of 2 and peak DSE graded only 434 of 992 (44%) segments as a score of 2 before the intravenous bolus of SonoVue or Optison because of an invisible or barely visible left ventricular endocardial border. After the intravenous administration of the contrast agents, at-rest echocardiography graded 974 of 992 (98%) segments as a score of 2 and peak DSE graded 942 of 992 (95%) segments as a score of 2 due to complete visualization of the endocardial border. The percentage increments of segments for a score of 2 at rest echocardiography and peak DSE were 81% and 116%, respectively. Rizzo et al (15) reported that 40 consecutive male master athletes were studied with contrast exercise echocardiography. After the administration of SonoVue, the percentage of wall segments judged as completely visualized at rest increased from 54.2% to 100%, and at peak stress, the percentage of wall segments completely visualized increased from 43.5% to 95.9%. The present study confirmed that the intravenous administration of contrast agents can enhance the left ventricular endocardial border in overweight or obese patients, thus optimizing the evaluation of left ventricular wall motion by at-rest echocardiography and DSE.
Takeuchi et al (16) reported that in 274 patients with known or suspected CAD who had undergone both contrast-enhanced DSE and coronary angiography, the sensitivity, specificity and accuracy of detecting left anterior descending coronary artery disease were 78%, 89% and 86%, respectively. Dolan et al (17) studied 229 patients prospectively, 112 of whom had good at-rest echocardiography results with no contrast, and 117 had poor at-rest echocardiography results with Optison injection during DSE. The percentages of endocardial border visualization, wall thickening, sensitivity and specificity were compared between both groups. Both groups were matched with respect to age, previous myocardial infarction, resting WMA, percentage of coronary stenosis, as well as the number of diseased coronary arteries. The results showed that Optison significantly improved endocardial border visualization, especially at peak stress. The ability to measure wall thickening was significantly higher in the contrast DSE group (89%) with suboptimal images versus the noncontrast group (71%) with optimal images (P=0.01). This resulted in comparable sensitivity (79% versus 71%, P not significant), specificity (76% versus 82%, P not significant) and diagnostic accuracy (80% versus 76%, P not significant). In the present study, the sensitivity, specificity and accuracy in detecting CAD with contrast were increased (70% versus 82%, 67% versus 78% and 69% versus 81%, respectively). Our results are concordant with these findings, in that the second generation of contrast agents increased sensitivity, specificity and accuracy of DSE for the detection of CAD.
As in other studies of stress echocardiography, the sensitivity, specificity and accuracy of the detection of patients with single-vessel disease were lower (71%, 78% and 74%, respectively) than for patients with multivessel disease (90%, 83% and 87%, respectively) (18). However, the differences in the study are not statistically significant (P>0.05). Further studies are needed to assess the diagnostic value of contrast DSE for single-vessel disease and multivessel disease in overweight or obese patients.
Seventeen direct comparisons of detecting CAD on stress echocardiography and single photon emission computed tomography (SPECT) were identified. Pooling of the data showed a slightly higher overall sensitivity for SPECT perfusion imaging compared with stress echocardiography (84% versus 80%, P<0.05). On the other hand, stress echocardiography was more specific than perfusion imaging (86% versus 77%, P=0.001) (19). In our study, the diagnostic value for the detection of CAD was comparable with that of SPECT.
Study limitations
Interpretation of DSE is partially subjective and strongly dependent on the skills of the reader. Strain rate imaging by tissue Doppler may provide objective evaluation of regional myocardial function. There is a potential limitation because the investigators were not blinded to the contrast-enhanced studies. The use of the coronary angiogram as the gold standard for ischemia has some limitations. It is well known that it is possible to have ischemia in specific areas without angiographically significant stenosis in the epicardial arteries supplying that area. This can be due to microvascular disease, diffuse atherosclerosis or coronary vasospasm, or other reasons.
CONCLUSIONS
The contrast agents SonoVue and Optison can enhance left ventricular endocardial border delineation in overweight or obese patients, optimizing the evaluation of left ventricular wall motion with at-rest echocardiography and during peak DSE. This increases the diagnostic value of DSE for the detection of CAD.
REFERENCES
- 1.Pellikka PA, Roger VL, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part II. Dobutamine stress echocardiography: Techniques, implementation, clinical applications, and correlations. Mayo Clin Proc. 1995;70:16–27. doi: 10.1016/S0025-6196(11)64660-0. [DOI] [PubMed] [Google Scholar]
- 2.Geleijnse ML, Fioretti PM, Roelandt JR. Methodology, feasibility, safety and diagnostic accuracy of dobutamine stress echocardiography. J Am Coll Cardiol. 1997;30:595–606. doi: 10.1016/s0735-1097(97)00206-4. [DOI] [PubMed] [Google Scholar]
- 3.Freeman AP, Giles RW, Walsh WF, Fisher R, Murray IP, Wilcken DE. Regional left ventricular wall motion assessment: Comparison of two-dimensional echocardiography and radionuclide angiography with contrast angiography in healed myocardial infarction. Am J Cardiol. 1985;56:8–12. doi: 10.1016/0002-9149(85)90556-9. [DOI] [PubMed] [Google Scholar]
- 4.Marwick T, D’Hondt AM, Baudhuin T, et al. Optimal use of dobutamine stress for the detection and evaluation of coronary artery disease: Combination with echocardiography or scintigraphy, or both? J Am Coll Cardiol. 1993;22:159–67. doi: 10.1016/0735-1097(93)90830-t. [DOI] [PubMed] [Google Scholar]
- 5.Kasprzak JD, Paelinck B, Ten Cate FJ, et al. Comparison of native and contrast-enhanced harmonic echocardiography for visualization of left ventricular endocardial border. Am J Cardiol. 1999;83:211–7. doi: 10.1016/s0002-9149(98)00826-1. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Mor-Avi V, Zoghbi WA, Senior R, Klein AL, Pearlman AS. The role of contrast enhancement in echocardiographic assessment of left ventricular function. Am J Cardiol. 2002;90:28J–34J. doi: 10.1016/s0002-9149(02)02945-4. [DOI] [PubMed] [Google Scholar]
- 7.Mulvagh SL, DeMaria AN, Feinstein SB, et al. Contrast echocardiography: Current and future applications. J Am Soc Echocardiogr. 2000;13:331–42. doi: 10.1067/mje.2000.105462. [DOI] [PubMed] [Google Scholar]
- 8.Arnese M, Fioretti PM, Cornel JH, Postma-Tjoa J, Reijs AE, Roelandt JR. Akinesis becoming dyskinesis during high-dose dobutamine stress echocardiography: A marker of myocardial ischemia or a mechanical phenomenon? Am J Cardiol. 1994;73:896–9. doi: 10.1016/0002-9149(94)90819-2. [DOI] [PubMed] [Google Scholar]
- 9.Timperley J, Mitchell AR, Thibault H, Mirza IH, Becher H. Safety of contrast dobutamine stress echocardiography: A single center experience. J Am Soc Echocardiogr. 2005;18:163–7. doi: 10.1016/j.echo.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Cerqueira MD, Weissman NJ, Dilsizian V, et al. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 11.Senior R, Andersson O, Caidahl K, et al. Enhanced left ventricular endocardial border delineation with an intravenous injection of SonoVue, a new echocardiographic contrast agent: A European multicenter study. Echocardiography. 2000;17:705–11. doi: 10.1111/j.1540-8175.2000.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 12.Reilly JP, Tunick PA, Timmermans RJ, Stein B, Rosenzweig BP, Kronzon I. Contrast echocardiography clarifies uninterpretable wall motion in intensive care unit patients. J Am Coll Cardiol. 2000;35:485–90. doi: 10.1016/s0735-1097(99)00558-6. [DOI] [PubMed] [Google Scholar]
- 13.Zilberman MV, Witt SA, Kimball TR. Is there a role for intravenous transpulmonary contrast imaging in pediatric stress echocardiography? J Am Soc Echocardiogr. 2003;16:9–14. doi: 10.1067/mje.2003.41. [DOI] [PubMed] [Google Scholar]
- 14.Mathias W, Arruda AL, Andrade JL, Filho OC, Porter TR. Endocardial border delineation during dobutamine infusion using contrast echocardiography. Echocardiography. 2002;19:109–14. doi: 10.1046/j.1540-8175.2002.00109.x. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo M, Vono MC, Toncelli L, et al. The feasibility and usefulness of contrast exercise echocardiography for the assessment of left ventricular function in master athletes. Eur J Echocardiogr. 2005;6:24–30. doi: 10.1016/j.euje.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi M, Miyazaki C, Yoshitani H, Otani S, Sakamoto K, Yoshikawa J. Which is the better method in detecting significant left anterior descending coronary artery stenosis during contrast-enhanced dobutamine stress echocardiography: Coronary flow velocity reserve or wall-motion assessment? J Am Soc Echocardiogr. 2003;16:614–21. doi: 10.1016/s0894-7317(03)00280-3. [DOI] [PubMed] [Google Scholar]
- 17.Dolan MS, Riad K, El-Shafei A, et al. Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients. Am Heart J. 2001;142:908–15. doi: 10.1067/mhj.2001.117608. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong WF, Zoghbi WA. Stress echocardiography: Current methodology and clinical applications. J Am Coll Cardiol. 2005;45:1739–47. doi: 10.1016/j.jacc.2004.12.078. [DOI] [PubMed] [Google Scholar]
- 19.Schinkel AF, Bax JJ, Geleijnse ML, et al. Noninvasive evaluation of ischaemic heart disease: Myocardial perfusion imaging or stress echocardiography? Eur Heart J. 2003;24:789–800. doi: 10.1016/s0195-668x(02)00634-6. [DOI] [PubMed] [Google Scholar]