Abstract
Congenital absence of the pericardium is a rare defect of which recognition is critical because it can be associated with catastrophic outcomes. While some carriers of this condition may present with a lethal complication, most are asymptomatic, and the defect is found incidentally. The case of a 49-year-old woman is described who presented with acute myocardial necrosis and absence of obstructive coronary artery disease, and in whom a complete left pericardial defect was found. An investigation was undertaken to determine the role of the defect in this patient’s presentation and to recommend the best possible therapy. Finally, a review of literature focusing on congenital absence of the pericardium, as well as a discussion of clinical presentation, imaging techniques and therapeutic options, is also presented.
Keywords: Congenital heart disease, Magnetic resonance imaging, Pericardium
Abstract
L’absence congénitale du péricarde est une malformation rare dont la reconnaissance est cruciale parce qu’elle peut donner lieu à des situations dramatiques. Certains porteurs peuvent connaître une issue fatale, mais la plupart des personnes atteintes ne présentent pas de symptômes, et la malformation est découverte par hasard. Suit la description du cas d’une femme de 49 ans, qui a consulté pour une nécrose aiguë du myocarde en l’absence d’une coronaropathie obstructive et chez qui on a découvert l’absence complète du péricarde gauche. On a donc entrepris une exploration afin de déterminer le rôle de la malformation sur les troubles ayant motivé la consultation et de recommander le meilleur traitement possible. L’article fera également état de l’examen de la documentation sur l’absence congénitale du péricarde et présentera une discussion sur le tableau clinique, les techniques d’imagerie et les différents traitements possibles.
Congenital absence of the pericardium is a rare condition (1). With contemporary imaging techniques, an increase of incidental pericardial defects has arisen. Significant concern comes from potential life-threatening complications. We report a case of congenital absence of the pericardium that was discovered in the throes of acute myocardial necrosis. Literature is reviewed, and investigative and therapeutic strategies are discussed.
CASE PRESENTATION
A 49-year-old woman required medical attention for an episode of acute respiratory distress leading to syncope. She reported a history of uncontrolled asthma in the prior weeks, which led her to excessively self-medicate with inhaled beta-adrenergic agonists. She nevertheless had excellent exercise capacity. She never felt any chest, jaw or left arm discomfort, or had any palpitations. The patient was an active smoker, but did not have other coronary risk factors. Her past medical history included mild asthma, panic disorder and depression. She also described syncope of an unknown origin, which was investigated when she was 20 years of age. At that time, she was informed that she had a congenital cardiac anomaly, but she refused to undergo investigation and left the hospital against advice. Her physical examination was normal. The initial electrocardiogram (ECG) indicated sinus rhythm with diffuse T wave inversion and a prolonged QT interval (corrected QT 560 ms). Levels of creatine kinase (876 U/L; normal less than 165 U/L) and troponin T (0.17 μg/L; normal 0.0 μg/L to 0.02 μg/L) were elevated. A D-dimer test was normal. The patient was diagnosed with acute non-ST segment elevation myocardial infarction, treated with standard pharmacological therapies and transferred to the Quebec Heart Institute at Laval Hospital (Quebec, Quebec).
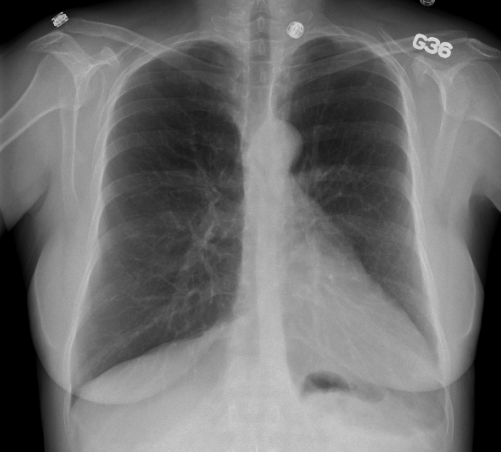
Urgent selective coronary angiography revealed right dominance and angiographically smooth coronary arteries, with Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow and normal blush. Ventriculography showed normal left ventricular ejection fraction (LVEF) and absence of mitral regurgitation, but a laterally displaced and vertically oriented heart. A follow-up ECG indicated the return of T waves to baseline, with slow R wave progression in the precordial leads; the QT interval also normalized. The chest x-ray (Figure 1) was notable for leftward displacement of the heart and rectification of the left heart border, suggestive of pericardial absence. Echocardiography was technically challenging because of poor echogenicity, unusual heart orientation within the chest cavity and significant cardiac motion. The patient’s regional LVEF was normal (55%).
Figure 1).
Posteroanterior chest roentgenogram showing the leftward displacement of the heart and rectification of the left heart border, suggestive of left pericardial absence
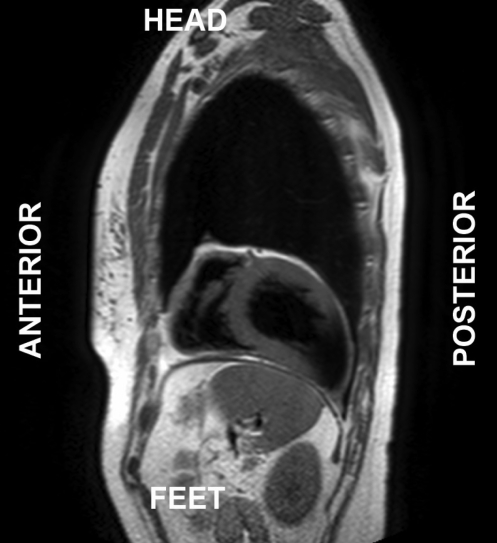
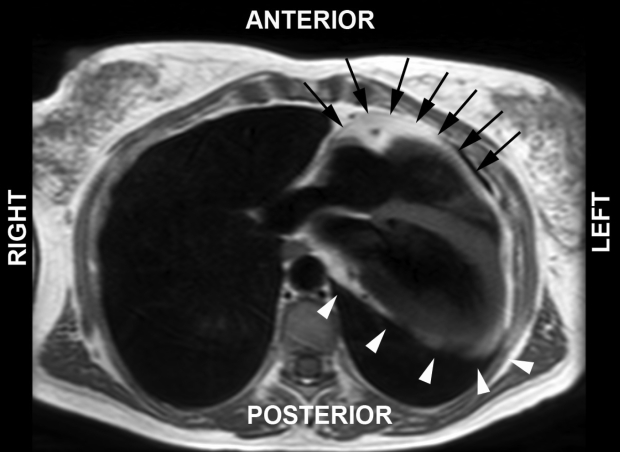
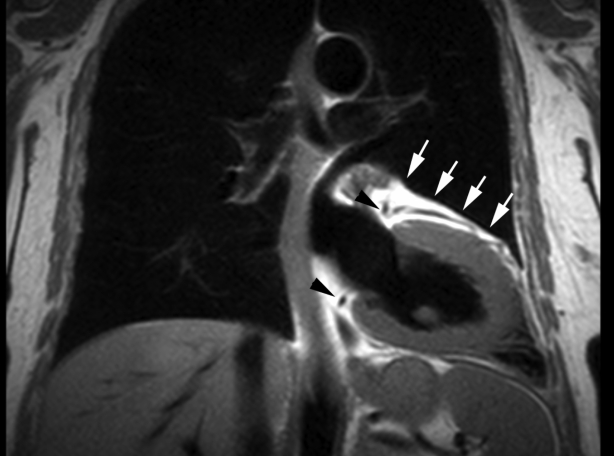
Cardiac magnetic resonance (CMR) imaging was performed to better define cardiac morphology and function. This confirmed the leftward position of the heart and found that the apex was orientated posteriorly (Figure 2). CMR imaging demonstrated the absence of pericardial recesses at the level of the great vessels. A lung was visible between the aorta and pulmonary artery, indicating the absence of pericardium at this level. The pericardium was visible only overlying the base and middle portions of the right ventricle, and was absent over the entire LV, indicating a total left absence of pericardium (Figures 3 and 4). No coronary artery anomalies or compression were noted. Left and right ventricular functions were within normal limits by volumetric analysis (LVEF 65%), as were cavity sizes. No defects of the atrial or ventricular septa were noted. No myocardial scar was observed on delayed-enhancement imaging. Multislice computed tomography (CT) was performed to exclude pulmonary embolism. The position of the heart had shifted from a posterior orientation of the apex on CMR imaging to an anterior orientation on CT, underlining the great mobility of the heart with a pericardial defect.
Figure 2).
T1-weighted cardiac magnetic resonance image in the sagittal view illustrating the apex oriented toward the posterior costodiaphragmatic sulcus. Pericardial absence allowed significant displacement of the heart within the chest cavity
Figure 3).
T1-weighted cardiac magnetic resonance image in the axial view indicating presence of the pericardium overlying the right ventricle (black arrows), while no pericardium is present overlying the left ventricle (white arrowheads). In this imaging sequence, the pericardium overlying the right ventricle is inferred from the dark stripe sandwiched between bright epicardial and pericardial fat. Both bright pericardial fat and dark pericardial stripes are absent overlying the left ventricle
Figure 4).
T1-weighted cardiac magnetic resonance image in the coronal view indicating the left anterior descending coronary artery coursing within epicardial fat (white arrows). In this imaging sequence, the pericardium would normally be inferred from a dark stripe sandwiched between bright epicardial and pericardial fat. The absence of pericardium here results in a single layer of fat, known to be epicardial because of the coursing coronary artery. Also note the normal positions of the left main and right coronary arteries in this patient (black arrowheads)
At this point in the investigation, a copy of the patient’s hospital chart was received from another admission for a similar episode 29 years earlier. The final diagnosis was listed as syncope of an unknown origin. Investigation at that time suggested a congenital pericardial defect.
During the course of the current hospitalization, the patient described recurrent episodes of dyspnea clearly related to psychological stressors. A psychiatric evaluation confirmed the presence of a panic disorder. Asthma was, in fact, excluded by a functional challenge. In an ultimate attempt to objectively clarify any limitations associated with the congenital absence of a pericardium, the patient performed an exercise stress test by the standard Bruce protocol, during which she completed 10 metabolic equivalents with no symptoms or ECG changes. Potential risks and benefits of surgical pericardiotomy and pericardioplasty were discussed with the patient. Watchful waiting was ultimately recommended, and the patient was discharged with regular follow-up.
DISCUSSION
We report the case of a patient who presented with acute dyspnea and syncope in the context of a panic attack and laboratory evidence of acute myocardial necrosis. The possibility of a pericardial defect was raised by a typical chest roentgenogram and coronary angiography. CMR imaging confirmed the diagnosis of a complete left pericardial defect. Extensive investigation did not identify any etiology for the myocardial damage in this patient. She was discharged with watchful waiting instead of proceeding to surgery after a careful assessment of risks and benefits. To our knowledge, this is the first report of a congenital pericardial defect presenting as acute myocardial necrosis in the absence of coronary artery disease or evidence of impingement by a pericardial rim. A congenital pericardial defect can be associated with catastrophic outcomes (1). For that reason, making the diagnosis is critical (2). In an era of modern imaging, pericardial defects are encountered with increasing frequency, and the possibility of a ‘red herring’ increases. Cardiologists can expect to be solicited for assistance in the diagnosis and management of this challenging condition.
Pericardial defects are rare. They are associated with other congenital abnormalities in 30% to 50% of patients, mostly cardiac and pulmonary in nature (atrial septal defect, patent ductus arteriosus, tetralogy of Fallot, bronchogenic cysts and pulmonary sequestrations) (1). Chest wall abnormalities have also been described. Pericardial absence is classified as a left or right defect. It is divided secondarily as being total or partial. The most commonly reported pericardial defect is by far left-sided, with left total defects more frequent than the left partial defects. Right-sided and bilateral absence have seldom been reported. The suspected etiology of a pericardial defect is the premature atrophy of the left common cardinal vein (duct of Cuvier). The consequent reduced blood supply to the left pleuropericardial membrane results in pericardial agenesis (3).
The clinical presentation is nonspecific. In most reported cases, the defect was discovered incidentally in an asymptomatic patient (3). For subjects who present with symptoms, atypical chest pain, dyspnea, trepopnea and palpitations are most commonly encountered. A physical examination may reveal a laterally displaced apex and systolic murmurs and clicks of undetermined origin (2). Patients may also present with complications clearly related to the defect. Most commonly cited are herniation and incarceration of the myocardium, predominantly the left atrial appendage (4–6). Ventricular herniation has also been described (5). Other complications include torsion of the great vessels secondary to increased cardiac mobility within the chest cavity, coronary artery compression by the edge of the pericardial defect, and cardiac traction due to adherence of the epicardium to other chest structures in the absence of the pericardial and pleural membranes (1). All these complications have been associated with presentations varying from chest pain to infarction, syncope (7), tricuspid regurgitation (1) and sudden death (8).
With such a wide range of nonspecific symptoms, diagnostic testing is the basis for raising suspicion and confirming the pericardial defect. ECG, chest x-ray, echocardiography (9,10), and even coronary angiography, may all serve as a basis for clinical suspicion, showing features mostly resulting from levoposition of the heart.
Although not pathognomonic, an ECG usually presents typical findings. The most common observation is right axis deviation, with complete or incomplete right bundle branch block or poor R-wave progression throughout the precordial leads. Classic features of the chest x-ray include levoposition of the heart, confirmed by the absence of the right heart border projecting on the right of the vertebral column, as well as left cardiac border straightening and elongation (‘Snoopy sign’), and the deep or very well-defined aortopulmonary window caused by the absence of pleura allowing lung to invaginate into this space. Echocardiographic findings are also related to cardiac levoposition and increased mobility within the chest cavity. These include unusual echocardiographic windows, marked change in cardiac position with changing patient position on the examination table (cardioptosis) and with the cardiac cycle (swinging heart), abnormal septal motion, and false-positive appearance of right cavity dilation (3,9).
Ultimately, the diagnosis is made by advanced imaging techniques such as CMR or CT (2,3,11,12). These modalities not only confirm the diagnosis, they also delineate the extent of the defect, which provides important information in the subsequent management of patients. Furthermore, advanced imaging allows exclusion of complications associated with the pericardial defect. CMR is the gold standard because it exquisitely visualizes the pericardium while providing additional information critical to decision making, most notably whether significant focal infarction has occurred. Delayed-enhancement CMR can detect infarction as light as 1.6 g, representing roughly 1.5% of the LV myocardial mass, even when no underlying function defect is identified (13). The features of congenital absence of the pericardium observed on CMR include left-sided shift of the heart, lung interposition between the aorta and pulmonary artery, as well as the precise location and extent of the pericardial absence.
Therapeutic options are chiefly based on small, retrospective series. It is recommended that patients presenting with complications be treated surgically (1,2,11,14). For patients in whom no complications have arisen, various strategies are proposed. A common approach is to estimate the risk of life-threatening complications (mostly herniation) based on the size of the pericardial defect. Total left defects, because they carry very little risk, usually warrant no surgical treatment (1). Left partial pericardial defects are more complex and controversial. While some authors consider all partial pericardial defects to be high-risk, irrespective of defect size, others divide the risk according to the size of the defect (15). Using this size-related approach, asymptomatic, large partial pericardial defects are not surgically treated because they are considered to carry a low risk of complications compared with complete defects (15). Small- and moderate-sized left pericardial defects are considered for prophylactic surgery by some, while others suggest treating only symptomatic patients (1,2,15). Watchful waiting appears to entail a good prognosis in such asymptomatic patients (1). Surgical options include pericardiotomy and pericardioplasty. The former enlarges the defect to reduce the risk of incarceration. Pericardioplasty attempts to achieve the same goal either by primary closure with simple suture or complete reconstruction with synthetic materials. Postpericardiotomy syndrome appears to be common following these surgeries (2).
In the present case, we were challenged by the unique situation of a patient with a total left pericardial defect who presented with myocardial necrosis, despite having normal coronary arteries and no evidence of impingement. Despite an extensive work-up, the etiology remained unclear. We considered several hypotheses to establish the relationship between the pericardial defect and myocardial necrosis: transient herniation of the left atrial appendage (4,6,16) or the LV (5), torsion of the great vessels due to cardiac hypermobility or impingement of a coronary artery by the edge of the pericardial defect (17,18). We also considered that these two conditions may be unrelated, and that the pericardial defect was solely an incidental finding. Myocardial necrosis might have resulted from coronary vasospasm or excessive demand on the myocardium associated with excessive beta-agonist use (19). The absence of focal necrosis on delayed-enhancement CMR, which can detect minuscule focal necrosis in the absence of a functional defect, indicates that causes related to the interruption of flow in a single coronary artery are highly unlikely (13). In this setting, the most likely cause remains excessive demand. No evidence of a clear link was found between myocardial necrosis and the pericardial defect. Keeping in mind that this patient presented with a similar episode 29 years earlier and did well in the interim, we decided to observe her with regular follow-up rather than consider surgery.
Footnotes
FUNDING: Dr Larose is supported, in part, by the Quebec Heart Institute Foundation, Quebec.
REFERENCES
- 1.Van Son JA, Danielson GK, Schaff HV, Mullany CJ, Julsrud PR, Breen JF. Congenital partial and complete absence of the pericardium. Mayo Clin Proc. 1993;68:743–7. doi: 10.1016/s0025-6196(12)60630-2. [DOI] [PubMed] [Google Scholar]
- 2.Gatzoulis MA, Munk MD, Merchant N, Van Arsdell GS, McCrindle BW, Webb GD. Isolated congenital absence of the pericardium: Clinical presentation, diagnosis, and management. Ann Thorac Surg. 2000;69:1209–15. doi: 10.1016/s0003-4975(99)01552-0. [DOI] [PubMed] [Google Scholar]
- 3.Victor AR, Osório P, Matos P, de Oliveira LM, Carrageta M. Congenital absence of left pericardium. Rev Port Cardiol. 2003;22:801–10. (Erratum in 2003;22:1272). [PubMed] [Google Scholar]
- 4.Robin E, Ganguly SN, Fowler MS. Strangulation of the left atrial appendage through a congenital partial pericardial defect. Chest. 1975;67:354–5. doi: 10.1378/chest.67.3.354. [DOI] [PubMed] [Google Scholar]
- 5.Saito R, Hotta F. Congenital pericardial defect associated with cardiac incarceration: Case report. Am Heart J. 1980;100:866–70. doi: 10.1016/0002-8703(80)90068-x. [DOI] [PubMed] [Google Scholar]
- 6.Finet G, Bozio A, Frieh JP, Cordier JF, Celard P. Herniation of the left atrial appendage through a congenital partial pericardial defect. Eur Heart J. 1991;12:1148–9. doi: 10.1093/oxfordjournals.eurheartj.a059850. [DOI] [PubMed] [Google Scholar]
- 7.Hoorntje JC, Mooyaart EL, Meuzelaar KJ. Left atrial herniation through a partial pericardial defect: A rare cause of syncope. Pacing Clin Electrophysiol. 1989;12:1841–5. doi: 10.1111/j.1540-8159.1989.tb01873.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones JW, McManus BM. Fatal cardiac strangulation by congenital partial pericardial defect. Am Heart J. 1984;107:183–5. doi: 10.1016/0002-8703(84)90160-1. [DOI] [PubMed] [Google Scholar]
- 9.Connolly HM, Click RL, Schattenberg TT, Seward JB, Tajik AJ. Congenital absence of the pericardium: Echocardiography as a diagnostic tool. J Am Soc Echocardiogr. 1995;8:87–92. doi: 10.1016/s0894-7317(05)80362-1. [DOI] [PubMed] [Google Scholar]
- 10.Letanche G, Gayet C, Souquet PJ, et al. [Agenesis of the pericardium: Clinical, echocardiographic and MRI aspects.] Rev Pneumol Clin. 1988;44:105–9. [PubMed] [Google Scholar]
- 11.Abbas AE, Appleton CP, Liu PT, Sweeney JP. Congenital absence of the pericardium: Case presentation and review of literature. Int J Cardiol. 2005;98:21–5. doi: 10.1016/j.ijcard.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Yamano T, Sawada T, Sakamoto K, Nakamura T, Azuma A, Nakagawa M. Magnetic resonance imaging differentiated partial from complete absence of the left pericardium in a case of leftward displacement of the heart. Circ J. 2004;68:385–8. doi: 10.1253/circj.68.385. [DOI] [PubMed] [Google Scholar]
- 13.Ricciardi MJ, Wu E, Davidson CJ, et al. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–3. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- 14.Funken JC, d’Udekem d’Acoz Y, Noirhomme P. Congenital pericardial defect. Cardiovasc Surg. 2002;10:618–9. doi: 10.1016/s0967-2109(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 15.Skalski J, Wites M, Haponiuk I, et al. A congenital defect of the pericardium. Thorac Cardiovasc Surg. 1999;47:401–4. doi: 10.1055/s-2007-1013186. [DOI] [PubMed] [Google Scholar]
- 16.Chapman JE, Rubin JW, Gross CM, Janssen ME. Congenital absence of pericardium: An unusual cause of atypical angina. Ann Thorac Surg. 1988;45:91–3. doi: 10.1016/s0003-4975(10)62407-1. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen DQ, Wilson RF, Bolman RM, Park SJ. Congenital pericardial defect and concomitant coronary artery disease. Ann Thorac Surg. 2001;72:1371–3. doi: 10.1016/s0003-4975(00)02705-3. [DOI] [PubMed] [Google Scholar]
- 18.Wolff F, Fritz A, Dumény P, Eisenmann B. [Diastolic coronary prolapse in partial left pericardial agenesis.] Arch Mal Coeur Vaiss. 1987;80:206–10. [PubMed] [Google Scholar]
- 19.Au DH, Curtis JR, Every NR, McDonell MB, Fihn SD. Association between inhaled beta-agonists and the risk of unstable angina and myocardial infarction. Chest. 2002;121:846–51. doi: 10.1378/chest.121.3.846. [DOI] [PubMed] [Google Scholar]