Abstract
BACKGROUND:
The present study compared computed tomo-graphic coronary angiography (CTA) and positron emission tomography (PET) for the detection of significant anatomical coronary artery stenosis as defined by conventional invasive coronary angiography (CICA).
METHODS:
The study protocol was approved by the local ethics board, and informed consent was obtained from all patients. Of the 26 patients (mean age 57±9 years, 18 men) who prospectively underwent CTA and rubidium-82 PET before CICA, 24 patients had a history of chest pain. Images were interpreted by expert readers and assessed for the presence of anatomically significant coronary stenosis (50% luminal diameter stenosis or greater) or myocardial perfusion defects. Diagnostic test characteristics were analyzed using patient-based, territory-based, vessel-based and segment-based analyses.
RESULTS:
In the 24 patients referred for chest pain, CTA had similar sensitivity to PET, but was more specific (sensitivity 95% [95% CI 72% to 100%] versus 95% [95% CI 72% to 100%], respectively; specificity 100% [95% CI 46% to 100%] versus 60% [95% CI 17% to 93%], respectively) in the detection of patients with anatomical coronary artery stenosis of 50% or greater. On a per-segment basis of all 26 patients, CTA had a sensitivity, specificity, positive predictive value and negative predictive value of 72%, 99%, 91% and 95%, respectively, in all coronary segments.
CONCLUSIONS:
Coronary CTA has a similar sensitivity and specificity to rubidium-82 PET for the identification of patients with significant anatomical coronary artery disease.
Keywords: Computed tomographic coronary angiography, Coronary artery disease, Diagnosis, Positron emission tomography
Abstract
CONTEXTE :
La présente étude visait à comparer la coronarographie par tomodensitométrie (CTDM) avec la tomographie par émission de positrons (TEP) en vue de la détection de sténoses anatomiques coronariennes importantes, confirmées par la coronarographie classique effractive (CCE).
MÉTHODE :
Le protocole de l’étude a reçu l’approbation du comité local d’éthique, et tous les sujets ont signé un consentement éclairé. Sur 26 patients (âge moyen : 57±9 ans; hommes : 18) qui ont subi, de façon prospective, la CTDM et la TEP au rubidium 82 avant la CCE, 24 avaient des antécédents de douleur thoracique. Les images ont été interprétées par des spécialistes, qui les ont examinées à la recherche de sténoses anatomiques coronariennes importantes, c’est-à-dire égales ou supérieures à 50 % de la lumière des vaisseaux, ou d’images lacunaires d’irrigation. Il y a eu analyse des caractéristiques des examens de diagnostic en fonction des patients, des territoires, des vaisseaux et des segments.
RÉSULTATS :
Chez les 24 patients dirigés en radiologie pour des douleurs thoraciques, la CTDM avait une sensibilité comparable à celle de la TEP mais une spécificité plus grande que celle-ci (sensibilité : 95 % [IC : 72 % à 100 %] contre 95 % [IC : 72 % à 100 %], respectivement; spécificité : 100 % [IC : 46 % à 100 %] contre 60 % [IC : 17 % à 93 %], respectivement) en ce qui concerne la détection des sténoses anatomiques coronariennes, égales ou supérieures à 50 %. Quant à l’analyse par segment chez les 26 sujets, la CTDM avait une sensibilité, une spécificité, une valeur prédictive positive et une valeur prédictive négative de 72 %, 99 %, 91 % et 95 % respectivement, et ce, dans tous les segments coronariens.
CONCLUSION :
La CTDM a une sensibilité et une spécificité comparables à celles de la TEP au rubidium 82 pour la détection des sténoses anatomiques coronariennes importantes.
Noninvasive coronary angiography using multidetector computed tomography (MDCT) has the ability to accurately (sensitivity 98%, specificity 86%) detect significant anatomical coronary artery stenosis as defined by conventional invasive coronary angiography (CICA) (1–20). While CT coronary angiography (CTA) may be an attractive alternative to CICA, it relies completely on the visualization of the coronary anatomy.
In contrast, traditional, noninvasive modalities (treadmill exercise stress test, myocardial perfusion imaging [MPI] and stress echocardiography) rely on the identification of stress-induced myocardial ischemia by way of electrocardiographic (ECG) changes, myocardial perfusion defects or impairment of left ventricular function. Of these traditional noninvasive modalities, rubidium-82 (Rb-82) positron emission tomography (PET) appears to have superior sensitivity (87% to 100%) and specificity (73% to 100%) for the identification of patients with significant anatomical coronary stenosis (21–24).
Despite the widespread availability and accuracy of traditional noninvasive modalities, up to 30% to 40% of patients still require CICA for the anatomical diagnosis of coronary artery disease (CAD) (25). Whether CTA (anatomical measure of coronary stenosis) and PET (functional assessment of myocardial ischemia) are mutually exclusive or complementary in the assessment of patients has not been studied. Thus, the objective of the present study was to compare the operating characteristics of coronary CTA and Rb-82 PET MPI for the identification of patients with significant anatomical CAD as defined by CICA.
METHODS
Study population and design
Between October 2004 and September 2005, 31 patients requiring CICA for either the diagnosis of CAD or anatomical risk stratification were referred to the study. Patients were excluded if they were younger than 18 years of age, if they had previous coronary revascularization, atrial fibrillation or other arrhythmias, lack of informed consent, renal insufficiency, or if they had a contraindication to iodinated intravenous contrast agents, radiation exposure, dipyridamole or atrioventricular nodal-blocking agents. The study protocol was approved by the local human research ethics board.
Of the 31 patients referred, three patients were not enrolled because of a previous revascularization (one patient) and frequent ventricular ectopy (two patients). Of the 28 patients prospectively enrolled into the single-centre study, two were excluded for having incomplete data (one patient refused PET due to claustrophobia and one patient had incomplete CICA data – the right coronary artery could not be cannulated due to a severely dilated aortic root).
CICA
The coronary arteries were visualized in multiple orthogonal views. A 17-segment model was used in the assessment of angiographic results (2,26), and quantitative coronary angiography (Inturis for Cardiology, version 1.1; Philips Medical Systems, Netherlands) was performed by an experienced observer blinded to all clinical data. Visual analysis of coronary stenosis was made in segments with a luminal diameter of less than 1.5 mm. Coronary artery stenosis was considered significant if the luminal diameter stenosis was 50% or greater. A luminal diameter stenosis of 70% or greater was used for the calculation of the Duke Jeopardy score (27). Disagreements with visual angiographic interpretations were resolved by consensus.
CTA
Before CTA, intravenous metoprolol or diltiazem (targeting a heart rate of less than 65 beats/min) and sublingual nitroglycerine (0.8 mg) were administered.
Retrospective ECG-gated data sets were acquired with the GE LightSpeed 16-slice MDCT (GE Healthcare, USA), with 16 mm × 0.625 mm slice collimation, gantry rotation of 500 ms (350 mA to 440 mA, 120 kV), scan interval of 0.625 mm, table speed of 1.25 mm and pitch of 0.3:1. A timing bolus was used to optimize the interval between intravenous contrast (iodixanol) infusion (4 mL/s) and image acquisition. The MDCT data sets were reconstructed using the cardiac phase(s) with the least cardiac motion.
CTA image analysis
ECG-gated MDCT images were processed using the Advantage Workstation (version 4.1, GE Healthcare, USA) and interpreted by two expert observers blinded to all clinical data. Each coronary artery was assessed using axial images, multiplanar reformations, curved multiplanar reformations and maximal-intensity projections. A 17-segment model of the coronary arteries (2,26), and four-point grading score (normal, mild [less than 50%], moderate [50% to 69%], severe [70% or greater]) was used for the evaluation of coronary stenosis. All coronary segments were assessed and graded, regardless of quality and ‘evaluability’. Disagreements with CTA image analysis were resolved by a third expert reader.
Dipyridamole stress Rb-82 PET
Dipyridamole (0.14 mg/kg/min) was infused over 5 min (28). Eight minutes after initiation of dipyridamole infusion, Rb-82 was administered, followed by aminophylline (2 mg/kg) at 12 min. Rest and dipyridamole stress Rb-82 PET MPI have been previously described (28,29). In brief, the PET images were acquired with an ECAT ART whole-body scanner (Siemens/CTI, USA). A 4 min cesium-137 transmission scan was acquired to confirm patient positioning and for attenuation correction of the rest Rb-82 data (30). After the acquisition of transmission data, Rb-82 (0.08 mCi/kg to 0.22 mCi/kg [3 MBq/kg to 8 MBq/kg]) was infused over 30 s at rest and with stress. A 10 min dynamic acquisition was obtained, and static uptake images were created by summing the last 7.5 min of dynamic data. Stress imaging was performed 10 min after completion of rest image acquisition. A second 4 min transmission scan was then acquired for attenuation correction of the dipyridamole stress scans.
PET image analysis
The PET images were assessed qualitatively by two expert observers blinded to all clinical data. Observers subjectively and semiquantitatively categorized each patient into presence or absence of significant CAD; single-, double- or triple-vessel disease; and involved vascular territories (left anterior descending, left circumflex and right coronary artery). Using a 17-segment model and a five-point grading system (0 – normal, 1 – mild, 2 – moderate, 3 – severe and 4 – absence of radiotracer uptake), summed stress score (SSS), summed rest score and summed difference scores were calculated (31,32). Patients were also categorized into the presence or absence of significant CAD based on SSS (CAD present if SSS was greater than 3.5) (33). All disagreements with PET image interpretation were resolved by a third expert reader.
Perfusion quantification
When the Rb-82 dose (3 MBq/kg) permitted the quantification of blood flow, Rb-82 retention was calculated using an automated program (34). Rb-82 retention was assessed according to vascular territories and significant abnormal flow reserve was deemed present if the defect size (more than 2 SD below normal) was 10% or more of the respective vascular territory (34).
Statistical analysis
SPSS version 11.5 (SPSS Inc, USA) was used for statistical analysis. Continuous variables were reported as frequencies and mean ± SD. Diagnostic test characteristics (sensitivity, specificity, positive predictive value [PPV] and negative predictive value [NPV]) are presented with 95% CI. Paired continuous variables were evaluated using the Pearson correlation and t test. Agreement of the CTA, PET and CICA categorical variables was assessed by Kappa analysis and the Z statistic.
RESULTS
Patient population
A total of 26 patients (mean age 57±9 years, 18 men) completed all three tests (Table 1). The mean time interval between CTA and CICA was 11.3±7.7 days, PET and CICA was 13.1±8.3 days, and CTA and PET was 2.7±5.5 days. Twelve patients were referred for CICA after MPI and seven after treadmill testing. Nineteen patients were found to have significant CAD on CICA, 10 patients had single-vessel disease and nine had multivessel disease (Figure 1). Seven patients did not have significant CAD. Twenty-four of the 26 patients referred for CICA had a history of chest pain, and two of the 26 patients with normal coronary arteries had nonischemic heart disease (dilated cardiomyopathy and severe aortic regurgitation).
TABLE 1.
Baseline demographics (n=26)
| Characteristic | |
|---|---|
| Mean age, years | 57±9 |
| Male sex, n (%) | 18 (69) |
| Mean body mass index, kg/m2 | 28.6±4.1 |
| Mean CTA heart rate, beats/min | 59±5 |
| Myocardial infarction, n (%) | 4 (15) |
| Cardiac risk factors, n (%) | |
| Diabetes | 4 (15) |
| Hypertension | 16 (62) |
| Dyslipidemia | 19 (73) |
| Current smoker | 4 (15) |
| Previous smoker | 14 (54) |
| Medications, n (%) | |
| Antiplatelet | 26 (100) |
| Beta-blocker | 18 (69) |
| ACEI/ARB | 18 (69) |
| Statin | 18 (69) |
| Indication for coronary angiography, n | |
| Chest pain | 24 |
| Typical angina | 17 |
| Valvular heart disease | 1 |
| Dilated cardiomyopathy | 1 |
| Noninvasive testing before coronary angiography, n | |
| None | 7 |
| Treadmill stress test | 7 |
| Myocardial perfusion imaging | 12 |
ACEI Angiotensin-converting enzyme; ARB Angiotensin receptor blocker; CTA Computed tomographic coronary angiography
Figure 1).
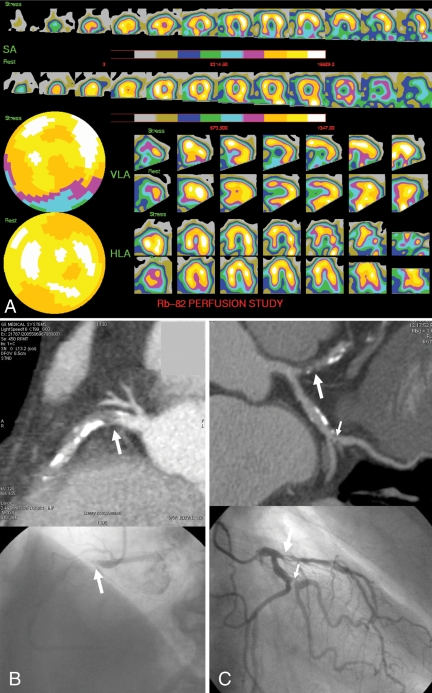
Positron emission tomography magnetic perfusion imaging, computed tomographic angiography and conventional invasive coronary angiography of a 73-year-old man with chest pain. A Positron emission tomography myocardial perfusion imaging: post-stress, a moderate (blue) reduction in rubidium-82 (Rb-82) uptake is present in the basal and mid-inferior wall, with normalization at rest. B Curved multiplanar reformation computed tomography and conventional invasive coronary angiography images demonstrating an occluded proximal right coronary artery (arrow). C Significant stenosis of the proximal left anterior descending coronary artery (large arrow) and the first marginal artery (small arrow). HLA Horizontal long axis; SA Short axis; VLA Vertical long axis
Patient-based analysis: Diagnosis of CAD
Among the 24 patients referred to CICA for chest pain, CTA appeared to have similar sensitivity and higher specificity (95% [95% CI 72% to 100%] and 100% [95% CI 46% to 100%], respectively) than dipyridamole stress Rb-82 PET (95% [95% CI 72% to 100%] and 60% [95% CI 17% to 93%], respectively) (Table 2) in the identification of patients with anatomically significant CAD. As well, the PPV and NPV appeared to be better with CTA (100% [95% CI 78% to 101%] and 83% [95% CI 36% to 99%]) than with PET (90% [95% CI 67% to 98%] and 75% [95% CI 22% to 99%], respectively). Using an SSS threshold of greater than 3.5, Rb-82 PET had a sensitivity of 89% (95% CI 64% to 98%) and specificity of 100% (95% CI 46% to 100%).
TABLE 2.
Patient-based analysis
| Diagnosis of coronary artery disease in 24 patients with chest pain
| ||||
|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | |
| CTA | 95% (18/19) | 100% (5/5) | 100% (18/18) | 83% (5/6) |
| PET (visual analysis) | 95% (18/19) | 60% (3/5) | 90% (18/20) | 75% (3/4) |
| PET (visual analysis with quantification, if available) | 95% (18/19) | 80% (4/5) | 95% (18/19) | 80% (4/5) |
| PET using SSS>3.5 | 89% (17/19) | 100% (5/5) | 89% (17/19) | 71% (5/5) |
|
Diagnosis of coronary artery disease in all patients | ||||
| CTA | 95% (18/19) | 100% (7/7) | 100% (18/18) | 88% (7/8) |
| PET (visual analysis) | 95% (18/19) | 43% (3/7) | 82% (18/22) | 75% (3/4) |
| PET (visual analysis with quantification, if available) | 95% (18/19) | 57% (4/7) | 86% (18/21) | 80% (4/5) |
| PET using SSS>3.5 | 89% (17/19) | 71% (5/7) | 89% (17/19) | 71% (5/7) |
| PET (excluding patients with nonischemic heart disease) | 95% (18/19) | 60% (3/5) | 90% (18/20) | 75% (3/4) |
| PET (excluding patients with nonischemic heart disease and patients with preceding MPI) | 90% (9/10) | 100% (2/2) | 100% (9/9) | 67% (2/3) |
Values in parentheses indicate the number of patients. CTA Computed tomographic coronary angiography; MPI Myocardial perfusion imaging; NPV Negative predictive value; PET Positron emission tomography; PPV Positive predictive value; SSS Summed stress score
The sensitivity and specificity of CTA were unchanged when all 26 patients were included in the analysis. Conversely, the sensitivity and specificity of Rb-82 PET fell when all 26 patients were included (95% and 43%, respectively, by visual analysis, and 95% and 71%, respectively, using SSS greater than 3.5). Quantification of coronary artery blood flow was available in 11 of all 26 patients. The addition of quantification information to visual PET interpretation did not change patient diagnosis in eight of the 11 patients. Two of the 11 patients who were initially misdiagnosed were correctly reclassified with the aid of quantification data, and one patient who was correctly diagnosed as having CAD by visual interpretation was incorrectly reclassified as having no CAD (Table 2).
Vessel-based and territory-based analysis
CTA was better than PET at identifying the territory of CAD (Table 3) and at identifying patients with no CAD, single-vessel disease and multivessel disease (Kappa 0.88 versus 0.46; P<0.01) (Table 4). With the addition of quantification data, there was a trend toward better agreement (Kappa 0.53) between PET and CICA in the identification of patients with no CAD, single-vessel disease and multivessel disease. Quantification of coronary blood flow also improved the detection of patients with multivessel disease (from six of nine patients to eight of nine patients) (Table 4). CTA was more successful than PET at identifying patients with none, single-, double- and triple-vessel disease (Kappa 0.73 versus 0.39; P=0.02).
TABLE 3.
Territory-based analysis: Identification of the territory of coronary artery disease with computed tomographic angiography (CTA) and positron emission tomography (PET)
| Left anterior descending artery
|
Left circumflex artery
|
Right coronary artery
|
||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |
| CTA | 92% (12/13) | 100% (13/13) | 88% (7/8) | 94% (17/18) | 81% (9/11) | 100% (15/15) |
| PET (all patients) | 77% (10/13) | 92% (12/13) | 63% (5/8) | 94% (17/18) | 91% (10/11) | 67% (10/15) |
| PET (24 patients with chest pain) | 77% (10/13) | 100% (11/11) | 63% (5/8) | 94% (15/16) | 91% (10/11) | 77% (10/13) |
Values in parentheses indicate the number of patients
TABLE 4.
Vessel-based analysis: Identification of coronary artery disease (CAD) by conventional invasive coronary angiography (CICA), computed tomographic coronary angiography (CTA) and positron emission tomography (PET)
| CTA versus CICA
| ||||
|---|---|---|---|---|
| CICA
|
||||
| No CAD | Single-vessel CAD | Multivessel CAD | ||
| No CAD | 7 | 1 | 0 | |
| CTA | Single-vessel CAD | 0 | 9 | 1 |
| Multivessel CAD | 0 | 0 | 8 | |
| Kappa 0.88 | ||||
| PET versus CICA
| ||||
|---|---|---|---|---|
| CICA
|
||||
| No CAD | Single-vessel CAD | Multivessel CAD | ||
| No CAD | 3 | 1 | 0 | |
| PET | Single-vessel CAD | 3 | 8 | 3 |
| Multivessel CAD | 1 | 1 | 6 | |
| Kappa 0.46; Z statistic 2.6; P<0.01 (PET versus CTA) | ||||
| PET (with quantification) versus CICA
| ||||
|---|---|---|---|---|
| CICA
|
||||
| No CAD | Single-vessel CAD | Multivessel CAD | ||
| No CAD | 4 | 1 | 0 | |
| PET | Single-vessel CAD | 3 | 6 | 1 |
| Multivessel CAD | 0 | 3 | 8 | |
| Values represent the number of patients | ||||
A total of 16 patients required revascularization (10 percutaneous coronary intervention [PCI], six coronary artery bypass grafting), 15 patients were successfully revascularized and one patient died while awaiting coronary artery bypass. Of the 28 vessels revascularized (12 by PCI, 16 by coronary artery bypass), 27 arteries were correctly identified with CTA and only one vessel (left circumflex artery) that underwent PCI was missed by CTA.
Segment-based analysis
All available coronary segments (398 segments; mean 15.3 segments per patient) in the 26 patients were assessed and scored (regardless of size and ‘evaluability’). The sensitivity, specificity, PPV and NPV for the detection of a significant (50% or greater) diameter luminal stenosis measured by quantitative coronary angiography was 72% (95% CI 59% to 83%), 99% (95% CI 97% to 100%), 91% (95% CI 78% to 97%), 95% (95% CI 93% to 97%). The ability of CTA to categorize stenosis (less than 50%, 50% to 69% and 70% or greater) was good (Kappa 0.69) (Table 5).
TABLE 5.
Segment-based analysis: Computed tomographic coronary angiography (CTA) versus conventional invasive coronary angiography
| Conventional invasive coronary angiography
|
|||||
|---|---|---|---|---|---|
| <50% | 50%–69% | ≥70% | Total | ||
| CTA | <50% | 336 | 5 | 11 | 352 |
| 50%–69% | 1 | 4 | 6 | 11 | |
| ≥70% | 3 | 3 | 29 | 35 | |
| Total | 340 | 12 | 46 | 398 | |
Values represent the number of identified segments. Kappa 0.69
Duke Jeopardy score
There was very good correlation (y=0.79x+0.19; r=0.87; P<0.001) between the calculated Duke Jeopardy score by CTA (3.2±3.4) and CICA (3.8±3.8).
Patient preference
On completion of all three tests (CTA, PET and CICA), patients were questioned as to which they would prefer “if one test needed to be repeated”. Of the three modalities, the majority of patients (24 of 26) preferred CTA; one patient preferred dipyridamole PET and the other preferred CICA. Both patients who preferred PET and CICA over CTA ranked CTA as their second-most preferred modality.
Hemodynamic significance
Eleven patients had quantification of coronary blood flow with PET. Excluding the two patients with nonischemic heart disease, the remaining two patients without significant CAD had no flow reserve defects (0±0%). In the seven patients with CAD, the flow reserve defect size in the 13 vascular territories supplied by stenotic arteries was 64.4±39.9% and in the eight territories without significant CAD, it was 18.6±27.3%. Among the 13 CAD vessels identified by CTA for which flow quantification was available, flow reserve was reduced in 11, confirming 85% of hemodynamically significant lesions.
DISCUSSION
We report a study that directly compared CTA with Rb-82 PET MPI. Our data suggest that 16-slice MDCT may have similar or superior accuracy to Rb-82 PET for the identification of patients with anatomical coronary stenosis and for the localization of CAD in patients without previous coronary revascularization.
CTA
The accuracy of 16-slice MDCT and 64-slice MDCT has previously been compared with CICA, and studies using the segment-based analysis have demonstrated that 16-slice CTA has an approximate sensitivity of 98% and specificity of 86% (1–20). However, many of the studies excluded ‘unevaluable’ segments and coronary arteries smaller than 2.0 mm in diameter, likely resulting in the overestimation of the accuracy of 16-slice CTA. Previously, readers have justified the exclusion of vessels with diameters larger than 2.0 mm, claiming that they may not be clinically significant. However, PCI and stenting is routinely feasible in vessels 2.25 mm in diameter or larger, and vessels 1.0 mm to 1.25 mm are considered graftable. We believe that if CTA is to become an effective and widely applicable noninvasive modality, it is important that all vessels and segments be included in the study analyses. The discrepancy in the segment analysis of our study, as well as others’, is attributed to the inclusion of all segments (regardless of size or ‘evaluability’) in our analysis.
Although 16-slice CTA may not be ready to replace coronary angiography, the present study suggests that CTA has high sensitivity and specificity for the detection of patients with significant CAD, and may be a potential alternative to other noninvasive techniques in select populations such as patients with valvular heart disease or dilated cardiomyopathy. This potential benefit of anatomical imaging may be accounted for by the heterogeneity of radiotracer uptake seen in patients with abnormal myocardium. This modality may also prove to be useful for the exclusion of CAD in patients with a previous noninvasive test result that is equivocal, nondiagnostic or discrepant with a physician’s clinical impression. These potential indications require future investigation.
Our study, along with others, confirms that CTA is highly accurate for the identification of patients with significant CAD (patient-based analysis) (1–20,35–38). Whether CTA can replace other noninvasive modalities is unknown, and larger studies are needed before any definitive conclusions can be made. Given the small sample size and excellent sensitivity and specificity of CTA in identifying patients with significant CAD, the potential incremental and complementary roles of PET could not be adequately assessed. However, CTA and MPI may have complementary roles with MPI used to assess functional stenosis identified by CTA and to assess patient prognosis.
CTA exhibits several advantages that make it an attractive non-invasive test for patients. Patients likely preferred CTA over PET and CICA because of the ease and rapidity of the CTA.
Although preferred by most patients, CTA may not be suitable for all patients, particularly those with irregular dysrrhythmias, heavily calcified native arteries, or those with a contraindication to intravenous contrast agents or radiation exposure. Patient radiation dose with CTA continues to be a concern. The total-body effective radiation doses of 16-slice and 64–slice MDCT angiography range between 4.0 mSv to 14 mSv and 4.8 mSv to 14 mSv, respectively (39), and are similar to technetium-99m single photon emission CT (9.2 mSv to 17.5 mSv) and Rb-82 PET (3.6 mSv to 21.2 mSv) (25,39–42). Given the limitations of CTA, further research is required to identify specific patient populations that may benefit most from CTA.
Currently, there are limited data supporting the prognostic value of CTA. Our study demonstrated very good correlation between CTA- and CICA-derived Duke Jeopardy scores. Because the Duke Jeopardy score has prognostic value, it suggests that CTA has the potential for patient prognostication (27,43). In the future, the ability of CTA to identify and characterize non-stenotic atherosclerotic plaque, which is not typically seen with traditional imaging, may enable further assessment of patient prognosis (38).
PET
Previous studies have demonstrated that the overall sensitivity and specificity of Rb-82 PET MPI are excellent (87% to 100% and 73% to 100%, respectively) (44). In our study, the specificity of Rb-82 PET (60%), at first glance, appeared to be low; however, this finding is consistent with previous MPI referral bias studies (45–47). Accounting for referral bias, the specificity (100%) of Rb-82 PET in our study was consistent with that of previous PET studies (21–24).
Two recent studies have examined the potential value of combining CTA with MPI. Hacker et al (48) compared CTA with MPI and demonstrated that MDCT detected reversible perfusion defects with a PPV of 29%, but this study did not confirm CTA findings with CICA (48). Namdar et al (49) reported that PET/CT enabled accurate detection of CAD and thus, may identify patients appropriate for revascularization. However, CTA interpreters were unblinded to the results of MPI, and CTA was not directly compared with MPI (49). Our study differs from the two aforementioned studies because we directly compared CTA with Rb-82 PET MPI.
Limitations
The present study was limited by its small sample size and potential patient referral bias. Because 12 of our enrolled patients were referred for CICA after MPI, this study suffered from referral bias, which may have biased the results in favour of CTA. In addition, using anatomical imaging (CICA) as the reference standard may also have biased the results in favour of CTA.
Our study also excluded patients with a history of revascularization; thus, our results may not necessarily be applicable to patients with previous coronary revascularization. Although it is often difficult to compare the two different techniques (anatomical versus functional imaging), diagnosing CAD and stratifying patients into the presence or absence of multivessel disease are important in clinical decision making, and this information may be obtained using either modality.
Because many previous CTA studies enrolled patients who were awaiting coronary angiography, CTA accuracy (patient-based analysis) was likely overestimated. A study assessing the normalcy rate of CTA is required.
The current study raised the possibility of CTA as an alternative to other noninvasive modalities in specific patient populations. The study was also limited by the use of 16-slice MDCT, with its suboptimal spatial resolution, temporal resolution (slower gantry rotation [500 ms]) and susceptibility to misregistration artifact (due to heart variability during long breath-holds). A larger diagnostic and prognostic study comparing 64-slice MDCT with conventional noninvasive modalities is required before CTA can be considered an alternative to other noninvasive modalities.
CONCLUSIONS
In a population of patients without previous coronary revascularization, CTA demonstrates a high level of accuracy in the detection of patients with significant anatomical coronary artery stenosis and may be a useful alternative to current noninvasive methods for the diagnosis of CAD.
Acknowledgments
The authors extend their gratitude to May Aung, Robert Chatelain, Sandina Cordani, Michael Donaldson, Linda Garrard, Michaela Garkish, Kim Gardner, Debbie Gauthier and Cathy Kelly for their dedication and technical expertise.
Footnotes
FUNDING: The study was supported, in part, by a grant from the American Society of Nuclear Cardiology/Bracco Diagnostics Inc Research Award, as well as the Ontario Research and Development Challenge Fund (Grant #00 May 0710).
REFERENCES
- 1.Schuijf JD, Bax JJ, Shaw LJ, et al. Meta-analysis of comparative diagnostic performance of magnetic resonance imaging and multislice computed tomography for noninvasive coronary angiography. Am Heart J. 2006;151:404–11. doi: 10.1016/j.ahj.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann U, Moselewski F, Cury RC, et al. Predictive value of 16-slice multidetector spiral computed tomography to detect significant obstructive coronary artery disease in patients at high risk for coronary artery disease: Patient-versus segment-based analysis. Circulation. 2004;110:2638–43. doi: 10.1161/01.CIR.0000145614.07427.9F. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann MH, Shi H, Schmitz BL, et al. Noninvasive coronary angiography with multislice computed tomography JAMA 20052932471–8.(Erratum in 2005;294:1208). [DOI] [PubMed] [Google Scholar]
- 4.Mollet NR, Cademartiri F, Nieman K, et al. Multislice spiral computed tomography coronary angiography in patients with stable angina pectoris. J Am Coll Cardiol. 2004;43:2265–70. doi: 10.1016/j.jacc.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Kuettner A, Trabold T, Schroeder S, et al. Noninvasive detection of coronary lesions using 16-detector multislice spiral computed tomography technology: Initial clinical results. J Am Coll Cardiol. 2004;44:1230–7. doi: 10.1016/j.jacc.2004.05.079. [DOI] [PubMed] [Google Scholar]
- 6.Nieman K, Cademartiri F, Lemos PA, Raaijmakers R, Pattynama PM, de Feyter PJ. Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation. 2002;106:2051–4. doi: 10.1161/01.cir.0000037222.58317.3d. [DOI] [PubMed] [Google Scholar]
- 7.Martuscelli E, Romagnoli A, D’Eliseo A, et al. Accuracy of thin-slice computed tomography in the detection of coronary stenoses. Eur Heart J. 2004;25:1043–8. doi: 10.1016/j.ehj.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Kuettner A, Beck T, Drosch T, et al. Diagnostic accuracy of noninvasive coronary imaging using 16-detector slice spiral computed tomography with 188 ms temporal resolution. J Am Coll Cardiol. 2005;45:123–7. doi: 10.1016/j.jacc.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 9.Mollet NR, Cademartiri F, Krestin GP, et al. Improved diagnostic accuracy with 16-row multi-slice computed tomography coronary angiography. J Am Coll Cardiol. 2005;45:128–32. doi: 10.1016/j.jacc.2004.09.074. [DOI] [PubMed] [Google Scholar]
- 10.Kefer J, Coche E, Legros G, et al. Head-to-head comparison of three-dimensional navigator-gated magnetic resonance imaging and 16-slice computed tomography to detect coronary artery stenosis in patients. J Am Coll Cardiol. 2005;46:92–100. doi: 10.1016/j.jacc.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 11.Heuschmid M, Kuettner A, Schroeder S, et al. ECG-gated 16-MDCT of the coronary arteries: Assessment of image quality and accuracy in detecting stenoses. AJR Am J Roentgenol. 2005;184:1413–9. doi: 10.2214/ajr.184.5.01841413. [DOI] [PubMed] [Google Scholar]
- 12.Cademartiri F, Marano R, Luccichenti G, et al. Image assessment with multislice CT coronary angiography. Radiol Med (Torino) 2005;109:198–207. [PubMed] [Google Scholar]
- 13.Cademartiri F, Runza G, Marano R, et al. Diagnostic accuracy of 16-row multislice CT angiography in the evaluation of coronary segments. Radiol Med (Torino) 2005;109:91–7. [PubMed] [Google Scholar]
- 14.Morgan-Hughes GJ, Roobottom CA, Owens PE, Marshall AJ. Highly accurate coronary angiography with submillimetre, 16 slice computed tomography. Heart. 2005;91:308–13. doi: 10.1136/hrt.2004.034892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgstahler C, Beck T, Kuettner A, et al. Image quality and diagnostic accuracy of 16-slice multidetector spiral computed tomography for the detection of coronary artery disease in elderly patients. J Comput Assist Tomogr. 2005;29:734–8. doi: 10.1097/01.rct.0000181720.95146.d4. [DOI] [PubMed] [Google Scholar]
- 16.Kuettner A, Beck T, Drosch T, et al. Image quality and diagnostic accuracy of non-invasive coronary imaging with 16 detector slice spiral computed tomography with 188 ms temporal resolution. Heart. 2005;91:938–41. doi: 10.1136/hrt.2004.044735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achenbach S, Ropers D, Pohle FK, et al. Detection of coronary artery stenoses using multi-detector CT with 16×0.75 collimation and 375 ms rotation. Eur Heart J. 2005;26:1978–86. doi: 10.1093/eurheartj/ehi326. [DOI] [PubMed] [Google Scholar]
- 18.Aviram G, Finkelstein A, Herz I, et al. Clinical value of 16-slice multi-detector CT compared to invasive coronary angiography. Int J Cardiovasc Intervent. 2005;7:21–8. doi: 10.1080/14628840510011207. [DOI] [PubMed] [Google Scholar]
- 19.Garcia MJ, Lessick J, Hoffmann MH, CATSCAN Study Investigators Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296:403–11. doi: 10.1001/jama.296.4.403. [DOI] [PubMed] [Google Scholar]
- 20.Hamon M, Biondi-Zoccai GG, Malagutti P, et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: A meta-analysis. J Am Coll Cardiol. 2006;48:1896–910. doi: 10.1016/j.jacc.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Go RT, Marwick TH, MacIntyre WJ, et al. A prospective comparison of rubidium-82 PET and thallium-201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med. 1990;31:1899–905. [PubMed] [Google Scholar]
- 22.Stewart RE, Schwaiger M, Molina E, et al. Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am J Cardiol. 1991;67:1303–10. doi: 10.1016/0002-9149(91)90456-u. [DOI] [PubMed] [Google Scholar]
- 23.Grover-McKay M, Ratib O, Schwaiger M, et al. Detection of coronary artery disease with positron emission tomography and rubidium 82. Am Heart J. 1992;123:646–52. doi: 10.1016/0002-8703(92)90502-m. [DOI] [PubMed] [Google Scholar]
- 24.Marwick TH, Nemec JJ, Stewart WJ, Salcedo EE. Diagnosis of coronary artery disease using exercise echocardiography and positron emission tomography: Comparison and analysis of discrepant results. J Am Soc Echocardiogr. 1992;5:231–8. doi: 10.1016/s0894-7317(14)80342-8. [DOI] [PubMed] [Google Scholar]
- 25.Chow BJ, Hoffmann U, Nieman K. Computed tomographic coronary angiography: An alternative to invasive coronary angiography. Can J Cardiol. 2005;21:933–40. [PubMed] [Google Scholar]
- 26.Kuettner A, Kopp AF, Schroeder S, et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in patients with angiographically proven coronary artery disease. J Am Coll Cardiol. 2004;43:831–9. doi: 10.1016/j.jacc.2003.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Graham MM, Faris PD, Ghali WA, et al. APPROACH Investigators (Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease Validation of three myocardial jeopardy scores in a population-based cardiac catheterization cohort. Am Heart J. 2001;142:254–61. doi: 10.1067/mhj.2001.116481. [DOI] [PubMed] [Google Scholar]
- 28.Chow BJ, Ananthasubramaniam K, dekemp RA, Dalipaj MM, Beanlands RS, Ruddy TD. Comparison of treadmill exercise versus dipyridamole stress with myocardial perfusion imaging using rubidium-82 positron emission tomography. J Am Coll Cardiol. 2005;45:1227–34. doi: 10.1016/j.jacc.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Klocke FJ, Baird MG, Lorell BH, et al. American College of Cardiology; American Heart Association; American Society for Nuclear ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging – executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003;42:1318–33. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Townsend DW, Beyer T, Jerin J, Watson CC, Young J, Nutt R. The ECAT ART scanner for positron emission tomography. 1. Improvements in performance characteristics. Clin Positron Imaging. 1999;2:5–15. doi: 10.1016/s1095-0397(98)00057-0. [DOI] [PubMed] [Google Scholar]
- 31.Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: Differential stratification for risk of cardiac death and myocardial infarction Circulation 199897535–43.(Erratum in 1998;98:190). [DOI] [PubMed] [Google Scholar]
- 32.Imaging guidelines for nuclear cardiology procedures, part 2. American Society of Nuclear Cardiology. J Nucl Cardiol. 1999;6:G47–84. [PubMed] [Google Scholar]
- 33.Yoshinaga K, Chow BJ, Williams K, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol. 2006;48:1029–39. doi: 10.1016/j.jacc.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Parkash R, deKemp RA, Ruddy TD, et al. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease J Nucl Cardiol 200411440–49.(Erratum in 2004;11:756). [DOI] [PubMed] [Google Scholar]
- 35.Leschka S, Alkadhi H, Plass A, et al. Accuracy of MSCT coronary angiography with 64-slice technology: First experience. Eur Heart J. 2005;26:1482–7. doi: 10.1093/eurheartj/ehi261. [DOI] [PubMed] [Google Scholar]
- 36.Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–23. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 37.Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–7. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 38.Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: A comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–54. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 39.Thompson RC, Cullom SJ. Issues regarding radiation dosage of cardiac nuclear and radiography procedures. J Nucl Cardiol. 2006;13:19–23. doi: 10.1016/j.nuclcard.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Radiation dose to patients from radiopharmaceuticals. Annals of the IRCP, vol 18/1—4. Elsevier, 1987 [PubMed]
- 41.Bacharach SL, Bax JJ, Case J, et al. PET myocardial glucose metabolism and perfusion imaging: Part 1 – guidelines for data acquisition and patient preparation. J Nucl Cardiol. 2003;10:543–56. doi: 10.1016/s1071-3581(03)00648-2. [DOI] [PubMed] [Google Scholar]
- 42.American Society of Nuclear Cardiology Updated imaging guidelines for nuclear cardiology procedures, part 1. J Nucl Cardiol. 2001;8:G5–G58. doi: 10.1067/mnc.2001.112538. [DOI] [PubMed] [Google Scholar]
- 43.Califf RM, Phillips HR, Hindman MC, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985;5:1055–63. doi: 10.1016/s0735-1097(85)80005-x. [DOI] [PubMed] [Google Scholar]
- 44.Schwaiger M, Ziegler S, Nekolla SG. PET/CT: Challenge for nuclear cardiology. J Nucl Med. 2005;46:1664–78. [PubMed] [Google Scholar]
- 45.Miller TD, Hodge DO, Christian TF, Milavetz JJ, Bailey KR, Gibbons RJ. Effects of adjustment for referral bias on the sensitivity and specificity of single photon emission computed tomography for the diagnosis of coronary artery disease. Am J Med. 2002;112:290–7. doi: 10.1016/s0002-9343(01)01111-1. [DOI] [PubMed] [Google Scholar]
- 46.Cecil MP, Kosinski AS, Jones MT, et al. The importance of work-up (verification) bias correction in assessing the accuracy of SPECT thallium-201 testing for the diagnosis of coronary artery disease. J Clin Epidemiol. 1996;49:735–42. doi: 10.1016/0895-4356(96)00014-5. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz RS, Jackson WG, Celio PV, Richardson LA, Hickman JR. Accuracy of exercise 201Tl myocardial scintigraphy in asymptomatic young men. Circulation. 1993;87:165–72. doi: 10.1161/01.cir.87.1.165. [DOI] [PubMed] [Google Scholar]
- 48.Hacker M, Jakobs T, Matthiesen F, et al. Comparison of spiral multidetector CT angiography and myocardial perfusion imaging in the noninvasive detection of functionally relevant coronary artery lesions: First clinical experiences. J Nucl Med. 2005;46:1294–300. [PubMed] [Google Scholar]
- 49.Namdar M, Hany TF, Koepfli P, et al. Integrated PET/CT for the assessment of coronary artery disease: A feasibility study. J Nucl Med. 2005;46:930–35. [PubMed] [Google Scholar]