Abstract
Tissue regeneration through stem cell activation and/or cell dedifferentiation is widely distributed across the animal kingdom. By comparison, regeneration in mammals is poor and this may reflect a limited dedifferentiation potential of mature cells. Because mammalian myotubes can dedifferentiate in the presence of newt blastema extract, the present study tested the dedifferentiation induction capability of the blastema from the teleost Sternopygus macrurus (SmBE). Our in vitro data showed that SmBE did not induce cell cycle reentry of myonuclei in myotubes. Instead, SmBE caused myotubes to detach and time-lapse imaging analyses characterized the cellular events before their detachment. Furthermore, SmBE enhanced myoblast proliferation and reversibly inhibited their differentiation. These data suggest the presence of protein factors in SmBE that regulate mammalian muscle physiology and differentiation, but do not support the conservation of a dedifferentiation induction capability by the blastema of S. macrurus.
Keywords: blastema, regeneration, dedifferentiation, electric fish, muscle differentiation
INTRODUCTION
Skeletal muscles in mammals have the ability to regenerate after injury or disease. This regenerative process is characterized by the proliferation and subsequent fusion of myogenic stem cells, that is, satellite cells, to form new myotubes and muscle fibers (Campion, 1984). Recent studies have revealed that adult stem cells not associated with muscle may also contribute to muscle fiber regeneration (reviewed in Asakura, 2003). Despite the activation of potentially different stem cell populations, the regenerative capacity of skeletal muscle in mammals is limited. This is evident in injuries or degenerative diseases where repeated cycles of degeneration and regeneration eventually exhaust the ability of muscle fibers to recover. In contrast, it is well known that less derived vertebrates display a very robust regeneration response to repeated tissue loss or injury. It is also becoming clear that in some vertebrates, mature cells can reenter the cell cycle, that is, dedifferentiate, and contribute to the regeneration process (Echeverri and Tanaka, 2002; Stocum, 2004). These data warrant an understanding of the cellular and molecular mechanisms underlying dedifferentiation, and redifferentiation of cells that occur during tissue restoration in highly regenerative vertebrate species because it has the potential to reveal correlate processes in mammalian systems.
Although most animals are capable of restoring lost tissues, Urodele amphibians have been an established regeneration model system and have yielded much information about cellular and molecular processes underlying epimormic regeneration. This regeneration process begins with the closing of a wound stump by migration of epithelial cells to form a wound epithelium followed by the formation of a blastema, that is, a specialized proliferative zone of mesenchymal cells forms beneath the epidermis at the wound site. The blastema gives rise to all tissue types lost during the regeneration process. Numerous studies on urodeles indicate that mature muscle cells near the injury site can lose their differentiated state, reenter the cell cycle, and be a source of blastemal cells (Tsonis, 2000; Brockes and Kumar, 2002; Echeverri and Tanaka, 2002; Nechiporuk and Keating, 2002; Nye et al., 2003; Stocum, 2004). The current thinking associates a greater potential for regeneration with a corresponding potential for cell dedifferentiation (Tsonis, 2000; Brockes and Kumar, 2002; Nye et al., 2003; Brockes and Kumar, 2005). This is further supported by observations showing that regeneration does not proceed in the absence of cell dedifferentiation (Brockes and Kumar, 2002; Echeverri and Tanaka, 2002; Stocum, 2004; Brockes and Kumar, 2005).
Whether cell dedifferentiation is the fundamental factor that triggers a robust regeneration capacity, and the loss or inhibition of this process explains the limited regeneration potential in other vertebrates is not known. Recent studies demonstrated that terminally differentiated mammalian myotubes can be induced to dedifferentiate when stimulated with the appropriate signals. Specifically, differentiated myotubes in culture responded to protein extracts from newt blastemas by myonuclei reentering the cell cycle (McGann et al., 2001). Some myotubes were also observed to cleave and produce smaller multinucleated myotubes or proliferating mononucleated cells in culture. These data suggest that mammalian muscle retains the capacity to dedifferentiate. The lack of cellular plasticity in mammalian myotubes may be due to the absence of signals that initiate the dedifferentiation process. The prevalence of tissue replacement and restoration by means of epimorphic regeneration among vertebrate groups that are less derived than mammals (Goss, 1968) suggested to us that the muscle dedifferentiation induction capability might be conserved in regeneration blastema from other species.
Electric fish are among the most highly regenerative teleost species known (Anderson and Waxman; 1983; Kirschbaum and Meunier, 1988; Patterson and Zakon, 1997). For example, the gymnotiform Sternopygus macrurus can inexhaustibly regenerate its spinal cord, skeleton, dermis, skeletal muscle and the muscle-derived electric organ (EO) within 3-4 weeks after repeated tail amputation without scar formation (Patterson and Zakon, 1993). Immediately after tail amputation, epidermal cells at the wound margin proliferate and cover the wound within 24 hr. Regeneration then proceeds by local formation of a blastema consisting of undifferentiated ependymal and mesenchymal progenitor cells. In the blastema, the regions closest to the intact tail contain the most differentiated cells, whereas the cells toward the tip of the tail are less differentiated and mitotically active. Within a week, the regeneration blastema is visible as a swelling at the end of the tail. Over the subsequent week, this swelling elongates and, from it, cells differentiate into distinct tissue types in a process that proceeds from the most ventral and anterior regions of the blastema (Patterson and Zakon, 1993). With subsequent differentiation, cells located more peripherally continue to mature into muscle cells, while those located more centrally (farthest from the skin) show several changes as they convert to electrocytes (Unguez and Zakon, 1998). Ependymal cells fill the central canal, and the ventral portion at some levels of the regenerating spinal cord is filled with neurites and capillaries as spinal cord regeneration proceeds in a rostral to caudal gradient.
Based on the similar blastema-dependent regeneration in S. macrurus and in urodeles, we anticipated that mammalian myotubes would alter their established differentiation phenotype and be induced to dedifferentiate in the presence of protein extracts from S. macrurus blastema (SmBE). Specifically, we presumed that myonuclei in differentiated muscle fibers would re-enter the cell cycle and/or myotubes would cleave into smaller multinucleated myotubes when exposed to SmBE. We cultured C2C12 myotubes in the presence of protein extract from S. macrurus blastema under culture conditions similar to those used by McGann et al. (2001) that induced dedifferentiation of myotubes in vitro. Our studies reveal that mouse myotubes also respond to SmBE based on phenotypic changes observed in proliferating myoblasts, differentiating myoblasts, and mature myotubes. Specifically, proliferation of myoblasts was enhanced, and expression of muscle differentiation markers and fusion of myoblasts were suppressed in the presence of SmBE. This inhibition effect was reversible as myoblasts were capable of differentiating after SmBE removal. Moreover, we show that SmBE does not induce cell cycle reentry of myonuclei in differentiated myotubes or cleavage of multinucleated cells. In contrast, SmBE treatment induced a rapid calcium transient that was followed by a contraction-like behavior in multinucleated myotubes. Although these data suggest the presence factors in SmBE that regulate mammalian muscle physiology and differentiation, the absence of cell dedifferentiation under the proposed culture conditions suggests that the dedifferentiation induction capability is not conserved in S. macrurus and may be unique to newt blastema cells.
RESULTS
Characterization of 1-week and 2-week Blastema
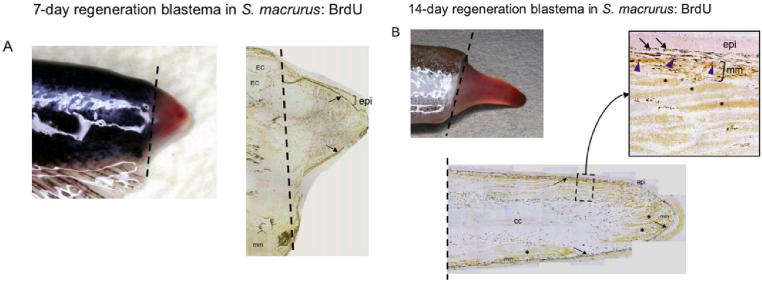
When the tip of the tail is amputated, epidermal cells at the wound margin rapidly proliferated and covered the wound within 24 hr. After 1 week, a blastema appeared as a small swelling at the end of the tail (Fig. 1A). At this stage, the blastema was generally less than 4 mm in length (Fig. 1A). Consistent with data from previous studies, the cell composition of a 1-week blastema was comprised primarily of undifferentiated mesenchymal cells (Patterson and Zakon, 1993) and bromodeoxyuridine (BrdU) -positive cells were located throughout the distal half to two-thirds of the blastema (Fig. 1A). Over the next week, this swelling elongated to an average of 7 mm in length (Fig. 1B). Within the 2-week blastema, some differentiated muscle fibers, electrocytes, and the central canal were apparent (Fig. 1B). Muscle fibers regenerated adjacent to the epithelium, whereas the larger muscle-derived electrocytes were located more central and farter away from the epithelium (Fig. 1B). These findings concur with those previously reported by Unguez and Zakon (1998). We detected a lower incidence of BrdU-positive cells in 2-week blastema compared with 1-week blastema (Fig. 1). Furthermore, unlike in 1-week blastema, BrdU-positive cells in 2-week blastema were predominantly located within muscle cells adjacent to the epithelium and not throughout the blastema (Fig. 1B, inset).
Fig. 1.
Regeneration blastemas 1-week and 2-week after tail amputation. A: Picture of a 1-week blastema formed at the base of the amputation plane (dashed line) that appears as a visible swelling at the end of the tail. A longitudinal cryosection (14 μm thick) of a 1-week blastema immunoreacted with anti-bromodeoxyuridine (BrdU) antibody shows a predominance of BrdU-positive cells in the distal half portion of the blastema. Mature electrocytes (EC) and muscle fibers (mm) are discerned proximal to the amputation plane (dashed line). B: Picture of a 2-week blastema formed at the base of the amputation plane (dashed line) that appears as a visible swelling at the end of the tail. Below the tail, we show a longitudinal cryosection (14 μm thick) of a 2-week blastema immunoreacted with anti-BrdU antibody. The presence of differentiated muscle fibers (mm) adjacent to the epithelium (epi) and more centrally located electrocytes (asterisks) was evident. A region containing BrdU-positive cells (blue arrowheads) is enlarged (dotted box) to clearly view the cells immunolabeled by anti-BrdU within the muscle fibers. The dark label adjacent to the epithelium (epi) in both A and B corresponds to melanocytes (arrows), which always display dark coloration.
Blastema Extract Does Not Induce Myotube Nuclei to Reenter the Cell Cycle
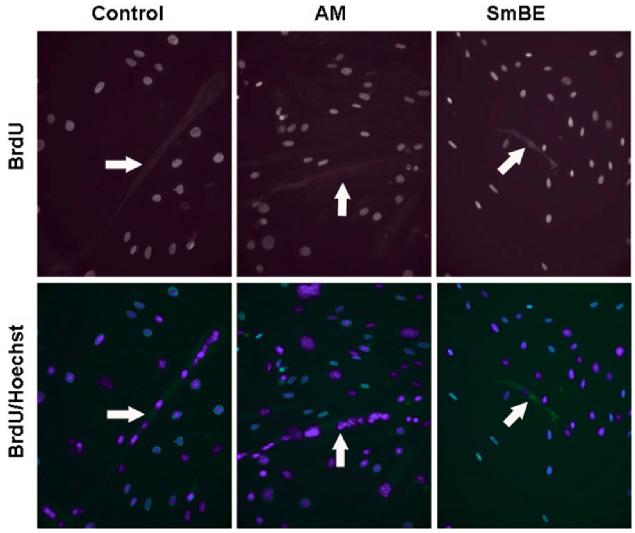
To determine whether mammalian muscle cells are capable of responding to the signals from S. macrurus regeneration blastema, C2C12 myotubes were treated with 1-week SmBE and tested for BrdU incorporation. After differentiation of C2C12 myoblasts in differentiation medium (DM) for 5 days, myotubes were re-plated at low density and incubated in the presence of either adult muscle extract (nonregenerating tissue) or SmBE at concentrations between 0.025 and 0.1 mg/ml for an additional 3-day period. The cultures were incubated with BrdU for the last 12 hr of the treatment period. We did not observe BrdU label in myonuclei of differentiated myotubes under any culture condition (Fig. 2). Our data showing that myonuclei in differentiated myotubes did not re-enter the cell cycle when cultured in SmBE, is in contrast to the response of myonuclei in myotubes cultured in the presence of newt blastema extract (McGann et al., 2001). In addition, we found that the 3-day treatment of myotubes with SmBE resulted in the detachment of most, if not all, myotubes. Treatment with adult muscle extract, or no extract at all had no effects on differentiated myotubes.
Fig. 2.
Protein extracts from S. macrurus blastema (SmBE) do not induce myotube nuclei to re-enter the cell cycle. After C2C12 myoblasts were induced to differentiate in differentiation medium (DM), they were re-plated at low density and treated with either DM only (control), adult muscle extract (AM) or blastema extract (SmBE) at 0.05 mg/ml for 3 days. For bromodeoxyuridine (BrdU) labeling, cells were incubated with BrdU for the last 12 hr of the 3-day treatment. BrdU labeled-nuclei were detected by immunofluorescence staining using anti-BrdU antibody and all cell nuclei were stained with Hoechst 33342 (blue). Myotubes are indicated by white arrows. Images were taken at ×100 magnification.
Characterization of the Effect of Blastema Extract on Myotubes
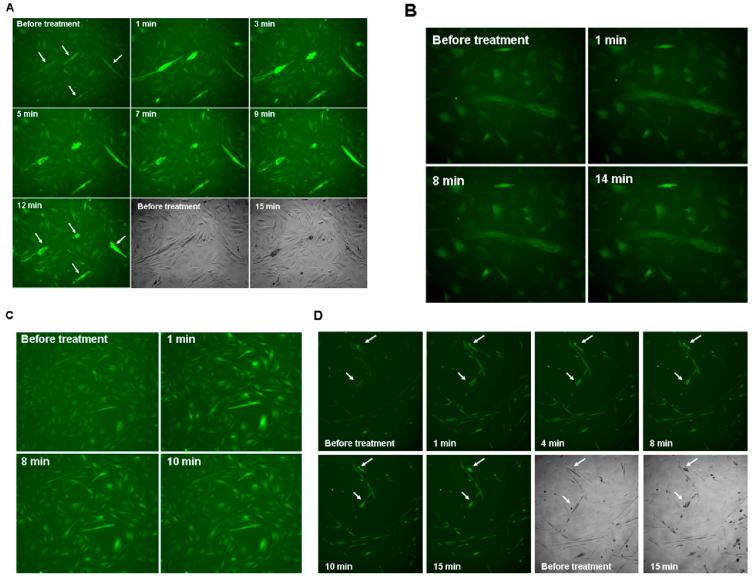
To characterize the events underlying the detachment of myotubes observed after the 3-day SmBE treatment, we performed time-lapse imaging after extract application. Although detachment of myotubes did not occur simultaneously, this effect was observed on all myotubes within 60 min of incubation with SmBE (Fig. 3A). As observed by time-lapse microscopy, the time of detachment of any given myotube could not be predicted, but it became apparent that some myotubes completely detached in less than 20 min after SmBE incubation (Fig. 3B). Myotubes characteristically shortened one end before proceeding to bulge and form an amorphous ball immediately before detachment (Fig. 3B).
Fig. 3.
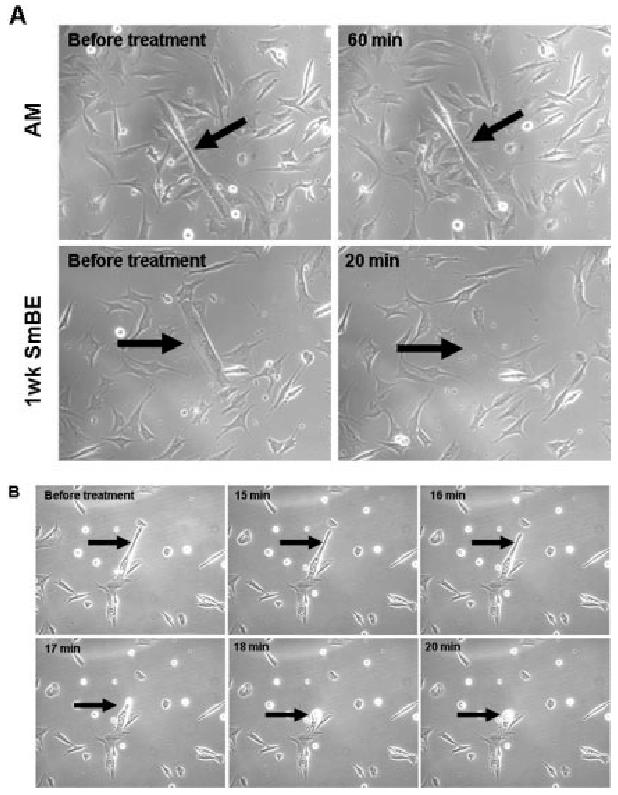
Protein extracts from S. macrurus blastema (SmBE) resulted in contraction of myotubes, leading to detachment. A: C2C12 myotubes in differentiation medium (DM) were either treated with adult muscle extract (AM: 0.025 mg/ml) or 1-week SmBE (0.025 mg/ml). Phase contrast microscopy was taken before the addition of extract to the medium (“before treatment”) and 20 min after blastema extract treatment (“20 min”) and 60 min after adult muscle extract treatment (“60 min”). B: Representative time lapse images of a myotube in culture treated with 1-week blastema extract (0.05 mg/ml) during 20-min period. Images were taken at ×200 magnification by phase contrast microscopy (Zeiss Axiovert 200M).
Given the contraction-like behavior of detaching myotubes, we studied the changes in cytosolic calcium levels in myotubes in all treatment conditions using the intracellular calcium indicator Fluo-4. Addition of SmBE to the culture caused an increase in Fluo-4 signal in most myotubes within 2 min (Fig. 4A), and this increase in signal was rapidly followed by myotube shortening (Fig. 4A). Although this effect was commonly observed in multinucleated myotubes, few mononucleate C2C12 cells also showed a similar time-dependent increase in Fluo-4 signal followed by contractions. Unlike with SmBE treatment, addition of adult muscle extract did not show considerable changes in Fluo-4 signal in myotubes over a 30-min incubation period (Fig. 4B). Of interest, addition of calcium chloride (final concentration: 20 mM vs 2 mM in DM) resulted in an increased Fluo-4 signal in both myotubes and mononucleate cells in the culture (Fig. 4C). Calcium chloride treatment did not replicate the rapid calcium transient followed by the apparent contraction of myotubes observed with SmBE treatment.
Fig. 4.
Protein extracts from S. macrurus blastema (SmBE) induced calcium transient in myotubes. Differentiated C2C12 cells were loaded with calcium indicator Fluo-4 for 1 hr before additional treatment for the indicated time periods. Time-lapse pictures were taken every minute. A: Representative time lapse images of Fluo-4 signal after addition of SmBE to the culture during 15-min period. The fluorescence signal was detected at excitation 485 nm and emission at 520 nm wavelength, and time-lapse images were taken at ×100 magnification. B: Representative time lapse images of Fluo-4 signal after addition of adult muscle extract to the culture during a 14-min period. C: Calcium chloride (20 mM) was added to C2C12 cells loaded with fluo-4. Representative time lapse images of 10 min period are shown. D: Caffeine (40 mM) was added to the culture of C2C12 cells loaded with fluo-4 and time lapse images were taken for 15-min period every minute. Representative images are shown. Arrows point individual myotubes that contracted after calcium transient. Images were taken at ×100 magnification.
Caffeine has been shown to induce rapid calcium transients in C2C12 myotubes by releasing calcium from the sarcoplasmic reticulum (SR) into the cytosol through the activation of ryanodine receptors (Lorenzon et al., 1997; Tarroni et al., 1997). To determine whether the effect of the blastema extract on myotubes is similar to that of caffeine, we replaced the differentiation medium with caffeine-containing medium (final caffeine concentration 40 mM). This led to a rapid (<1 min) increase in Fluo-4 signal and a subsequent contraction within 15 min in most myotubes (Fig. 4D). In general, caffeine did not affect the Fluo-4 signal in the mononucleated C2C12 cell population. These findings suggest that SmBE might contain a factor(s) that releases calcium from the SR of C2C12 cells which leads to contractions in those cells with a more differentiated contractile apparatus.
Proliferative Effect of Blastema Extract on C2C12 Myoblasts
In contrast to detachment of myotubes after SmBE treatment, most mononucleated C2C12 cells in differentiation medium remained attached, showing no distinct morphological differences from C2C12 cells in control conditions. After 3 days of SmBE treatment, mononucleated cells incorporated BrdU, and the BrdU immunolabeling pattern was heterogenous among the cultured cells. Moreover, both 1-week and 2-week SmBE treatments resulted in an increase in BrdU-positive cell number (∼15% increase, P value < 0.05; Fig. 5). Increases in cell proliferation in the presence of 1- or 2-week SmBE were similar (Fig. 5). In contrast, adult muscle extract treatment did not have an effect on the number of BrdU-positive cells compared with controls (DM only). These data show that SmBE treatment did not induce dedifferentiation in C2C12 myotubes, but it did enhance cell proliferation of C2C12 myoblasts.
Fig. 5.
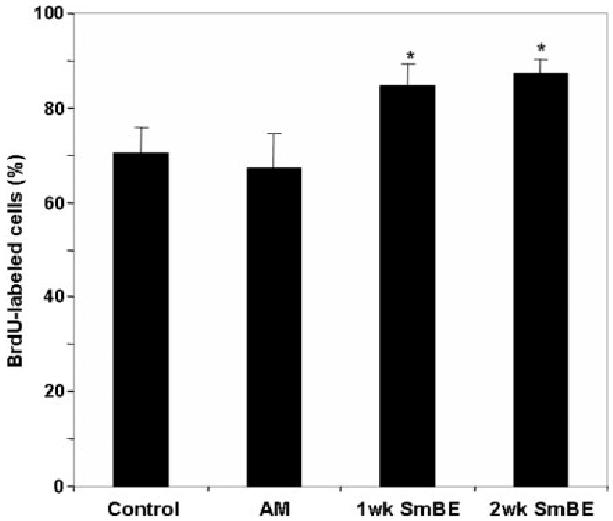
Protein extracts from S. macrurus blastema (SmBE) enhances the proliferation of C2C12 myoblasts. Quantification of bromodeoxyuridine (BrdU) -labeled cells. After C2C12 myoblasts were induced to differentiate in differentiation medium (DM, they were re-plated at low density and treated with either DM only (control), adult muscle extract (AM), or SmBE (1-week and 2-week) at 0.05 mg/ml for 3 days. Cells were incubated with BrdU for the last 12 hr of the 3-day treatment in each condition. Cells from 10 different microscopic fields with ≥ 100 cells per field were counted and the percentage of BrdU-labeled cells was calculated in each field. Data are the means ± SD. Student t-test was used to compare the effect of extract with control. Asterisk (*) indicates a difference at P < 0.05.
Inhibition of C2C12 Differentiation by Blastema Extract
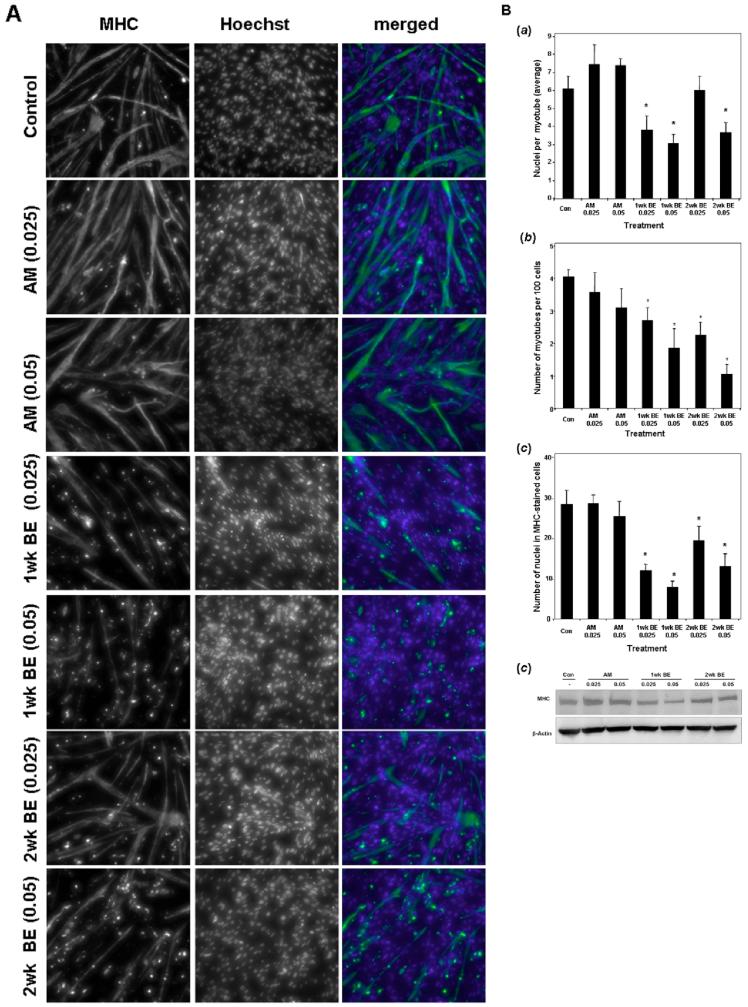
To test whether SmBE modulates the muscle differentiation program of C2C12 mononucleate cells, cells were induced to differentiate in the presence of SmBE for 5-6 days. The effect of SmBE on C2C12 differentiation was based on the expression of a muscle differentiation marker (e.g., MHC), cell fusion, and myotube formation (cells with at least two nuclei were counted as myotubes). SmBE treatment reduced the number of MHC-expressing mononucleate cells and myotubes in a dose-dependent manner, whereas adult muscle extract had no effect on either MHC expression or myotube formation (Fig. 6A). Our quantitative analysis also demonstrated that both 1-week and 2-week SmBE significantly decreased the number of MHC-positive cells (Fig. 6Ba) and myotubes (Fig. 6Bb). However, the inhibitory effect of 1-week SmBE on both aspects of differentiation was more potent than that obtained with 2-week SmBE treatment (Fig. 6B). Inhibition of C2C12 differentiation was also reflected on the number of nuclei incorporated into myotubes (Fig. 6Bc). Specifically, myotubes had significantly fewer nuclei when culture in the presence of 1-week SmBE. Similar results were observed with 2-week SmBE treatment, but only at the higher concentration (Fig. 6Bc).
Fig. 6.
Protein extracts from S. macrurus blastema (SmBE) inhibits differentiation of C2C12 myoblast cells. Myoblasts were induced to differentiate for 5-6 days in the presence of differentiation medium (DM) only (Control), adult muscle extract (AM), or SmBE (1-week and 2-week) at concentrations of 0.025 mg/ml (0.025) and 0.05 mg/ml (0.05). A: Cells were fixed and immunostained using MF20 (green in merged images). Cell nuclei were stained with Hoechst 33342 (blue in merged images). B: Quantitative analysis for the effects of SmBE on muscle differentiation. a: Effect of SmBE on MHC expression. b: Effect of SmBE on myotube formation. c: Effect of SmBE on cell fusion measured by number of nuclei per myotube. Data are the means ± SD. Student t-test was used to compare the effect of extract with control. Asterisk (*) indicates a difference at P < 0.05. C: Western blot analyses of C2C12 myoblasts cultured in DM for 5-6 days in the presence of different protein extracts. Total protein (50 μg) extracted from myoblasts were resolved by 4-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride (PVDF) membrane, and probed with MF20. The membrane was also probed with monoclonal β-actin antibody to demonstrate equal loading of protein.
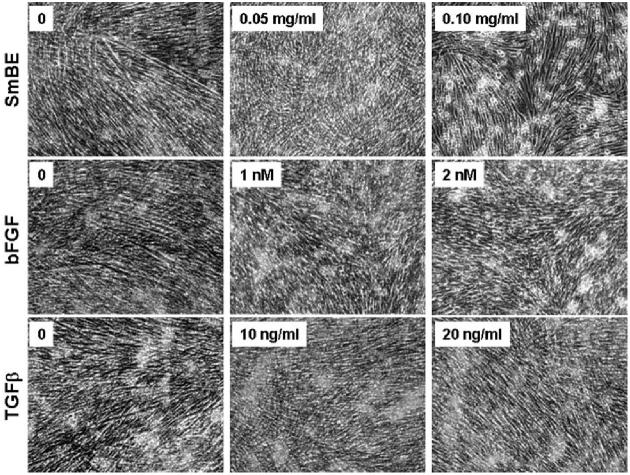
The SmBE-induced suppression of MHC expression in C2C12 cells was further confirmed by Western blot analysis (Fig. 6C). As shown in Figure 6C, the level of MHC protein was lower in SmBE-treated cells. Consistent with immunolabeling studies (Fig. 6A), the suppression effect on MHC levels was dependent on dosage and age of the blastema, that is, 1-week SmBE treatment was more potent than 2-week SmBE treatment. Similar results were obtained with titin immunofluorescence, that is, the number of C2C12 cells expressing titin was lower in the presence of 1- or 2-week SmBE compared with treatments with adult muscle extract or DM only (data not shown). In addition, the staining intensity for titin immunolabeling of C2C12 cells markedly decreased in cells treated with SmBE (data not shown). No differences in the number of MHC-positive or titin-positive cells were observed between cultures treated with adult muscle extract and control (DM only) cultures. We compared the effect of SmBE treatment with those effects with basic fibroblast growth factor (bFGF) and transforming growth factor-beta (TGFβ) treatments on differentiating C2C12 myoblasts. The growth factors bFGF and TGFβ have a negative effect on differentiation of myoblasts in vitro (Massagué et al., 1986; Olson et al., 1986; Spizz et al., 1986; Rao and Kohtz, 1995). Like SmBE, both bFGF and TGFβ markedly inhibited the formation of myotubes at concentrations ≥1 nM and ≥10 ng/ml, respectively (Fig. 7).
Fig. 7.
Protein extracts from S. macrurus blastema (SmBE) -induced inhibition of differentiation is similar to the inhibition caused by transforming growth factor-beta (TGFβ) and basic fibroblast growth factor (bFGF). C2C12 myoblasts were cultured in differentiation medium (DM) for 5 days in the presence of bFGF or TGFβ at concentrations indicated in each panel. A parallel culture was maintained in the presence of SmBE to compare the phenotype. Phase contrast images were taken at ×100 magnification.
Effect of Blastema Extract on Muscle Differentiation Is Reversible
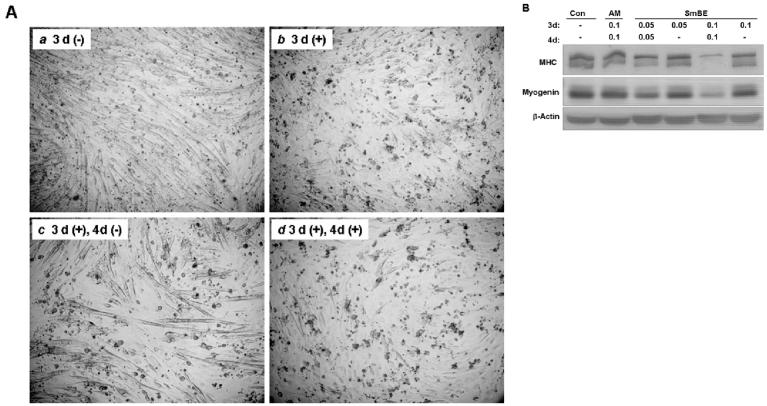
We tested whether the inhibitory effect of SmBE on myoblast differentiation could be reversed after withdrawal of the blastema extract from DM. To address this question, C2C12 myoblasts were cultured for 3 days in the presence of SmBE, and then incubated in DM without SmBE for a subsequent period of 4 days. Differentiation of C2C12 was suppressed by SmBE after 3 days (Fig. 8Aa, Ab). However, myoblasts differentiated when SmBE was removed from DM, resulting in formation of readily detectable myotubes (Fig. 8Ac). By comparison, myotube formation was noticeably suppressed in parallel cultures that continued to be treated with SmBE for 7 days (Fig. 8Ad). Western blot analysis of C2C12 myoblasts for MHC and myogenin proteins also demonstrated that myoblasts resume differentiation after removal of SmBE (Fig. 8B).
Fig. 8.
Inhibition of myoblast differentiation by Protein extracts from S. macrurus blastema (SmBE) is reversible. A: C2C12 myoblasts were cultured in differentiation medium (DM) only (a), or in DM with SmBE (b) for 3 days. Myoblasts treated with SmBE (0.1 mg/ml) for 3 days (b) were replaced with DM only and incubated for 4 additional days (c). To compare the effect of SmBE removal from DM, myoblasts were continued to be incubated in DM with SmBE for 4 additional days (d), in parallel with (c). Phase contrast images were taken at ×100 magnification. 3d(−): DM only for 3 days; 3d(+): DM and SmBE for 3 days; 3d(+), 4d(−): DM and SmBE for 3 days followed by DM only for 4 days; 3d(+), 4d(+): DM and SmBE for 7 days. B: Western blots of C2C12 myoblasts cultured in different conditions for 7 days using antibodies against sarcomeric MHC, myogenin and β-actin. Total protein (50 μg) extracted from myoblasts was resolved by 4-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride (PVDF) membrane, and probed with antibodies. β-actin immunoblot was done to demonstrate equal loading. Con, DM only; AM, adult muscle extract; SmBE, S. macrurus blastema extract. Concentrations of protein extract were 0.05 mg/ml and 0.10 mg/ml.
Effects of Blastema Extract on the Expression of Differentiation Regulators
To further characterize some of the molecular changes that are associated with the SmBE-induced inhibition of C2C12 myoblast differentiation, the expression of proteins known to be involved in skeletal muscle differentiation were studied. Myoblasts were incubated in DM with different tissue extracts for 1 or 2 days and then assayed by Western blot analysis. Incubation of C2C12 myoblasts for 1-2 days in DM alone, or in adult muscle extract showed an increase in MHC expression compared with that observed in myoblasts maintained in GM. Treatment with SmBE (1-week, 0.05 mg/ml) for 1 day resulted in no expression of MHC in C2C12 myoblasts (Fig. 9). Although MHC expression was detected in myoblasts after two days of SmBE treatment, MHC expression levels were lower than those observed in control or adult muscle treated myoblasts (Fig. 9).
Fig. 9.
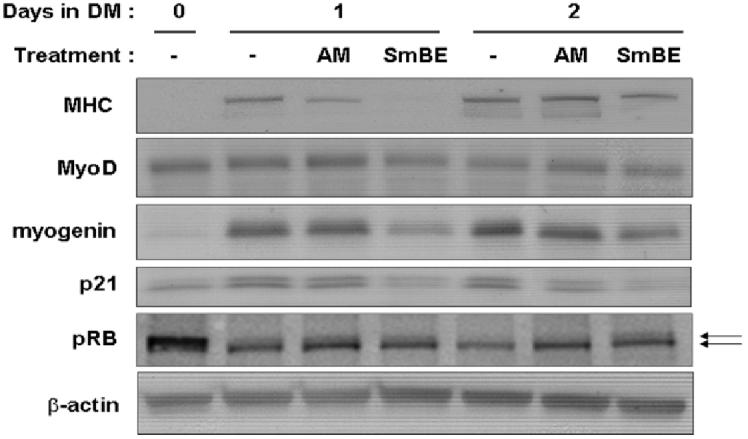
Protein extracts from S. macrurus blastema (SmBE) modulates the expression of differentiation regulators and markers. Western blot analyses of C2C12 myoblasts cultured in differentiation medium (DM) with extract from adult muscle extract (AM) or 1-week blastema (SmBE) at 0.05 mg/ml or without extract (−) for 0, 1 and 2 days. Total protein (50 μg) was resolved by 4-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a PVDF membrane and probed with antibodies against MHC, MyoD, myogenin, Myf5, p21, cyclin D1, and pRb antigens. Top arrow indicates hyperphosphorylated pRb and bottom arrow indicates hypophosphorylated pRb. Membranes were also probed with anti-β-actin antibody to demonstrate equal loading.
Muscle regulatory factors MyoD and myogenin are essential for the commitment and differentiation of cells to the skeletal muscle lineage (Pownall et al., 2002; Tapscott, 2005). We observed MyoD protein levels to be greater in C2C12 cells on day 0 in DM than in GM (data not shown). No changes were detectable in the protein levels of MyoD during early differentiation for at least 2 days with any treatment (Fig. 9). Myogenin protein also increased after 1 day in DM, but not in SmBE-treated myoblasts (Fig. 9). The onset of C2C12 differentiation is accompanied by changes in the expression of cell cycle regulatory proteins, that is down-regulation of cyclin D, up-regulation of cyclin-dependent kinase (CDK) inhibitor p21, and increase in pRb activity, leading to cell cycle exit (Kitzmann and Fernandez, 2001; DeFalco et al., 2006). As predicted, p21 was upregulated in myoblasts treated with DM only and adult muscle extract for 1 and 2 days (Fig. 9). In contrast, p21 protein levels did not change in SmBE-treated myoblasts (Fig. 9). In low serum conditions, pRb is hypophosphorylated and this is the active form that helps to induce cell cycle exit and initiate the differentiation program. Myoblasts increased their levels of hypophosphorylated pRb after 1 day in DM (Fig. 8). After 2 days in DM, only the hypophosphorylated form of pRb was detectable in myoblasts in DM only or in DM with adult muscle extract. This expression pattern of different pRb conformations was affected by SmBE treatment. Both hypo- and hyperphosphorylated forms of Rb were detected in C2C12 cells treated with SmBE and their levels did not change after 2 days of treatment (Fig. 9).
Effect of Heat-Inactivated Blastema Extract on Differentiating C2C12 Cells
To determine whether factor(s) in SmBE that affected the C2C12 cell phenotype was heat sensitive, SmBE was boiled for 5 min, and precipitate cleared from SmBE by brief centrifugation before adding to the myoblasts or myotubes. Under these conditions, SmBE at concentrations of 0.10 and 0.20 mg/ml had no effect on the phenotypic properties of myoblasts or myotubes that were investigated in this study. C2C12 cells treated with heat-inactivated SmBE could not be distinguished from C2C12 cells cultured in DM only or in adult muscle extract (data not shown).
DISCUSSION
Based on a blastema-dependent regeneration process in S. macrurus to replace its tail after amputation, we predicted that mouse muscle cells would alter their phenotype and be induced to dedifferentiate by proteins extracts from S. macrurus blastema (SmBE). In this report, we demonstrate that SmBE has different effects on C2C12 myoblasts at different stages of differentiation. Specifically, SmBE treatment (1) does not induce dedifferentiation of C2C12 myotubes, (2) reversibly inhibits the differentiation of myoblasts, (3) enhances proliferation of undifferentiated myoblasts in low serum conditions, and (4) results in a rapid calcium transient in multinucleated myotubes that is followed by a contraction-like behavior and detachment. These data show that SmBE contains molecular factors that regulate the muscle differentiation program in mammals, but does not express signals that induce dedifferentiation of mammalian myotubes.
Blastema Extract Does Not Induce Myotube Nuclei to Re-enter the Cell Cycle
Previous studies have shown that terminally differentiated mammalian myotubes can dedifferentiate under certain conditions. For example, when SV40 T antigen is conditionally expressed in terminally differentiated C2C12 myotubes, it induces myotubes to reenter the cell cycle upon serum stimulation (Gu et al., 1993). Dedifferentiation of myotubes is also reported to occur when Msx1 is ectopically expressed in myotubes under growth-promoting conditions (Odelberg et al., 2000). Treatment of myotubes in vitro with newt regeneration extract shows that cellular events suggestive of myotube dedifferentiation can occur, and these include cell cycle re-entry, reduced levels of muscle differentiation markers, and/or mononucleation (McGann et al., 2001), suggesting that mammalian myotubes can respond to newt blastema by activating a dedifferentiation program.
In the present study, we did not find BrdU-labeled nuclei in myotubes treated with SmBE, indicating that no myonuclei were detected to re-enter the cell. In addition, evidence of myotube cleavage or mononucleation after SmBE treatment was not observed. Absence of myotube dedifferentiation under the same culture conditions as those used to test the effect of newt extract (McGann et al., 2001) might be due to the lack of similar dedifferentiation inducing factor(s) in SmBE. Alternatively, dedifferentiation factors(s) may be present in SmBE but their effect on mammalian cells might not be functionally conserved.
Dedifferentiation of myotubes is believed to be the predominant mechanism by which muscle stem cells are generated during newt regeneration (Straube and Tanaka, 2006). In contrast, the cellular and molecular bases for tail regeneration in S. macrurus have yet to be fully determined. However, the incidence of satellite cells in adult muscle fibers and electrocytes, the increased proliferation of satellite cells after tail amputation, and their contribution to the regeneration of muscle and EO (unpublished data) indicate that pre-existing myogenic precursor cells contribute to the regeneration of tissue in the muscle lineage in S. macrurus. Moreover, preliminary analyses of cell morphology do not indicate changes that are suggestive of cellularization of either muscle fibers or electrocytes in regions proximal to the transection site (unpublished data). Taken together, our in vivo (unpublished data) and in vitro (present study) data may be indicative of differences to which dedifferentiation and stem cell activation contribute to the regeneration process in S. macrurus vs. Urodeles amphibians.
Characterization of the Detachment Effect of Blastema Extract on Myotubes
In our study, myotubes detached from the plate within a short period of time after SmBE addition to the culture medium. Time-lapse imaging showed that this detachment was coupled to a rapid and transient calcium increase that was followed by the shortening of myotubes. Increases in intracellular calcium levels have been studied in C2C12 cells in vitro. Caffeine treatment is known to cause the release of calcium from the ryanodine-sensitive stores of the sarcoplasmic reticulum in C2C12 myoblasts and myotubes (Lorenzon et al., 1997; Tarroni et al., 1997). During C2C12 differentiation, the number of acetylcholine receptors increases in C2C12 cells (Inestrosa et al., 1983). Treatment of C2C12 cells with acetylcholine (ACh) or ACh agonists raises the intracellular calcium levels by inducing calcium influx through transmitter-gated and voltage-gated channels, and by activating the inositol 1, 4, 5-triphosphate second messenger system (Giovannelli et al., 1991; Grassi et al., 1993; Ogilvie et al., 2000). Given the similar response in intracellular calcium levels in C2C12 cells to SmBE treatment, it is possible that SmBE contains a factor analogous to caffeine that mobilizes the calcium from the intracellular calcium storages into the cytosol leading to myotube contraction. SmBE might also contain ACh-like factors that can bind to the receptors on differentiated myotubes (and some differentiated mononucleated cells) and induce calcium mobilization from intracellular storage in myotubes. The variability among myotubes in the onset of contraction and strength of contraction after SmBE exposure could be attributed to different expression levels of ACh receptors and muscle contractile proteins within each myotube.
Inhibition of Skeletal Muscle Differentiation by S. macrurus Blastema Extract
Our data showed that SmBE markedly inhibits the formation of myotubes in a dose-dependent manner, and showed that the inhibitory effect of SmBE on skeletal muscle differentiation was reversible. The potential to reversibly inhibit muscle differentiation is not unique to SmBE, but is shared with several molecules including growth factors (Massagué et al., 1986; Olson et al., 1986; Spizz et al., 1986; Allen and Boxhorm, 1987; Olwin and Rapraeger, 1992; Rao and Kohtz, 1995) and cytokines (Langen et al., 2001; Zorzano et al., 2003). Hence, the presence of a factor in SmBE that can behave as a ligand, reversibly bind to its receptor on the cell membrane, and activate a specific intracellular signaling cascade leading to inhibition of muscle differentiation would be consistent with previous studies. If true, we speculate the muscle inhibition signal in SmBE to be analogous to soluble extracellular proteins such as cytokines and growth factors because denaturation by boiling the soluble fraction of SmBE completely abolished the inhibitory effect on myoblast differentiation (data not shown).
TGFβ and FGFs inhibit muscle differentiation in different mammalian myogenic cell lines in vitro (Massagué et al., 1986; Olson et al., 1986; Spizz et al., 1986; Rao and Kohtz, 1995). Inhibition by TGFβ is thought to occur by directly suppressing the transcriptional activity of MyoD and myogenin (Martin et al., 1992; Liu et al., 2001; Kollias and McDermott, 2008). In addition to its mitogenic effect on myoblasts, FGF is also believed to inhibit differentiation in a proliferation-independent mechanism (Spizz et al., 1986; Tortorella et al., 2001). Similarly to TGFβ, FGF can suppress the activity of MyoD and myogenin (Li et al., 1992). In our study, we did not detect changes in the protein levels of MyoD in C2C12 myoblasts treated with SmBE. However, a decrease in the levels of transcriptional targets of MyoD (e.g., p21, myogenin and MHC) was detected during early differentiation in SmBE-treated myoblasts.
Our data demonstrated that protein extract from 1-week SmBE was more potent than that from 2-week SmBE. Blastemas at different stages of regeneration are expected to vary in their composition and abundance of regulatory factors of cell proliferation and differentiation. One-week blastema tissue is likely to have a higher concentration of proliferation promoting factors than 2-week blastema tissue, resulting in a greater inhibitory effect by 1-week than 2-week SmBE on differentiation of C2C12 myoblasts. Clear histological differences between 1- and 2-week blastema tissues concur with this idea. For instance, 1-week blastema is composed mostly of loose connective tissue with small vessels, a high proportion of proliferating cells (Fig. 1), and cell clusters of unknown origin or phenotype (Patterson and Zakon, 1993; Unguez and Zakon, 1998). In contrast, the 2-week blastema contains a predominance of cells at different stages of differentiation and these different cell types are in the early stages of organization into cartilage, muscle, electric organ, and spinal cord (Patterson and Zakon, 1993; Unguez and Zakon, 1998) and relatively fewer proliferating cells compared with 1-week blastema (Fig. 1).
Given the apparently greater number of vessels in 1-week than 2-week blastema, there may be more angiogenic factors present in 1-week blastema tissue. In this regard, the roles of bFGF and erythropoietin on angiogenesis and their inhibitory effect on differentiation of myogenic cells are consistent with our findings using blastema extract. FGF promotes proliferation of muscle precursor cells during early regeneration and also has been suggested to play a role in the revascularization process during muscle regeneration through its angiogenic properties (Lefaucheur et al., 1996). Likewise, erythropoietin, a cytokine required for the development and maturation of erythrocytes, has been demonstrated to stimulate the proliferation while inhibiting differentiation of satellite cells and C2C12 cells (Ogilvie et al., 2000; Kertesz et al., 2004). Previous studies also demonstrated expression of erythropoietin receptors in nonerythroid cell lineages including endothelial cells (Anagnostou et al., 1994; Vacca et al., 1999). Therefore, it is speculated that erythropoietin is another putative functionally conserved cytokine that might be more abundant in 1-week than 2-week SmBE and induces neo-vascularization and proliferation of muscle stem cells during regeneration in S. macrurus.
Proliferative Effect of Blastema Extract on C2C12 Myoblasts
The significant increase in BrdU incorporation among mononucleated cells in SmBE-treated cultures is similar to the proliferative effect of newt regeneration blastema extract on dedifferentiated C2C12 mononucleated cells reported by McGann et al. (2001). However, our study demonstrates the proliferation enhancing effect of SmBE on myoblasts in low serum conditions. C2C12 myoblasts differentiate into myotubes when they are cultured in low serum conditions (i.e., differentiation promoting conditions) after they reach confluence. It has been shown that there is a subset of myoblasts called “reserve cells” that withdraw from the cell cycle, but do not undergo differentiation (Yoshida et al., 1998; Carnac et al., 2000). SmBE treatment may have provided the reserve cells with a mitogenic signal analogous to TGFβ or FGF, inducing them to enter the cell cycle and promoting the proliferation of this subset of cells.
Effect of Blastema Extract on Cell Cycle Regulators
Skeletal muscle differentiation is a highly coordinated event that requires withdrawal from the cell cycle, expression of muscle regulatory factors, and fusion of mononucleated cells to form multinucleated myotubes. Cell cycle withdrawal involves the up-regulation of negative cell cycle regulators like p21 and active pRb proteins. p21 and pRb repress G1 to S phase transition (Nevins, 1992; Sherr and Roberts, 1995; Kouzarides, 1995; De Falco et al., 2006). In addition, crosstalk between cell cycle regulators and myogenic regulatory factors—particularly MyoD—has been well established in cell cycle withdrawal. For example, independent of its role as a transcriptional activator of muscle specific genes, MyoD can induce p21 during myogenic differentiation (Martelli et al., 1994; Halevy et al., 1995; Guo et al., 1995; Kitzmann and Fernandez, 2001). In this regard, our data showing lower levels of p21 in SmBE-treated cells could be due to lower activity levels of MyoD, although MyoD protein levels do not appear to change with SmBE treatment. Lower p21 levels can lead to higher levels of pRb in the hyperphosphorylated state (inactive form; Walsh and Perlman, 1997), and hyperphosphorylated pRb is associated with G1 to S phase transition. The expression pattern of pRb in C2C12 cells cultured with SmBE suggests the presence of mitogenic signals in SmBE that suppresses cell cycle exit and differentiation.
MyoD regulates the expression of myogenin and sarcomeric MHC genes by activating their promoters during muscle differentiation (Tapscott, 2005). In culture conditions with DM only or adult muscle extract, muscle cell differentiation was evident by increases in both myogenin and MHC protein levels (Fig. 9). In contrast, culture conditions with SmBE contained lower levels of myogenin and MHC proteins indicating an inhibition of this aspect of the muscle differentiation process. A decrease in myogenin and MHC levels could result from a decrease in MyoD activity levels. However, it remains to be determined if the activity of MyoD is affected by blastema extract. In addition, the molecular mechanisms involved in other aspects of the differentiation process that are affected by SmBE, that is, myoblast fusion, will be considered in further investigations. In summary, we suggest that SmBE suppresses skeletal muscle differentiation by the promotion of cell proliferation and/or a mechanism by which differentiation is directly inhibited.
EXPERIMENTAL PROCEDURES
Animals and Tissue Collection
Adult S. macrurus, a fresh-water species of knife fish native to South America, were obtained commercially from Segrest Farms (Gibsonton, FL). Fish of both sexes were anesthetized in 2-phenoxyethanol (1:1,500 in tank water) and the tail segment distal to the ventral fin was amputated. One or 2 weeks after tail amputation, fish were re-anesthetized and their regeneration blastema removed, frozen directly in liquid nitrogen and stored at −80°C until protein isolation. Similar procedures were followed to dissect and freeze ventral skeletal muscle from adult control fish that were kept at −80°C until protein isolation. After each surgery, fish were returned to their tanks, treated with Stress Coat, and monitored until they recovered from surgery. All procedures used in this study followed the American Physiological Society Animal Care Guidelines and were approved by the Animal Use Committee at New Mexico State University.
Preparation of Blastema and Muscle Extracts
Protein extracts from regeneration blastema and ventral muscle were prepared as described in McGann et al. (2001). All procedures were carried out at 4°C or on ice. Approximately 1 g of blastema or muscle tissue was placed into 5 ml of DMEM (Dulbecco’s Modified Eagle Medium; Invitrogen, #21063, Carlsbad, CA) containing protease inhibitors (2 μg/ml leupeptin, 2 μg/ml aprotinin, and 1 mM phenylmethylsulphonylfluoride [PMSF]). Tissues were ground with an electric homogenizer for 2-3 min and then hand-homogenized for 15 min. Cell debris that remained insoluble in DMEM was removed in two subsequent centrifugation steps. The homogenate was first centrifuged at 2,000 × g for 25 min and the supernatant was centrifuged again at 100,000 × g for 60 min. The supernatant was filter-sterilized through a 0.22-μm syringe filter. Total protein content was assayed with the Bradford assay kit (Bio-Rad, Hercules, CA) and stored at −80°C until use.
Cell Culture and Reagents
The murine C2C12 myoblast cell line was purchased from American Type Cell Culture (Manassas, VA) and maintained in growth medium (GM: DMEM supplemented with 10 % fetal bovine serum, 100 units/ml penicillin G and 100 μg/ml streptomycin). To induce muscle differentiation, C2C12 myoblasts were allowed to grow to approximately 90% confluence in GM and switched to differentiation medium (DM: DMEM supplemented with 2% horse serum, 100 units/ml penicillin G, and 100 μg/ml streptomycin). Cell culture reagents (DMEM, serum, antibiotics, etc.) were purchased from Invitrogen unless otherwise indicated.
Tissue Extract Treatments
We determined the effects of S. macrurus tissue extracts on the phenotype of both fully differentiated C2C12 myotubes by incubating them in extracts from either adult skeletal muscle or blastema (0.025-0.10 mg/ml) for up to 3 days after re-plating them at low density in multi-well plates coated with 0.1% gelatin (Sigma, G1890). Effects of protein extracts on myoblast differentiation were also studied by culturing C2C12 cells in different conditions for 5 days and then processed for phenotypic assays. Treatment conditions included muscle, 1-week, and 2-week blastema extract at concentrations of 0.025, 0.05, and 0.10 mg/ml. Control treatments did not include tissue extracts.
BrdU Incorporation and Immunofluorescence Assays
To determine changes in cell proliferation, C2C12 cells cultured under all treatments described above were incubated in 5-bromo-2′-deoxyuridine (BrdU; 10 μM) for 12 hr. BrdU incorporation was detected by immunofluorescence using the BrdU Detection Kit I (Roche Applied Science, Indianapolis, IN) following the manufacturer’s instructions. Markers of the differentiated muscle phenotype included antibodies specific to sarcomeric MHCs (MF20; 1:5) and Titin (9D10; 1:5) that were purchased from the Developmental Hybridoma Bank (University of Iowa, IA). Briefly, C2C12 cells were fixed in cold methanol at −20°C for 15 min and permeabilized in phosphate buffered saline containing 0.1% Triton X-100 (PBS-T). After incubation in blocking buffer (3% bovine serum albumin in PBS-T), cells were incubated with primary antibodies diluted in blocking buffer for 1 hr at 37°C. The cells were then incubated with AlexaFluor488-conjugated secondary antibodies (antimouse, 1:200; Molecular Probes, Eugene, OR). Cell nuclei were stained using Hoechst 33342 (Molecular Probes). Images of immunolabeled cells were captured using a Zeiss Axiovert 200M interfaced with a CCD fluorescent camera and equipped with Axiovision software version 4.5. (Carl Zeiss microimaging, Thornwood, NY).
Fluo-4-AM Loading and Imaging
C2C12 myoblasts were loaded with 5 μM of the Ca2+ indicator Fluo-4-AM (Molecular Probe; F14217) for 1 hr at 37°C and washed twice with indicator-free DM before additional incubation in DM for 30 min. The fluorescence signal was detected at excitation 485 nm and emission at 520 nm wavelength by using a Zeiss Axiovert 200M equipped with a CCD fluorescent camera, and the images were captured using Axiovision software (version 4.5). For BAPTA (Calbiochem, 196419) pretreatment, cells were incubated in DM containing BAPTA (30 mM) for 1 hr before either caffeine (Sigma; C0750) or extract treatment. BAPTA stock solution (5 mM) was prepared in dimethyl sulfoxide and kept at −20°C until use. Caffeine was solubilized in culture medium at working concentrations before use.
Quantification of the Effect of Blastema on C2C12 Differentiation
The effect of blastema extract on C2C12 differentiation was assessed using three different parameters: (1) expression of a muscle differentiation marker (e.g., MHC), (2) degree of cell fusion in myotubes, and (3) frequency of myotube formation. The extent of cell fusion under different culture conditions was estimated based on the mean number of nuclei per myotube, whereas the frequency of myotube formation was determined by calculating the mean number of myotubes formed per 100 nuclei. C2C12 cells were induced to differentiate in DM containing extract from either adult muscle or blastema (0.025 or 0.05 mg/ml) for 5 days and processed for immunofluorescence staining using antibodies against MHC or titin. To quantify immunolabeling patterns obtained after each treatment, four microscopic fields were randomly selected containing approximately 2,000-2,500 nuclei. The number of nuclei in MHC-positive cells (both mononucleated cells and myotubes) was compared with the total number of nuclei present in each microscopic field in each treatment group. Cells with at least two nuclei were defined to be myotubes. We also calculated the mean percent of nuclei in MHC positive cells compared with total number of nuclei in each field (number of nuclei in MHC-stained cells divided by total number of nuclei) to determine the effect of blastema extract on the differentiated phenotype.
Western Blot Analysis
Total cell lysate from C2C12 cells were prepared using lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM Na2 ethylenediaminetetraacetic acid [EDTA], 1 mM ethyleneglycoltetraacetic acid [EGTA], 1 % Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin and 1 mM PMSF and 1× protease inhibitor cocktail [Roche, Indianapolis, IN]) on ice for 5 min. The cell lysate was scraped off the plate and incubated on ice for 15 min with intermittent vortexing followed by centrifugation at 13,000 rpm for 15 min at 4°C. Supernatant was collected and assayed for total protein concentration by using the Bradford assay kit (Bio-Rad). Total protein (50 μg) was separated by means of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4-12%), transferred onto PVDF membranes, and visualized by Ponceau S staining to ensure equal loading and transfer of proteins to the membranes. Membranes were washed twice with TBS-T (0.1 % Tween-20 in Tris buffered saline [TBS]), and incubated in blocking buffer (5% nonfat dry milk in TBS-T) for 1 hr at room temperature. Membranes were then incubated with primary antibody diluted in 5% BSA in TBS-T overnight at 4°C. Primary antibodies included: monoclonal mouse anti-MyoD (1:500, BD Pharmingen), polyclonal rabbit anti-Myf5 (1:500, Santa Cruz Biotechnology), monoclonal mouse anti-myogenin (F5D, 1:20, Hybridoma Bank), monoclonal mouse anti-myosin heavy chain (MF20, 1:20, Hybridoma Bank), monoclonal rabbit anti-phospho-p38 MAPK (1:1,000, Cell Signaling), polyclonal rabbit anti-p38 MAPK kinase (1:1,000, Cell Signaling), monoclonal mouse anti-p21 (1:500, Santa Cruz), monoclonal mouse anti-cyclin D1 (1:500, Santa Cruz), monoclonal mouse anti-Rb (1:250, BD Pharmingen), and monoclonal mouse anti-β-actin (1:1,000, Santa Cruz) antibodies. Membranes were washed with PBS-T, incubated with anti-mouse or anti-rabbit horseradish peroxidase (HRP) -conjugated secondary antibodies (1:5,000, Bio-Rad) for 1 hr at room temperature, and then exposed to the Opti-4CN substrate (Bio-Rad) to visualize the primary-secondary antibody complex.
BrdU Immunolabeling of Tissue Sections
Fish were given intraperitoneal injections of BrdU (Sigma) at a dose of 100 mg/kg immediately after tail amputation and the regeneration blastema was removed 7 or 14 days after for BrdU immunofluorescence. Longitudinal sections of regeneration blastema 1-week and 2-week after tail amputation were cut at 14-μm thickness in a cryostate at −20°C, mounted on glass slides (Superfrost Plus, Fisher, Pittsburgh, PA), and air-dried at room temperature. Slides were immersed in 0.1M PBS for 5 min followed by incubation in HCl solution (1.5 ml HCl/23.5 ml ddH2O) for 30 min at 37°C. Slides were rinsed in PBS, incubated in 0.1 M sodium borate (pH 7-8) for 10 min at room temperature, and then immersed in 1% blocking solution (Invitrogen) for 30 min before incubation in mouse anti-BrdU antibody (BD biosciences, San Jose, CA; 1:20) for 15-20 hr at room temperature. Primary antibody was visualized using a biotinylated secondary antibody (Vectastain ABC kit, Vector Labs) and HRP reaction was run to amplify the signal by use of diaminobenzidine and hydrogen peroxidase. Brown precipitate indicated a positive label. Images of immunolabeled tissue sections were visualized and captured on a CCD camera (ORCA-100, Hamamatsu, Japan) controlled by OpenLab imaging software (Improvision, Lexington, MA). Final images were represented using Adobe Photoshop (version 7.0; Adobe Systems, Inc., San Jose, CA).
ACKNOWLEDGEMENTS
We thank the NMSU-INBRE Cell and Organismal Culture Facility at New Mexico State University for its cell culture services, Vince Gutschick for live fish photography, and two anonymous reviewers for providing critical suggestions.
Grant sponsor: National Institutes of Health; Grant numbers: S06-GM008136; RR16480; U56-CA96286; R25GM061222; GM007667-30S1
REFERENCES
- Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J Cell Physiol. 1987;133:567–572. doi: 10.1002/jcp.1041330319. [DOI] [PubMed] [Google Scholar]
- Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ, Waxman SG. Regeneration of spinal neurons in inframammalian vertebrates: morphological and developmental aspects. J Hirnforsch. 1983;24:371–398. [PubMed] [Google Scholar]
- Asakura A. Stem cells in adult skeletal muscle. Trends Cardiovasc Med. 2003;13:123–128. doi: 10.1016/s1050-1738(03)00024-0. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol. 2002;3:566–574. doi: 10.1038/nrm881. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Campion DR. The muscle satellite cell: a review. Int Rev Cytol. 1984;87:225–251. doi: 10.1016/s0074-7696(08)62444-4. [DOI] [PubMed] [Google Scholar]
- Carnac G, Fajas L, L’honoré A, Sardet C, Lamb NJ, Fernandez A. The retinoblastoma-like protein p130 is involved in the determination of reserve cells in differentiating myoblasts. Curr Biol. 2000;10:543–546. doi: 10.1016/s0960-9822(00)00471-1. [DOI] [PubMed] [Google Scholar]
- De Falco G, Comes F, Simone C. pRb: master of differentiation. Coupling irreversible cell cycle withdrawal with induction of muscle-specific transcription. Oncogene. 2006;25:5244–5249. doi: 10.1038/sj.onc.1209623. [DOI] [PubMed] [Google Scholar]
- Echeverri K, Tanaka EM. Mechanisms of muscle dedifferentiation during regeneration. Semin Cell Dev Biol. 2002;13:353–360. doi: 10.1016/s1084952102000915. [DOI] [PubMed] [Google Scholar]
- Giovannelli A, Grassi F, Mattei E, Mileo AM, Eusebi F. Acetylcholine induces voltage-independent increase of cytosolic calcium in mouse myotubes. Proc Natl Acad Sci U S A. 1991;88:10069–10073. doi: 10.1073/pnas.88.22.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss RJ. Inhibition of growth and shedding of antlers by sex hormones. Nature. 1968;220:83–85. doi: 10.1038/220083a0. [DOI] [PubMed] [Google Scholar]
- Grassi F, Giovannelli A, Fucile S, Eusebi F. Activation of the nicotinic acetylcholine receptor mobilizes calcium from caffeine-insensitive stores in C2C12 mouse myotubes. Pflugers Arch. 1993;422:591–598. doi: 10.1007/BF00374007. [DOI] [PubMed] [Google Scholar]
- Gu W, Schneider JW, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Guo K, Wang J, Andrés V, Smith RC, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol. 1995;15:3823–3829. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Miller JB, Silberstein L, Ziskind-Conhaim L, Hall ZW. Developmental regulation of 16S acetylcholinesterase and acetylcholine receptors in a mouse muscle cell line. Exp Cell Res. 1983;147:393–405. doi: 10.1016/0014-4827(83)90221-5. [DOI] [PubMed] [Google Scholar]
- Kertesz N, Wu J, Chen TH, Sucov HM, Wu H. The role of erythropoietin in regulating angiogenesis. Dev Biol. 2004;276:101–110. doi: 10.1016/j.ydbio.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Kirschbaum F, Meunier FJ. South American gymnotiform fishes as model animals for regeneration experiments? Monogr Dev Biol. 1988;21:112–123. [PubMed] [Google Scholar]
- Kitzmann M, Fernandez A. Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell Mol Life Sci. 2001;58:571–579. doi: 10.1007/PL00000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias HD, McDermott JC. Minireview: transforming growth factor-{beta} and myostatin signaling in skeletal muscle. J Appl Physiol. 2008;104:579–587. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Transcriptional control by the retinoblastoma protein. Semin Cancer Biol. 1995;6:91–98. doi: 10.1006/scbi.1995.0012. [DOI] [PubMed] [Google Scholar]
- Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 2001;15:1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Gjata B, Lafont H, Sebille A. Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-beta 1. J Neuroimmunol. 1996;70:37–44. doi: 10.1016/s0165-5728(96)00099-9. [DOI] [PubMed] [Google Scholar]
- Li L, Zhou J, James G, Heller-Harrison R, Czech MP, Olson EN. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell. 1992;71:1181–1194. doi: 10.1016/s0092-8674(05)80066-2. [DOI] [PubMed] [Google Scholar]
- Liu D, Black BL, Derynck R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15:2950–2966. doi: 10.1101/gad.925901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzon P, Giovannelli A, Ragozzino D, Eusebi F, Ruzzier F. Spontaneous and repetitive calcium transients in C2C12 mouse myotubes during in vitro myogenesis. Eur J Neurosci. 1997;9:800–808. doi: 10.1111/j.1460-9568.1997.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Martelli F, Cenciarelli C, Santarelli G, Polikar B, Felsani A, Caruso M. MyoD induces retinoblastoma gene expression during myogenic differentiation. Oncogene. 1994;9:3579–3590. [PubMed] [Google Scholar]
- Martin JF, Li L, Olson EN. Repression of myogenin function by TGF-beta 1 is targeted at the basic helix-loop-helix motif and is independent of E2A products. J Biol Chem. 1992;267:10956–10960. [PubMed] [Google Scholar]
- Massagué J, Cheifetz S, Endo T, Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986;83:8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann CJ, Odelberg SJ, Keating MT. Mammalian myotube dedifferentiation induced by newt regeneration extract. Proc Natl Acad Sci U S A. 2001;98:13699–13704. doi: 10.1073/pnas.221297398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechiporuk A, Keating MT. A proliferation gradient between proximal and msxb-expressing distal blastema directs zebrafish fin regeneration. Development. 2002;129:2607–2617. doi: 10.1242/dev.129.11.2607. [DOI] [PubMed] [Google Scholar]
- Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Nye HL, Cameron JA, Chernoff EA, Stocum DL. Regeneration of the urodele limb: a review [review] Dev Dyn. 2003;226:280–294. doi: 10.1002/dvdy.10236. [DOI] [PubMed] [Google Scholar]
- Odelberg SJ, Kollhoff A, Keating MT. Dedifferentiation of mammalian myotubes induced by msx1. Cell. 2000;103:1099–1109. doi: 10.1016/s0092-8674(00)00212-9. [DOI] [PubMed] [Google Scholar]
- Ogilvie M, Yu X, Nicolas-Metral V, Pulido SM, Liu C, Ruegg UT, Noguchi CT. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem. 2000;275:39754–39761. doi: 10.1074/jbc.M004999200. [DOI] [PubMed] [Google Scholar]
- Olson EN, Sternberg E, Hu JS, Spizz G, Wilcox C. Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol. 1986;103:1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwin BB, Rapraeger A. Repression of myogenic differentiation by aFGF, bFGF, and K-FGF is dependent on cellular heparan sulfate. J Cell Biol. 1992;118:631–639. doi: 10.1083/jcb.118.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JM, Zakon HH. Bromodeoxyuridine labeling reveals a class of satellite-like cells within the electric organ. J Neurobiol. 1993;24:660–674. doi: 10.1002/neu.480240510. [DOI] [PubMed] [Google Scholar]
- Patterson JM, Zakon HH. Transdifferentiation of muscle to electric organ: regulation of muscle-specific proteins is independent of patterned nerve activity. Dev Biol. 1997;186:115–126. doi: 10.1006/dbio.1997.8580. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Gustafsson MK, Emerson CP., Jr. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol. 2002;18:747–783. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- Rao SS, Kohtz DS. Positive and negative regulation of D-type cyclin expression in skeletal myoblasts by basic fibroblast growth factor and transforming growth factor beta. A role for cyclin D1 in control of myoblast differentiation. J Biol Chem. 1995;270:4093–4100. doi: 10.1074/jbc.270.8.4093. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Spizz G, Roman D, Strauss A, Olson EN. Serum and fibroblast growth factor inhibit myogenic differentiation through a mechanism dependent on protein synthesis and independent of cell proliferation. J Biol Chem. 1986;261:9483–9488. [PubMed] [Google Scholar]
- Stocum DL. Amphibian regeneration and stem cells. Curr Top Microbiol Immunol. 2004;280:1–70. doi: 10.1007/978-3-642-18846-6_1. [DOI] [PubMed] [Google Scholar]
- Straube WL, Tanaka EM. Reversibility of the differentiated state: regeneration in amphibians. Artif Organs. 2006;30:743–755. doi: 10.1111/j.1525-1594.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- Tarroni P, Rossi D, Conti A, Sorrentino V. Expression of the ryanodine receptor type 3 calcium release channel during development and differentiation of mammalian skeletal muscle cells. J Biol Chem. 1997;272:19808–19813. doi: 10.1074/jbc.272.32.19808. [DOI] [PubMed] [Google Scholar]
- Tortorella LL, Milasincic DJ, Pilch PF. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J Biol Chem. 2001;276:13709–13717. doi: 10.1074/jbc.M100091200. [DOI] [PubMed] [Google Scholar]
- Tsonis PA. Regeneration in vertebrates. Dev Biol. 2000;221:273–284. doi: 10.1006/dbio.2000.9667. [DOI] [PubMed] [Google Scholar]
- Unguez GA, Zakon HH. Phenotypic conversion of distinct muscle fiber populations to electrocytes in a weakly electric fish. J Comp Neurol. 1998;399:20–34. [PubMed] [Google Scholar]
- Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, Albini A, Bussolino F, Dammacco F. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93:3064–3073. [PubMed] [Google Scholar]
- Walsh K, Perlman H. Cell cycle exit upon myogenic differentiation. Curr Opin Genet Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Yoshida S, Koishi K, Masuda K, Nabeshima Y. Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates ‘reserve cells’. J Cell Sci. 1998;111:769–779. doi: 10.1242/jcs.111.6.769. [DOI] [PubMed] [Google Scholar]
- Zorzano A, Kaliman P, Gumà A, Palacín M. Intracellular signals involved in the effects of insulin-like growth factors and neuregulins on myofibre formation. Cell Signal. 2003;15:141–149. doi: 10.1016/s0898-6568(02)00081-5. [DOI] [PubMed] [Google Scholar]