Abstract
Ultraviolet-B (UVB) (290–320 nm) radiation-induced cyclobutane pyrimidine dimers within the DNA of epidermal cells are detrimental to human health by causing mutations and immunosuppressive effects that presumably contribute to photocarcinogenesis. Conventional photoprotection by sunscreens is exclusively prophylactic in nature and of no value once DNA damage has occurred. In this paper, we have therefore assessed whether it is possible to repair UVB radiation-induced DNA damage through topical application of the DNA-repair enzyme photolyase, derived from Anacystis nidulans, that specifically converts cyclobutane dimers into their original DNA structure after exposure to photoreactivating light. When a dose of UVB radiation sufficient to induce erythema was administered to the skin of healthy subjects, significant numbers of dimers were formed within epidermal cells. Topical application of photolyase-containing liposomes to UVB-irradiated skin and subsequent exposure to photoreactivating light decreased the number of UVB radiation-induced dimers by 40–45%. No reduction was observed if the liposomes were not filled with photolyase or if photoreactivating exposure preceded the application of filled liposomes. The UVB dose administered resulted in suppression of intercellular adhesion molecule-1 (ICAM-1), a molecule required for immunity and inflammatory events in the epidermis. In addition, in subjects hypersensitive to nickel sulfate, elicitation of the hypersensitivity reaction in irradiated skin areas was prevented. Photolyase-induced dimer repair completely prevented these UVB radiation-induced immunosuppressive effects as well as erythema and sunburn-cell formation. These studies demonstrate that topical application of photolyase is effective in dimer reversal and thereby leads to immunoprotection.
Because of increased leisure time, the growing popularity of staying outdoors, and of holidays in the sun, it has become more and more important to study the molecular and cellular effects that ultraviolet radiation exposure to the sun exerts on human skin (1). In this context, a critical observation has been the appreciation that the induction of DNA damage by ultraviolet-B (UVB) radiation (290–320 nm) is detrimental to human health. Among the DNA lesions induced by UVB irradiation, cyclobutane pyrimidine dimers predominate. Dimer formation is thought to be of crucial importance for the initiation of skin cancer, because it was found to be closely linked to the generation of mutations in tumor-suppressor genes expressed in UV-induced skin cancer (2). From recent animal work, it has been concluded that dimers also contribute to photocarcinogenesis through suppression of the skin's immune system, allowing transformed cells to grow unimpeded (3, 4). Strategies directed at the prevention of detrimental effects resulting from dimers in human skin are, thus, of paramount concern for human health.
Sunscreens provide protection against erythema of human skin through absorption or reflection of UV radiation (5). This widely used photoprotective approach, however, is not effective once damage to skin cells has been generated after sun exposure. In healthy human skin, cyclobutane pyrimidine dimers are repaired through nucleotide excision repair. In addition to nucleotide excision repair, cyclobutane pyrimidine dimers in some biological systems may be effectively removed from cellular DNA by the DNA-repair enzyme photolyase (6, 7). Photolyase recognizes and specifically binds to cyclobutane pyrimidine dimers, and exposure of the photolyase–dimer complex to photoreactivating light (300–500 nm) converts the dimerized pyrimidines to their monomeric form. Dimer-specific photolyase is present in an active form in numerous prokaryotes and certain eukaryotes, including fish and marsupials.
The existence of photoreactivation in humans is controversial. Although recent reports have presented evidence of photoreactivating activity in human white blood cells (8) and fibroblasts (9), many negative reports have questioned the validity of these findings (ref. 10, see review in ref. 11). The recent discovery of a human homologue of a Drosophila photolyase gene with unknown function (12, 13) has again raised the possibility of photoreactivation in human skin.
In this paper, we have assessed whether dimer repair in UVB-irradiated human skin can be enhanced through topical application of a cyclobutane pyrimidine dimer-specific photolyase that has been prepared from Anacystis nidulans and encapsulated into liposomes. Because immunosuppression, in contrast to mutagenesis, can be detected in UVB-irradiated skin within hours after UVB radiation exposure, we also wanted to determine whether this approach was capable of providing immunoprotection.
Materials and Methods
Human Subjects.
The local ethical committee (Ethikkommission der Medizinischen Einrichtungen der Heinrich-Heine-Universität, Düsseldorf, Germany) approved the protocol, advertisements, and consent document for the study. Nineteen healthy, adult human volunteers were studied. Individuals were of skin type II or III (14), without a history of chronic disease, and not currently on medication.
UVB Radiation.
For administration of biologically equivalent doses, the individual minimal erythema dose (MED) was determined for each subject 24 h after irradiation by exposing previously unirradiated buttock skin to increasing doses of UVB radiation from a UVB phototherapy device containing six FS-40 bulbs (Westinghouse Electric, Pittsburgh), which are known to emit primarily in the UVB range (280–320 nm). The UVB output was monitored by means of an IL1700 research radiometer and SEE240 UVB photodetector (International Light, Newburyport, MA) and was approximately 0.24 mW/cm2 at a source-to-skin distance of 22 cm. For all individuals included in the study, the MED was in the normal range (30–50 mJ/cm2 UVB), and in particular, no abnormal reaction to UVB radiation was observed. To assess the effect of UVB radiation on intercellular adhesion molecule-1 (ICAM-1) expression in human keratinocytes, previously unirradiated buttock skin (16 cm2) subsequently was exposed to 1 MED of UVB radiation or sham-irradiated. Except for the 16-cm2 exposure areas, all areas of the body were draped.
Immunostaining of Dimers in Human Skin Sections.
Biopsies were taken as indicated, either immediately after photoreactivation or 24 h after UVB irradiation, and skin samples were embedded in paraffin. Sections (5 μm) were deparaffinized by subsequent 2-min incubations in xylene (two times), 100% ethanol (two times), 96% ethanol, 70% ethanol, and PBS. The slides were then boiled for 10 min in 10 mM citrate buffer (pH 6.0), rinsed with PBS (two times), and used for immunostaining. The skin sections were stained with monoclonal antibodies against cyclobutane thymine dimers (hybridoma clone H3) and goat anti-mouse IgG fluorescein-labeled secondary antibodies. The H3 mouse monoclonal antibody has been developed against a TT-containing oligonucleotide (15) and has high affinity for 5′-T-containing dimers, e.g., TT and TC (L.R. and A.A.V., unpublished data). These photoproducts are representative for the cyclobutane pyrimidine dimers induced by UV radiation. Nuclei of skin cells were counterstained with propidium iodide. Nuclear green fluorescence in the epidermal cells proportional to the level of thymine dimers was assessed with a scanning laser microscope (Zeiss LSM-41) by using image processing and image analysis. The procedure for the immunostaining and measurement of the fluorescence has been described (15, 16). Photographs were taken on 35-mm 400 ASA Kodak Elite chrome film for color slides.
In Vivo Photoreactivation.
For in vivo photoreactivation, liposomes containing functionally active DNA-photolyase were employed (Photosomes, a gift from Applied Genetics, Freeport, NY). Briefly, DNA-photolyase was prepared from Escherichia coli expressing the photolyase from Anacystis nidulans and subsequently incorporated into liposomes. The liposome component was formed from the lipids egg phosphatidylcholine, egg phosphatidylethanolamine, and oleic acid, and from the membrane stabilizer cholesterol hemisuccinate.
Photolyase-containing liposomes were applied immediately after UVB irradiation as a lotion [Photosome Daytime Formula without sunscreen (1% DNA-photolyase-containing liposomes in a 1% hydrogel)] onto UVB-irradiated human skin (0.5 ml/cm2) for 60 min to achieve sufficient penetration of the DNA-repair enzyme into human skin. The treated skin area subsequently was covered with aluminum foil to prevent photoreactivation at this time point. After 1 h, the aluminum foil was removed and the skin was exposed to photoreactivating radiation from a UVASUN 3000 irradiation device (340–450 nm, maximum at 365 nm; Muzhas, Munich, Germany) for 30 min at 9.6 mW/cm2. As controls, selected UVB-irradiated skin areas first were exposed to photoreactivating radiation and subsequently were treated with Photosome lotion as described. To limit the degree of spontaneous photoreactivation to a minimum, Photosome-treated human skin was either kept in the dark or, for obtaining biopsies, exposed to yellow light only.
Assessment of Keratinocyte ICAM-1 Expression.
To induce the expression in keratinocytes of ICAM-1, sham-irradiated or UVB-irradiated areas of buttock skin were intracutaneously (i.c.) injected with 38,000 units of recombinant human (rh) IFN-γ (a gift from Dr. Rentschler, Laupenheim, Germany; specific activity, 1.2 × 107 units/mg). This dose was applied because it previously had been shown to induce intense ICAM-1 expression on the surface of most keratinocytes 24 h after application (17). At the indicated time points, 6-mm punch biopsies were obtained, snap frozen immediately, and stored in liquid nitrogen.
For immunohistochemical detection of keratinocyte ICAM-1 surface expression, frozen specimens were embedded in Optimum Cutting Medium (OCT, Miles), and 5-μm serial sections were prepared using a Cryocut 2000 (Reichert & Jung, Nußbach, Germany). For immunohistochemistry, the labeled streptavidin–biotin method was used, employing a commercially available kit (Dako) and a 1:400 dilution of monoclonal anti-ICAM-1 antibody 84H10 (mIgG1; Immunotech, Marseille, France) or an equivalent concentration of a mIgG1 isotype control antibody (Immunotech). After staining, the slides were mounted with aqueous mounting gel (Permafluor, Immunotech). Positive staining was identified by light microscopy as brown reaction products.
Patch Testing.
Three male subjects (mean age, 32±12 years) hypersensitive to nickel sulfate (NiSO4), as proven by earlier patch testing, were enrolled. Irradiated (2 MED) and nonirradiated skin sites were always tested in parallel. For the evaluation of delayed hypersensitivity reactions, nickel sulfate was applied immediately after UVB irradiation to the skin of the back by using Finn chambers (18). The test chambers were removed 24 h after application, and the test reaction was evaluated 48 h later and scored as positive if there were erythema, infiltration, and papules. From each test site, 4-mm punch biopsies were obtained, fixed in formalin, embedded in paraffin, stained with hematoxylin-eosin, and subsequently histologically evaluated. For semiquantitative assessment of lymphocytes in the epidermis and dermis, three serial sections per specimen were analyzed, and the number of lymphocytes in three high-power (×200) view fields was counted (19).
Results
Removal of Cyclobutane Pyrimidine Dimers from UVB-Irradiated Human Skin by Photoreactivation.
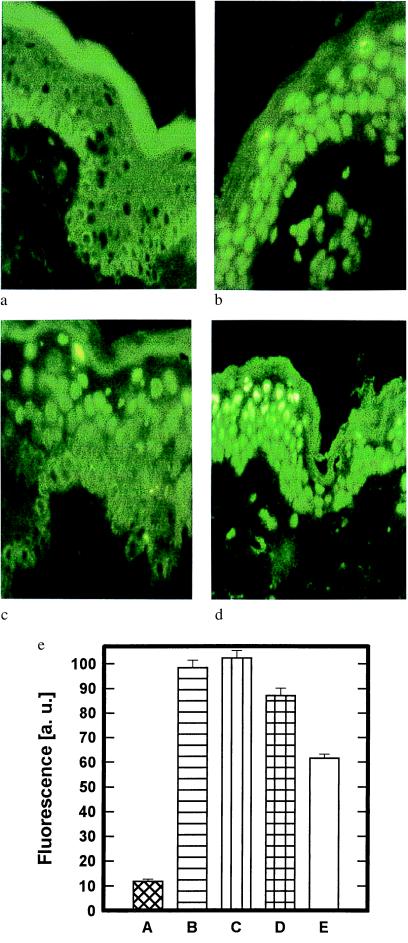
Cyclobutane pyrimidine dimer formation was studied in previously sunprotected human buttock skin 24 h after exposure to 1 MED of UVB radiation (Fig. 1). Significant cyclobutane pyrimidine dimer formation was observed in biopsies, which were obtained from UVB-irradiated skin areas (Fig. 1b), but could not be detected in unirradiated skin areas (Fig. 1a). The administration of photolyase encapsulated in liposomes to UVB-irradiated human skin and the subsequent exposure to photoreactivating radiation reduced the number of UVB radiation-induced cyclobutane pyrimidine dimers within the epidermis (Figs. 1c and 2g). In contrast, no significant decrease in the number of epidermal cyclobutane pyrimidine dimers was observed in UVB-irradiated skin areas, which had first been exposed to photoreactivating radiation and subsequently treated with photolyase (Figs. 1d and 2g). Thus, we did not detect endogenous photoreactivation under conditions in which exogenous photoreactivating enzyme was active in irradiated skin. When the time of exposure to photoreactivating light was increased from 0 to 30 min, a time-dependent increase in the removal of cyclobutane pyrimidine dimers was observed (Fig. 1e). This effect was maximal after a 30-min exposure to photoreactivating light; by this time 40–45% of cyclobutane pyrimidine dimers were removed from UVB-irradiated human skin. Therefore, a 30-min photoreactivation period was used for all further experiments. The efficacy of photolyase-induced dimer removal was essentially identical immediately after (Fig. 1e, ≈42%) or 22.5 h after (Fig. 2g, ≈44%) photoreactivation, indicating that the repair effect was already maximal at the end of the photoreactivating period.
Figure 1.
Removal of pyrimidine dimers from UVB-irradiated human skin through photoreactivation. (a–d) Human buttock skin was left untreated (a), UVB-irradiated (1 MED) (b), UVB-irradiated and subsequently treated with photolyase-containing liposomes followed by a 30-min exposure to photoreactivating light (c), or UVB-irradiated and subsequently exposed to photoreactivating light for 30 min and then treated topically with photolyase-containing liposomes (d). The presence of cyclobutane pyrimidine dimers was assessed 22.5 h after photoreactivation by immunofluorescence microscopy with mAb H3 as described in Materials and Methods. Data represent one of seven essentially identical experiments. (e) Dose response of photoreactivation-induced dimer removal from UVB-irradiated human skin. To assess the dose response between photoreactivation and dimer removal, human buttock skin was either left unirradiated (A) or exposed to 2 MED of UVB radiation and subsequently left untreated (B), UVB-irradiated and treated with photolyase-containing liposomes plus photoreactivating light for 1 min (C), 5 min (D), or 30 min (E). The presence of dimers in these skin areas was assessed immediately after photoreactivation in triplicates and quantified by immunfluorescence microscopy with mAb H3 as described in Materials and Methods. Data are given as histograms of specific fluorescence in arbitrary units (a. u.) as described (16) versus skin area and represent mean values ± SD of three experiments.
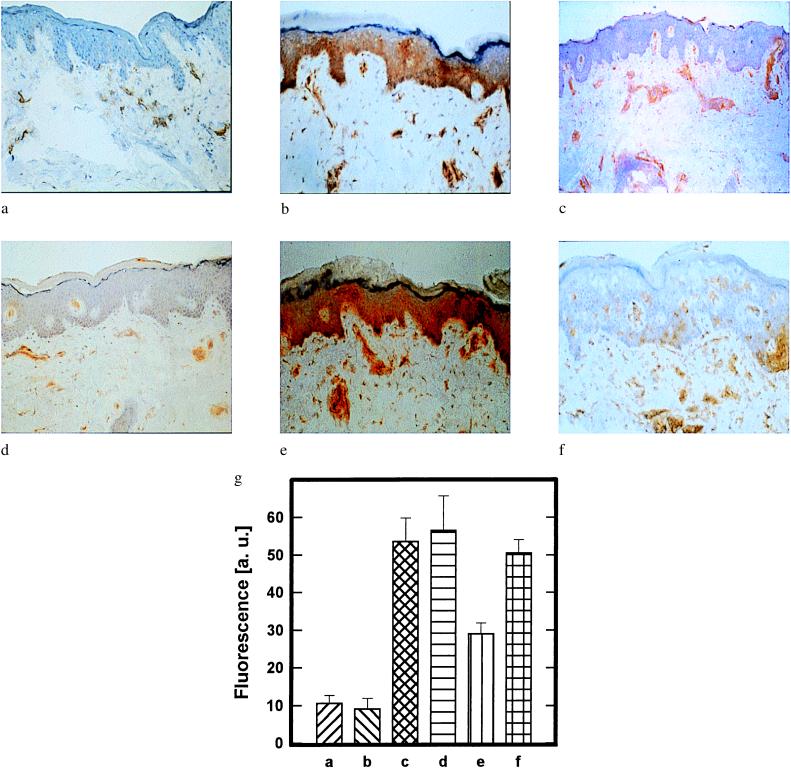
Figure 2.
Restoration of UVB radiation-induced inhibition of IFN-γ-induced keratinocyte ICAM-1 expression by photoreactivation. (a–f) Keratinocyte ICAM-1 surface expression in human buttock skin that had been left untreated (a); i.c. injected with rh IFN-γ (b); UVB-irradiated (1 MED) (c); UVB-irradiated and subsequently i.c. injected with rh IFN-γ (d); UVB-irradiated, then topically treated with photolyase-containing liposomes, followed by exposure to photoreactivating light and subsequent i.c. injection with rh IFN-γ (e); or UVB-irradiated, then exposed to photoreactivating light, followed by topical treatment with photolyase-containing liposomes and subsequent i.c. injection with rh IFN-γ (f). ICAM-1 surface expression was detected by immunohistochemistry with mAb 84H10 as described in Materials and Methods. Data represent one of three essentially identical experiments. (g) Semiquantitative analysis of epidermal pyrimidine dimer content in skin areas a–f. The presence of pyrimidine dimers was assessed 22.5 h after photoreactivation and quantified by immunfluorescence microscopy with mAb H3 as described in Materials and Methods. Data are given as histograms of specific fluorescence in arbitrary units (a. u.) as described (16) versus skin area and represent mean values ± SD of three experiments.
Photoreactivation Prevents UVB Radiation-Induced Suppression of IFN-γ-Mediated Keratinocyte ICAM-1 Expression.
We next asked whether the induction of cyclobutane pyrimidine dimers after exposure to 1 MED of UVB radiation was capable of exerting immunosuppressive effects in human skin. For this purpose, we assessed the capacity of UVB radiation to suppress IFN-γ-induced keratinocyte ICAM-1 expression in vivo in human skin. Buttock skin was exposed to 1 MED of UVB radiation, subsequently i.c. injected with rh IFN-γ, and, 24 h later, IFN-γ-induced keratinocyte ICAM-1 protein expression was assessed by immunohistochemistry. In control biopsy specimens obtained from vehicle-injected skin or UVB-irradiated skin, positive staining for ICAM-1 was limited to dermal endothelial cells (Fig. 2 a and c) as previously described (17). Injection of rh IFN-γ induced intense ICAM-1 expression on the surface of almost all keratinocytes, and staining was maximal along the basal cell layer (Fig. 2b). Injection of the identical concentration of rh IFN-γ into a buttock skin area of the same individual, which prior to cytokine stimulation had been exposed to UVB radiation, did not induce or only weakly induced keratinocyte ICAM-1 surface expression (Fig. 2d). These data indicate that in vivo exposure of human skin to 1 MED of UVB radiation is effective in suppressing IFN-γ-induced keratinocyte ICAM-1 surface expression.
This observation allowed us to ask whether removal of cyclobutane pyrimidine dimers from UVB-irradiated human skin through photoreactivation was capable of restoring IFN-γ-induced keratinocyte ICAM-1 expression. Administration of rh IFN-γ into UVB-irradiated skin, which had been treated with photolyase-containing liposomes followed by exposure to photoreactivating radiation, induced keratinocyte ICAM-1 expression (Fig. 2e). In contrast, IFN-γ-induced keratinocyte ICAM-1 expression was inhibited in UVB-irradiated skin areas that had been left untreated (Fig. 2b) or that had been exposed first to photoreactivating radiation and then treated with photolyase (Fig. 2f).
To further determine the specificity of the photolyase treatment, the number of dimers present in the epidermal compartment of these same skin areas was simultaneously measured. Intracutaneous injection of rh IFN-γ did not affect the formation of dimers in UVB-irradiated skin (Fig. 2g). Restoration of IFN-γ-mediated keratinocyte ICAM-1 expression in UVB-irradiated skin areas that had been treated with photolyase-containing liposomes followed by exposure to photoreactivating light was associated with a reduction of epidermal cyclobutane pyrimidine dimer expression by about 44% (Fig. 2g). In contrast, no significant dimer reduction was observed in UVB-irradiated skin areas that had been exposed first to photoreactivating light, followed by topical treatment with photolyase-containing liposomes and subsequent i.c. injection of rh IFN-γ (Fig. 2g).
Photoreactivation Prevents UVB Radiation-Induced Suppression of Elicitation Phase of Contact Hypersensitivity Response to Nickel Sulfate in Human Skin.
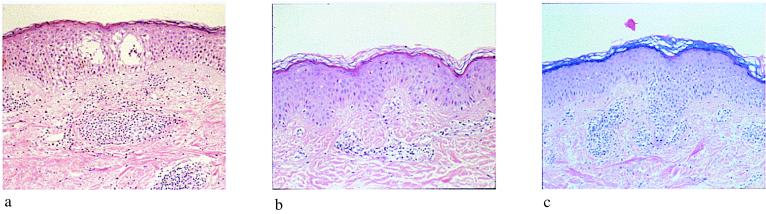
In subjects hypersensitive to nickel sulfate, positive patch test reactions were observed if patch testing was performed in unirradiated skin areas (data not shown). Histological examination of the same skin area revealed spongiosis and the presence of an intraepidermal and intradermal inflammatory infiltrate consistent with a delayed-type hypersensitivity reaction (Fig. 3a). Elicitation of this hypersensitivity reaction was completely prevented in the same individuals if patch tests were applied to a skin area immediately after exposure to 2 MED of UVB radiation. Histological examination did not indicate the presence of a significant inflammatory infiltrate in UVB-irradiated, erythematous skin, but revealed the presence of sunburn cells (Fig. 3b). Topical treatment of UVB-irradiated skin areas with photolyase-containing liposomes followed by irradiation with photoreactivating light restored the clinical as well as histological (Fig. 3c) features of the hypersensitivity response to nickel sulfate in UVB-preirradiated human skin. The major factor responsible for retention of lymphocytes in the epidermis during inflammatory and immunological reactions, including allergic contact dermatitis, is ICAM-1 expression on keratinocytes. To provide a functional correlate of restoration of allergic contact dermatitis with restoration of keratinocyte ICAM-1 expression in UVB-irradiated skin areas after treatment with photolyase-containing liposomes and photoreactivating light, the number of epidermal and dermal lymphocytes in Fig. 3 was quantified (Table 1). UVB radiation induced a marked decrease in the number of dermal and epidermal lymphocytes, which could be reversed partially by treating UVB-irradiated skin with photolyase and photoreactivating light. In addition to the effects on the lymphocytic infiltrate, photoreactivation also prevented UVB radiation-induced erythema (data not shown) and sunburn-cell formation (Fig. 3c).
Figure 3.
Restoration of the elicitation phase of the contact-hypersensitivity response to nickel sulfate in UVB-irradiated human skin through photoreactivation. Histological pictures (×100) of patch test reactions to nickel sulfate in human skin. Skin areas had been left unirradiated (a); UVB-irradiated (2 MED) before patch testing (b); or UVB-irradiated, then topically treated with photolyase-containing liposomes, followed by exposure to photoreactivating light before patch testing (c). Biopsies were taken 48 h after application of the patch test.
Table 1.
Semiquantitative assessment of lymphocytic infiltration in Fig. 3
| Localization | Cell count after treatment
|
||
|---|---|---|---|
| NiSO4 | NiSO4, UVB | NiSO4, UVB, photolyase-containing liposomes, UVA | |
| Epidermis | 12 ± 2 | 5 ± 1 | 9 ± 2 |
| Upper dermis | 48 ± 7 | 24 ± 4 | 35 ± 5 |
The number of epidermal and dermal lymphocytes was quantified as described in Materials and Methods. Data represent the mean cell count (±SD) from three view fields in three serial sections.
Discussion
In this study, it has been demonstrated that topical treatment of human skin with liposomes containing biologically active photolyase and subsequent exposure to photoreactivating radiation is effective in partially removing UVB radiation-induced cyclobutane pyrimidine dimers from the epidermis of treated skin areas, thereby diminishing erythema, sunburn-cell formation, and suppression of ICAM-1, a molecule required for immunity and inflammatory events in the epidermis. Photolyase is a DNA-repair enzyme that is functionally defined by its capacity to monomerize pyrimidine dimers in cellular DNA through a repair mechanism that requires exposure of the enzyme–DNA complex to radiation at 300–500 nm and has therefore been termed photoreactivation (6, 7). The recent cloning of a human photolyase gene homologue and reports of photoreactivation in human white blood cells and fibroblasts have suggested that endogenous photoreactivation may offer a natural means of photoprotection in human skin (6, 8, 9, 13). However, we found no evidence of endogenous photoreactivation, because exposure of UVB-irradiated human skin to photoreactivating radiation before topical treatment with photolyase-containing liposomes (Fig. 1d) or treatment of human skin with empty liposomes followed by exposure to photoreactivating radiation (data not shown) did not decrease the number of cyclobutane pyrimidine dimers present in UVB-irradiated human skin. This discrepancy might be because of the possibility that wavelengths and doses used to effect photoreactivation by Anacystis nidulans might differ from those used by human skin. In addition, using different techniques to measure dimers might give different results. In the present study, the only condition under which photoreactivation was observed was when exogenous photolyase in liposomes (Photosomes) was applied before photoreactivating light. These studies demonstrate that UVB radiation-induced DNA damage can be efficiently repaired in normal human skin, after it has been exposed to UV radiation, through the topical administration of photolyase, a DNA repair enzyme that, under our experimental conditions, cannot be detected in human skin. Similarly, topical application of the DNA-repair enzyme T4 endonuclease V was shown previously to effect dimer repair in skin of patients suffering from the DNA-repair deficiency syndrome xeroderma pigmentosum (20). In these previous studies, the average dimer removal enhanced by T4 endonuclease V treatment was approximately 20% in 6 h, whereas in the present study, photolyase treatment resulted in removal of 40–45% of cyclobutane pyrimidine dimers present in UVB-irradiated normal human skin immediately after photoreactivation, indicating that the latter approach has a greater efficiency. Also, instant reversal of cyclobutane pyrimidine dimers by photolyase treatment might prevent saturation of the natural repair systems and thereby facilitate or enhance nucleotide excision repair. At the dose used in our experiment, however, this was not the case, and the dimer-repair rates in UVB-exposed skin either treated with photolyase or untreated are very similar. Consequently, the relative reductions in DNA-damage content immediately after photolyase treatment and after 24 h were similar.
Removal of cyclobutane pyrimidine dimers from UVB-irradiated human skin through photoreactivation restored the capacity of keratinocytes to up-regulate expression of the adhesion molecule ICAM-1 on stimulation with rh IFN-γ. ICAM-1 expression on human skin cells is thought to be of critical importance for the elicitation of cell-mediated immune responses in human skin (21). In previous studies, the induction of ICAM-1 expression in human cells, including keratinocytes, has been shown to be suppressed upon exposure to sublethal doses of UVB radiation (22). This immunosuppressive effect of UVB irradiation was found to be dose-dependent, and this assay system has therefore been used as a photoimmunological endpoint to assess the susceptibility of human skin cells toward UVB radiation-induced immunosuppression (23). The present study demonstrated that, similar to the in vitro situation, UVB radiation is capable of suppressing IFN-γ-induced ICAM-1 expression in vivo as well. Moreover, restoration of IFN-γ-induced keratinocyte ICAM-1 expression in UVB-irradiated human skin through photoreactivation strongly suggests that this immunosuppressive effect is causally linked to the formation of dimers within UVB-irradiated skin areas.
The UVB doses required to achieve suppression of IFN-γ-induced keratinocyte ICAM-1 up-regulation in human skin were essentially identical to those that previously were found to suppress T cell-mediated immune responses against contact allergens in human skin (24). In those experiments, UVB radiation-induced reduction of immunization rates in human skin was found to be MED-dependent, and significant immunomodulatory effects could be observed at levels of in vivo UVB exposure that were equivalent to those used in the present study for inhibition of IFN-γ-induced keratinocyte ICAM-1 expression. Cyclobutane pyrimidine dimer formation in human keratinocytes in UVB-irradiated human skin not only may be relevant for UVB radiation-induced suppression of keratinocyte ICAM-1 expression, but also may be of general importance for the capacity of UVB radiation to affect the immune function of keratinocytes and thereby the generation of cell-mediated immune responses in human skin.
One consequence of this general immunosuppressive ability of UVB radiation is that exposure of human skin to 2 MED of UVB radiation effectively inhibited the elicitation phase of the delayed-type hypersensitivity reaction to nickel sulfate in sensitized individuals (25–27). This immunosuppressive effect could be overcome through the topical application of photolyase and the subsequent exposure of treated skin areas to photoreactivating radiation. These data indicate that DNA damage is linked to UVB radiation-induced suppression of the elicitation phase of allergic contact dermatitis. Photolyase treatment also inhibited the formation of UVB-induced erythema as well as of sunburn cells. These results clearly link DNA damage with the clinical symptoms of immunosuppression and erythema. They extend observations made in the opposum that photoreactivation of pyrimidine dimers reduced UV radiation-induced erythema (28). These data explain why the action spectrum for human erythema so closely parallels the action spectrum for the induction of cyclobutane dimers in DNA (29).
In aggregate, the present study indicates that topical application of photolyase encapsulated into liposomes to UVB-irradiated normal human skin followed by exposure of these skin areas to photoreactivating radiation was efficient in providing DNA repair and immunoprotection. Interestingly, this approach provided complete restoration of keratinocyte ICAM-1 expression, whereas the number of epidermal thymine dimers within the same skin areas was reduced by only 40–45%. A similar discrepancy between complete immunoprotection and incomplete dimer removal had been noted previously after treatment of mouse skin with T4 endonuclease V-containing liposomes (4). A possible explanation for this discrepancy might be that not all dimers are equal, but that repair of a fraction of dimers might have disproportionate effects on biological responses. This hypothesis is supported by the recent observation that only photoproducts in actively transcribed DNA, which are preferentially repaired (30) and constitute only a small fraction of the total DNA, are important in signal transduction (31). Perhaps DNA regions involved in the transcriptional regulation of the human ICAM-1 gene may be repaired more rapidly than others may. Alternatively, UVB radiation effects may require a critical number of cyclobutane pyrimidine dimers within epidermal keratinocytes, and if the number of dimers is reduced below this threshold, complete protection against UVB radiation-induced immunomodulation and apoptosis may be achieved. This would also imply that there is no linear correlation between the number of dimers and the development of immunosuppression. In support of this view, UVB radiation-induced suppression of immune responses in human skin was shown to be a function of biologically rather than physically equalized UVB radiation doses (24). Moreover, recent evidence demonstrates that keratinocyte apoptosis induced by UV (sunburn cells) is mediated by DNA damage and can be prevented even if not all cyclobutane pyrimidine dimers in DNA are repaired (32).
In conclusion, the present study indicates that topical application of exogenous photolyase to human skin is an approach that is highly efficient in protecting human skin from the deleterious effects that result from the presence of UVB radiation-induced pyrimidine dimers. Exogenous application of photolyase differs from conventional photoprotection through its ability to remove damage that has already occurred. This enzyme therapy approach could thus be ideally combined as an after-sun strategy with conventional sunscreens to provide photoprotection and repair at the same time.
Acknowledgments
We thank Paula van den Berg for her excellent technical assistance and Dr. Mossad Megahed of Düsseldorf, Germany, and Dr. Daniel Yarosh of Applied Genetics for helpful discussions. This work was supported by grants from the European Commission (ENV4-CT95–0174a); the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (BMBF 07UVB570); and the Biologisch-Medizinische Forschungszentrum of the Heinrich Heine University, Düsseldorf, Germany.
Abbreviations
- ICAM-1
intercellular adhesion molecule-1
- i.c.
intracutaneously
- MED
minimal erythema dose
- rh
recombinant human
- UVB
ultraviolet-B
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030528897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030528897
References
- 1.Coldiron B M. J Am Acad Dermatol. 1992;27:653–662. doi: 10.1016/0190-9622(92)70233-6. [DOI] [PubMed] [Google Scholar]
- 2.Elmets C A, Mukhtar H. Prog Dermatol. 1996;30:1–16. [Google Scholar]
- 3.Applegate L A, Ley R A, Alcalay J, Kripke M L. J Exp Med. 1989;170:1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vink A A, Strickland F M, Bucana C, Cox P A, Roza L, Yarosh D B, Kripke M L. J Exp Med. 1996;183:1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasparro F P, Mitchnick M, Nash J F. Photochem Photobiol. 1998;68:243–256. [PubMed] [Google Scholar]
- 6.Sutherland B M, Hacham H, Gange R W, Maytum D, Sutherland J C. In: DNA Damage and Repair in Human Tissues. Sutherland B M, Woodhead A D, editors. New York: Plenum; 1990. pp. 149–159. [DOI] [PubMed] [Google Scholar]
- 7.Sancar G B. Mutat Res. 1990;236:147–160. doi: 10.1016/0921-8777(90)90002-m. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland B M, Bennett P V. Proc Natl Acad Sci USA. 1995;92:9732–9736. doi: 10.1073/pnas.92.21.9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zölzer F, Belisheva N K, Levin V L, Samoilova K A. J Photochem Photobiol B. 1993;18:87–89. doi: 10.1016/1011-1344(93)80044-a. [DOI] [PubMed] [Google Scholar]
- 10.de Gruijl F R, Roza L. J Photochem Photobiol B. 1993;10:367–372. doi: 10.1016/1011-1344(91)80022-a. [DOI] [PubMed] [Google Scholar]
- 11.Ley R D. Proc Natl Acad Sci USA. 1993;90:4337. doi: 10.1073/pnas.90.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanai S, Kikuno R, Toh H, Ryo H, Todo T. J Mol Evol. 1997;45:535–548. doi: 10.1007/pl00006258. [DOI] [PubMed] [Google Scholar]
- 13.Todo T, Tsuji H, Otoshi E, Hitomi K, Kim S-T, Ikenaga M. Mutat Res. 1997;384:195–204. doi: 10.1016/s0921-8777(97)00032-3. [DOI] [PubMed] [Google Scholar]
- 14.Pathak M A, Nghiem P, Fitzpatrick T B. In: Fitzpatrick's Dermatology in General Medicine. Freedberg I M, Eisen A Z, Wolff K, Austen K F, Goldsmith L A, Katz S I, Fitzpatrick T B, editors. New York, NY: McGraw–Hill; 1999. pp. 1598–1607. [Google Scholar]
- 15.Roza L, Van der Wulp K J M, Macfarlane S J, Lohman P H M, Baan R A. Photochem Photobiol. 1988;48:627–634. doi: 10.1111/j.1751-1097.1988.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 16.Roza L, de Gruijl F R, Bergen Henegouwen J B A, Guikers K, Van Weelden H, Van Der Schans G P, Baan R A. J Invest Dermatol. 1991;96:903–907. doi: 10.1111/1523-1747.ep12475429. [DOI] [PubMed] [Google Scholar]
- 17.Barker J N W N, Allen M H, MacDonald D M. J Invest Dermatol. 1989;93:439–442. doi: 10.1111/1523-1747.ep12284016. [DOI] [PubMed] [Google Scholar]
- 18.Rietschel R L, Fowler J F, editors. Fisher's Contact Dermatitis. Baltimore: Williams & Wilkins; 1995. pp. 11–32. [Google Scholar]
- 19.Morita A, Werfel T, Stege H, Ahrens C, Karmann K, Grewe M, Grether-Beck S, Ruzicka T, Kapp A, Klotz L O, et al. J Exp Med. 1997;186:1763–1768. doi: 10.1084/jem.186.10.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarosh D, Klein J, Kibitel J, Alas L, O'Connor A, Cummings B, Grob D, Gerstein D, Gilchrest B A, Ichihashi I, et al. Photodermatol Photoimmunol Photomed. 1996;12:122–130. doi: 10.1111/j.1600-0781.1996.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 21.Nickoloff B J. In: The Skin Immune System. Bos J D, editor. Boca Raton, FL: CRC; 1989. pp. 49–74. [Google Scholar]
- 22.Krutmann J, Köck A, Schauer E, Parlow F, Kapp A, Förster E, Schöpf E, Luger T A. J Invest Dermatol. 1990;95:127–131. doi: 10.1111/1523-1747.ep12477839. [DOI] [PubMed] [Google Scholar]
- 23.Ahrens C, Grewe M, Berneburg M, Grether-Beck S, Quilliet X, Mezzina M, Sarasin A, Lehmann A R, Arlett C F, Krutmann J. Proc Natl Acad Sci USA. 1997;94:6837–6841. doi: 10.1073/pnas.94.13.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper K D, Oberhelman L, Hamilton T A, Baadsgaard O, Terhune M, LeVee G, Anderson T, Koren H. Proc Natl Acad Sci USA. 1992;89:8497–8501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalimo K, Koulu K, Jansen C T. Arch Dermatol Res. 1983;275:374–378. doi: 10.1007/BF00417336. [DOI] [PubMed] [Google Scholar]
- 26.Sjovall P, Christensen O B. Acta Derm Venereol. 1986;66:290–294. [PubMed] [Google Scholar]
- 27.Damia D L, Halliday G M, Barnetson R S. J Invest Dermatol. 1997;109:146–151. doi: 10.1111/1523-1747.ep12319200. [DOI] [PubMed] [Google Scholar]
- 28.Ley R. Proc Natl Aacd Sci USA. 1985;82:2409–2411. doi: 10.1073/pnas.82.8.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hacham H, Freeman S E, Gange R W. Photochem Photobiol. 1991;53:559–565. doi: 10.1111/j.1751-1097.1991.tb03671.x. [DOI] [PubMed] [Google Scholar]
- 30.Hanawalt P C. Science. 1994;266:1957–1958. doi: 10.1126/science.7801121. [DOI] [PubMed] [Google Scholar]
- 31.Blattner C, Bender K, Herrlich P, Rahmsdorf H J. Oncogene. 1998;16:2827–2834. doi: 10.1038/sj.onc.1201827. [DOI] [PubMed] [Google Scholar]
- 32.Kulms D, Pöppelmann B, Yarosh D, Luger T A, Krutmann J, Schwarz T. Proc Natl Acad Sci USA. 1999;96:7974–7979. doi: 10.1073/pnas.96.14.7974. [DOI] [PMC free article] [PubMed] [Google Scholar]