Abstract
Inhaled nitric oxide (iNO) has many extrapulmonary effects. As the half-life of NO in blood is orders of magnitude less than the circulation time from lungs to the brain, the mediator of systemic effects of iNO is unknown. We hypothesized that concentrations of nitrite, a circulating byproduct of NO with demonstrated NO bioactivity, would increase in blood and cerebrospinal fluid (CSF) during iNO therapy. iNO (80ppm) was given to six newborn lambs and results compared to six control lambs. Blood and CSF nitrite concentrations increased two-fold in response to iNO. cGMP increased in blood but not CSF suggesting brain guanylate cyclase activity was not increased. When sodium nitrite was infused intravenously blood and CSF nitrite levels increased within 10 min and reached similar levels of 14.6±1.5 µM after 40 min. The reactivity of nitrite in hemoglobin-free brain homogenates was investigated, with the findings that nitrite did not disappear nor did measurable amounts of s-nitroso, n-nitroso, or iron-nitrosyl-species appear. We conclude that although nitrite diffuses freely between blood and CSF, due to its lack of reactivity in the brain, nitrite’s putative role as the mediator of the systemic effects of iNO is limited to intravascular reactions.
Keywords: nitrite, methemoglobin, whole blood clearance rate, neuroprotection
Inhaled nitric oxide (iNO) is now a well-established therapy for the treatment of pulmonary hypertension in term infants (1–3). Although the vasodilatory effects of iNO are largely selective to the pulmonary vasculature, peripheral actions are also observed throughout the body (4–6). iNO therapy has been shown to reduce the incidence of severe intracerebral hemorrhage and periventricular leukomalacia in select groups of premature infants (7, 8) and cerebral palsy (9) and improves neurodevelopmental outcome at 18 months of age (10). These studies indicate that NO bioactivity can be taken up by blood flowing through the lungs and carried systemically. However, NO reacts rapidly with hemoglobin and its half-life in blood is only milliseconds (11). Therefore, attention has been directed towards more stable circulating metabolites of NO that might confer the systemic bioactivity of iNO.
The nitrite anion, NO2−, meets many of the criteria for being a mediator of the systemic effects of iNO. Nitrite, produced in the plasma from the oxidation of NO (12), is present in the blood of mammals at mid-nanomolar concentrations (13). Although it was thought until recently to be biologically inert at these concentrations, there is now evidence that under hypoxic/ischemic conditions nitrite becomes a significant source of NO by a number of biochemical pathways. Evidence that nitrite is a potent cytoprotective source of NO during hypoxic/ischemic stress is provided by animal studies in the liver, heart, brain and kidney (see review by Lundberg et al (14)).
Based on evidence of systemic effects of iNO and bioactivity of nitrite, we hypothesized that the neuroprotective effects of iNO are mediated by its conversion to nitrite in the pulmonary capillaries followed by transport of nitrite into the brain where it would be converted back into NO. To investigate this possibility, experiments were carried out to detect changes in the concentrations of nitrite and cGMP in the blood and CSF of newborn lambs in response to treatment with iNO. In addition, the kinetics of nitrite movement between the blood and CSF, and metabolic clearance rate of NO2− from blood in vivo were determined. Finally, the bioactivity of NO2− in hemoglobin-free brain homogenates was assessed.
Methods
Newborn lambs at ten to twenty days of age were used for these studies. Protocols were approved by the Loma Linda University Institutional Animal Care and Use Committee. Additional details regarding the methods used herein may be found online at www.pedresearch.org in the Supplemental Materials.
The lambs were anesthetized with 10 mg/kg of short-acting intravenous barbiturate, an endotracheal tube inserted, and anesthesia then continued with 1.5 % halothane in O2 from a volume respirator. Lambs were instrumented with bilateral brachial arterial and femoral venous catheters and a rectal thermistor for monitoring arterial blood pressure and heart rate, drug infusions, blood sampling, and temperature measure. A catheter was placed in the 4th ventricle by passage from the suboccipital regions for CSF sampling. The concentration of O2 utilized during the instrumentation period was 1.0. Inhalation anesthesia was then discontinued and anesthesia was maintained thereafter by morphine given intravenously (bolus 0.5 mg kg−1 and continuing infusion of 0.1 mg kg−1 hr−1) in association with vecuronium (0.1 mg kg−1 hr−1). This regime was used to mimic clinical maintenance of newborn humans receiving NO therapy. Upon discontinuation of inhalation anesthesia, FiO2 was also decreased to 0.30 for the remainder of the experiment.
Inhaled NO
A total of twelve lambs were randomly assigned to either a control (n=6) or iNO (n=6) group. To exclude endogenous NO production, N-nitro-L-arginine-methyl-ester (L-NAME) was administered (60 mg•kg−1 bolus iv followed by 2 mg•kg−1•h−1 continuous infusion) beginning 15 min before of the start of iNO treatment. Following a baseline period, iNO (80ppm) was administered for 30 min (INOvent®, iNO Therapeutics®, Clinton, NJ) followed by a 1-h recovery period. As discussed later, this dose of iNO was chosen to ensure detectable increases in blood nitrite concentrations against which CSF could be compared. Blood and CSF samples were collected simultaneously at baseline (prior to initiation of L-NAME), one min before initiation of iNO, and 15, 30, 60, and 90 min following initiation of iNO. A control group of lambs was also studied in which L-NAME was administered but iNO was withheld.
Sodium nitrite infusion
In nine of the twelve lambs studied above (six from the control group and three from the iNO group), and on completion of the recovery period of the iNO protocol, sodium nitrite was infused intravenously for 120 min (0.6 to 2.5 mg•kg−1 bolus followed by 1 mg•kg−1•h−1 infusion). Blood and CSF samples were collected simultaneously at baseline, 10, 20, 40, 60, 80, 100, and 120 min during the infusion and 30 min following termination of the infusion.
Blood and CSF sample processing
Two-milliliter arterial blood samples were collected in heparinized syringes for determination of blood gases, pH, hemoglobin, oxyhemoglobin, and methemoglobin concentrations (ABL5 and OSM3 hemoximeter, Radiometer, Copenhagen). An aliquot of the sample was added to a nitrite stabilization solution for measurement of nitrite by triiodide chemiluminescence method (15). CSF and plasma cGMP concentrations were determined via competitive EIA (Cayman Chemical, Ann Arbor, MI).
Calculation of a blood-CSF transfer coefficient
A transfer coefficient (tc) for the movement of nitrite between the blood and CSF was determined by fitting experimental data to a two-compartment model of blood-to-CSF transfer during constant infusion of nitrite. Details of this model, written in BASIC, are found in the Supplemental Materials.
Nitrite metabolism in brain homogenates
To investigate nitrite metabolism in brain tissue, homogenates of adult ovine brain were studied in vitro. After both carotids had been perfused with l liter of cold saline to remove blood from the cerebral circulation brain samples were excised, mixed with 2 volumes of TRIS-buffered saline, and homogenized (Polytron, Brinkman Instr, Westury, NY). Oxygen tensions were reduced to 10–20 Torr tonometrically by equilibration with 3% CO2 in a balance of N2, and homogenates were transferred anaerobically to sealed vials and warmed to 39 C.
Sodium nitrite was injected to achieve an initial concentration of 100 µM. Samples were collected over time for measurement of nitroso species including s-nitrosothiols, nitrosylated iron, and n-nitrosamines as previously described (16), as well as nitrite. Total nitrite and nitroso-species concentrations were determined by injection of the first aliquot into the triiodide solution in line with a chemiluminescence NO analyzer. The second aliquot was treated with acid sulfanilamide to remove free nitrite before chemiluminescence detection. The third aliquot was treated with both sulfanilamide and HgCl2 to remove both nitrite and s-nitrosothiols for measurement of iron-nitrosyl and n-nitrosamine species.
Statistical analysis
One-way ANOVA was used to detect significant changes from baseline measurements over time. Two-way ANOVA with repeated measures was used to detect differences between control and iNO groups. Bonferroni’s post hoc test was used to detect differences at specific data points. Means are presented with their standard errors. The software GraphPad Prism v5.0 for Macintosh (GraphPad Software, Inc, San Diego, CA) was used for statistical analyses.
Results
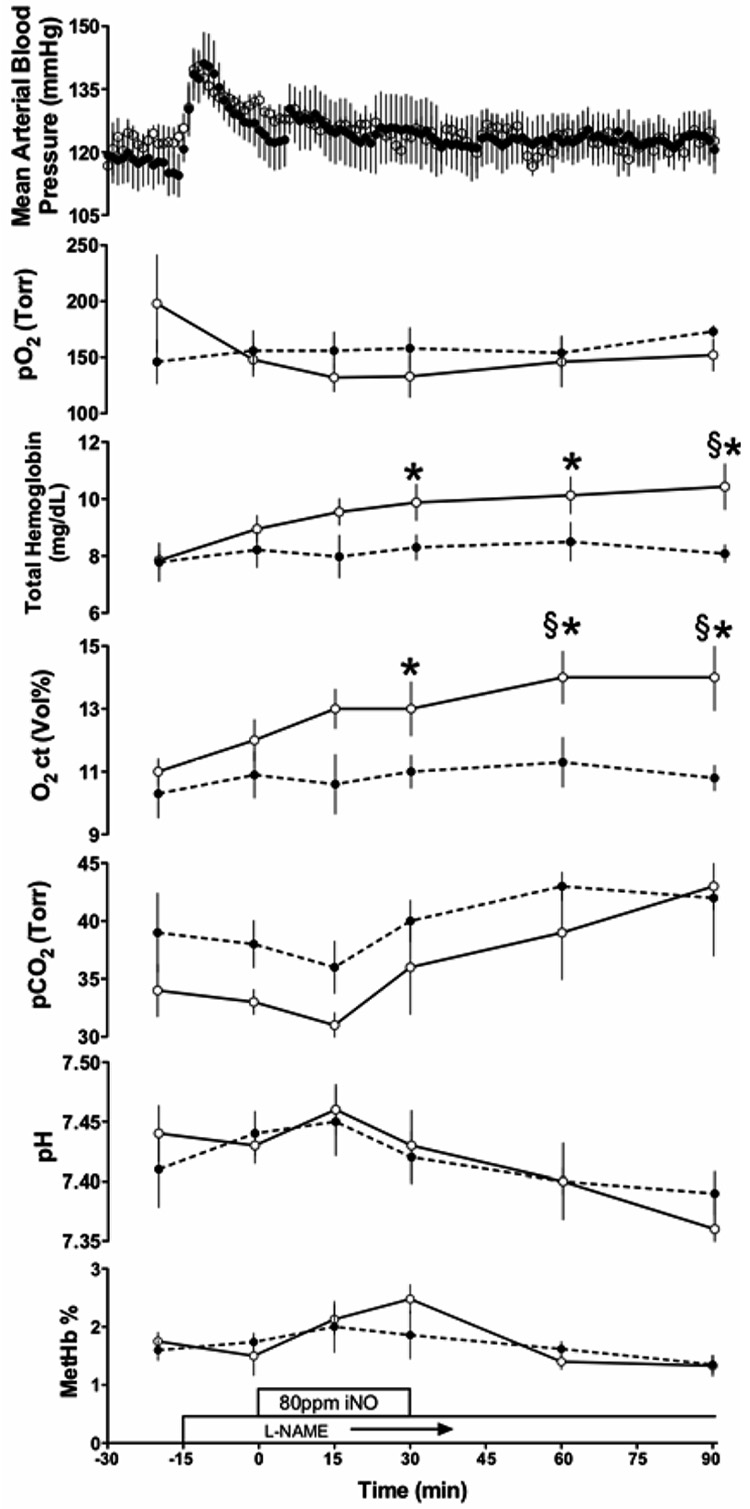
The weight of the twelve lambs studied averaged 5.5 ± 0.5 kg, with no significant baseline differences between the iNO and control groups. Baseline blood gases, methemoglobin levels and arterial blood pressures are provided in Fig 1 and were in the normal range.
Fig 1.
Time course of changes in mean arterial blood pressure and arterial PO2, oxygen content (O2 ct), PCO2, pH, and methemoglobin (MetHb) concentrations. Both control (●) and iNO (○) animals received L-NAME infusion. * indicates significant difference from baseline values (p ≤ 0.01); § indicates significant difference between control and experimental values (p ≤ 0.01).
Effects of iNO
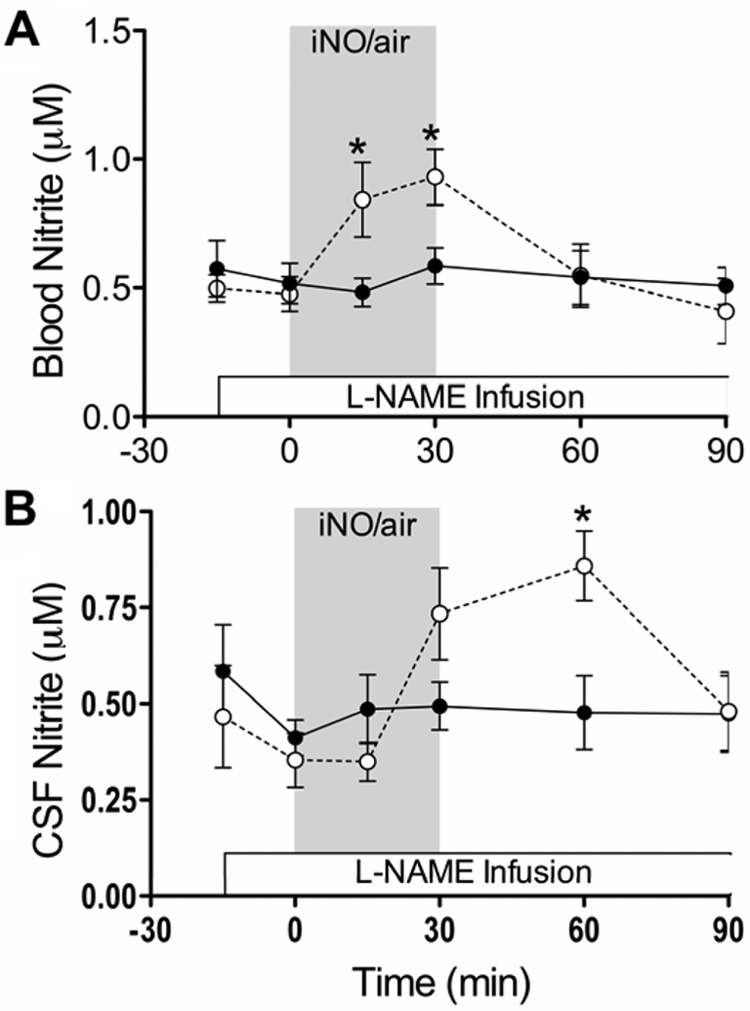
Administration of iNO resulted in significant increases in blood nitrite after 15 and 30 min of inhalation, with a return to baseline values within 30 min after the end of iNO treatment (Fig 2). Nitrite concentrations in the CSF increased more slowly and did not become significantly elevated above baseline levels until 30 min after the end of the iNO treatment period, and then returned to baseline levels 30 min later.
Fig 2.
Time profile of whole blood and CSF nitrite concentrations in response to L-NAME and addition of 80 ppm NO to inspired air (○) compared to controls (●). * indicates significant difference from baseline reached after L-NAME infusion (p ≤ 0.01).
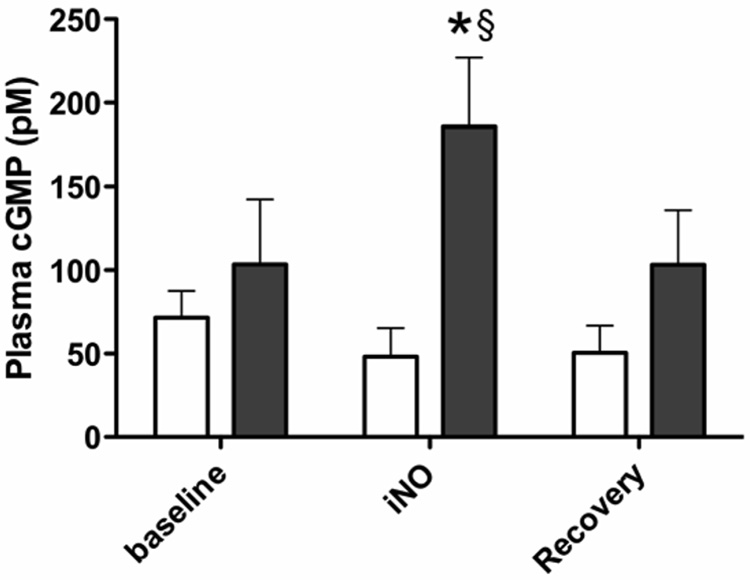
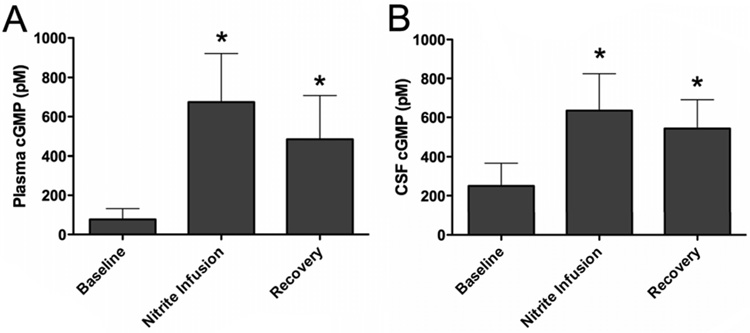
Plasma cGMP concentrations increased significantly above baseline during iNO treatment and also above levels in control animals (Fig 3). cGMP concentrations in the CSF were measurably higher than plasma levels at all time points and remained stable throughout the experiments.
Fig 3.
Plasma and CSF cGMP concentrations before, during, and after 80ppm iNO (■) compared to controls (□). Baseline samples were collected following initiation of L-NAME infusion but before initiation of iNO. * indicates significant difference from baseline values (p ≤ 0.01); § indicates significant difference from control values (p ≤ 0.01).
Methemoglobin concentration increased from baseline levels of 1.7 ± 0.2 % to reach a peak of 2.5± 0.2 after 30 min of iNO, a change that did not reach significance.
Results of intravenous infusion of nitrite
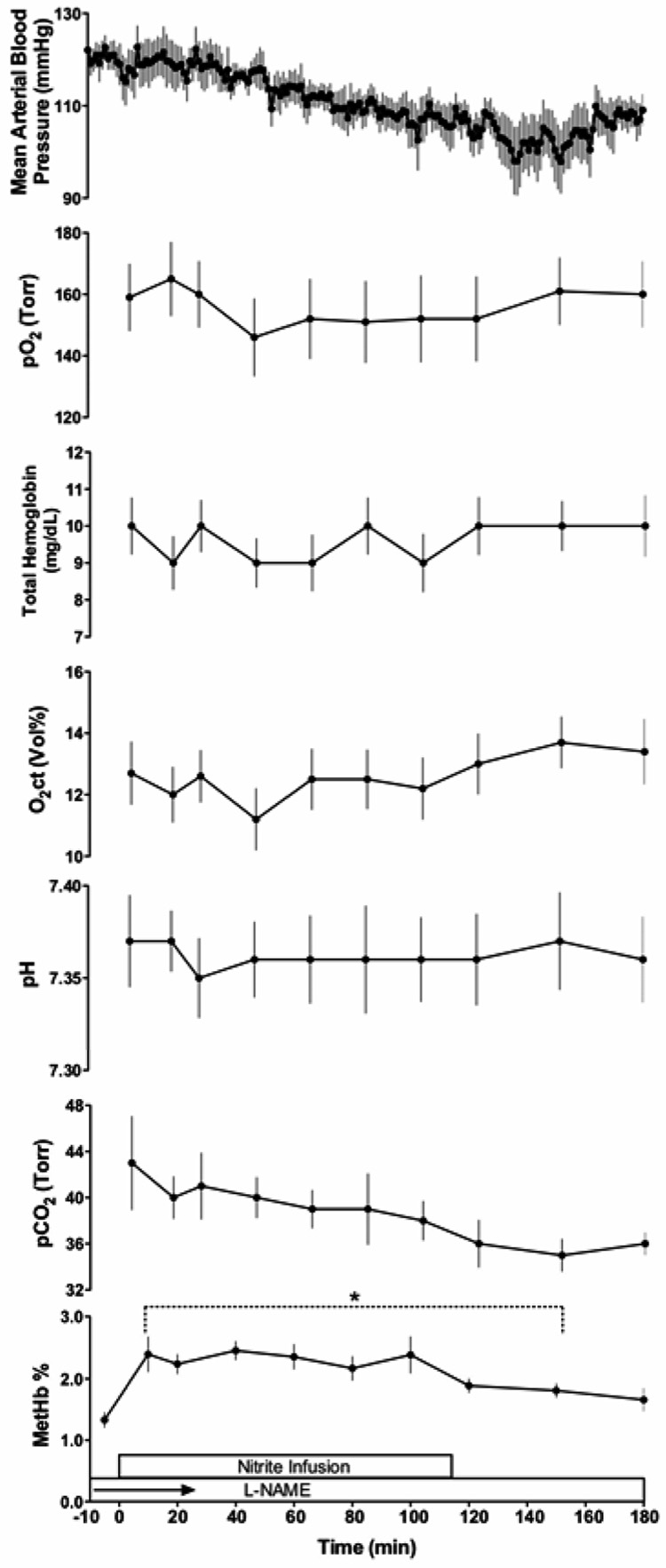
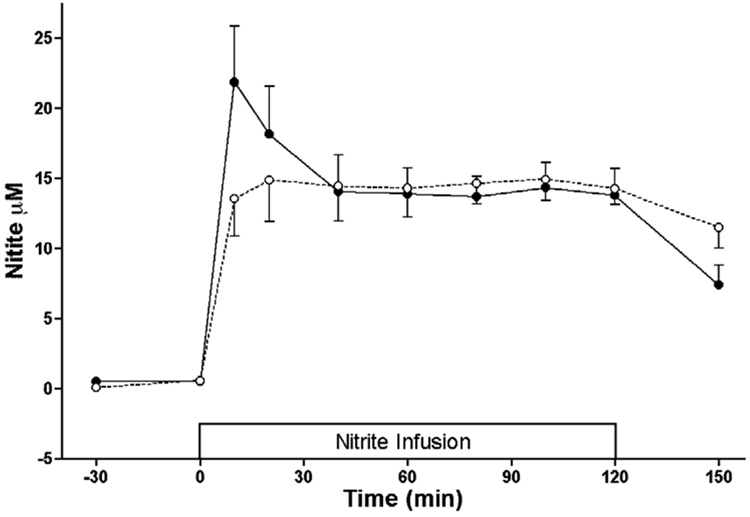
Sodium nitrite was infused to measure the tc of nitrite between blood and CSF. Physiological responses to the nitrite infusion are shown in Figure 4, and blood and CSF nitrite concentrations are shown in Figure 5. After about 45 min, blood and CSF nitrite concentrations were stable and no longer measurably different from one another. The average blood level thereafter was used to calculate the clearance rate for each lamb. Blood nitrite concentrations averaged 14.6±1.5 µM and the metabolic clearance rate was then readily calculated as the rate of nitrite infusion (0.24 µmoles•min-1•kg-1) divided by this blood level. The result averaged 16.4± 1.7 mL•min−1•kg−1 for these lambs weighing 5.5 kg. After stopping the infusion, whole blood nitrite concentrations decreased with an apparent half time of 28.3±3.6 min, somewhat longer than found earlier after bolus injections (17), suggesting nitrite had entered slower equilibrating compartments during the prolonged infusion. In the CSF, nitrite levels fell with an apparent half-life of 61±9 min.
Fig 4.
Time course of changes in mean arterial blood pressure, PO2, oxygen content (O2 ct), PCO2, pH, and methemoglobin (MetHb) levels before and during a 120-min intravenous infusion of nitrite. * indicates significant difference from baseline values (p ≤ 0.01).
Fig 5.
Blood (●) and CSF (○) nitrite responses to an initial bolus and then continuing infusion of nitrite, showing apparent steady state after ~40 min. Average values thereafter were used to calculate the metabolic clearance rate of nitrite.
The highly diffusible compound urea was used as a time marker for passage from the fourth ventricle to sampling site. After an intravenous bolus (0.2 g•kg−1), its concentration had peaked in less than five min and thereafter declined (n=3, data not shown). Because this time interval is appreciably less than the 15 min that elapsed before the first collections were made for nitrite infusion, it was felt justified not to correct for delays occasioned by transit of nitrite from the CSF-formation site to the sampling catheter.
Nitrite infusion resulted in significant increases in both plasma and CSF cGMP concentrations. Levels remained elevated for 30 min after the end of nitrite infusion, as shown in Figure 6. The blood-to-CSF transfer coefficient, tc, averaged 3.7±0.6×10−4 micromoles of nitrite flux per min per unit micromolar blood-to-CSF difference.
Fig 6.
Blood (A) and CSF (B) cGMP concentrations before, during, and after continuous intravenous infusion of nitrite. * indicates significant difference from baseline reached after L-NAME infusion (p ≤ 0.01).
Nitrite metabolism in homogenates of ovine brain tissue
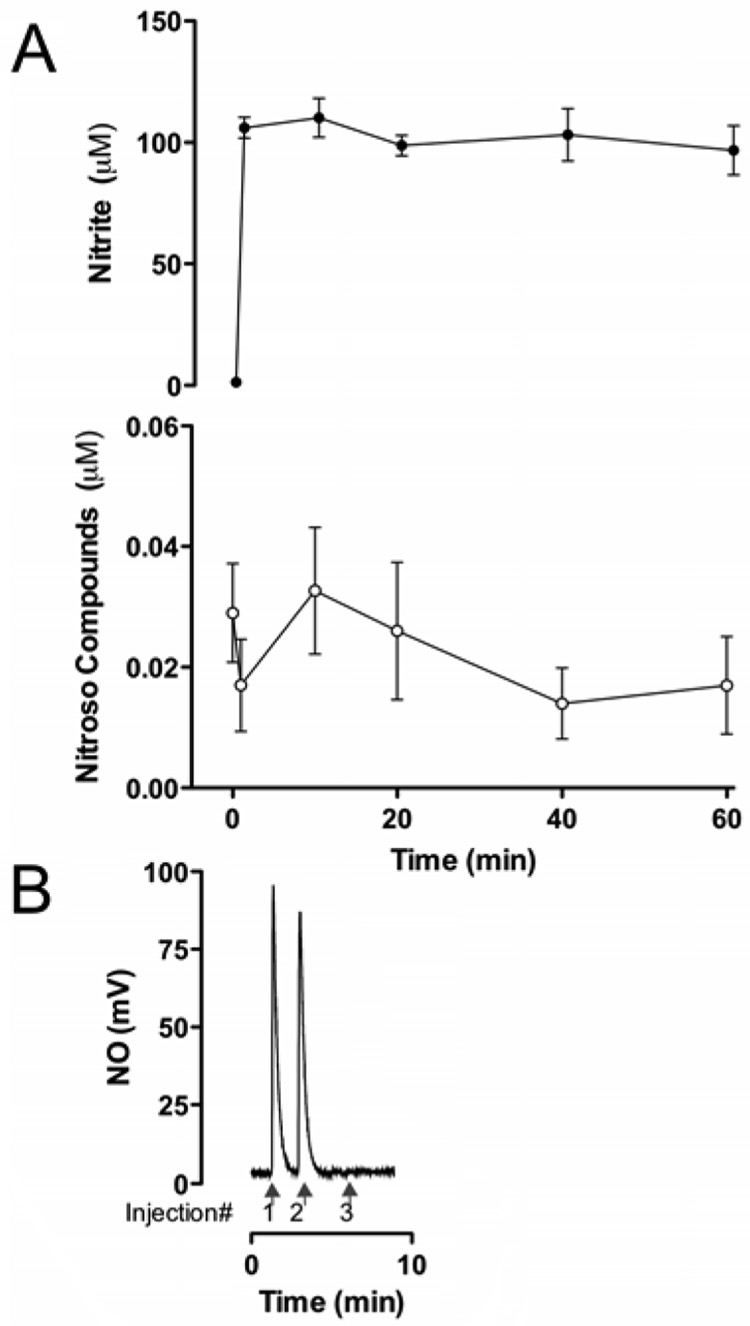
After addition of sodium nitrite to an initial concentration of 100 µM no significant changes in nitrite concentrations were observed during a 60-min period of observation. Nor was there significant production of nitroso species during this time. The average results for three adult sheep are shown in Fig 7A. A demonstration of the specificity of the analytical method for SNO is shown in Fig 7B.
Fig 7.
A) Time course of changes in nitrite and nitroso-species concentrations in brain homogenate following addition of 100µM nitrite (n = 3). B) Demonstration of the selectivity of the assay for nitrosothiols. Nitrosated cysteine standard was injected into the triiodide purge vessel either untreated (Injection #1), following treatment with acidified sulfanilamide to remove free nitrite (Injection #2), or following treatment with HgCl2 and acidified sulfanilamide to convert s-nitrosothiols to nitrite which is then removed from detection by the sulfanilamide (Injection #3).
Discussion
The present experiments provide evidence that nitrite levels increase in blood and CSF because of iNO treatment in newborn lambs. Circulating concentrations of cGMP, a downstream product of NO-mediated activation of guanylate cyclase, also increase two-fold as a result of iNO. The experimental findings also demonstrate for the first time that circulating nitrite, provided either by intravenous infusion or indirectly by inhalation of NO, freely, rapidly and reversibly enters the CSF and reaches levels comparable to those in the blood. These observations of the movement of nitrite between the blood and brain are consistent with a neuroprotective role for iNO acting via nitrite. However, the experiments provide no evidence of NO production from nitrite within the brain, suggesting any neuroprotective role for nitrite may be limited to vascular regulation.
Previous studies have shown that the effects of iNO therapy are not limited to the lungs. Administration of iNO has been shown to increase renal blood flow and glomerular filtration rate during iNO (40 ppm) in pigs (4), decrease platelet aggregation and increase aortic cGMP content in the rat (6), increase mesentery flow in cats (18), decrease peripheral resistance in sheep (19), and increase forearm blood flow in humans (5). A number of clinical studies have also investigated the possible neuroprotective effects of iNO in the premature newborn with conflicting results. iNO is associated with decreased incidence of severe intraventricular hemorrhage and periventricular leukomalacia in subgroups of preterm infants (7), improved neurodevelopmental outcome at 18 months of age (10), decreased incidence of intracranial hemorrhage and ventriculomegaly (8), and decreased incidence of cerebral palsy (9). In contrast, the use of iNO in extremely premature infants (<1000g) has also been shown to increase the incidence of intraventricular hemorrhage (20) with no effect on neurodevelopmental outcome (21). Thus, further controlled clinical trials are needed to investigate the potential neurological benefit of iNO administration to the premature infant. Nonetheless, much evidence indicates NO bioactivity can be transported throughout the body. However, hemoglobin rapidly scavenges free NO so that the half-life of NO in whole blood is about 10 msec (22). Therefore, iNO entering the pulmonary blood is likely metabolized before leaving the lung, suggesting iNO’s systemic effects are mediated by metabolites of NO.
In recent years, nitrite has been proposed as a circulating reservoir of NO bioactivity. It is normally present at mid nanomolar concentrations in plasma (13), and is formed there as an oxidation product of endogenously released NO (12). Nitrite can be reduced back to NO by a number of different biochemical pathways, most of which are favored in acidic and hypoxic tissues (see review by Lundberg et al (14)).
In this study, we hypothesized that iNO would be converted to nitrite in the lung, carried to the brain via the blood, and enter the CSF and brain tissue. This hypothesis is supported by our observations of the effects of iNO on blood and CSF nitrite concentrations. We found that iNO does result in increased concentrations of nitrite in the blood. Furthermore, the current experiments demonstrate for the first time that iNO can result in increased nitrite concentrations within the brain itself. That nitrite moves freely between the blood and CSF is further supported by the current measurements of increased CSF nitrite concentrations during intravenous nitrite infusion.
There is a growing consensus that nitrite’s bioactivity begins with its conversion to NO, supported by numerous studies showing that the cytoprotective effects of nitrite are inhibited by carboxy-PTIO-(2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5,-tetramethyl-1 H-imidazolyl-1-oxy-3-oxide (PTIO) (14). The reduction of nitrite to NO occurs by a number of biochemical pathways including reaction with deoxyhemoglobin (23), myoglobin(24), non-enzymatic disproportionation (25), and reactions catalyzed by both xanthine oxidase (26) and nitric oxide synthase (27) under hypoxic conditions.
In order to assess the potential NO-bioactivity of nitrite, cGMP concentrations were measured in the blood and CSF of the current study as a surrogate marker for the presence of increased systemic and brain NO concentrations, respectively. cGMP concentrations became elevated in blood during iNO treatment, but did not change significantly in the CSF, suggesting the increase in CSF nitrite did not result in increased cerebral NO. In contrast, intravenous infusion of nitrite to blood concentrations approximately 15-fold higher than baseline levels resulted in a significant increase in both blood and CSF cGMP concentrations. These latter data are consistent with the concept that nitrite serves as a source of NO in the body, with subsequent activation of guanylate cyclase. However, it cannot presently be determined whether the increase in CSF cGMP we observed was a result of nitrite reduction to NO within the brain itself, reduction of nitrite to NO in the blood vessels followed by diffusion of NO into the brain, or by diffusion of cGMP itself from the blood into the CSF.
Other possible carriers of NO bioactivity
Nitrite is not the only potential carrier of NO bioactivity from the lungs to peripheral organs. Other possibilities include s-nitrosated proteins (RSNOs) (28, 29), and iron-nitrosyl hemoglobin, HbNO (5). Although HbNO has traditionally been considered biologically inert due to its stable nature, recent evidence now suggests that HbNO may release NO in the arterial vasculature (5, 30). RSNOs are formed by NO binding reversibly to the cysteine residues of circulating proteins such as albumin and hemoglobin, resulting in a circulating pool of RSNO capable of releasing NO in the resistance vessels and capillaries (28, 29). These carriers, as well as nitrite, may play a part and the findings in the present study do not exclude them.
It is also worth noting that the dose of iNO administered in clinical practice (5 to 20 ppm) is significantly lower than that employed in the present study (80ppm). Dose-ranging studies in both the human (23, 31) and mouse (32) demonstrate the vasoactive and cytoprotective effects of nitrite are potent, with physiological effects observed with increases of blood nitrite concentrations as small as 20%, which approaches the precision limits of the nitrite assay. Our decision to use 80ppm was based on the desire to ensure measurable increases in blood nitrite concentrations in a minimal number of lambs. Given our observation of increased CSF nitrite concentrations after a 30-min treatment with 80ppm iNO, it is not unreasonable to assume effective changes in nitrite concentrations are observed in the blood and CSF of patients who are typically treated at lower doses but for periods of hours to days. In addition, the lack of increase in CSF cGMP concentrations at 80ppm suggests clinical doses of iNO are not likely to activate brain cGMP.
Metabolism of nitrite in brain tissue homogenates
Based on reports that intravascular nitrite is an important regulator of vascular tone following conversion to NO by reaction with deoxyhemoglobin, it is possible that the neuroprotective effects of nitrite are mediated by direct vasoregulation. However, circulating nitrite may also alter organ function via production of s-nitrosated, heme-nitrosylated, or n-nitrosaminated proteins (33). In the present study, we observed no metabolism of nitrite added to brain tissue homogenates in vitro, nor any evidence of NO-modified protein production. These findings are consistent with those of Bryan et al, who observed increased nitrosated protein concentrations in a number of organs, but not in the brain, following intraperitoneal administration of nitrite to mice (33). While further studies are needed to evaluate the effects of nitrite on living brain tissue, the current study offers no evidence of direct metabolism of nitrite within the brain.
Effect of iNO on blood oxygen content
As demonstrated in Fig. 1, we observed a significant increase in blood oxygen content of iNO-treated lambs, a result of increased blood hemoglobin concentrations. Given the potent effects of iNO on stimulating renal blood flow and glomerular filtration rates, this may have been caused by decreased plasma volume due to increased glomerular filtration (4), or due to release of erythrocytes into the circulation. In any case, if one assumes no decrease in cardiac output during iNO administration (4), the increase in oxygen-carrying capacity observed in the present studies would equate to a ~20% increase in systemic oxygen delivery and bears further examination as a possible extrapulmonary benefit of iNO in its own right.
Conclusions and perspectives
The finding that iNO results in extrapulmonary NO-mediated effects has heightened interest in the endocrine role of NO and its byproduct nitrogen-oxide species. Growing evidence now suggests nitrite plays a protective role under conditions of hypoxic stress. The results of the present study confirm that iNO treatment results in increased nitrite concentrations in both the blood and CSF. We were unable to observe any reactivity of nitrite within the brain tissue, suggesting any effects of nitrite are mediated by regulation of blood flow from within the vascular lumen as opposed to reactions within the brain tissue.
Given the high prevalence of neurological sequelae in preterm infants and the recent evidence that they may be prevented by iNO treatment, further study of the metabolism of nitrite and other putative mediators of the extrapulmonary effects of iNO carries great therapeutic potential.
Acknowledgments
We are grateful to Shannon Bragg, Jennifer File, and Janet R. Ninnis for their help with these experiments and manuscript preparation.
Financial Support: This study was supported in part by grant R01-HL65494 to GGP from the National Institutes of Health.
Abbreviations
- iNO
inhaled nitric oxide
- CSF
cerebrospinal fluid
- HbNO
iron nitrosyl hemoglobin
Footnotes
Supplementary material contained in this article is available online at www.pedresearch.com.
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts JD, Jr, Fineman JR, Morin FC, 3rd, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, Heymann MA, Zapol WM. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336:605–610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 2.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 3.Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia The Neonatal Inhaled Nitric Oxide Study Group (NINOS) Pediatrics. 1997;99:838–845. doi: 10.1542/peds.99.6.838. [DOI] [PubMed] [Google Scholar]
- 4.Troncy E, Francoeur M, Salazkin I, Yang F, Charbonneau M, Leclerc G, Vinay P, Blaise G. Extra-pulmonary effects of inhaled nitric oxide in swine with and without phenylephrine. Br J Anaesth. 1997;79:631–640. doi: 10.1093/bja/79.5.631. [DOI] [PubMed] [Google Scholar]
- 5.Cannon RO, 3rd, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kermarrec N, Zunic P, Beloucif S, Benessiano J, Drouet L, Payen D. Impact of inhaled nitric oxide on platelet aggregation and fibrinolysis in rats with endotoxic lung injury Role of cyclic guanosine 5'-monophosphate. Am J Respir Crit Care Med. 1998;158:833–839. doi: 10.1164/ajrccm.158.3.9709097. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349:2099–2107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 8.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, George TN, Southgate WM, Carriedo H, Couser RJ, Mammel MC, Hall DC, Pappagallo M, Sardesai S, Strain JD, Baier M, Abman SH. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka Y, Hayashi T, Kitajima H, Sumi K, Fujimura M. Inhaled nitric oxide therapy decreases the risk of cerebral palsy in preterm infants with persistent pulmonary hypertension of the newborn. Pediatrics. 2007;119:1159–1164. doi: 10.1542/peds.2006-2269. [DOI] [PubMed] [Google Scholar]
- 10.Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. N Engl J Med. 2005;353:23–32. doi: 10.1056/NEJMoa043514. [DOI] [PubMed] [Google Scholar]
- 11.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 12.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 13.Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 15.Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, Gladwin MT, Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med. 2006;41:541–548. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Feelisch M, Rassaf T, Mnaimneh S, Singh N, Bryan NS, Jourd'Heuil D, Kelm M. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. FASEB J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 17.Blood AB, Power GG. In vitro and in vivo kinetic handling of nitrite in blood: effects of varying hemoglobin oxygen saturation. Am J Physiol Heart Circ Physiol. 2007;293:H1508–H1517. doi: 10.1152/ajpheart.01259.2006. [DOI] [PubMed] [Google Scholar]
- 18.Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi Y, Kobayashi H, Tanaka N, Sato T, Takizawa N, Tomita T. Nitrosyl hemoglobin in blood of normoxic and hypoxic sheep during nitric oxide inhalation. Am J Physiol. 1998;274:H349–H357. doi: 10.1152/ajpheart.1998.274.1.H349. [DOI] [PubMed] [Google Scholar]
- 20.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, Perritt R, Higgins RD, Oh W, Hudak ML, Laptook AR, Shankaran S, Finer NN, Carlo WA, Kennedy KA, Fridriksson JH, Steinhorn RH, Sokol GM, Konduri GG, Aschner JL, Stoll BJ, D'Angio CT, Stevenson DK. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005;353:13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 21.Hintz SR, Van Meurs KP, Perritt R, Poole WK, Das A, Stevenson DK, Ehrenkranz RA, Lemons JA, Vohr BR, Heyne R, Childers DO, Peralta-Carcelen M, Dusick A, Johnson YR, Morris B, Dillard R, Vaucher Y, Steichen J, Adams-Chapman I, Konduri G, Myers GJ, de Ungria M, Tyson JE, Higgins RD. Neurodevelopmental outcomes of premature infants with severe respiratory failure enrolled in a randomized controlled trial of inhaled nitric oxide. J Pediatr. 2007;151:16–22. doi: 10.1016/j.jpeds.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim-Shapiro DB, Gladwin MT, Patel RP, Hogg N. The reaction between nitrite and hemoglobin: the role of nitrite in hemoglobin-mediated hypoxic vasodilation. J Inorg Biochem. 2005;99:237–246. doi: 10.1016/j.jinorgbio.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 24.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim Biophys Acta. 1999;1411:250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Naughton D, Winyard PG, Benjamin N, Blake DR, Symons MC. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem Biophys Res Commun. 1998;249:767–772. doi: 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]
- 27.Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci. 2007;64:96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc. 2006;3:153–160. doi: 10.1513/pats.200507-066BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng ES, Jourd'heuil D, McCord JM, Hernandez D, Yasui M, Knight D, Kubes P. Enhanced S-nitroso-albumin formation from inhaled NO during ischemia/reperfusion. Circ Res. 2004;94:559–565. doi: 10.1161/01.RES.0000117771.63140.D6. [DOI] [PubMed] [Google Scholar]
- 30.Grubina R, Huang Z, Shiva S, Joshi MS, Azarov I, Basu S, Ringwood LA, Jiang A, Hogg N, Kim-Shapiro DB, Gladwin MT. Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy- and oxyhemoglobin. J Biol Chem. 2007;282:12916–12927. doi: 10.1074/jbc.M700546200. [DOI] [PubMed] [Google Scholar]
- 31.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 32.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]