Abstract
The rates of transmethylation and transsulfuration of methionine were quantified using [1-13C]methionine and [C2H3]methionine tracers in newborn infants born at term gestation and in prematurely born low birth weight infants. Whole body rate of protein breakdown was also measured using [2H5]phenylalanine. The response to enteral formula feeding and parenteral nutrition was examined in full term and prematurely born babies, respectively. The relative rates of appearance of methionine and phenylalanine were comparable to the amino acid composition of mixed body proteins. Rates of transmethylation were high, both in full term infants (fast 32±14 µmoles kg−1 h−1; fed 21.7±3.2) and in preterm infants (57.2±14.8). Significant flux through the transsulfuration pathway was evident (full term: fast 6.0±4.4, fed 4.1±2.1; preterm: 24.9±9.9 µmoles kg−1 h−1). Transsulfuration of methionine is evident in the human newborn in the immediate neonatal period, suggesting that cysteine may not be considered a “conditionally” essential amino acid for the neonate. The high rate of transmethylation may reflect the high methylation demand, while high rates of transsulfuration in premature babies may be related to high demands for glutathione and to the amounts of methionine in parenteral amino acid mixtures.
Keywords: methionine, transmethylation, transsulfuration, homocysteine, newborn infant, prematurity, LBW Infants
Methionine, an essential amino acid, is also a source of methyl groups for a number of methylation reactions such as methylation of nucleic acids, proteins, biogenic amines, phospholipids, etc. Methionine is also a source for the cysteine required for the synthesis of glutathione (1,2). Interest in the metabolism of methionine has remained high ever since it was observed that a key enzymes involved in the formation of cysteine from homocysteine (transsulfuration), cystathionine γ lyase, is absent in the fetal liver and its activity appears for the first time in the immediate neonatal period (3–7). It has been suggested that the human fetus and neonate is unable to convert cystathionine to cysteine in significant quantities. Thus, cysteine has been suggested to be a “conditionally essential” amino acid for the neonate, and is often added to the parenteral amino acid mixtures, especially for prematurely born infants (8,9). However, the development of the activity of cystathionine γ lyase after birth, the impact of premature birth and of nutrient interventions on the transsulfuration of methionine is unknown.
The synthesis of cysteine from homocysteine and serine is regulated by an individual’s nutrient state and by the relative concentration of insulin, glucagon and adrenal corticosteroids (10–12). Insulin has a repressive effect on hepatic cystathionine γ lyase and on cystathionine β synthase, while glucagon and glucocorticoids increase the hepatic activity of these enzymes. Transition to extrauterine life is characterized by a decrease in plasma levels of insulin and a surge in plasma glucagon and catecholamines (13). In contrast, parenteral nutrition (amino acids plus glucose) increases plasma insulin and decreases the concentration of glucagon. The impact of adaptation during transition to extrauterine life and the effect of nutrient interventions on methionine metabolism have not been evaluated. In the present study, we have quantified the kinetics of methionine and its metabolism in healthy full term and prematurely born infants. Our data show that the human newborn develops the capacity to metabolize methionine via transsulfuration rapidly after birth.
Methods
The study protocol was approved by the Institutional Review Board of MetroHealth Medical Center, Cleveland, Ohio. All studies were carried out after obtaining verbal assent from the attending neonatologist. Written informed consent was obtained from the parent(s) after fully explaining the procedure. The studies in full term infants were performed in the General Clinical Research Center, while studies in premature infants were performed in the neonatal intensive care unit.
Full term appropriate- for- gestation newborn infants (n=18) were recruited from the newborn nursery (Table 1). They were all healthy and had no antenatal or neonatal problems. Their Apgar scores were within normal range and none were receiving antibiotics. All of them were receiving formula (Similac®, Ross Pharmaceuticals, Columbus, Ohio) ad libitum every 3h from birth.
Table 1.
Characteristics of subjects and nutritional data
| Gestational Age | Birth Wt | Age at Study | Weight at Study | Protein* | Phenylalanine* | Methionine* | |
|---|---|---|---|---|---|---|---|
| (wks) | (gm) | (h) | (gm) | (g.kg.d) | (µmol.kg−1.h−1) | (µmol.kg−1h−1) | |
| Full term | 39.1 ± 1.5 | 3315 ± 503 | 39 ± 10 | 3277 ± 516 | 2.4 ± 0.1 | 21.6 ± 0.6 | 14 ± 0.4 |
| (n=18) | |||||||
| Preterm | 28.6 ± 1.7 | 1230 ± 248 | 73 ± 14 | 1155 ± 248 | 3.1 ± 0.5 | 30 ± 5 | 24 ± 0.4 |
| (n=9) |
Data are mean ± SD
Full term infants were fed commercial formula (SimilacR) while preterm infants were receiving parenteral nutrition (TrophamineR) with added cysteine (40 mg/g protein or 42 ± 7 μmole.kg−1.h−1) and intralipid.
Nine prematurely born neonates were recruited from the neonatal intensive care unit. Their Apgar scores were >7 at 5 min and the median score for neonatal acute physiology (SNAP), a marker of acuity of illness at birth (14), was 11 (25th –75th percentile: 6–16). The infants were all clinically stable, were either on minimal ventilator support or were receiving supplemental oxygen via nasal cannula. None were receiving vasopressors or glucocorticoids. All prematurely born babies were given ampicillin and gentamicin for 48 h for presumed sepsis.
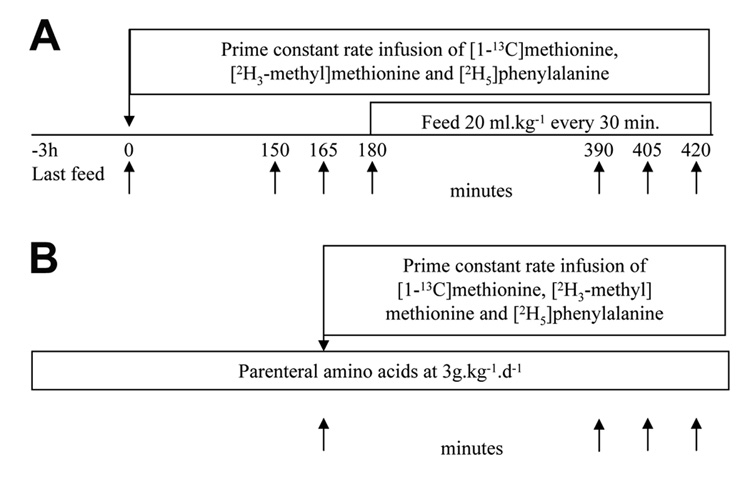
Full term infants (Figure 1A)
Figure 1.
Study design. (A) Full term infants. (B) Preterm infants.
Three hours after their last feed, two indwelling vascular catheters were placed, one on the dorsum of the hand for infusion of the tracer solution, and the other in the saphenous vein. After obtaining basal sample (time 0), tracer amino acid solution was infused for 7h. Blood and breath samples were obtained during the basal state and following feeding. Every 30 minutes, infants were fed 20ml.kg−1 of infant formula (Similac®) in six equal aliquots. The formula provided methionine at 14±0.4 µmol.kg−1.h–1 and phenylalanine at 21.6±0.6 µmol.kg−1.h−1. Breath samples were collected in a small anesthesia bag using a facemask and a low resistance Rudolph valve (15) and then transferred into the sampling tube. The rate of carbon dioxide production (VCO2) was measured during the basal period and after enteral feeding by using a DeltaTrac II indirect calorimeter (SensorMedics; Yorba Linda, CA) (15,16).
Premature infants (Figure 1B)
As per the clinical practice, premature infants were started on 10% dextrose water at birth and changed to parenteral nutrition between 24–36h. Babies were on parenteral amino acid solution (Troph Amine ®, B. Braun Medical, Irvine, CA) at 3g kg−1 d−1 for 24h before the tracer study. Cysteine hydrochloride was added at 40 mg g−1 amino acid. Preexisting indwelling vascular catheters, placed for clinical reasons, were used to infuse the tracers and to collect blood samples. After obtaining the basal samples, the tracer solutions were infused for 5h. Breath samples were collected by placing a plastic cannula attached to a 20 ml syringe near the external nares. The expired air was drawn in the syringe and transferred into a sampling tube. During the tracer study, babies received parenteral amino acids 3.1±0.5g kg−1 d−1, methionine 24±0.4 µmol kg−1 h−1, phenylalanine 30±5 µmol kg−1 h−1, and cysteine 33±6 µmol kg−1 h−1.
The tracers were infused as follows: [1-13C] methionine (Prime: 2.9 µmole kg−1; Constant rate 1.8µmol kg−1 h−1), [2H3 methyl] methionine (Prime: 2.9µmol kg−1; Constant rate 1.8µmol kg−1 h−1) and [2H5] phenylalanine (Prime: 4µmol kg−1; Constant rate 4µmol kg−1 h−1). A priming dose of 60µmol of NaH[13C]O3 was given to achieve an early isotopic steady state in the bicarbonate pools. Initial data showed that this priming dose of sodium bicarbonate was high for the fasting state and therefore the dose was reduced to 25 micromoles in subsequent (n=5) studies. Two full term infants were studied without a priming dose of sodium bicarbonate. Complete fasting and feeding data on transsulfuration was obtained in seven full term infants, while data in the fed state could be calculated on all infants. In full term infants, blood and breath samples were collected every 15 min between 150–180 min (fast) and between 390–420 min (feed). Samples from premature infants were collected every 30 minutes between 240–300 min. Samples of the tracer infusates were obtained for quantitative analysis and to test for sterility.
Analytical procedures
Blood glucose was measured by the glucose oxidase method using a glucose/lactate analyzer (Yellow Springs Instruments, Yellow Springs, OH). The concentration of total homocysteine, total cysteine and of amino acids in the plasma and infusates were measured by HPLC (15,17). Plasma insulin levels were determined using a human plasma insulin ELISA kit (Millipore; Billerica, MA).
Gas chromatography-mass spectroscopy analysis
The methodology used to measure the enrichment of amino acid tracers in the plasma has been described (15–17). The m/z 250 (m+0) and 255 (m+5) were monitored to measure the enrichment of [2H5]phenylalanine. The mass-to-charge (m/z) ratios 234 (m+0), 235 (m+1), 236 (m+2), 237 (m+3) and 238 (m+4) were monitored to quantify unlabeled and labeled methionine. The mass 235 (m+1) represented the enrichment of [1-13C]methionine and the mass 237 (m+3) represented the enrichment of [2H3 methyl]methionine. Multiple linear regression analyses were performed in order to calculate the relative enrichments and correction for natural abundance of m+1 ([1-13C] tracer) and m+3 ([C2H3]methyl)methionine (18,19), using an in-house developed software (by J. Kim). Enrichment of 13C in the carbon dioxide, which was quantified by isotope ratio mass spectrometry (Metabolic Solutions; Nashua, NH). The 13C enrichment of homocysteine in plasma was measured as described by Davis et al (20).
The rate of appearance (Ra) of phenylalanine was calculated by tracer dilution during isotopic steady state (21). Phenylalanine Ra from protein breakdown was calculated by subtracting the exogenously administered phenylalanine from total phenylalanine Ra.
The various components of methionine metabolism were calculated as described by Storch et al and McCoss et al (19,22,23). The Ra of methionine estimated from the dilution of carboxyl labeled tracer (Qc) represents methionine entering the circulation from proteolysis and from exogenous, enteral or parenteral, source. The carboxyl label is retained during the conversion of methionine to homocysteine (transmethylation) or back to methionine (remethylation). In contrast, the methyl label is lost during transmathylation and replaced by unlabeled methyl group during remethylation. Therefore, the Ra of methionine estimated from the dilution of [C2H3]methionine is a sum of methionine released from protein breakdown, and the methionine that is exogenously administered plus the amount that is synthesized by methylation of homocysteine.
Ra (carboxyl tracer) or QC = B+I, where B is the appearance of methionine via protein breakdown and I is the methionine administered exogenously.
Ra (methyl tracer) or QM = B+I+RM, where RM is the rate of remethylation of methionine from homocysteine. The difference between QM and QC represents the rate of remethylation: QM-QC=RM.
The rate of transsulfuration was assumed to be equal to the rate of oxidation of methionine and was estimated from the rate of appearance of 13C of [1-13C]methionine tracer in the expired CO2 (22,23). We could not perform satisfactory respiratory calorimetry estimates in the LBW infants. Therefore, the average VCO2 data (6 ml.kg−1.min−1−) from the literature (24–26) were used. The calculations for the rate of oxidation have been described (16). It is assumed that during the formation of cysteine, an equimolar quantity of alpha-ketobutyrate is formed and is oxidized to CO2 in the tricarboxylic acid cycle (19,23).
We did not correct the kinetic data for the intracellular enrichments of methionine; therefore, our estimates of transmethylation and transsulfuration are lower than actual. We did measure the plasma homocysteine enrichments as an index of intracellular [1-13C]methionine enrichment. However, homocysteine enrichments were not measured at all time points and, therefore, cannot be used as a measure of isotopic plateau.
We did not adjust for possible CO2 retention, because our previous data and those of others from healthy adults showed no significant retention of tracer during parenteral glucose infusion (27). The use of a 20% tracer retention factor would only increase our estimates of transsulfuration.
Statistical analysis
All data are presented as mean±SD. Group comparisons were made using parametric and nonparametric statistical methods with Statistix software (Analytical Software; La Jolla, CA).
Results
All infants were in stable clinical state and tolerated the procedures well.
Plasma amino acids and insulin concentrations
In full term babies, in response to feeding there was a significant increase in the concentration of essential amino acids (leucine, isoleucine, valine, phenylalanine, methionine and arginine), and of certain non-essential amino acids, i.e. ornithine, citrulline, alanine aspartate and glutamate (Table 2). The plasma amino acid concentrations of preterm infants were significantly higher when compared with full term infants in the fed state, except for glutamine and alanine, which were significantly lower.
Table 2.
Plasma amino acid concentration in full term and premature infants
| Full term babies | Premature infants | ||
|---|---|---|---|
| Fast | Feed | During Parenteral Nutrition | |
| (n=18) | (n=18) | (n=9) | |
| Glutamate | 22 ± 7 | 24 ± 6 | 35 ± 16 |
| Aspartate | 48 ± 11 | 52 ± 9* | 10 ± 2 |
| Serine | 141 ± 43 | 152 ± 41 | 194 ± 70 |
| Glutamine | 770 ± 146 | 808 ± 147 | 450 ± 150 |
| Glycine | 263 ± 83 | 262 ± 73 | 266 ± 78 |
| Histidine | 63 ± 19 | 67 ± 18 | 94 ± 28 |
| Threonine | 129 ± 44 | 137 ± 42 | 200 ± 102 |
| Citrulline | 17 ± 7 | 14 ± 5** | 23 ± 13 |
| Alanine | 181 ± 55 | 217 ± 57** | 157 ± 40 |
| Arginine | 55 ± 20 | 61 ± 19** | 143 ± 57 |
| Tyrosine | 69 ± 21 | 75 ± 18* | 117 ± 75 |
| Valine | 110 ± 25 | 123 ± 18* | 221 ± 48 |
| Taurine | 41 ± 16 | 42 ± 14 | 37 ± 12 |
| Tryptophan | 31 ± 5 | 31 ± 5 | 38 ± 10 |
| Methionine | 31 ± 6 | 35 ± 5** | 63 ± 30 |
| Phenylalanine | 61 ± 11 | 66 ± 8* | 84 ± 28 |
| Isoleucine | 36 ± 9 | 44 ± 7** | 74 ± 15 |
| Leucine | 64 ± 13 | 73 ± 11** | 144 ± 25 |
| Ornithine | 42 ± 17 | 56 ± 22** | 153 ± 75 |
| Lysine | 115 ± 32 | 133 ± 33** | 202 ± 56 |
Mean ±SD
Fast vs. fed
p<0.05;
p<0.01;
Premature infants amino acid concentrations were significantly different (p<0.05 to p<0.001) compared with full term infants.
The plasma concentration of homocysteine (fast 5.0±1.2, fed 5.2±1.3 µmole.L−1), cysteine (fast 311.0±39.2, fed 321.5±31.1 µmole.L−1), and taurine did not change in response to formula feeding.
The concentration of plasma insulin of full term infants was 3.5±2.1 and 4.2±2.3 µU/ml before and during feeds, respectively. In preterm infants, the insulin levels were 11.1±7.0 µU/ml.
Amino acid kinetics
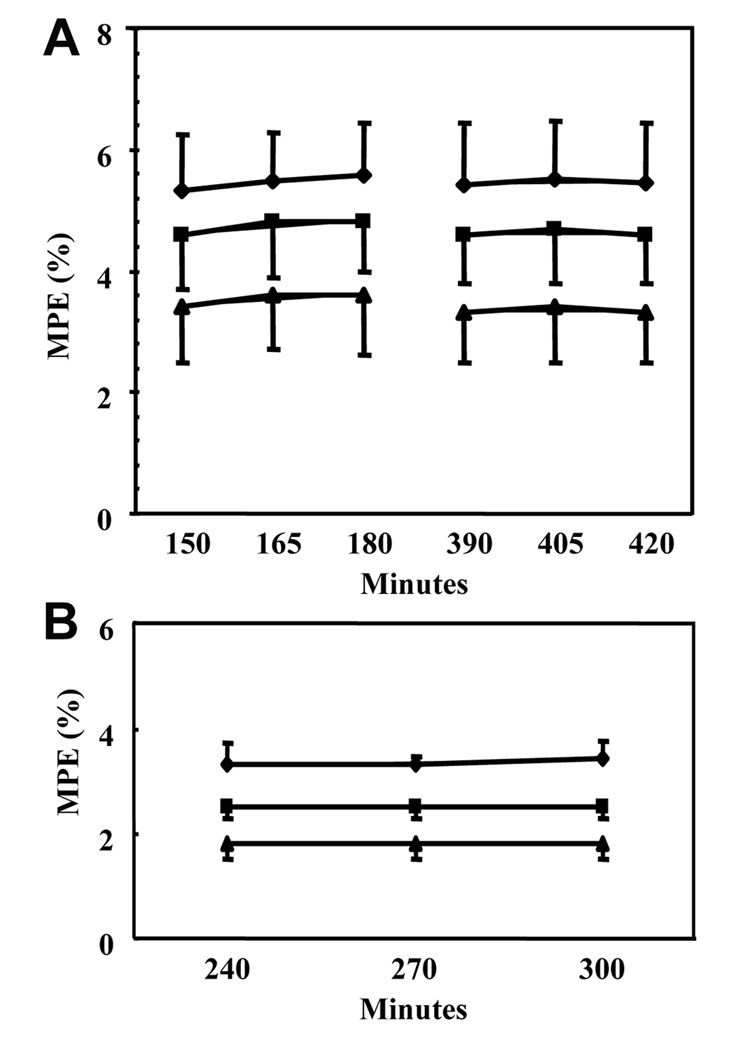
Isotopic tracer plateaus were reached in the plasma, for methionine and phenylalanine both in preterm and full term infants (Figure 2).
Figure 2.
Isotopic tracer enrichments in the plasma in full term babies (A) and preterm babies (B). MPE: moles % excess. ♦ [2H5]phenylalanine, ■ [1-13C]methionine, ▲[C2H3]methionine.
Phenylalanine
The Ra of phenylalanine in full term infants during fasting (72.6±10.8 µmole.kg−1.h−1) was similar to that reported by us previously (Table 3) (15,28). The total Ra of phenylalanine remained unchanged during feeding (Table 3). Assuming complete absorption of phenylalanine from the gut, the calculated endogenous Ra of phenylalanine was significantly less than that during fasting, suggesting a suppression of whole body proteolysis.
Table 3.
Phenylalanine and methionine kinetics
| Term | Preterm | |
|---|---|---|
| (n=18) | (n=9) | |
| RA Phenylalanine | ||
| Fast | 72.6 ± 10.8 | - |
| Fed (total) | 73.2 ± 13.3 | 128.9 ± 34.9 |
| Fed (endo) | 52.3 ± 13.3* | 98.7 ± 37.6** |
| Ra Methionine ([1-13C] tracer) | ||
| Fast | 36.2 ± 7.3 | - |
| Fed (total) | 37.0 ± 7.0 | 75.4 ± 24.1 |
| Fed (endo) | 23.4 ± 7.1* | 54.4 ± 25.8** |
| Ra Methionine ([C2H3] tracer) | ||
| Fast | 53.3 ± 12.5 | - |
| Fed (total) | 55.9 ± 14.2 | 100.1 ± 19.31 |
| Fed (endo) | 42.4 ± 14.3* | 76.3 ± 20.4** |
Mean ± SD, µmoles.kg−1.h−1
(endo) = endogenous
significantly different than fast, paired t < 0.001
significantly different from full term babies, p<0.006
In contrast to the full term babies, the Ra’s of phenylalanine, both total and endogenous, was significantly higher in the preterm infants, suggesting a higher rate of protein breakdown.
Methionine
Using [1-13C]methionine tracer, the Ra of methionine in full term infants during fasting was 36.2±7.3 µmole.kg−1.h−1. Mixed nutrient feeding did not have any significant impact on the total Ra of methionine. The calculated Ra of methionine from proteolysis was significantly less in the fed state. Ra methionine estimated using [C2H3]methionine tracer was higher than that estimated using [1-13C]methionine tracer (Table 3). The Ra of methionine was significantly higher in preterm infants, using either tracer.
The ratio of endogenous methionine:phenylalanine Ra for both preterm and full term infants, ~0.5, was similar to that reported in mixed animal proteins, ~0.48 (29).
13C enrichment of plasma homocysteine
The 13C enrichment of plasma homocysteine was measured in ten full term infants in order to examine the relation between the intracellular and extracellular enrichments of methionine (22). 13C enrichment of total homocysteine in the plasma at 180 and 420 minutes of tracer infusion was 1.37±0.37 and 2.32±0.48 moles % excess, or 30% and 53%, respectively, of the corresponding plasma methionine enrichment. In the preterm infants, plasma homocysteine enrichment at 4.5 hours of tracer infusion was 1.15±0.14 moles % excess, or 44% of the 13C enrichment of plasma methionine.
Transmethylation and transsulfuration
The rate of methylation of homocysteine (RM) was variable (range 4–38 µmole.kg−1.h−1). It was 17.1±12.1 µmole.kg−1.h−1 (n=17) during fasting in the full term babies and did not change during feeding (Table 3). The rate of methylation of homocysteine (35±18 µmole.kg−1.h−1) was significantly higher (p<0.03) in the prematurely born infants.
Because of the low contribution of 13CO2 from methionine to expired carbon dioxide, reliable data could be obtained in only six infants during fasting (Table 4). An isotopic steady state in expired CO2 was evident during the fed state in all infants. Oxidation of methionine contributed less than 0.04% to the expired CO2. The rate of oxidation of methionine, or transsulfuration, was 6.0±4.4 (n=6) and 4.1±2.1 (n=14) µmole kg−1 h−1 during fasting and feeding, respectively. One full term baby showed no evidence of transsulfuration. The contribution of methionine to expired CO2 was higher (0.18%) in preterm infants. We calculated the rate of transsulfuration using the average reported value of VCO2 [(6 ml kg−1 min−1, (24–26)). The rate of transsulfuration was higher in low birth weight infants as compared with those born at term gestation.
Table 4.
Transmethylation and transsulfuration of methionine in the newborn infant
| Methylation | VCO2 | CO2 from methionine | Transsulfuration | Transmethylation§ | |
|---|---|---|---|---|---|
| µmole.kg−1.h−1 | mmole.kg−1.h−1 | % | µmole.kg−1.h−1 | µmole.kg−1.h−1 | |
| Full term | |||||
| Fast (6) | 26.0 ± 10.7 | 17.3 ± 2.9 | 0.039 ± 0.03 | 6.0 ± 4.4 | 32 ± 14 |
| Fed (14) | 18.8 ± 13.5 | 18.7 ± 3.1 | 0.029 ± 0.01 | 4.1 ± 2.1 | 21.7 ± 13.2 |
| Preterm | |||||
| TPN (7) | 35.0 ± 17.6 | 16.1* | 0.18 ± 0.07 | 24.9 ± 9.9 | 57.2 ± 14.8 |
Discussion
Our data show that transsulfuration of methionine was evident in healthy newborn infants born at term gestation and that the rate of transsulfuration is high in prematurely born neonates receiving parenteral amino acids. The rates of remethylation and transmethylation of methionine were high in newborn babies compared with those reported in adults (19,23).
Our kinetic measurements are based upon quantification of tracer enrichments in the plasma compartment. Since the intracellular enrichments of methionine are likely to be less than that in the plasma, our measurements of transmethylation and transsulfuration are less than the actual rates. Although the enrichment of 13C in homocysteine was measured and showed a significant plasma to intracellular gradient, we could not calculate intracellular kinetics, since a steady state enrichment of tracer in homocysteine could not be confirmed.
The Ra of essential amino acids in the plasma reflects the respective amino acid composition of the body proteins (29). We compared the relative Ra of phenylalanine with Ra of methionine measured by [1-13C] tracer. Both in the full term babies and in low birth weight babies, the ratio of Ra methionine/Ra phenylalanine was ~0.5. The similarity of this ratio to the reported amino acid composition of mixed body proteins ~0.45 (30) provides credence to our measurements. As reported by us (15,28), the rates of phenylalanine turnover and hence the rate of whole body protein turnover were high in the neonate when compared with those in adults.
The rate of transsulfuration was estimated by the appearance of 13C of carboxyl carbon of methionine in CO2 (19). Although methionine could also be decarboxylated via s-adenosyl methionine decarboxylase, the contribution of this pathway to mammalian methionine metabolism has been suggested to be negligible (2). Therefore, the oxidation of methionine reflects, for the most part, the transsulfuration pathway. The rate of transsulfuration was high in the low birth weight infants who were receiving parenteral amino acids with methionine when compared with the full term babies. These data are of interest when examined in the context of the expression and appearance of the enzymes, cystathione β synthase and cystathione γ lyase during development (3–7). Studies by Sturman and Gaull (3,7) had shown that the cystathione gamma lyase activity was not detectable in the liver of the human fetus, but it was present in significant quantities in both prematurely born infants as well as those born at term gestation (5,7). Cystathione γ lyase activity could be induced by cAMP, glucagon and dexamethasone in vitro in explants of liver obtained from human fetuses in the second trimester (4). A recent study has confirmed these observations and shown that although the gene for cystathione γ lyase is expressed in the liver in the human fetus, (mRNA was detected), but there was no CGL enzyme activity and the protein was not detectable in fetal, premature and full term neonatal liver tissue (6). Cystathionine γ lyase is present in fetal kidney but whether the renal activity can substitute for the lack of activity in the liver has not been determined (5). Thus, the gene for cystathione γ lyase is transcribed in the liver during fetal life, but there is no enzyme activity until after birth. This is probably due to an inhibition of translation of cystathionine γ lyase mRNA in the liver before birth. The mechanism that is responsible for this unusual type of regulation is not clear. The enzyme data thus suggest a low transsulfuration activity in the human newborn, which may be significant for premature infants. Our data show that in healthy full term infants, there is significant transsulfuration during the first 48 hours after birth. The magnitude of transsulfuration was variable although it approximated that reported in healthy adults (19). This was likely related to the variability of the expression of enzymes involved because of difference in nutrient intake at this stage after birth.
The data in the premature infants are significant in that the magnitude of transsulfuration was five-fold higher than was noted in full term babies. This high rate may be related to the large amount of methionine in the parenteral nutrition and represents the irreversible disposal of “unbalanced” methionine administered to these babies. Alternatively, it may also be related to the possible high demands for glutathione and creatine and may be controlled by the redox sensitive regulation of cystathionine β synthase (31).
The rates of methylation of homocysteine were higher in the neonates when compared with those reported in healthy adults (19,22). The rates of remethylation and transmethylation were even higher in prematurely born infants. The high rates may be related to the high demands for methylation required for cell proliferation, growth, polyamine, and DNA synthesis (32). The data of the premature infants are particularly significant since both transmethylation and transsulfuration were high in these babies. Similar high rates of transmethylation and transsulfuration were seen in patients with severe brain injury (33), a protein catabolic state, and during parenteral amino acid administration (33). A high methionine load is expected to increase the concentration of s-adenosylmethionine (SAM). SAM is an allosteric activator of the CBS reactions (31,34). Parenteral methionine infusion would therefore increase transsulfuration, as was seen in our study. SAM is also inhibitory for enzymes involved in methylation of homocysteine, i.e. betaine homocysteine methyltransferase (BHMT) and methylene tetrahydrofolate reductase (MTHFR) (35,36). Therefore, the high rate of methylation of homocysteine was surprising. In this context, MTHFR activity was reported to be higher in the second trimester in human fetal liver and kidney as compared with adults, and BHMT specific activity was lower in fetal liver than in mature liver (37).
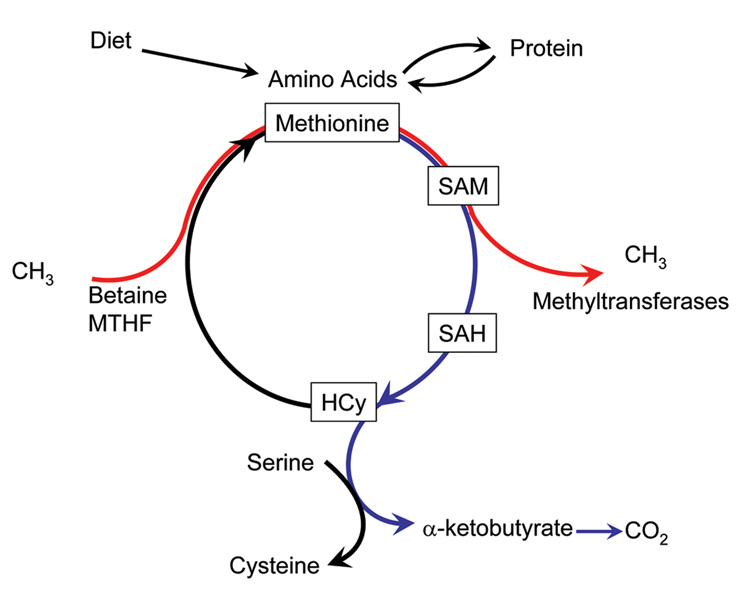
Transmethylation and remethylation of homocysteine in the methionine cycle does not result in net gain of methionine in the body. We propose that the high rate of remethylation of homocysteine to form methionine in the human newborn is aimed at shuttling of the methyl groups from methyl donors, methylene tetrahydrofolate and betaine to SAM for various methyltransferase reactions (Figure 3), while the transsulfuration is aimed at meeting the cysteine and glutathione requirement, and for the disposal of “excess” methionine.
Figure 3.
Methionine metabolism in-vivo. Red indicates transfer of methyl groups from methyl donors; blue indicates the catabolism of methionine.
Clinical implications
As discussed above, data from previous studies had suggested that the activity of both CGL and CBS were absent or low in the human fetus and in the newborn. In the prematurely born infant, the low activity was associated with higher concentrations of cystathionine and lower levels of cysteine in the plasma (38). In addition, red blood cells isolated from the premature infants at less than 32 weeks of gestation synthesized glutathione from added methionine at a lower rate when compared with red cells from full term infants (39). Based upon such data L-cysteine has been suggested to be “conditionally” essential amino acids for neonates. However, cysteine supplementation in clinical studies has not been shown to impact nitrogen balance, weight gain or other clinical parameters (40). The negative clinical results of cysteine supplementation can be explained from our data showing significant rates of transsulfuration in the neonate. These data suggest that cysteine may not be a “conditionally” essential amino acid for the newborn. However, because of the marked variability in the rate of transsulfuration and because of its role in increasing the solubility of Ca and P, it may be appropriate to continue to provide cysteine in parenteral nutrition.
Acknowledgment
The authors thank the staff of both the General Clinical Research Center and the Neonatal Intensive Care Unit for their help and support. The secretarial assistance from Mrs. Joyce Nolan is gratefully appreciated.
We thank Jaeyeon Kim for the use of IsoMet, software used for the calculation of relative enrichment of tracer isotopomer data. The software is available from jae.y.kim@case.edu.
Financial Support: This work was supported by NIH grants RO1 HD042154, and MO1 RR00080.
Abbreviations
- CBS
Cystathionine β synthase
- CGL
Cystathionine γ lyase
- Ra
Rate of appearance
- SAM
S-adenosylmethionine
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr. 2006;136:1636S–1640S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- 2.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 3.Sturman JA, Gaull G, Raiha NC. Absence of cystathionase in human fetal liver: is cystine essential? Science. 1970;169:74–76. doi: 10.1126/science.169.3940.74. [DOI] [PubMed] [Google Scholar]
- 4.Heinonen K, Räihä NC. Induction of cystathionase in human foetal liver. Biochem J. 1974;144:607–609. doi: 10.1042/bj1440607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlotkin SH, Anderson GH. The development of cystathionase activity during the first year of life. Pediatr Res. 1982;16:65–68. doi: 10.1203/00006450-198201001-00013. [DOI] [PubMed] [Google Scholar]
- 6.Levonen A-L, Lapatto R, Saksela M, Raivio KO. Human cystathionine γ-lase: developmental and in vitro expression of two isoforms. Biochem J. 2000;347:291–295. [PMC free article] [PubMed] [Google Scholar]
- 7.Gaull G, Sturman JA, Raiha NC. Development of mammalian sulfur metabolism: absence of cystathionase in human fetal tissues. Pediatr Res. 1972;6:538–547. doi: 10.1203/00006450-197206000-00002. [DOI] [PubMed] [Google Scholar]
- 8.van Goudoever JB, Sulkers EJ, Timmerman M, Huijmans JG, Langer K, Carnielli VP, Sauer PJ. Amino acid solutions for premature neonates during the first week of life: the role of N-acetyl-L-cysteine and N-acetyl-L-tyrosine. JPEN J Parenter Enteral Nutr. 1994;18:404–408. doi: 10.1177/0148607194018005404. [DOI] [PubMed] [Google Scholar]
- 9.Helms RA, Christensen ML, Storm MC, Chesney RW. Adequacy of sulfur amino acid intake in infants receiving parenteral nutrition. J Nutr Biochem. 1995;6:462–466. [Google Scholar]
- 10.Jacobs RL, Stead LM, Brosnan ME, Brosnan JT. Hyperglucagonemia in rats results in decreased plasma homocysteine and increased flux through the transsulfuration pathway in liver. J Biol Chem. 2001;276:43740–43747. doi: 10.1074/jbc.M107553200. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs RL, House JD, Brosnan ME, Brosnan JT. Effects of streptozotocin-induced diabetes and of insulin treatment on homocysteine metabolism in the rat. Diabetes. 1998;47:1967–1970. doi: 10.2337/diabetes.47.12.1967. [DOI] [PubMed] [Google Scholar]
- 12.Ratnam S, MacLean KN, Jacobs R, Brosnan ME, Kraus JP, Brosnan JT. Hormonal regulation of cystathionine b-synthase expression in liver. J Biol Chem. 2002;277:42912–42918. doi: 10.1074/jbc.M206588200. [DOI] [PubMed] [Google Scholar]
- 13.Kalhan SC, Parimi PS. Metabolism of glucose and methods of investigation in the fetus and newborn. In: Polin RA, Fox WW, editors. Fetal and Neonatal Physiology. Philadelphia, PA: WB Saunders & Co.; 2002. pp. 357–372. [Google Scholar]
- 14.Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics. 1993;91:617–623. [PubMed] [Google Scholar]
- 15.Parimi PS, Gruca LL, Kalhan SC. Metabolism of threonine in newborn infants. Am J Physiol Endocrinol Metab. 2005;289:E981–E985. doi: 10.1152/ajpendo.00132.2005. [DOI] [PubMed] [Google Scholar]
- 16.Denne SC, Kalhan SC. Leucine metabolism in normal newborns. Am J Physiol. 1987;253:E608–E615. doi: 10.1152/ajpendo.1987.253.6.E608. [DOI] [PubMed] [Google Scholar]
- 17.Kalhan SC, Parimi PS, Gruca LL, Hanson RW. Glutamine supplement with parenteral nutrition decreases whole body proteolysis in low birth weight infants. J Pediatr. 2005;146:642–647. doi: 10.1016/j.jpeds.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Brauman JI. Least squares analysis and simplification of multiple-isotope mass spectra. Anal Chem. 1966;38:607–610. [Google Scholar]
- 19.Storch KJ, Wagner DA, Burke JF, Young VR. [1-13C; methyl-2H3]methionine kinetics in humans: methionine onservation and cystine sparing. Am J Physiol. 1990;258:E790–E798. doi: 10.1152/ajpendo.1990.258.5.E790. [DOI] [PubMed] [Google Scholar]
- 20.Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF., 3rd Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab. 2004;286:E272–E279. doi: 10.1152/ajpendo.00351.2003. [DOI] [PubMed] [Google Scholar]
- 21.Tserng K-Y, Kalhan SC. Calculation of substrate turnover rate in stable isotope tracer studies. Am J Physiol. 1983;245:E308–E311. doi: 10.1152/ajpendo.1983.245.3.E308. [DOI] [PubMed] [Google Scholar]
- 22.MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab. 2001;280:E947–E955. doi: 10.1152/ajpendo.2001.280.6.E947. [DOI] [PubMed] [Google Scholar]
- 23.Storch KJ, Wagner DA, Burke JF, Young VR. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol. 1988;255:E322–E331. doi: 10.1152/ajpendo.1988.255.3.E322. [DOI] [PubMed] [Google Scholar]
- 24.Thureen PJ, Phillips RE, DeMarie MP, Hoffenberg A, Bronstein MN, Spedale SB, Hay WW., Jr Technical and methodological considerations for performance of indirect calorimetry in ventilated and non ventilated preterm infants. Crit Care Med. 1997;25:171–180. doi: 10.1097/00003246-199701000-00031. [DOI] [PubMed] [Google Scholar]
- 25.Fok TF, Gu J-S, Lim CN, Ng PC, Wong HL, So KW. Oxygen consumption and resting energy expenditure during phototherapy in full term and preterm newborn infants. Arch Dis Child Fetal Neonatal Ed. 2001;85:F49–F52. doi: 10.1136/fn.85.1.F49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer J, Maier K, Linderkamp O, Hentschel R. Effect of caffeine on oxygen consumption and metabolic rate in very low birth weight infants with idiopathic apnea. Pediatrics. 2001;107:660–663. doi: 10.1542/peds.107.4.660. [DOI] [PubMed] [Google Scholar]
- 27.Glamour TS, McCullough AJ, Sauer PJ, Kalhan SC. Quantification of carbohydrate oxidation by respiratory gas exchange and isotopic tracers. Am J Physiol. 1995;268:E789–E796. doi: 10.1152/ajpendo.1995.268.4.E789. [DOI] [PubMed] [Google Scholar]
- 28.Parimi PS, Devapatla S, Gruca L, O’Brien AM, Hanson RW, Kalhan SC. Glutamine and leucine nitrogen kinetics and their relation to urea N in newborn infants. Am J Physiol Endocrinol Metab. 2002;282:E618–E625. doi: 10.1152/ajpendo.00403.2001. [DOI] [PubMed] [Google Scholar]
- 29.Bier DM. Intrinsically difficult problems: The kinetics of body proteins and amino acids in man. Diabetes Metab Rev. 1989;5:111–132. doi: 10.1002/dmr.5610050203. [DOI] [PubMed] [Google Scholar]
- 30.Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr. 1994;124:906–910. doi: 10.1093/jn/124.6.906. [DOI] [PubMed] [Google Scholar]
- 31.Prudova A, Bauman Z, Braun A, Vivitsky V, Lu SC, Banerjeec R. S-adenosylmethionine stabilizes cystathionine β-synthase and modulates redox capacity. Proc Natl Acad Sci USA. 2006;103:6489–6494. doi: 10.1073/pnas.0509531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stead LM, Jacobs RL, Brosnan ME, Brosnan JT. Methylation demand and homocysteine metabolism. Adv Enzyme Regul. 2004;44:321–333. doi: 10.1016/j.advenzreg.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y-M, Burke JF, Young VR. A kinetic study of L-2H3-methyl-1-13C methionine in patients with severe burn injury. J Trauma. 1993;35:1–7. doi: 10.1097/00005373-199307000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Miles EW, Kraus JP. Cystathionine β-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem. 2004;279:29871–29874. doi: 10.1074/jbc.R400005200. [DOI] [PubMed] [Google Scholar]
- 35.Ou X, Yang H, Ramani K, Ara AI, Chen H, Mato JM, Lu SC. Inhibition of human betaine-homocysteine methyltransferase expression by S-adenosylmethionine and methylthioadenosine. Biochem J. 2007;401:87–96. doi: 10.1042/BJ20061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jencks DA, Matthews RG. Allosoteric inhibition of methylenetetrahydrofolate reductase by adenosylmethionine. J Biol Chem. 1987;262:2485–2493. [PubMed] [Google Scholar]
- 37.Gaull GE, von Berg W, Raiha NC, Sturman JA. Development of methyltransferase activities of human fetal tissues. Pediatr Res. 1973;7:527–533. doi: 10.1203/00006450-197305000-00006. [DOI] [PubMed] [Google Scholar]
- 38.White CW, Stabler SP, Allen RH, Moreland S, Rosenberg AA. Plasma cysteine concentrations in infants with respiratory distress. J Pediatr. 1994;125:769–777. doi: 10.1016/s0022-3476(94)70077-x. [DOI] [PubMed] [Google Scholar]
- 39.Viña J, Vento M, García-Sala F, Puertes IR, Gascó E, Sastre J, Asensi M, Pallardó FV. L-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am J Clin Nutr. 1995;61:1067–1069. doi: 10.1093/ajcn/61.4.1067. [DOI] [PubMed] [Google Scholar]
- 40.Soghier LM, Brion LP. 2006 Cysteine, cysteine or N-acetylcysteine supplementation in parenterally fed neonates. Cochrane Database Syst Rev. 2006;4:CD004869. doi: 10.1002/14651858.CD004869.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]