Abstract
The etiology of multiple sclerosis (MS) is unknown but it manifests as a chronic inflammatory demyelinating disease in the central nervous system (CNS). During chronic CNS inflammation, nicotinamide adenine dinucleotide (NAD) concentrations are altered by (T helper) Th1-derived cytokines through the coordinated induction of both indoleamine 2,3-dioxygenase (IDO) and the ADP cyclase CD38 in pathogenic microglia and lymphocytes. While IDO activation may keep auto-reactive T cells in check, hyper-activation of IDO can leave neuronal CNS cells starving for extracellular sources of NAD. Existing data indicate that glia may serve critical functions as an essential supplier of NAD to neurons during times of stress. Administration of pharmacological doses of non-tryptophan NAD precursors ameliorates pathogenesis in animal models of MS. Animal models of MS involve artificially stimulated autoimmune attack of myelin by experimental autoimmune encephalomyelitis (EAE) or by viral-mediated demyelination using Thieler's murine encephalomyelitis virus (TMEV). The WldS mouse dramatically resists razor axotomy mediated axonal degeneration. This resistance is due to increased efficiency of NAD biosynthesis that delays stress-induced depletion of axonal NAD and ATP. Although the WldS genotype protects against EAE pathogenesis, TMEV-mediated pathogenesis is exacerbated. In this review, we contrast the role of NAD in EAE versus TMEV demyelinating pathogenesis to increase our understanding of the pharmacotherapeutic potential of NAD signal transduction pathways. We speculate on the importance of increased SIRT1 activity in both PARP-1 inhibition and the potentially integral role of neuronal CD200 interactions through glial CD200R with induction of IDO in MS pathogenesis. A comprehensive review of immunomodulatory control of NAD biosynthesis and degradation in MS pathogenesis is presented. Distinctive pharmacological approaches designed for NAD-complementation or targeting NAD-centric proteins (SIRT1, SIRT2, PARP-1, GPR109a, and CD38) are outlined towards determining which approach may work best in the context of clinical application.
Keywords: Multiple sclerosis; nicotinamide adenine dinucleotide; Wallerian degeneration; indoleamine 2,3-dioxygenase; ADP cyclase; SIRT1; PARP-1; CD200
1. INTRODUCTION
This manuscript describes clinical multiple sclerosis (MS) and animal models thereof with a renewed focus of nicotinamide adenine dinucleotide (NAD)-centric pathways. Bioenergetic links (Fig. 2) and potential tissue specific NAD sinks (Fig. 3) are described in the context of biochemical pathways where the greatest emphasis is placed on pharmacological complementation of possible MS-induced NAD deficiencies (Fig. 4). A model of tissue specific MS pathogenesis is presented, concluding with a discussion of the potential involvement of NAD in stem cell biology and possible dietary connections related to MS etiology.
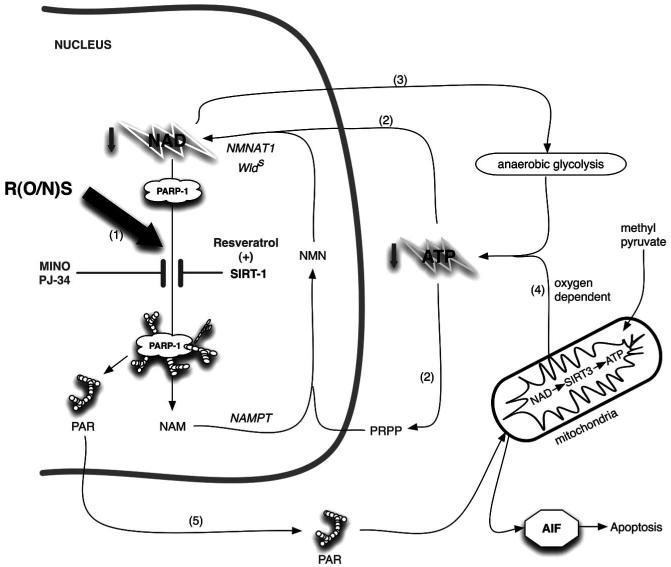
Fig. (2).
A nuclear SIRT1 mechanism for delaying NAD-ATP depletion through inhibition of PARP-1 is presented. The NAD salvage pathway uses nicotinamide (NAM) as a substrate. NAMPT1 converts nicotinamide (NAM) to nicotinamide mononucleotide (NMN) which via nicotinamide adenylyl-transferase (NMNAT) gets converted to NAD. Both of these processes require ATP. Reactive oxygen/nitrogen species (R(O/N)S) causing DNA damage causes activation of PARP-1, which leads to NAD depletion as NAD is used as a substrate in the polymerization reaction. The salvage pathway is simultaneously activated by (R(O/N)S), which leads to a futile NAD-recycling process that ultimately depletes ATP. Similar lesser characterized ATP depleting pathways are predicted specifically in axonal cytosol and mitochondria where NMNAT2 and NMNAT3 enzymes have been colocalized respectively with Sirtuin and PARP family members. R(O/N)S cause damage to DNA, which activates PARP-1 leading to NAD depletion through use of NAD as a substrate to generate poly(ADP)ribose, PAR (1). Nicotinamide (NAM) is used by nicotinamide phosphoribosyltransferase (NAMPT) to make nicotinamide mononucleotide (NMN). This is then converted to NAD via nicotinamide nucleotide adenylyltransferase 1 (NMNAT1), part of the genomic triplication responsible for the slow Wallerian degeneration mouse (Wlds) phenotype. The NAD salvage pathway repeatedly uses ATP to replenish NAD (2). The NAD-dependent mitochondrial deacetylase, SIRT3, functions as a master regulator of ATP levels. Under ischemic conditions NAD is required to maintain production of ATP from anaerobic glycolysis. The increased salvage pathway efficiency of the Wlds mouse is probably able to provide NAD to maintain ATP generation by anaerobic glycolysis during oxidative stress (3). Otherwise ATP can be supplied via oxidative phosphorylation (4). The futile cycle continues until PAR formation triggers AIF-dependent apoptosis or ATP stores are so depleted that necrosis happens (5). PARP-1 activity can be inhibited directly (e.g. minocycline and PJ-34 are nanomolar affinity inhibitors) or indirectly through activation of SIRT-1 (e.g. resveratrol; 6). Either PARP-1 inhibitory approach can significantly delay NAD and ATP depletion similar to the Wlds mouse. Alternatively pharmacological administration of NAD or precursors can help prevent deficiencies. All of the pharmacological approaches described here have been shown to ameliorate EAE pathogenesis in published reports. Neurons are particularly susceptible to depletion of NAD owing to their apparent lack of a fully functional salvage pathway (see text).
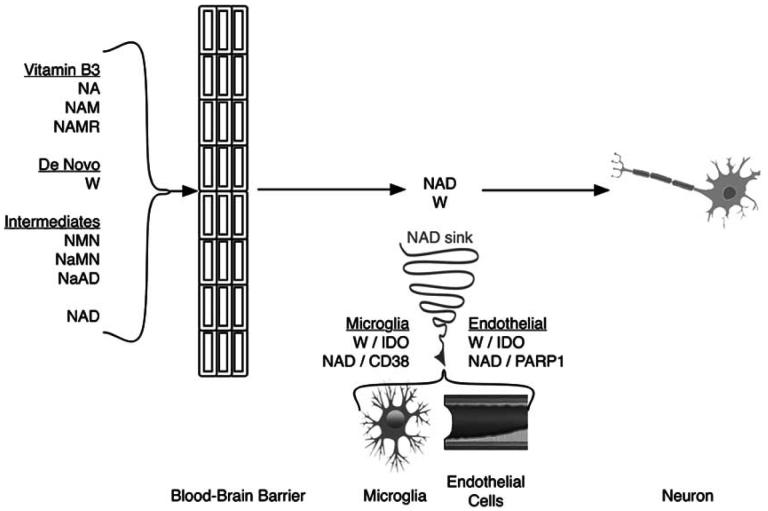
Fig. (3).
The route from dietary NAD precursor to neuron is shown with NAD sinks developing during multiple sclerosis. Astrocytes readily use NAD precursors to generate NAD and can directly transport NAD across the plasma membrane directly via the adenosine receptor P2XY7R (NA, nicotinic acid; NAM, nicotinamide; NAMR, nicotinamide riboside; W, tryptophan; NaMN, nicotinic acid mononucleotide; NMN, nicotinamide mononucleotide; NaAD, nicotinic acid adenine dinucleotide). By contrast neurons are innefficient in this energy dependent process. Similar to several other pathways, it appears as though glia are likely to serve prominent roles in supplying NAD to neurons. With the sudden appearance of lymphocytes in the CNS of the MS patient, exceptional efforts are apparently made to remove lymphocytes by high level IDO induction in microglia and endothelial cells. This decreases the available extracellular tryptophan (W). Additionally, CD38 is highly induced by TNF alpha in professional antigen presenting cells (PAPCs; macrophages, dendritic cells, or microglia) during MS. TNF alpha activation of CD38 leads to degradation of NAD which generates products that stimulate calcium signaling pathways mediating PAPC chemotaxis and activation. PARP-1 activity increases in endothelial cells juxtaposed next to microglial cells in EAE models of MS. The IDO induction in these cells can provide complementary NAD that is lost by CD38 mediated depletion in PAPCs. This persistent inner battle to control the immune system creates a NAD sink that consumes that renders neurons exceptionally vulnerable to stress-induced cell death. Low levels of NAD typically leads to neurodegeneration.
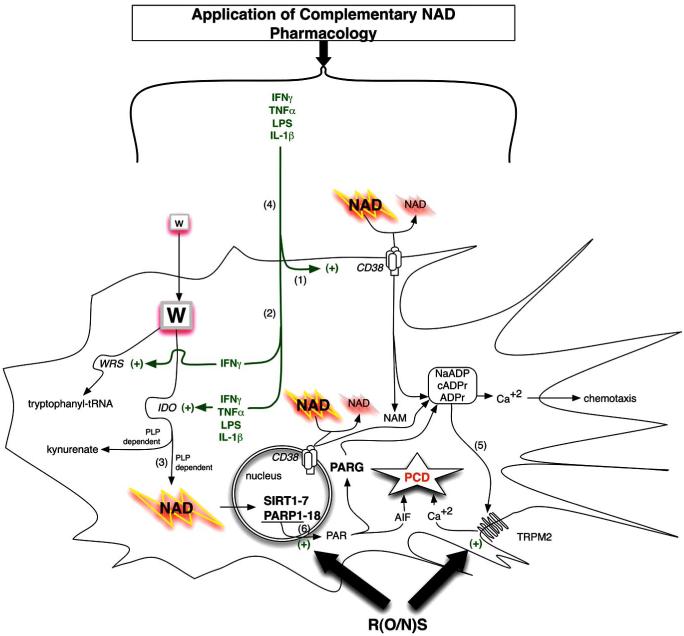
Fig. (4).
Enzymes controlling NAD metabolism in professional antigen presenting cells (PAPCs; microglia, macrophages, or dendritic cells) are shown with consideration of pharmacological administration of complementary NAD precursors or effectors of NAD utilizing enzymes (SIRT1 activators / PARP-1 inhibitors) towards rescue NAD deficiency arising from chronic inflammatory disease. Immunomodulatory factors exert a coordinated regulation of NAD levels during autoimmune disease or infection. PAPCs including microglia act as sinks acquiring de novo pathway NAD precursor (tryptophan) or degrading NAD directly via activation of IDO and CD38 respectively. CD38 activity is required for chemotaxis (1). Thus, IFNγ activates IDO to increase intracellular NAD while simultaneously activating tryptophanyl-tRNA (encoded by WRS) to maintain essential tryptophan-dependent protein synthesis (2). All three IFNγ-mediated inductions occur in professional antigen presenting cells. IFNγ-mediated activation of IDO leads to complementary increases in NAD levels in a pyridoxyl phosphate (PLP; derived from vitamin B6) co-factor dependent fashion. The anti-epileptic molecule kynurenate is also produced through this pathway. In the end this pathway is predicted to affect global chromatin structure through NAD dependent SIRT-1 and PARP-1 mediated activities as well as other effects through related Sirtuin/PARP family member proteins (3). During chronic inflammation local extracellular NAD sources become deficient (tryptophan and NAD) and this exerts both an anti-proliferative immunotoleragenic effect on T cells but also decreases PAPC chemotaxis while making neighboring cells more vulnerable (4). Accordingly pharmacological application of NAD precursors provides tremendous cytotrophic benefit in numerous models of autoimmune disease. ADPR from either ROS-PARP1-PAR-PARG or excessive CD38 activity can lead to persistent activation of TRPM2 leading to programmed cell death, PCD (5). Excessive CD38 activity has been observed in type 1 diabetics via excessive autoreactive anti-CD38. Highly expressed in the brain and clearly important in immune function, the role of CD38 in MS is completely unexplored. Peroxynitrate can activate PARP1 leading to nuclear PAR formation that translocates to the mitochondria to promote AIF release which also leads to programmed cell death (PCD). Two R(O/N)S sensitive pathways shown at the bottom include DNA damage-PARP1 activation along with direct activation of the redox sensitive TRPM2 divalent cation channel. (6) Administration of pharmacologic doses of NAD precursors (nicotinic acid/niacin, nicotinamide/niacinamide, or nicotinamide riboside) or pharmacologic targeting of NAD-dependent targets (SIRT1 activators or PARP1 inhibitors) may complement the NAD deficiencies arising from immune activation of IDO and CD38.
2. CONTROLLING NAD LEVELS IN NEURONS: ANOTHER POTENTIAL SUPPORTIVE FUNCTION FOR GLIA
2.1. Enzyme Reactions Using NAD
An essential molecule, nicotinamide adenine nucleotide (NAD) is required in more enzymatic reactions than perhaps any other small molecule. NAD(P(H)) functions as a co-factor in over 200 redox reactions or as a substrate in three categorical reactions (http://lpi.oregonstate.edu/infocenter/vitamins/niacin/). Generally, NAD functions as a co-factor in energy-producing catabolic reactions, such as the degradation of carbohydrates, fats, proteins, and alcohol, whereas NADP functions in anabolic reactions, such as the synthesis of cellular macromolecules including fatty acids and cholesterol [1]. As a co-factor NAD participates in oxidation-reduction (redox) reactions as hydride donor (NADH and NADPH) and acceptor (NAD and NADP). Of all the NAD((P)H) specific molecular isoforms, it is specifically NAD and not NADH, NADP, nor NADPH that is the molecule most susceptible to deficiency under niacin-limiting conditions in bone marrow cells subjected to common oxidative stress [2].
The redox reactions are not accompanied by any net consumption of the nucleotides. However, cells require ongoing NAD synthesis because NAD is consumed as a substrate by three categorical enzymes that break the glycosidic bond between the nicotinamide (NAM) moiety and ADP ribose moiety: 1) ADP-ribose transferase (ARTs) or poly(ADP-ribose) polymerases (PARPs), 2) cADPR-ribose synthases (CD38 and CD157) and 3) sirtuins (class III protein lysine deacetylases) [3]. A common feature of these reactions is that NAD donates its ADP-ribose group, which breaks the glycosidic bond between nicotinamide and ribose, destroying the parent NAD molecule [4].
2.2. Biosynthesis of NAD
Twentieth century man is particularly susceptible to dietary induced NAD deficiency. The most devastating nutritional deficiency disease in the history of the United States of America was the NAD deficiency disease pellagra, an epidemic which killed over 120,000 people in the first two decades of the 1900s [5]. NAD precursors are distinguished as one of the few known molecules identified as important enough to be forced into the public diet for improved health by the U.S. government [6]. It should be realized however, that niacin requirements of individuals can vary depending on genetics and stress. Significantly, disease pathology is well known to actively promote NAD depletion through poly(ADP)ribose polymerase (PARP-1) activation. Recently, new NAD metabolic pathways have gained recognition for their important roles in MS. For example, the NAD precursor nicotinamide (NA) can ameliorate MS in the experimental autoimmune encephalomyelitis (EAE) animal model [7]. Thus, we focus here on NAD biosynthesis to understand the potential role of NAD depletion in MS pathogenesis.
In all vertebrates, NAD can be synthesized by two pathways: de novo synthesis from tryptophan [3, 4] and/or from vitamin precursors in the diet: NA, nicotinamide (NAM), and nicotinamide riboside (NAMR). The pathway from tryptophan to the NAD precursor nicotinic acid mononucleotide (NaMN) requires four enzymes encoded by the genes KMO, KYNO, HAAO, and QPRT. NaMN is then converted to NAD via three reactions catalyzed by NARPRT, NMNAT, and NADS. For the de novo pathway to be completed it is necessary to have adequate riboflavin (vitamin B2), pyridoxyl phosphate (vitamin B6), and ascorbate (vitamin C). Deficiencies in any of these can lead to the accumulation of “kynurenine pathway” intermediates (Fig. 1). NAD can also be synthesized from three different essential non-amino acid precursors in the diet: nicotinic acid (NA), nicotinamide (NAM), and nicotinamide riboside (NAMR). Yeast with an inactivated de novo pathway may use any of the salvageable precursors (NA, NAM, NAMR, or nicotinic acid riboside) to support life [8]. Nicotinic acid riboside has only been examined in yeast. These non-amino acid dietary NAD precursors are collectively termed vitamin B3 and require two (NAM, NAMR) or three (NA) steps respectively to generate NAD [1].
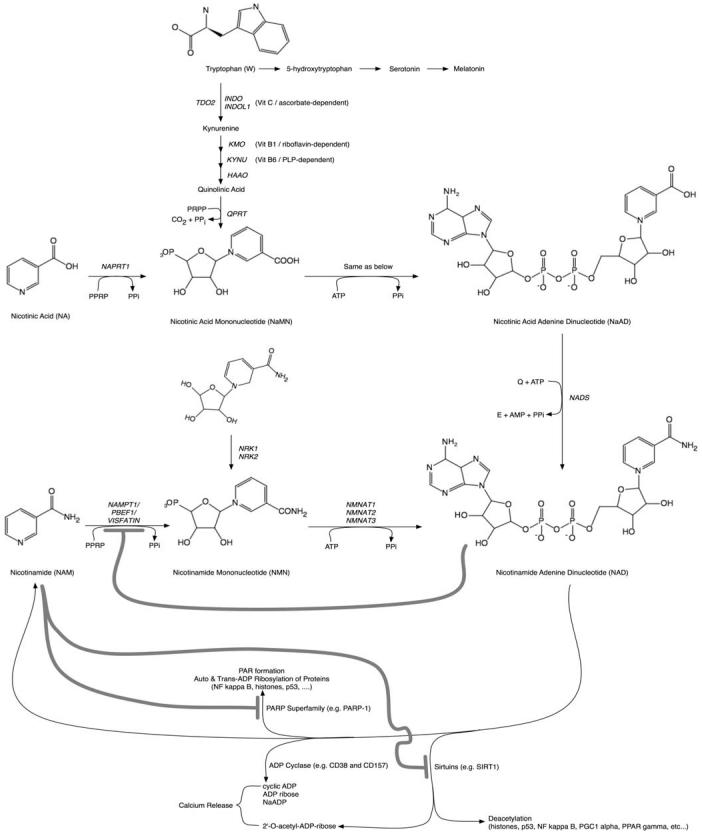
Fig. (1).
The pathway from NAD precursors to NAD biosynthesis is shown with molecular structures and abbreviations used throughout the manuscript. Also shown, are the reactions that use NAD as a substrate, not a co-factor. Products of ADP cyclase and Sirtuin catalyzed reactions generate products causing release of calcium. CD38 is involved in the persistent release of intracellular calcium connected with hematopoietic chemotaxis and microglial activation, both of which are likely to be of great importance in MS pathogenesis. Feedback inhibition loops are drawn with heavy lines. Additional co-factors required for completion of de novo pathway NAD biosynthesis include ascorbate (vitamin C), pyridoxyl phosphate (vitamin B6), and riboflavin (vitamin B2). Of likely significance to nicotinic acid distinguished capacity as a provider of NAD, NAPRT is not inhibited by NAD feedback inhibition (see text for reference), while NAMPT is inhibited by NAD.
It should be realized that not every cell is capable of converting each precursor to NAD at all times although NAD is essential to survival. Because tissue and cell-type specific enzyme expression differences exist in metazoans, the precursors are differentially utilized in the gut, brain, blood and other organs [1] (Table 1). The de novo pathway is clearly active in liver, neuronal and immune cells. The pathway from NA is expressed in the liver, kidney, heart and intestine. While, vertebrates do not possess the nicotinamidase enzyme that converts NAM to NA, intestinal bacteria in the vertebrate gut use nicotinamidase to convert NAM to NA, which in turn is used to synthesize NAD via the Preiss and Handler salvage pathway. The NAMR salvage pathway is expressed in neurons and in cardiac and skeletal muscle [1].
Table 1.
NAD Biosynthctic Pathways
| NAD Precursor | Pathway | Requirement | Tissue Specificity |
|---|---|---|---|
| Tryptophan (W) | de novo | Eight-step pathway | liver, neuronal, and immune cells |
| Nicotinic acid (NA) | Salvage | Three-step Preiss-Handler pathway | liver, kidney, heart, intestine |
| Nicotinamide (NAM) | Salvage | Two-step Nampt pathway | adipose tissue, liver, kidney and immune cells |
| Nicotinamide riboside (NAMR) | Salvage | Nrk pathway or nucleoside phosphorylated and Nampt pathway | neurons, cardiac and skeletal muscle |
Collectively, experimental observations support the idea that by increasing NAD, we may be able to prevent or reduce the effects of MS pathogenesis. Mammals elevate their NAD during autoimmune encephalomyelitis [7, 9], while tryptophan levels are decreased in the cerebrospinal fluid (CSF) and sera of MS patients [10]. Caloric restriction (CR) raises NAD levels [11] and provides protection against EAE-mediated pathogenesis [12-14]. CR reduces inflammation, demyelination, and neurodegeneration, but does not suppress immune function. CR increases both the expression of the rate-limiting enzyme controlling NAD biosynthesis [15] and lifespan in a SIRT1 dependent manner [16]. Consequently, the body may synthesize NAD to help control autoimmune encephalomyelitis.
The enzymes required to initiate de novo NAD biosyn-thesis are nicotinamide phosphoribosyltransferase (NAMPT), nicotinic acid phosphoribosyltransferase (NAPRT), indole-amine 2,3-dioxygenase (IDO/INDOL and IDO2/INDOL2), or tryptophan dioxygenase (TDO2). The NAD salvage pathway recycles NAM back to NAD starting with the rate-limiting enzyme NAMPT [17]. The NAD recycling pathway responds to stress conditions in a highly evolutionarily conserved manner, by increasing expression of nicotinamidase (PNC) in prokaryotes or by increasing NAMPT in vertebrates (for a review [18]).
NAMPT requires the substrate NAM, which is rapidly generated following PARP-1 activation in response to genomic damage. PARP-1 activity may also change in direct interferon-γ (IFNγ) causes robust induction of IDO activity in response to local changes in NAD concentration-an active area of research investigation in vivo [19]. Although inflammation-mediated PARP-1 activation can lead to a rapid NAD depletion, NAD can be quickly restored through the NAD salvage pathway. The NAD salvage pathway recycles NA to NAD. Induction of NAMPT or increased NMNAT in WldS dramatically increases NAD levels during times of PARP-1 activation. Other distinct pathways to increase levels of tissue NAD include activation of either TDO2 or IDO. While TDO2 has traditionally been understood to restricted in expression in the liver, it is in fact also expressed in neurons and glia [20]. IDO expression is highly induced in professional APCs and neurons by the immune system. IDO is also detectable in endothelial cells [21]. In neurons interferon-γ (IFNγ) causes robust induction of IDO activity in primary human neurons and a slight reduction of TDO2 activity [22]. Although NAD levels were not measured in these experiments, similar IFNγ induction of IDO has been shown to increase NAD levels in macrophages [23, 24] and glia [25, 26]. It is unclear whether IFNγ directly increased NAD in neurons. Other experiments suggest that the tryptophan de novo biosynthesis pathway is not efficient enough to provide neuroprotection [27]. By contrast, other mononucleotide containing precursors in the salvage pathway including NaMN or nicotinamide mononucleotide (NMN) are neuroprotective in the Wallerian degeneration assay.
2.3. Changes in NAD Levels in Animal Models of MS and NAD Precursors
Under conditions of NAD deficiency, neurons are exceptionally vulnerable to the degeneration characteristic of MS [28]. The EAE model of MS indicates that endogenous levels of NAD are elevated in the CNS [7, 9]. This presumably occurs due to the combined increases in lymphocyte infiltration, hematopoietic cell proliferation, and IDO activity in professional APCs commonly observed in MS.
While net increases in NAD in the CNS are observed in EAE models, we predict that these increases are largely restricted to the immune system and come at the risk of NAD deficiency in neurons. Extracellular levels of the precursor used for de novo biosynthesis (tryptophan) are significantly decreased in the serum and CSF of MS patients [10]. Fortunately, this loss of available NAD precursor can be partially rescued either by administrating pharmacological doses of nicotinamide or by enforced caloric restriction [11]. Both approaches lead to increased NAD levels and profoundly ameliorate EAE pathogenesis [7, 12-14]. By contrast, administration of tryptophan is known to increase lymphoproliferation [29, 30]. Thus high dose tryptophan administration may be expected to exacerbate immune-mediated demyelinating pathogenesis, actually promoting neurodegeneration due to hyper activation of the immune system (discussed in greater detail in section 8.1.1). Similarly, inhibition of IDO, which can increase tryptophan levels, exacerbates EAE pathogenesis [31-33]. The latest research indicates that stem cell mediated prevention and recovery from EAE requires IDO activity [34]. Thus it is important to limit the NAD available to lymphocytes but not at the expense of starving neighboring cells of NAD.
2.4. Glia as Suppliers of NAD to Neurons
Of likely significance to MS pathogenesis, the efficiency de novo and salvage NAD pathways is different in glia compared to neurons [35]. Neuronal explant experiments performed by Sasaki et al. reveal neurons are inefficient at de novo and salvage NAD biosynthesis. By contrast all other downstream intermediates (NaMN, NaAD, or NMN; Fig. 2) as well as NAD itself could provide potent delay in neuronal Wallerian degeneration assays [27, 36]. Significantly, addition of NA or NAM to these neuronal explants failed to delay Wallerian degeneration unless the salvage pathway enzymes NAMPT or NMNAT were ectopically expressed. However, Wang et al. revealed that NAM considerably delayed Wallerian degeneration when supplied at higher concentrations of 5-25 mM [37]. Further, NAMPT1 or NAPRT1, which initiate NAD biosynthesis using NAM or NA respectively, were both transcriptionally induced after sciatic nerve transection in mice, where glia are present. These studies clearly revealed the rate-limiting nature of the NAM-NAMPT and NA-NAPRT pathways in controlling NAD biosynthesis specifically in neurons [27]. Meanwhile, NAM has been repeatedly shown to be potently neuroprotective when administered in vivo using animal models of disease [7, 38-42]. Thus, surrounding glial cells may synthesize and supply NAD to neurons in vivo when NAM is administered at pharmacological doses.
Examination of NAD biosynthesis in glial cells further supports this idea. In contrast to neurons, glial cells possess efficient de novo and salvage pathways. Glial cells use NA with more than a 250-fold greater efficiency than NAM or quinolinate [43]. Furthermore, astrocytes are able to transport NADH via P2X7R in both directions [44, 45]. Astrocytes form the blood-brain barrier (BBB), are the first cells of the CNS exposed to dietary or pharmacologic NAD precursors, and are the most abundant cell type in the brain. Current data strongly support the notion that glia may serve important roles in both the synthesis of NAD from tryptophan, NA, or NAM, and the delivery of NAD to neurons (Fig. 3).
There are three established examples for a division of labor reserved for glial cells but not neurons that are of particular significance to MS. These processes include myelin biosynthesis, cholesterol biosynthesis, and the lactate shuttle. Both myelin and cholesterol synthesis require high levels of NADPH, while NADP is required for lipid anabolic metabolism. Myelin is composed of over 80% phospholipids, while cholesterol synthesis starts with just two carbon substrates to build the twenty seven-carbon sterol. Furthermore, cholesterol supplied by glia are absolutely required for synapto-genesis, connecting neuron to neuron [46]. Accordingly, pathological analysis of alcoholic pellagrans clearly reveals demyelination [47]. Perhaps part of the dramatic recovery seen with administration of NAD precursors to pellagrans in the 1940s involves remyelination. Interestingly, administration of pharmacological doses of NAM profoundly prevents demyelination in the EAE model [7]. More research is needed to determine whether pharmacological doses of NAD precursors can affect remyelination.
Lastly, the lactate shuttle functions to provide energy to neurons that is particularly essential to neuronal survival during ischemic shock ([48] for a review see [49]). When oxidative phosphorylation is unavailable NAD is required to maintain ATP synthesis by anaerobic substrate level to maintain ATP synthesis by anaerobic substrate level phosphorylation (Fig. 2). Neurons receiving lactate from glia can immediately use lactate to generate both pyruvate and NADH for ATP generation [50, 51]. NAD clearly serves critical bioenergetic functions particularly important to neuronal survival during ischemic shock.
Given the divisions of labor reserved for glia, it makes sense that glia are likely more efficient at NAD biosynthesis than neurons and consequently serve critical functions as providers of NAD to neurons. Collectively experimental data supports the notion that glial may be required for the essential delivery of NAD to neurons starting from tryptophan, nicotinic acid/niacin, or nicotinamide/niacinamide, but not nicotinamide riboside [1].
3. CLINICAL MULTIPLE SCLEROSIS AND ANIMAL MODELS
3.1. Pathology of MS
MS is the most common inflammatory demyelinating disease of the central nervous system (CNS) [52, 53]. Affecting more than 2.5 million people worldwide, 30% of MS patients develop paralysis becoming wheelchair bound for the rest of their lives [54, 55].
The brain is an immunologically privileged site that is largely protected by the tight junctions of the blood-brain barrier (BBB). Generally, lymphocytes are not present in the brain. However, when health problems promote BBB disruption, lymphocytes can infiltrate the CNS. Accordingly, disruption of the BBB is believed to play an important role in MS pathogenesis.
Histological MS lesions can be divided into three components; 1) inflammation due to immune cell infiltration, 2) demyelination and axonal degeneration, and 3) activation of microglia (resident CNS macrophage) and astrocytes, the latter of which causes gliotic or “sclerotic” lesions. Inflammation (the first component) can function as an effector to cause CNS damage that results in clinical neurological deficits. Inflammatory lesions are composed of T cells, B cells (antibody-producing cells), and macrophages (antigen presenting cells, APC). These immune cells can be myelin specific autoimmune cells or non-myelin specific immune cells. Myelin-specific autoimmune cells may attack the myelin sheath directly, while nonspecific inflammation causes myelin damage in a bystander fashion. CNS inflammation can lead to demyelination and/or damage in myelin forming cells, the oligodendrocytes, as well as axonal degeneration (the second component).
The third component, gliosis, can be regarded as a reactive response to the other two components, and is similar to scar formation in the periphery. Although this last step may be considered a process of healing and repair leading to neuronal regeneration in the peripheral nervous system (PNS), regeneration of neuronal cell bodies and processes (axons and dendrites) is in general impossible in the CNS. Although remyelination and regeneration of myelin-forming cells can occur in the CNS, it is a much less active process there than in the myelin-forming Schwann cells of the periphery. In addition gliosis is considered to hinder neuronal regeneration in the CNS.
3.2. Clinical Course of MS
MS can be characterized by either episodic acute periods of worsening (relapses, exacerbations, bouts, attacks), gradual progressive deterioration of neurologic function, or combinations of both. The clinical course of MS is classified into four forms: relapsing-remitting (RR), primary progressive (PP), secondary progressive (SP), and progressive relapsing (PR) [56]. RR-MS is defined by disease relapses with full recovery or with sequelae. PP-MS progresses continuously from the onset. Initial RR disease is often followed by progression (SP-MS).
Clinical and immunological profiles can be different among different forms of MS or even among the same form of MS. However, in general, attack by inflammatory cells in the CNS results in disease onset, exacerbation/relapse and disease progression (Table 2), while inhibition of autoimmune reactions lead to disease remission. Demyelination and axonal degeneration result in clinical attack and disease progression. Irreversible axonal damage and lack of remyelination contribute to a lack of recovery. Since remyelination in the CNS, if at all, is a very slow process, recovery from clinical signs should be attributed to subsiding inflammatory responses, but not necessarily neuro-regeneration. Gliosis may further inhibit regeneration processes in the CNS. In section 9 we will later discuss that all three components are connected, and targeting one component can affect the other component in some cases.
Table 2.
Pathology of MS
| Pathology | Cell Types | Role | Clinical Course | NAD Involvement |
|---|---|---|---|---|
| Inflammation | Lymphocytes, macrophages (APC) | Effector | Disease onset, progression, relapse (attack) | IDO |
| Demyelination / Axonal degeneration | Oligodendrocytes (myelin sheath), axons (neuronal processes) | CNS damage, neurological deficits | Disease attack and progression, lack of remission | NMNAT, SIRT |
| Gliosis / Sclerosis | Microglia, astrocytes | Repair? CNS damage? |
Inhibition of regeneration? Disease progression? |
IDO (CD200) |
3.3. Etiology of MS
Whether infectious agents, environmental toxins, mutagenesis, or nutrient deficiencies cause MS is uncertain. Inheritance studies of MS have found genetic links affecting disease susceptibility, but have not revealed any single genetic mutation [57]. Given the high frequency of MS in the population, it seems most likely that more than one factor is involved. For example, geographical epidemiology and animal models clearly reveal important roles for vitamin D signaling [58], while deficiencies of vitamin B12 [59-61] or sadenosylmethionine [62, 63] can directly cause demyelination. Meanwhile recurrent reactivation of a latent herpes infection resembles relapsing-remitting MS and is commonly found in the CNS, where its potential role in MS pathogenesis is difficult to resolve owing to its nearly ubiquitous presence [64].
The autoimmune etiology of MS is supported by 1) evidence of immune cells infiltrating the CNS, 2) the presence of anti-myelin T cell and antibody responses, 3) a susceptibility associated with major histocompatibility complex (MHC) haplotypes, and 4) altered clinical signs after immunomodulation therapy [52]. Currently, type I interferon (IFNα and β), glatiramer acetate, and antibody against very late antigen (VLA)-4 have been shown to suppress clinical signs of MS.
It has been proposed that MS is mediated by anti-myelin helper T cell type (Th1 or Th2) responses. The Th1 cells produce cytokines such as IL-1, IFNγ, tumor necrosis factor-α/β (TNFα and TNFβ/lymphotoxin-α). These cytokines help are involved in cellular immune responses including delayed type hypersensitivity (DTH). The Th2 cells produce cytokines including interleukin (IL)-4, 5, 6, and 10, which contribute to antibody production. This is partly because a human clinical trial of IFNγ has been reported to exacerbate MS [65, 66]. Although the study was controversial (the observation period, small number of patients, clinical outcome of follow up period, etc), no large clinical trial using IFNγ has been tried since then. In fact, there is some evidence to support that Th1 responses are protective in the early stages of the disease and IFNγ treatment early on can prevent the development of MS [67, 68].
IFNγ clearly exerts a protective role in viral animal models of MS [69]. Perhaps most significantly with respect to NAD metabolism, the IFNγ has been shown to require indoleamine 2,3-dioxygenase (IDO) activity to exert many of its effects including anti-viral (herpes [70, 71] and CMV [72]), anti-parasitic (malaria [73]), anti-bacterial (streptococci [74]), and anti-intracellular pathogen (T. gondii [75, 76] and Chlamydia [77]).
3.4. Animal Models for MS
The two most commonly used animal models of MS are experimental autoimmune encephalomyelitis (EAE) and Theiler's murine encephalomyelitis virus-induced demyelinating Disease (TMEV-IDD). Here we briefly review the unique differences of each model. Autoimmune etiology of MS has been supported by EAE [78, 79]. EAE is induced by injection of brain homogenate, myelin proteins, or related peptides. EAE manifests as an autoimmune response against myelin antigens in the CNS. EAE is most frequently induced by myelin basic protein (MBP), myelin proteolipid protein (PLP), or myelin oligodendrocyte glycoprotein (MOG) [80]. Adoptive transfer of myelin-specific IFNγ-producing Th1 cells also induces EAE. Interestingly, IFNγ has been shown to play a protective role in several EAE models. Because clinical and immunological profiles of EAE are sometimes quite different between models, it is not always appropriate to use evidence from one model and applied for all EAE models [81].
A viral etiology for MS is supported by the fact that 1) exacerbation/relapse is triggered by infection, 2) detection of virus itself or anti-viral immune responses, 3) epidemiological evidence, and 4) animal models [64]. TMEV is one of most commonly used viral models for MS [79, 82]. TMEV belongs to the family Picornaviridae, and intracerebral injection of TMEV causes chronic inflammatory demyelinating disease, similar to MS. Although the precise mechanism of demyelination is controversial, viral persistence in the CNS as well as immune responses, including T cells, antibodies, and macrophages, have been shown to play key roles in demyelination. Immune modulation treatments have been shown to influence the clinical outcome of TMEV infection.
Comparing the same immunomodulation therapy between models of EAE and TMEV-IDD, some therapies, such as depletion of T cells, inhibit both EAE and TMEV-IDD, but other therapies, such as anti-chemokine therapy, were effective only in one model but not the other [83]. Thus, although two models share many immunological profiles, these two models are not immunologically identical [78].
4. CONTRASTING ROLES OF WLDS IN ANIMAL MODELS OF MS
4.1. Slow Wallerian Degeneration Mice (WldS)
When an axon is cut, the distal part of the axon, which is disconnected from the neuronal cell body, breaks up into fragments; this classical type of axonal degeneration is called Wallerian degeneration [84]. Wallerian degeneration is a fundamental reaction of the nervous system to loss of continuity of axons as a result of traumatic, ischemic, toxic, metabolic or other injury. C57BL/WldS (Wallerian degeneration slow mutant, originally described as C57BL/6/Ola) (WldS) mice, are a substrain of C57BL/6 (B6) mice [85]. WldS mice have prolonged survival of the distal stumps of transected axons both in the peripheral nervous system (PNS) and the CNS [86-89]. Transected axons from wild type neurons degenerate in less than 24 hours. By contrast transected WldS neurons survive for up to 4 weeks and support action potentials for at least 2 weeks ! Axons continue anterograde and retrograde transport of proteins for similar amounts of time as well [90-92]. WldS mice are protected from Wallerian degeneration by a mutation, an 85-kb tandem triplication on mouse chromosome 4. The mutation generates a novel chimeric fusion protein composed of the complete sequence of nicotinamide mononucleotide adenylyltransferase1 (NMNAT1), localized in the nucleus, the N-terminal 70 amino acids of ubiquitin fusion degradation protein 2 (Ufd2), and a short peptide sequence conjugating the two proteins [93, 94]. The mechanism of WldS action is still enigmatic, and the nature of the WldS gene product suggested two hypothesis for their mode of action: 1) a dominant negative effect of the ubiquitination factor fragment, and 2) activity of NMNAT1 via NAD biosynthesis [93, 95]. While there is data supporting either hypothesis, we will mainly discuss the latter in this review since there is evidence that NAD or NAD precursors exert dramatic survival protection from stressed neurons in vivo [7, 37, 48, 49] or in neuronal explant assays [27, 36, 37].
NMNAT1 has been proposed as responsible for the preservation of axons and SIRT1 is the downstream effector of increased NMNAT activity that leads to axonal protection [27, 36]. By RNA interference-mediated gene silencing of each SIRT1 member (SIRT1-7) in dorsal root ganglion (DRG) neurons, Araki et al. [36] showed that only SIRT1 is involved in mediating the protective effects of NMNAT1 and NAD. In contrast, Wang et al. [36, 37] found no significant difference in the extent of axonal degeneration in DRG axons treated with NAD or in NMNAT- or WldS-overexpressing DRG axons between DRG explant cultures from wild-type and SIRT1 knockout mice, which were subjected to axonal transection. Thus NAD was capable of protecting neurons independent of SIRT1 activity. Furthermore at these higher concentrations it was no longer necessary to incubate for greater than 8 hours. A more detailed explanation for this is provided in section 8.3. Wang et al. also found that neither sirtinol (SIRT1 inhibitor) nor resveratrol (SIRT1 activator) affected the protective effects of NAD and WldS/NMNAT1 on axonal degeneration in the same assay. Wang et al. [37] suggested that local bioenergetics are primarily responsible for NMNAT1/NAD-mediated neuroprotection, since 1) NAD levels decrease in degenerating axons and 2) exogenous application of NAD or nicotinamide prevented degeneration of transected axons by delaying the decrease of NAD and ATP levels. Further support for a NAD-dependent mechanism of action has been repeatedly provided upon examination of pharmacological administration of NAD precursors [7, 38, 42, 96].
4.2. Amelioration of EAE Pathogenesis in WldS Mice
Two independent research groups have tested WldS mice in animal models of MS [37, 97, 98]. Tsunoda et al. [98] induced EAE in WldS mice and their parent strain, C57BL/6 (B6) mice, using MOG35-55 peptide. Both mouse groups showed a similar disease onset, MOG-specific lymphoproliferative responses in the periphery, and CNS inflammation and demyelination at 2-week post immunization (p.i.) with MOG. Similar levels of peripheral immune responses between two mouse groups were expected, since no immunological deficit has been reported in WldS mice. However, WldS mice showed complete recovery with a decline in MOG-specific T cell responses. In contrast, wild-type B6 mice showed disease progression clinically with high levels of axonal degeneration, demyelination, and MOG-specific lymphoproliferative responses. Chitnis et al. [97] used identical mouse strains and EAE generating protocols and obtained similar results with WldS dramatically delaying EAE disease progression. Further Chitnis et al. noted a reduction in macrophage accumulation and activated microglia for the WldS mouse.
Similarly, Kaneko et al. [7] demonstrated an attenuation of clinical signs of MOG35-55-induced EAE in WldS mice compared with wild-type B6 mice. Histologically, at 2 weeks post-inoculation (p.i.), B6 mice showed more severe axonal degeneration with increased microglia/macrophage infiltration [97] than WldS mice, while no significant difference was seen in the extent of demyelination or CD4+ and CD8+ T cell infiltration in the CNS between the two groups at 2 weeks p.i. In addition, there were no differences between the two mouse groups in MOG induced T cell infiltration, proliferation, delayed type hypersensitivity (DTH) responses, IL-5, 6, 10, and IFNγ production, at 2 weeks p.i., demonstrating that there is no deficit in priming in WldS mice [97]. NAD levels were preserved only in WldS mice at 2 and 4 weeks, p.i. Daily administration of 500 mg/kg (40g/170lb human equivalent) nicotinamide to EAE mice from the day of MOG immunization or from day 10 pi., reduced all pathology parameters. This included reductions in axonal loss, T cell infiltration and demyelination. NAD levels were quantitatively increased in the spinal cord by the nicotinamide administration. In that study, autoimmune Th1 immune response against MOG was not examined in the nicotinamide treatment group, although others showed that nicotinamide can modulate IFNγ-mediated reactions, and that NAD itself can cause cell death of T cells, particularly CD4+CD25+ regulatory T (Treg) cells [100, 101]. Thus, it is not clear whether or not amelioration of EAE in the nicotinamide treatment group is solely attributed to preservation of axons.
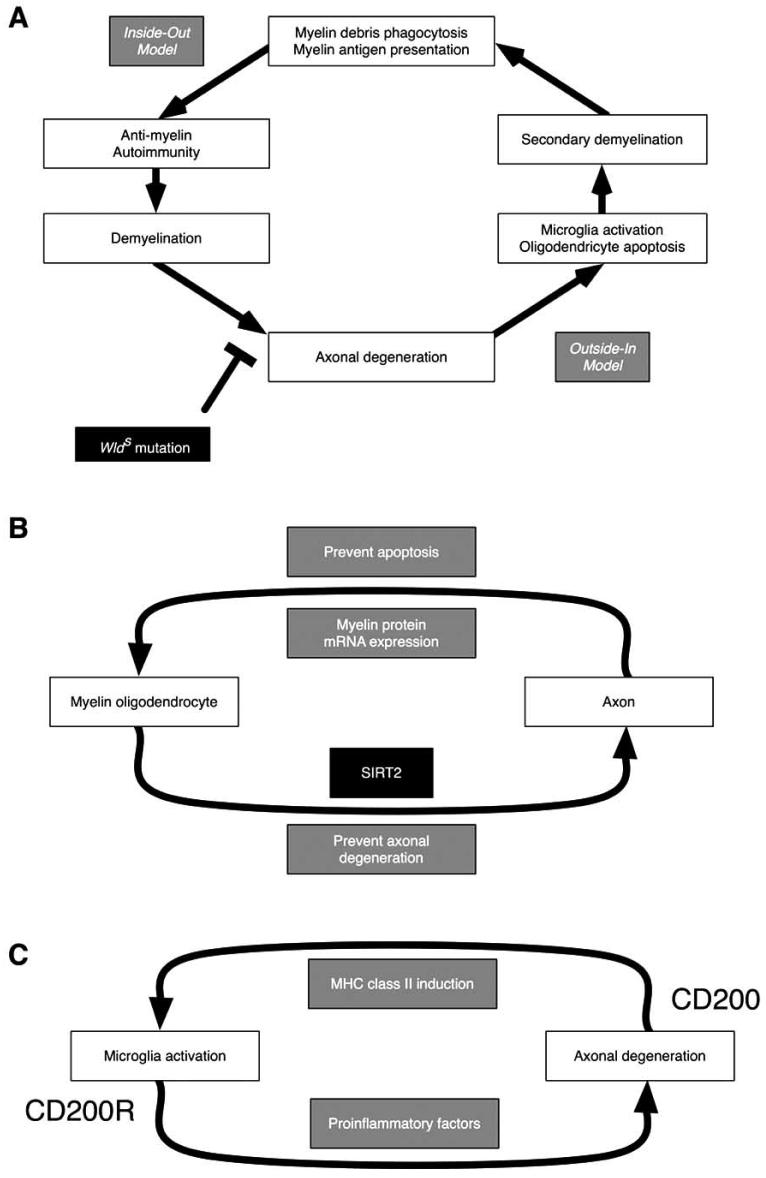
While it is beyond the scope of this review to discuss the role of the Ufd2 aspect of the WldS gene, Ufd2 regulates multi-ubiquitination of proteins targeted for degradation by the 26S proteasome complex. Chitnis et al. [97] demonstrated elevated expression of CD200 in neurons and axons in the CNS of naïve WldS mice and WldS mice with EAE, which was associated with decreased ubiquitination of CD200, most likely caused by the Ufd2 portion of WldS gene. Administration of CD200 antibody into WldS mice with EAE exacerbated clinical signs of EAE, suggesting that the CD200-CD200R pathway plays a role in attenuating EAE in WldS mice. CD200-CD200R interactions play a role in the control of myeloid cellular activity. Interestingly, activation of the CD200 receptor with CD200-Ig in mouse plasmacytoid dendritic cells causes an increase IDO expression and function that exerts a toleragenic effect on autoreactivity [102]. Conversely, IDO inhibition exacerbates EAE pathogenesis prevents stem cell rescue of EAE [31, 33, 34]. Thus, we may expect that CD200-Ig mediated increases in IDO may ameliorate the clinical symptoms of MS. In section 10 we discuss the recent discovery of another soluble factor (TREM-2) that controls IDO expression and has been determined to be present at exceptionally high levels in the cerebrospinal fluid of MS patients [103].
SIRT1 is a member of a highly conserved gene family, the Sirtuins, encoding NAD dependent deacetylases, which deacetylate histones leading to increased DNA stability and prolonged survival in yeast and higher organisms, including mammals. Neuroprotection mediated by SIRT1 activation has been demonstrated in axotomized dorsal root ganglion neurons [36]. Shindler et al. [104] tested whether two SIRT1 activators, nicotinamide riboside (NAMR, SRT647) and resveratrol (SRT501) protects from optic neuritis in EAE mice sensitized with myelin proteolipid protein (PLP)139-151 peptide. Intravitreal injection of NAMR or resveratrol prevents loss of retinal ganglion cells (RGC), but does not prevent inflammation or clinical signs of EAE. The neuroprotective activity was blocked by sirtinol, a SIRT1 inhibitor. Although specific substrates of SIRT1-mediated RGC survival are not known in this study, apoptotic proteins can be candidates for the downstream targets of SIRT1-mediated RGC neuroprotection, since SIRT1 is known to act on proteins involved in apoptosis. In addition, as Bogan et al. [1] proposed, the specific utilization of NAMR by neurons may provide qualitative advantages over niacins in promoting function in the central and peripheral nervous system [1].
In contrast, Suzuki and Koike [105] showed that resveratrol diminished resistance of cerebellar granule cells of WldS mice to axonal degeneration induced by colchicine. The authors showed that resveratrol promotes tubulin deacetylation by acting directly on SIRT2, and SIRT2 silencing restores the resistance to axonal degeneration in resveratrol-treated WldS neurons.
4.3. Exacerbation of TMEV-IDD Pathology in WldS Mice
Although axonal preservation is beneficial in EAE, a lack of delay of axonal degeneration has been shown to be detrimental in WldS mice infected with TMEV [98]. TMEV infection was also compared between WldS mice and wild-type B6 mice [98]. Since B6 mice are resistant to TMEV-IDD, TMEV-infected B6 mice showed no clinical disease. In contrast, TMEV-infected WldS mice developed paralysis with increased inflammation and virus antigen positive cells in the CNS. This suggests that the slowed axonal degeneration in WldS mice favors virus spread in the CNS, since TMEV is known to use axons to disseminate in the CNS. This suggests that axonal degeneration in B6 mice might be a beneficial mechanism that limits the virus spread in the CNS [106]. In this experiment, neither WldS mice nor B6 mice developed demyelination.
Roussarie et al. [107] demonstrated that intraocular injection of TMEV into wild-type B6 mice and WldS mice resulted in infection of CNPase+ oligodendrocytes (65% in B6 and 75% in WldS) and glial fibrillary acidic potein (GFAP)+ astrocytes in the ipsilateral optic nerve. There was no infection in the contralateral pre-chiasmatic segment of the optic nerve. Virus most likely spread using axonal transport, not the hematogenous route, although virus infection in the axon was not shown in this experiment. In the optic nerve, axonal degeneration was observed only in the B6 mice, but not in WldS mice by immunostaining against nonphosphorylated neurofilament or electron microscopy. The authors suggested that in WldS mice TMEV is able to traffic from the axon into the surrounding myelin in the absence of membrane lysis, by some cellular exit route, possibly via the autophagosome-like membranes induced by picornavirus infection [108].
5. BIOENERGETIC NAD-ATP LINK IN AUTOIMMUNE-INDUCED DEMYELINATION
Reactive oxygen or nitrogen species, R(O/N)S, that damage DNA immediately activate the poly(ADP)ribose polymerase-1 (PARP-1) catalyzed enzymatic polymerization reaction using NAD is used as the substrate. The products are poly(ADP)ribose (PAR) and nicotinamide, where PAR can be transferred to proteins including PARP-1 itself. High levels of DNA damage lead to excessive PARP-1 mediated depletion of NAD. The NAD salvage pathway becomes activated in response to this in an attempt to regenerate the PARP-1 product, nicotinamide, back to NAD. ATP is required indirectly for the NAMPT reaction and directly for the NMAT catalyzed reaction. This causes a lowering of ATP levels since the NAD salvage pathway uses ATP to regenerate NAD starting with the product of the PARP-1 catalyzed reaction, nicotinamide (Fig. 2). With excessive DNA damage this becomes a futile cycle that depletes energy reserves to such an extent that total bioenergetic collapse occurs with necrosis (for a review [109]). Ultimately, accumulated PAR formed from PARP-1 can promote apoptosis to insure cells with severely damaged DNA are elegantly killed, thus decreasing the likelihood for uncontrolled tumorigenesis.
Cells are particularly vulnerable to lethal ischemic shock when PARP-1 is activated, most immediately, simply because NAD is an essential requirement for generation of ATP by substrate level phosphorylation (glycolysis) during anaerobic conditions. Significantly, inhibition of PARP-1 has the dramatic effect of increasing cell survival. PARP-1 inhibitors do delay Wallerian degeneration [36]. Furthermore application of NAD or activation of SIRT1 has both been shown to inhibit PARP-1 as well and increase cell survival [110-112]. Thus one can envisage a pathway for WldS extending: NMNAT1-NAD-SIRT1-PARP-1 inhibition-delayed Wallerian degeneration. However, whether or not PARP-1 inhibition is aprt of the mechanism of action of WldS remains to be determined. Accordingly, pharmaceutical companies have invested heavily in PARP-1 inhibitors for diseases ranging from acute (myocardial infarction, acute respiratory syndrome, endotoxic shock, aneurysm) to the chronic (colitis, arthritis diabetes; for a review [113]) disease conditions. An alternative approach is to simply supply elevated levels of NAD as a means of preventing PARP-1 mediated deficiency. With this approach, very significant cytotrophic cellular survival results have been seen in response to otherwise lethal shocks in numerous instances when NAD levels have been increased [48, 114-118].
NAMPT is the rate-limiting enzyme controlling the rate of enzymatic activity in the NAD salvage pathway [17]. Thus NAMPT is the most highly transcriptionally regulated enzyme in the NAD salvage pathway. Meanwhile the NMNAT enzymes control the efficiency of the NAMPT initiated salvage pathway during stress responses, where the effect of increased gene dosage of NMNAT1 (nuclear) or NMNAT3 (mitochondrial), but not NMNAT2 (cytosolic/golgi) isoforms has been shown to protect against neurodegeneration [27]. Significantly Sasaki et al. [27] reveal that NAMPT and NAPRT may be generally lacking or inefficient in neurons.
Addition of NAD or nicotinamide prevents Wallerian degeneration [36, 37]. Significantly, Wang et al. showed that immediate benefit could be derived in axons by the addition of NAD (5 or 20mM) or the NAD precursor nicotinamide (5 or 25mM) in a SIRT1 independent fashion. Previously it was determined that preincubation at lower concentrations of NAD (1mM) required at least 8 hours (preferably 24 hours) of incubation and the presence of nuclear SIRT1 [36]. This data strongly suggested that the effect was at the transcriptional level since SIRT1 is nuclear and the axon is completely separated from the nucleus in the Wallerian degeneration assay. Furthermore, the much higher concentrations of NAD or NAM used in the SIRT1 independent phenomena underscore the importance of bioenergetic mechanisms at work in the higher concentrations. Significantly, higher levels of NAD were able to confer protection in an otherwise immediately lethal situation.
If SIRT1 is not involved, then what else may be responsible? The most likely explanation may involve a bioenergetic mechanism. NAD levels are intimately connected to substrate level phosphorylation, the only means of generating ATP/energy under ischemic conditions. Inflammation can lead to ischemic shock within inflamed target tissues. For the essential glycolytic generation of ATP to persist under anaerobic conditions, NAD is needed. During anaerobic glycolysis, NAD is obtained via the additional lactate dehydrogenase reaction step. The lactate produced can also be shuttled from glia to neuron via the “lactate shuttle [51].” Then the reaction proceeds backwards to produce pyruvate for the TCA cycle and NADH for oxidative phosphorylation. By using this strategy the neuron is better able to obtain from glia the energy supplies needed to repeatedly repolarize essential ion gradients.
Other possibilities, besides the bioenergetic model, include a potential role for a primarily mitochondrial NAD dependent deacetylase, SIRT3. SIRT3 is now known to be a master regulator controlling ATP generation [119] and global acetylation in the mitochondria [120]. Consistent with the idea of a role for SIRT3, resveratrol has been shown to increase SIRT3 [121] and was previously shown to delay Wallerian degeneration using 10 or 100μM [36]. While resveratrol did fail to provide protection in the acute time frames [37], it remains possible that resveratrol may work at higher concentrations. However solubility become an issue for resveratrol at high concentrations since it is not dissolvable in water.
Alternatively, related possible NAD responsive targets present in the axon include the cytosolic SIRT2, is known to deacetylate tubulin or the mitochondrial Sirtuins (SIRT3, SIRT4, and SIRT5). More genetic work is needed to determine the protein(s) responsible for the immediate protective effect of high dose NAD in Wallerian degeneration model. To conclude, NAD would seem to have great promise in acute care critical medicine in particular and perhaps acute MS attacks as well.
6. IDO AND EAE
Tryptophan is most immediately essential as the sole known precursor for endogenous de novo NAD biosynthesis in mammals. Plasma tryptophan concentrations are depressed in most autoimmune diseases and is often diagnostic of poor disease prognosis [122]. There are three different enzymes that catabolize tryptophan. These are tryptophan dioxygenase (TDO/TDO2), indoleamine 2,3-dioxygenase (IDO/INDO), and indoleamine 2,3-dioxygenase-2 (IDO2/INDOL1) [123, 124]. TDO2 is popularly understood to be restricted to the liver, where it regulates tryptophan serum levels. However, TDO2 protein and mRNA are also expressed in neurons and astrocytes [20]. Furthermore, interferon causes simultaneous decreases in TDO2 and increases in IDO expression levels in neurons [22]. Interestingly, TDO2 expression levels are elevated in schizophrenia [20], a disease known to involve neurodegeneration. Nothing is known regarding the function of TDO2 in the nervous system but based on the commonly accepted hepatic function of TDO2, we expect TDO2 to serve critical functions in NAD biosynthesis starting from tryptophan in other tissues.
Indoleamine 2,3-dioxygenase (IDO) activity is inducible in various cell types, including macrophages and dendritic cells, by the pro-inflammatory cytokine IFNγ, while IDO activity is enhanced by the synergistic effects of either TNFα or lipopolysaccharide (LPS) [21, 125]. The IDO gene promoter contains multiple sequence elements that confer responsiveness to type I interferons (α/μ) and the more potent type II interferon (γ). IDO production by syncytiotrophoblasts, macrophages, and dendritic cells has been demonstrated to result in the inhibition of T cell proliferation due to tryptophan depletion by IDO.
Juxtaposed in series on human chromosome 8 and possibly sharing common cis-regulatory elements, IDO and IDO2 are expressed in many of the same tissues [126]. IDO and IDO2 are just 43% identical. New questions are being raised regarding isoform specific functions [123]. IDO2 was only recently discovered and nothing is known regarding potential IDO2 specific function.
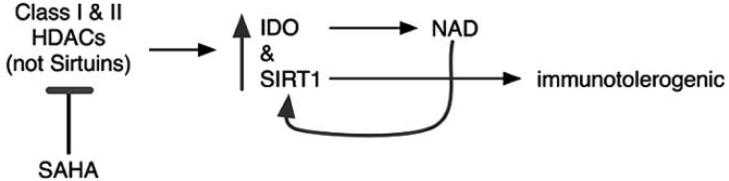
All of this occurs due to selective control of the endogenous NAD precursor and substrate, tryptophan. Expression of IDO is highly regulated within professional antigen presenting cells (professional APCs; macrophages, microglia, or dendritic cells). The general function of IDO in professional APCs is to limit autoreactive T cell proliferation, thus exerting anti-inflammatory activity but at the potential risk of NAD deficiency for local host tissues. IDO regulation occurs via Th1-derived cytokines including IFNγ, interleukin-1, and TNFα. While these cytokines are commonly connected with inflammation, their activities through IDO are anti-inflammatory and function to resolve inflammatory processes. IFNγ-IDO signaling serves as a negative SIRT loop to limit the inflammatory signaling event. This occurs through IDO catalyzed enzymatic depletion of tryptophan, the only NAD precursor for de novo synthesis in vertebrates. Th1-derived cytokines also increase activity of CD38, which also functions to decrease extracellular NAD levels. The general idea is to limit NAD levels to prevent immune cell hyperproliferation. This is an ancient mechanism for competition of resources that probably evolved for use in the metazoan immune system.
IDO is responsible for the most dramatic example of immunotolerance, fetal acceptance (for a review [21]). During pregnancy IDO is expressed at high levels in the cells surrounding the foreign antigen or fetus. Here the IDO enzyme catabolizes local tryptophan, thus preventing lymphocytes from proliferating. When IDO is inhibited it immediately results in catastrophic rejection of the fetus [127]. This same basic process applies within the adaptive immune system in general, where cytokines commonly stimulating proliferation of lymphocytes, also activate IDO. Eventually extracellular tryptophan levels are so decreased that the initial proliferation is halted. SIRT loop that ultimately limiting the extent of proliferation through eventual depletion of the extracellular NAD precursor tryptophan.
Current publication literature suggests that IDO serves primarily immune functions, while TDO2 has more critical functions in NAD biosynthesis. However, given the new-found realization that TDO2 is expressed in neurons [20] and that TDO2 is distinctly up regulated in schizophrenia [128], one wonders whether TDO2 also serves critical roles in controlling the levels of the endogenous precursor tryptophan. The observation that large numbers of schizophrenics respond favorably when treated with high doses of NAD precursors [129] adds further support to the potential existence of a TDO2 mediated pathway to prevent NAD depletion. No examinations of IFNγ effects on neuronal NAD levels have been reported. However, for macrophages [23] and glia [25] IFNγ treatment causes increased IDO activity and increased NAD levels.
In the adoptive transfer model of EAE induced by MBP-specific T cell transfer, IDO mRNA expression and kynure- ine-to-tryptophan ratio, which reflect IDO activity, are higher during the remission phase [31]. IDO inhibitor, 1-methyl-tryptophan (1-MT) slightly decreased MBP-specific lymphoproliferation in vitro, and in vivo treatment of 1-MT resulted in mild exacerbation of clinical and histological EAE (1-MT-treated mice showed delayed remission). Similarly, Kwidzinski et al. [33] demonstrated an increase in IDO activity and a decreased kynurenine/tryptophan ratio in SJL/J mice after EAE induction with PLP139-151 peptide. In this study, IDO was expressed in macrophage/microglia, and 1-MT treatment exacerbated EAE. The authors hypothesized that IFNγ producing-Th1 cells induce expression of IDO in macrophage/microglia, which decreases tryptophan levels, inducing T cell apoptosis (self-limiting negative SIRT mechanism induced by T cells).
IDO ameliorates PLP139-151-induced EAE by transfer of pluripotent lineage negative Sca1+ bone marrow stem cells in mice [34]. Stem cell-treated mice showed elevated IFNγ production with induction of IDO in dendritic cells. The IDO inhibitor, 1-MT, abrogated amelioration of EAE by stem cell transfer.
Estrogen-induced IDO expression on dendritic cells can suppress T cell proliferation and cytokine production. This has been suggested to play a role in estrogen-induced suppression of EAE [130] as well as the reduced relapse rate of MS during pregnancy [131]. Glucocorticoids, as the potent known anti-inflammatory molecules, are the most commonly used therapeutic approach for treating acute attacks of multiple sclerosis. The mechanism of action of steroidal anti-inflammatories is unknown. Recent discovery shows that steroids work in part via a requisite dramatic induction of IDO [132, 133]. Whether or not IDO mediated changes in NAD levels are central to this aspect of the glucocorticoid mechanism of action is unknown as well. Nonetheless, IDO appears to be basic to the mechanisms of action for the most potent and commonly used immunosuppressant for treating acute attacks of MS.
Given the known ineffectiveness of steroidal approaches for long term therapy combined with their risk for serious adverse events, it makes sense to give greater consideration to targeting IDO activity as a potentially safer means of therapy. TDO2 has glucocorticoid response elements in its promoter, where the potential involvement of TDO2 in the anti-inflammatory action of glucocorticoids is unknown. Nonetheless, consistent with such regulatory relationship, schizophrenics have elevated cortisol levels [134] and TDO2 levels [20, 135], where the long-term effect may be result in neurodegeneration. By contrast IDO generally decreases lymphocyte proliferation [29, 30] while ameliorating the clinical symptoms of MS in EAE models [31-33]. When targeting IDO activation however, it is important to realize that too much IDO activity in the absence of pharmacological NAD precursor administration may yield terrible pathological results due to NAD deficiency in host cells even though autoreactive T cells may be diminished.
7. IMMUNOMODULATORY PATHWAYS REGULATING CELLULAR NAD LEVELS
7.1. Introduction
Inflammation causes a localized depletion of cellular NAD primarily by activating three different enzymes including indoleamine 2,3-dioxygenase (IDO), CD38, and PARP-1. PARP-1 and CD38 degrade NAD itself, while IDO degrades the extracellular de novo pathway NAD precursor tryptophan (Fig. 4). In professional APCs IFNα-CD38 pathways have been shown to decrease NAD [136] while IFNγ-IDO (and glucocorticoid-IDO) pathways have been shown to increase intracellular NAD [23, 24] at the expense of extracellular NAD precursor (tryptophan). IFNγ-IDO pathways operate similarly in glial cells as well [25, 26, 43]. During chronic inflammatory lesions in the CNS, lymphocyte proliferation and microglial activation can occur in a CD38-mediated NAD-depleting fashion, while IDO may be activated. PARP-1 activation occurs as a result of R(O/N)S stress connected with inflammation. The roles of NAD depleting enzymes in the immune system are increasingly being appreciated, while the potential for NAD as a second messenger stimulating the persistent release of intracellular calcium to control lymphocyte chemotaxis [137] or microglia activation [138, 139]. Given the importance of these three reactions in controlling NAD levels during autoimmune disease we review these enzymes with the ultimate goal of achieving greater understanding of potential complementary NAD-centric molecular therapeutic approaches to treating MS.
7.2. IDO
The enzyme indoleamine 2,3-dioxygenase (IDO) mediates the extracellular depletion of the sole endogenous precursor of NAD biosynthesis (tryptophan). Accordingly, serum tryptophan concentrations are consistently low and directly linked to poor prognosis in autoimmune diseases in general starting from the persistent Th1 cytokine activation of IDO [122]. Tryptophan levels are decreased during MS [10, 140] and novel assays are being developed for monitoring immune mediated changes in tryptophan levels for consideration in the context of multiple sclerosis [141]. Activation of IDO exerts the therapeutically beneficial effects of decreasing autoreactive T cell proliferation as well as exerting antimicrobial effects. However, persistent activation of IDO in a conflicted immune system can lead to NAD depletion in otherwise healthy neighboring collateral tissues (reviewed [142]).
Professional APCs including microglia, macrophages, and dendritic cells quickly begin to express large amounts of active IDO when exposed to the Th1-derived cytokines including IFNγ and IFNα. Depletion of extracellular tryptophan by professional APCs causes autoreactive T cells to decrease in number [29, 30]. Dendritic cells are considered the most potent modulator of the adaptive immune system [143]. This is consistent with the exceptionally highly level of IDO inductions commonly seen in dendritic cells. While most typically studied in the context of skin, dendritic cells are also in fact present in the CNS [144] where they are being considered for their potential role in the breakdown of myelin autoantigenicity seen in MS (for a review [145]). Finally, stem cells preventing EAE pathogenesis require inducible IDO function [34]. All observations indicate that professional APCs expressing IDO play a role in controlling multiple sclerosis disease progression.
Similar to professional APCs, neurons exposed to IFNγ also increase IDO expression, while repressing the expression of tryptophan dioxygenase (TDO2) [22]. However, the functional significance of IFNγ-mediated IDO induction and whether NAD levels are actually increased remains to be determined. Both IDO and TDO2 use tryptophan as a substrate to commit to the de novo NAD biosynthetic pathway. Alternatively, when the pathway is not completed, intermediates may be formed. This is known as the kynurenine pathway. Accumulation of these intermediates most frequently occurs during times of co-factor deficiency when nutrition is lacking. IDO activity is highly regulated by cytokines where it primarily functions in regulating the immune system. Meanwhile TDO2 is mostly known for controlling organismal NAD biosynthesis in the liver. In fact however, very little is known with respect to the importance of IDO mediated NAD biosynthesis or extra-hepatic TDO2 function respectively. The functional purpose of this contrasting IFNγ-mediated transcriptional regulation in neurons remains to be determined [22].
EAE models indicate NAD depleting pathways are activated based on detection of increased PAR formation in EAE models [9, 146]. In summation, we expect the concentration of NAD within professional APCs to be elevated during inflammation such that a net increase in NAD is detected, but at likely expense of NAD in other cells, where neurons are well known to be particularly vulnerable to neurodegeneration due to NAD deficiency as witnessed in the classic NAD deficiency disease pellagra [147, 148].
While increased IDO activity causes depletion of the extracellular NAD precursor tryptophan, it can increase intracellular NAD levels. This gives the host immune cells directly combating infectious pathogens (e.g. macrophages), a competitive survival advantage. Interferon causes significant increases in NAD levels in astrocytes [25, 26] or macrophages [23, 24]. Inhibition of IDO [23, 25, 26] prevents IFNγ-mediated increases in NAD; by contrast inhibition of PARP-1 [23, 24] increases IFNα stimulated NAD production in glia and macrophages. Whether or not IFNα can cause an increase in neuronal NAD levels however, is unknown. Many of interferon's activities are completely dependent on the strong interferon-mediated induction of IDO activity [70-77]. Furthermore IFNγ-IDO mediated increases in NAD levels can provide protection against otherwise lethal hydrogen peroxide treatment in glial cells [25], our understanding of the IFNγ-IDO mediated NAD biosynthetic pathway is severely limited.
Interestingly, the addition of the de novo NAD biosynthetic pathway intermediate kynurenine can also actively exert anti-inflammatory effects [149, 150]. It is possible that kynurenine is being used in neighboring cells as a NAD precursor, such that the active IDO-mediated generation of NAD as a second messenger may be more important to immunosuppression than the depletion of tryptophan. Consistent with this idea is the newly appreciated role of IDO-mediated signaling from professional antigen presenting cells that stimulates T regulatory cells to send toleragenic signals to other T cells [150]. Accordingly it may be more the active generation of NAD as a second messenger in professional antigen presenting cells that is more important in immunosuppression than the depletion of NAD precursors locally. Ultimately more research is needed to increase our basic understanding of IDO-mediated immunosuppression.
7.3. CD38
CD38 is an ectozyme with ADP cyclase activity that uses NAD to generate cyclic ADP ribose (cADPR), nicotinic acid dinucleotide phosphate (NAADP), or ADP ribose (ADPR) (for a review [151]). These products then act as potent second messenger activators of calcium channels to control chemotaxis of dendritic cells [152] and activation of microglia [138]. All studies to date indicate that CD38 enzyme activity is constitutive, such that changes in CD38 activity are primarily mediated through transcriptional regulation or subcellular location. CD38 is expressed highly expressed in the brain as well as a variety of blood cells including T cells, B cells, monocytes, red blood cells, and platelets. CD38 is also expressed in the pancreas, prostate, spleen, and heart. While CD157 is CD157 is a related family member, we are limiting this discussion to CD38 since far less is known regarding CD157, but the potentially redundant role of CD157 in controlling NAD levels is completely unknown.
CD38 uses extracellular NAD to generate second messengers that open intracellular calcium stores to control lymphocyte chemotaxis. CD38 exerts significant control over total tissue NAD levels in animal [153-155]. TNFα signaling in macrophages causes a dramatic up-regulation of CD38 that leads to a decrease in NAD levels. This is likely to be a part of TNFα-mediated chemotaxis originating from the Th1 cell that are newly present in MS pathogenesis. Thus, the characteristic CNS lymphoproliferation is likely to be sending signals to microglia via Th1-TNFα binding to TNFα receptor on microglia which through CD38 promotes gliosis (third histological component of MS pathogenesis described in section 2.1). Thus, inhibition of CD38 can be expected to minimize gliosis as well as increase NAD levels. However, this may come at the expense of increased susceptibility to infection. The increased lymphocyte proliferation and infiltration seen in MS is likely to contribute to appreciable losses in neuronal NAD. There are many reasons to consider the function of CD38 as related to MS. First, CD38 is highly expressed in the afflicted and pathogenic tissues of the MS of brain and lymphocytes respectively (for a review [151]). Similarly, CD38 is well established for its likely involvement in possible autoimmune pathogenesis of type II diabetes [156]. Significantly, CD38 appears to be one of the primary regulators of NAD levels in tissue [136, 153-155]. The original defining function of CD38 was determined to lie in controlling innate and adaptive immune functions [152, 157]. Meanwhile, recent studies reveal that by altering NAD levels, CD38 exerts strong control over bioenergetic aspects of metabolism particularly in the context of obesity [155]. Such control over NAD-related bioenergetics may play a role in MS pathogenesis as has described in the bioenergetics section above linking maintenance of NAD levels to maintenance of ATP levels with resistance to EAE-mediated neurodegeneration [37].
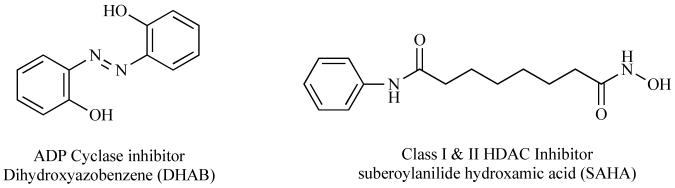
The products of CD38 catalyzed degradation of NAD are among the most potent known molecules controlling the release of intracellular calcium stores ([158-160]). These products are cyclic ADP ribose (cADPR) or nicotinic acid adenine dinucleotide phosphate (NAADP) or ADP ribose (ADPR), each of which is produced with differing degrees of reaction efficiency. The CD38 inhibitor dihydroxyazobenzene (DHAB) has been shown to exert cytotrophic effects, keeping cells alive through inhibition of persistent increases in intracellular calcium (Fig. 5; [161]). Microglial cell activation [138] and dendritic cell migration [152] can be controlled by CD38 dependent calcium signaling pathways. All of these observations support the notion that CD38 is highly likely to play important roles in controlling MS pathogenesis. However, no research has been reported describing the potential importance of CD38 in MS. Thus, more research is needed regarding determining the potential importance of CD38 mediated NAD biology in MS pathogenesis. Here we review the functions of CD38 with special consideration devoted to its ability to control NAD levels.
Fig. (5).
Structures of a new ADP cyclase inhibitor and the first clinical trial inhibitor of constitutive HDACs, not Sirtuins are shown.
Knockout studies indicate that CD38 exerts dominant control of basal tissue NAD levels. Aksoy et al. propose that CD38 is the major enzyme negatively affecting tissue NAD levels [153]. Absence of CD38 results in high increases in steady state NAD levels. Elevations in lung and muscle are particularly dramatic with increases of approximately 10 [154] and 5 fold [155] respectively. Estimates of changes in brain tissue NAD levels range from approximately 2 [154] to 10 fold [153]. Other tissues possess as much as 20 fold greater levels of NAD [153]. Changes in NAD levels are typically fractional for most stress responses with the exception of PARP-1 activation by mutagen treatment, which can lead to decreases in NAD levels by over an order of magnitude [162]. However, increases in NAD levels of this magnitude are rarely if ever observed. It should also be realized that CD38 has long been used as a marker for hematopoietic stem cells, and has recently been determined to be constitutively present in the nucleus of a wide range of hematopoietic cells [163].
Where the first observations of the CD38 knockout mouse led to the understanding of CD38 as important for innate and adaptive immune function, the recent application of a high fat diet to the CD38 deficient mouse revealed profound roles for CD38 in controlling metabolic bioenergetics [155]. Barbosa et al. discovered that CD38 deficient mice fed a high fat diet do not gain weight. This was then determined to be due to increased mitochondrial biogenesis through the pathway extending: NAD-SIRT1-PGC1alpha-mitochondrial number. Accordingly, the direct application of NAD is now being considered in the context of obesity drug discovery research [164]. The metabolic implications of increasing NAD levels to allow the body to distribute NAD as needed may have tremendous therapeutic potential in the context of disease treatment.
As mentioned above, CD38 activity is constitutive where it is primarily regulated through changes in expression levels and perhaps sub-cellular location. Activation of CD38 by Th1-derived cytokines can cause decreases in available NAD. Immunomodulatory factors strongly regulate the expression level of CD38. Given the ability of CD38 strongly regulates basal NAD levels, we may predict that Th1-derived cytokines may cause a lowering of extracellular NAD levels. This may play a particularly important role in pathogenesis especially given that NAD has a much greater ability to protect neurons specifically in axotomy experimentation [27, 36]. TNFα-mediated changes in NAD levels appear to occur through altering CD38 activity more so than through altering IDO or other potential pathways [136, 165]. TNFα treatment causes a slight decrease in NAD levels of approximately 10% after 12 hours [136]. TNFα dramatically regulates CD38 expression levels more than any other NAD metabolizing enzyme (+100 fold within 6 hours), while NAMPT expression is elevated by approximately 10 fold. The pathways and mechanisms regulating cellular NAD supplies during inflammation are described in further detail below. Significantly, TNFα-mediated decreases in NAD levels are achieved through regulation of CD38 activity in macrophages [136]. Striking increases in CD38 expression have been seen in immunosuppressive B cells [166] and T cells [167]. Thus, CD38 in coordination with IDO is capable of mediating immunotoleragenic effects.
Another theory proposed is that CD38 may be functioning as a chemosensor for plasma NAD that has leaked from infected and damaged cells. Leucocytes respond by moving towards the NAD gradient. CD38 activity is required for proper lymphocytes chemotaxis. Similarly LPS stimulates CD38, which via the production of cADPR causes calcium release, which increases iNOS, and TNFα release from these activated microglia [139]. Inhibition of the CD38 activity prevents the entire process. It is known that approximately 100 NAD molecules are degraded per each individual cADPR molecule that is produced [153].
One of the first lines of defense preventing CNS disease is the blood-brain barrier (BBB). The brain is considered an anatomic site of immune privilege, whereby lymphocytes are not typically found in the brain. Disruption of lymphocyte trafficking across the blood brain barrier is considered as an attractive therapeutic goal [168, 169]. However, animal models of MS as well as in patients reveal the presence of high levels of lymphocytes in the brain. These cells are known to express the neuronal NAD-depleting enzymes IDO and CD38, the latter of which is required for microglial activation and dendritic cell migration.
Significantly, TNFα leads to decreases in NAD through increased expression of CD38 [136]. Microglial IDO is similarly regulated in response to LPS and TNFα, where IDO is known to decrease the concentration of extracellular NAD precursor tryptophan (for a review see [142]). The importance of CD38 mediated NAD depletion in microglial mediated pathogenesis is likely to be very significant indeed.
Anti-CD38 antibodies most of which activate calcium signaling have been observed in a significant number of type I and type II diabetics (for a review [170]). Similarly over-expression of CD38 compromises beta cell functioning. Collectively it would appear that auto-reactive CD38 antibodies might promote autoimmune disease. The importance of CD38 in MS pathogenesis is unexplored, but may be important in MS through Th1 cytokine-increased CD38-decreased NAD/increased calcium signaling-dependent pathways.
7.4. PARP-1
Poly(ADP)ribose polymerase-1 (PARP-1) activated by reactive oxygen species generated by lymphocytes or activated microglial cell can actively deplete NAD by using NAD as a substrate in a polymerization reaction that generates the polynucleotide Poly(ADP)ribose (PAR). Pharmacological inhibition of PARP-1 is known to protect cells from death in a wide variety of otherwise lethal stress situations. NAD depleting pathways are activated during chronic inflammatory diseases. PARP-1 activation is estimated to be responsible for greater than 85% of PARP-1 activity [171, 172], where PAR formation in brain injury is almost completely due to PARP-1 activity based on knockout studies [173, 174]. Thus, while there are now known to be 18 related family members in ADP-ribosylation family based on sequence gazing, it is clear that PARP-1 is responsible for the bulk of the NAD-depleting activity. PARP-1 uses NAD as a substrate for a polymerization reaction that generates poly(ADP)ribose (PAR; Fig. 2). PARP-1 is activated by DNA damage, where cytokines can stimulate production of free radicals that can subsequently activate PARP-1.
A generalized endogenous pathway for immune-mediated activation of PARP-1 corresponds to Th1 cytokines leading to increased NADPH oxidase (NOX) which, uses SOD to produce hydrogen peroxide which can then become peroxynitrate via nitric oxide synthase catalyzed reactions. Both hydrogen peroxide and peroxynitrate are potent activators of PARP-1 activity (for a review [175]). The hydrogen peroxide produced by this process can then activate PARP-1 leading to depletion of NAD and ATP by extension. EAE animal models of MS reveal a high amount of PARP-1 activity in the CNS [9, 146].
Oxidative stress causes a rapid depletion of NAD levels via activation of the PARP-1 enzyme. PAR is then converted to ADP ribose (ADPr), which activates TRPM2. This also leads to the production of cyclic ADP ribose, which activates the divalent cation channel TRPM2 (Fig. 4 [172]). Accordingly, inhibition of PARP-1 activity is well known to prevent neutrophil activation and to protect against septic shock [176-178]. Similarly, TRPM2 is an attractive target for drug development currently being considered [179]. TRMPM2 is most highly expressed in the central nervous system but has to date been mostly studied in the context of immune function. Serving important function in inflammation TRPM2 may be important in controlling MS pathogenesis.
Investments in the development of PARP-1 inhibitors have been remarkably successful (reviewed [113]) and continue to be a highly active area of drug development today. Studies utilizing PARP-1 inhibitors in the context of animal models of MS are described in greater detail below.
8. POTENTIAL NAD-RELATED PHARMACOTHERAPEUTIC STRATEGIES FOR TREATING MS
8.1. NAD Precursor Complementation
8.1.1. Pharmacokinetics and BBB
Experimental observations suggest that expansion of the immune system during chronic inflammation may create NAD sinks in glial cells that deplete NAD stores otherwise available to neurons (Fig. 3). There are three classical NAD precursors obtained from the diet. These are nicotinic acid, nicotinamide, or nicotinamide riboside, where these three are collectively referred to as vitamin B3. Tryptophan, while not a vitamin, but rather an essential amino acid, can in fact be converted to NAD by de novo biosynthesis. A deficiency in either vitamin B3 or tryptophan results in pellagra like symptoms that are ultimately lethal. During autoimmune diseases [122] including MS [10, 140], the levels of tryptophan are dramatically depressed likely primarily due to increases in IDO expression that are used by the body to dampen the autoimmune response. Unfortunately this can cause collateral damage to tissues by causing deficiencies in tissue NAD levels. Neurons are particularly exquisitely susceptible to NAD deficiency induced cell death. Complementation through tryptophan administration however, is likely not to be advisable in large part because tryptophan deprivation is a basic mechanism that is used to limit inflammation. Also, tryptophan in high doses is the most toxic of all amino acids [180], it is mitogenic for T cells [30, 31], and tryptophan may be involved in causing deadly eosinophilia myalgia syndrome (EMS [181]). The FDA banned tryptophan shortly after it was linked to an outbreak of EMS in 1989, a disease involving hyperproliferation of eosinophils. Similarly, patients taking gram quantities of tryptophan in attempts to elevate serotonin for combating depression often have eosinophilia [182]. Finally and most tellingly, prevention of the normal mechanism of IDO mediated tryptophan depletion, exacerbates EAE pathogenesis [31].
Fortunately, addition of alternative NAD precursors has been repeatedly shown to provide tremendous apparent therapeutic benefit in a wide variety of autoimmune diseases observed in both animal models and in the clinic (for a review see [142]).
It is important to consider drug delivery aspects of NAD precursor administration so that we can determine optimal clinical applications of NAD pharmacotherapeutics particularly related to critical care acute MS attacks or more preventative situations as with relapsing MS. The basic therapeutic potential for the application of high doses of non-tryptophan NAD precursors for the treatment of autoimmune diseases still remains unstudied in a systematic fashion. Given the tremendous apparent potential for such approaches, we review NAD precursor aspects of blood brain barrier (BBB) penetration and NAD pharmacokinetics for each precursor. We also review the latest knowledge of individual NAD precursors related specifically to MS.
Nicotinamide permeates the mammalian BBB based on previous positron emission tomography studies performed in mice [183, 184] and injected radiolabelled precursors [185]. Mice subcutaneously injected with nicotinamide receive profound protection against behavioral defects, demyelination and death from EAE and high increases in NAD [7]. Nicotinic acid is rapidly converted to NAD in the brain within just 20 minutes [185]. High doses of oral nicotinic acid are well established in their ability to rescue pellagric dementia and to provide benefit in many cases of schizophrenia [129, 186, 187]. Thus either oral nicotinic acid or nicotinamide can readily provide therapeutic benefit in the CNS and likely MS as well. However, more research is needed to determine the optimal therapeutic concentration window, route, and frequency of administration.
We may expect several hours are needed from the time of administration of precursor until the actual endogenous synthesis of NAD in order to achieve maximal benefit. Nicotinic acid injected in the brain results in detectable synthesis of NAD within just 20 minutes [185] while increases in NAD synthesis in glial cell culture are detectable within just 2 hours [43]. Moreover, nicotinamide [188] or NAD [48] treatment provides immediate cytotrophic activity in acute time frames in a whole animal model of stroke. For the nicotinamide study the drug was applied after initiation of ischemic shock, while the other study involved application of NAD as an intranasal spray less than 20 minutes prior to transient focal ischemia. Nicotinic acid and nicotinamide riboside appear to be largely unexplored in the context of potential acute critical medicine scenarios.
Interestingly, it was previously determined that at least 8 hours were needed for NAD to delay Wallerian degeneration in a SIRT1 dependent manner [36]. Collectively this supports the notion that optimal cytotrophic activities are more likely to be achieved after giving the cell or animal time to respond at the transcriptional level through NAD-SIRT1-histone dependent pathways. When using NAD precursors this is likely to require several hours more time than the 8 hours seen using NAD on neuronal explants. Experiments performed using zebrafish larvae exposed to an otherwise lethal anoxic shock reveal that NAD precursors provide far greater protection after a 12 hour preincubation of NAD precursors than when performing a co-incubation of precursors simultaneous with ischemic shock (unpublished observations of Penberthy et al).
The most likely mechanism of action for nicotinamide in time ranges less than an hour may involve the direct enzymatic inhibition of PARP-1 mediated NAD depletion. Inhibition of PARP-1 enzyme activity is well known to protect against a wide array of otherwise lethal shock situations [113, 118], where the survival benefits of PARP-1 inhibition are so profound that entire companies have been created with this focus [189]. Neurodegeneration induces expression of NAD salvage pathway enzymes [27] as well which by themselves would use both the applied nicotinamide as well as the PARP-1 catalyzed reaction product (nicotinamide) to generate more NAD. Accordingly, PARP-1 inhibitors maintain otherwise depleted NAD levels after application of the stress. Thus, acute phase effects of nicotinamide treatment may involve inhibition of NAD depletion more so that complementation of NAD loss of function.
There are two primary factors promoting confusion regarding NAD precursors ability to function as a neuroprotectant. First, of all time is needed for the cell to make NAD. Nicotinamide can function in the short incubations, however, this is primarily in vitro, may not be physiologically relevant, and seems most likely to be working through inhibition of PARP-1 activity. Second, for neurons it seems that other cell types may be required for NAD precursors to exert their neuronal protect. Much more basic research is needed regarding glial versus neuron specific NAD conversion from precursors. Ultimately, NAD precursors may be pharmacologically superior for delivering NAD. Otherwise, for MS it may be best to provide pharmacologic doses of NAD precursors as a means of maintaining elevated NAD levels for preventing any NAD depletion, which could be connected to relapses of MS attacks. Given the rapid clearance of NAD precursors in general it seems best to repeatedly administer NAD precursors towards maintaining elevated NAD levels with the ultimate goal of tightening the plethora of NAD-dependent cellular chemistries potentially needed for halting the immune system and stimulating recovery.
As discussed in greater detail below, there was a wide range of recovery rates when patients were first treated with niacin during the pellagra epidemics of the 1940's [5]. This ranged from individuals that recovered from catatonic conditions literally overnight to others that required a month of niacin application. Still others had suffered irreversible damage and could not be rescued. Abram Hoffer observed similar response phenomena in the treatment of the neurodegenerative disease of schizophrenia [187, 190]. The general idea had been to attempt to keep NAD levels elevated. Accordingly, high doses of niacin were administered by ingesting gram of niacin up to 5 times a day. It seems likely that this approach to persistently elevating NAD may similarly serve to cultivate remyelination. Administration of NAD precursor administration may be necessary to perform similar repeated administrations towards maintaining persistently elevated levels of NAD to facilitate MS recovery.
8.1.2 Nicotinic Acid
Administration of pharmacologic doses of NA is able to raise high-density lipoprotein (HDL) more than any known pharmaceutical while simultaneously decreasing triglycerides, VLDL, and cholesterol. HDL is anti-inflammatory, antithrombogenic, where low levels of HDL is considered one of the greatest indicators of cardiovascular disease [191, 192]. With the statins as the most commercially successful class of pharmaceuticals in the world, yet nicotinic acid still outperforms them in spite of unparalleled investments to develop something better, this is truly remarkable. Thus, nicotinic acid is the gold standard for correcting dyslipidemia on the whole. Given the ever increasing appreciation of the critical function of lipids in CNS regeneration and function [193-196], there are likely to be many more potential therapeutically beneficial applications to pharmacological nicotinic acid administration that have yet to be characterized. Nicotinic acid is distinguished among the NAD precursors for several reasons of which we will briefly cover here.
Nicotinic acid appears to be preferred over nicotinamide as a NAD precursor primarily when it is applied at the high concentrations [43, 197, 198]. Hara et al. [199], discovered that NAPRT is not inhibited by NAD. By contrast, NAMPT is inhibited by NAD (Fig. 1). Nicotinic acid is the more preferred substrate in evolution based on experimental radioactive labeling [43, 197, 198] and comparative genomic studies examining to date (presentation by Mathias Ziegler at PARP 2008 meeting, Tucson, Arizona, U.S.A.). In glia, nicotinic acid provides greater levels of NAD biosynthesis per mole than nicotinamide or tryptophan by 200 and 500 fold respectively [43]. This also appears to be true for glia. However, neurons are distinctively inefficient in their ability to convert dietary NAD precursors to NAD [27]. As described above this may be another biosynthetic job for glia like that of cholesterol biosynthesis.
With a healthy diet the human body routinely keeps the lipid profile within acceptable levels. However, with a high fat diet the lipid profile is skewed. Under these conditions the pharmacological dosage of nicotinic acid is known to correct HDL, triglycerides, cholesterol, and VLDL while minimal vitamin level concentrations are insufficient. This is an active process that is not commonly conceptualized as a prevention of a deficiency, but rather as a drug-like approach. The process by which nicotinic acid does this is likely to involve not only boosting of NAD levels, but also other signaling pathways through the high affinity nicotinic acid receptor GPR109a described in greater detail below. Ultimately, target genes affected by nicotinic acid administration include CETP [200, 201], CD36 [202], ABCA1 [202], LXR [203], and many others [204]. Attempts to out-perform nicotinic acid for correcting lipodystrophy via CETP inhibitors have been fraught with failure due to increased mortality [205-207]. Thus, the mechanisms of action for nicotinic acid continue to be an active area of basic research investigation.
Secondly, nicotinic acid binds to a high affinity receptor (GPR109a) that is expressed specifically on professional APCs including macrophages, dendritic cells, and microglia. GPR109a is also highly expressed in adipocytes, neutrophils, and a variety of other cells. GPR109a is responsible for nicotinic acid-mediated inhibition of lipolysis based on murine knockout studies [208] and is likely to be involved in nicotinic acid mediated elevation of HDL as well, an activity not observed with high dose nicotinamide therapy.
Nicotinic acid activation of GPR109a causes a massive production of the cytotrophic prostaglandin PGD2 in professional APCs. PGD2 is converted to PGJ2, which then activates PPAR gamma [202, 209]. This aspect of the nicotinic acid mechanism of action resembles that of thiazolidinediones for correcting lipodystrophy. PGJ2 itself has been shown to suppress microglial activation [210], ameliorate EAE pathogenesis [210-212], and is a general cytotrophic agent protecting against endotoxic shock [213]. The niacin associated flush appears to be mediated via the massive production of prostaglandins PGD2 and PGE2, which via G-PCR signaling through DP1 and EP2 respectively mediate the flush response [214].
The current best guess for an endogenous molecular activator of GPR109a is believed to be the ketone body, beta-hydroxybutyrate [215]. Elevated during starvation, beta-hydroxybutyrate activation of GPR109a signals to inhibit lipolysis, thus conserving essential energy supplies. Interestingly, beta-hydroxybutyrate is potently neuroprotective, where the potential for dependence through activation of GPR109a is unknown. Interestingly, PGE2 is used to promote differentiation of monocytes to toleragenic IDO expressing cells [216]. Interferon is known to induce expression of GPR109a and IDO, where IDO is known to be required for many interferon-dependent activities. Thus, it is possible that the GPR109a activation may exert toleragenic processes. GPR109a almost certainly serves important functions in the immune system and it is readily activated using nicotinic acid. More research is needed to examine the potential of nicotinic acid-mediated activation of GPR109a for stimulating immunotolerance and whether this effect is beneficial controlling autoimmune aspects MS pathogenesis.
Glial cells seem likely to control delivery of NAD to neurons. The persistent increases in NAD of WldS seem to help against acute attacks of MS causing neurodegeneration (based on EAE model) but they exacerbate pathology in chronic relapsing based on comparative EAE and TMEV-IDD animal model research respectively. By contrast, it makes best sense to consider pharmacological administration of NAD precursors to give glia the control of NAD delivery or NAD restriction with respect to neurons in MS. Increases in NAD(P(H)) concentrations ultimately has the potential to tighten catabolic and anabolic chemical homeostasis. This seems likely to be particularly important for myelination, a process where greater than 80% of myelin is known to be synthesized phospholipid. Accordingly administration of pharmacological doses of nicotinic acid should be considered for the correcting dysregulated NAD homeostasis in MS.
Most curiously, while nicotinic acid is well known for providing tremendous relief in animal models of atherosclerosis, it can actually increase the levels of a biomarker known to be directly correlated with increased risk of atheroslerosis (homocysteine) in rat studies as well as lowering of pyridoxyl phosphate (vitamin B6) and cobalamin (vitamin B12) [217]. Increases in plasma homocysteine have also been observed in patients treated with high doses of niacin [218]. Interestingly, the additional application of pyridoxyl phosphate [217] or methionine [219] would correct the elevation in homocysteine while still exerting the positive effects on lipid profile. These observations suggest that once the body has enough NAD to perform most of its desired chemistry, then one the next co-factors to become rate-limiting for performing of endogenous biochemistry is pyridoxyl phosphate (vitamin B6) or cobalamin (vitamin B12). This makes sense given that NAD is perhaps followed by pyridoxyl phosphate in the shear total number of known reactions requiring these co-factors respectively. Furthermore, it is well known that deficiencies of cobalamin lead to demyelination and such deficiencies have on occasion been observed in MS (reviews [60, 220, 221]). Thus, in order to maintain optimal NAD function it seems ideal to include increased administration of pyridoxyl phosphate and cobalamin.
8.1.3. Nicotinamide
In contrast to nicotinic acid, nicotinamide has no appreciable binding affinity for GPR109a, and accordingly does not cause a skin flush response. Nicotinamide is incapable of exerting the profound benefits correcting lipodystrophy. Sometimes nicotinamide is referred to as no-flush niacin, however this can be misleading as it suggests that it may be useful in correcting bad lipid profiles. More basic research has been performed using nicotinamide than nicotinic acid for many years. Still, there unique aspects of nicotinamide activated signal transduction continue to be realized. This includes nicotinamide signaling through modification of nuclear receptor chromatin remodeling independent of SIRT1 and PARP-1 [222] as well as Akt/Protein kinase B activating pathways [96, 223].
Nicotinamide when administered daily causes a dramatic protection against demyelination and neurological signs in MOG-induced EAE [7]. This is accompanied by a persistent elevation in whole body NAD levels, where untreated mice suffered severe depletion of NAD levels. It is essential that the nicotinamide be administered with regularity in order to maintain the elevated levels of NAD. At the highest nicotinamide concentrations it prevented death and was capable of delaying onset of behavioral deficits from 8 days to 26 days. No adverse reactions were noted upon administration of nicotinamide, which is on the whole a highly safe molecule even when administered at high doses.
8.1.4. Nicotinamide Riboside
Nicotinamide riboside was only recently discovered in 2004 ([224], reviewed in [1]). The other vitamin B3 NAD precursors nicotinic acid and nicotinamide were discovered roughly 70 years ago now. The mandatory fortification of bread using niacin in the 1940's was a tremendous public health success story and high dose niacin therapy remains the most effective therapeutic for correcting dyslipidemia in many respects. Thus there is a long history of clinical use for high doses of nicotinic acid and nicotinamide. By contrast, nothing is known regarding the utility of nicotinamide riboside in the clinic.
Of potentially great clinical therapeutic significance nicotinamide riboside is distinguished as the only NAD precursor capable of providing NAD directly to neurons [1] and delaying Wallerian degeneration assay [27]. Furthermore, the induction of the initiating NAMR utilizing enzyme NRK2 was more dramatic than any other enzyme after sciatic nerve transection thus suggesting an important function for NAMR and the potential existence of another yet to be characterized salvage pathway [27]. This tremendous induction or NRK2 after razor axotomy suggests that there is an mechanism of regulation inherent to the neuron itself. Furthermore, the apparent dependence of neurons on glia for NAD biosynthesis from NAD precursors supports a complementary medical approach may be therapeutically beneficial for treating MS.
More clinical research is needed given the tremendous potential therapeutic benefit of nicotinamide riboside therapy for treating neurodegenerative diseases. In particular, it would be most interesting to determine the effects of nicotinamide riboside administration in the animal models of MS, acute (EAE) and chronic (TMEV). Given that nicotinamide riboside delays neurodegeneration similar to the WldS mouse, perhaps there will be undesirable activities in the TMEV-IDD model similar to that seen in the WldS background [98]. However, since there is a neuronal inherent regulatory mechanism of action regulating NRK2 expression this seems unlikely. Thus we may expect therapeutic benefits from nicotinamide riboside administration in both MS models. Perhaps, the effect is more immediately beneficial to the neuron since glia are not apparently required for synthesis of NAD from nicotinamide riboside.
8.1.5. Nicotinamide Mononucleotide
Nicotinamide mononucleotide (NMN) is distinguished in that the enzymes that make use of NMN are intracellular, while the NMN generating enzyme (NAMPT/PBEF/Visfatin) is also expressed in the sera where it is being considered as a diagnostic biomarker of inflammatory disease [225]. Accordingly, by applying NMN, cells can take up NMN to generate NAD using the enzymes NMNAT1, NMNAT2, or NMNAT3. Administration of NMN bypasses the NAMPT/PBEF/Visfatin step, to allow the cells to make NAD as desired. Already beneficial phenomena have been demonstrated for this precursor in the context of diabetes [226]. Furthermore NMN has been shown to delay Wallerian degeneration [27], an activity that is well correlated with amelioration from EAE clinical symptoms [37, 97, 98]. All indications are that NMN has great therapeutic potential in the clinic; however, to date nothing is known regarding its clinical potential, as it has never been tested in man.
8.2. IDO Induction
Approaches to targeting IDO for increasing immunotolerance are extensively described elsewhere [142]. Thus, we only briefly describe a novel approach involving the first clinically available histone deacetylase (HDAC) inhibitor suberoylanilide hydroxamic acid (SAHA; Figs. 5, 6; reviewed [227]). SAHA was recently determined to increase IDO production, decrease cytokine production, decrease T cell proliferation, and to ultimately provide immunosuppression in the context of transplantation [228]. These effects were genetically determined to be dependent on increased IDO activity. SAHA is an inhibitor that works against the HDACs (class I and II) with constitutive activity, but not the dynamic NAD-dependent class III Sirtuins HDACs. SAHA is known to increase expression of neuronal SIRT1 [229]. Collectively, this suggests the potential existence of a HDAC rescuing SIRT loop whereby the cell responds to loss of constitutive HDAC activity by increasing SIRT1 expression, IDO activity, and therefore likely increasing NAD which may then increase SIRT1 activity (Fig. 6). This is a unique example connecting increased IDO activity with increased SIRT1. Ultimately, SAHA-mediated IDO-NAD-SIRT1 pathways may provide benefits in the context of MS.
Fig. (6).
Inhibition of the constitutive HDACs leads to increased transcriptional expression of SIRT1 and IDO. The increased IDO activity can increase NAD levels which can then further increase SIRT1 activity. Ultimately, SAHA increases immunotolerance. Thus SAHA may have potential in the context of controlling autoreactive pathogenesis in MS.
8.3. SIRT1 Activation
Activation of SIRT1 using resveratrol/SRT501 [104, 230], or nicotinamide riboside/SRT647 [104] has been shown to minimize the clinical signs and inflammatory response in EAE, while treatment with the SIRT1 inhibitor Sirtinol would prevent these beneficial effects [104]. Here we briefly review the current state of Sirtuin knowledge with emphasis on potential targeting in the context of MS. The Sirtuins are histone-deacetylating enzymes that exert global changes in chromatin structure in response to changes in either the NAD concentration or the redox status of the cell as measured by NAD/NADH ratio. Whereas most other deacetylases are considered to possess constitutive activity that is generally heritable, the Sirtuins are distinguished by their ability respond to metabolic fluctuations in NAD levels. One particularly exciting prospect from a pharmacological standpoint is the following. While we cannot change our genetics, we can change some of our epigenetics. Most epigenetic modifications are heritable and not alterable either. However, as the defining class III histone deacetylases, the Sirtuins can be targeted by pharmacologically modifying NAD levels. Functionally, the Sirtuins are extremely highly conserved evolution. They serve pivotal roles exerting control of metabolic functions in response to nutrient availability as observed in all examined organisms ranging from man to bacteria [231] !
In man there are seven different Sirtuins. SIRT1, SIRT6, and SIRT7 are primarily nuclear. SIRT3, SIRT4, and SIRT5 are primarily mitochondrial. SIRT2 is primarily cytosolic [232]. However, SIRT3 is also detectable in the nucleus and is the only Sirtuin family member clearly implicated in controlling human lifespan to date [233], while SIRT1 is also observed at lower levels in the cytoplasm [234]. As the founding member of the Sirtuin family, SIRT1 is by far the most studied isoform to date.
SIRT3 continues to emerge as a tremendously interesting Sirtuin family member. It is primarily expressed in the mitochondria where it functions in controlling both total cellular ATP levels [119] and global mitochondrial acetylation [120]. SIRT3 activity can protect against stress-induced cardiomyocyte cell death [233]. SIRT3 is predominately expressed in the mitochondria and has also been detected in the nucleus [233, 235, 236], however, other focused studies suggest SIRT3 is exclusively mitochondrial [232, 237]. In either case, by increasing SIRT3 expression in an NAD dependent fashion we expect to the level of ATP produced by the mitochondria to be increased based on previous studies [119]. The classic SIRT1 activator resveratrol is now known to also increase SIRT3 expression [121]. Accordingly it stands to reason that the ability of high dose nicotinamide to provide NAD and subsequently ATP may involve the NAD mediated activation of SIRT3 leading to sustained levels of ATP. Given that nicotinamide when applied at high concentrations is protective against Wallerian degeneration and EAE, greater attention should be directed at considering nicotinamide in the context of clinical MS.
Originally, the Sirtuins came into the limelight when yeast sir2 was identified as playing central roles in controlling caloric restriction (CR) mediated increases in lifespan [238]. CR represents 60% of normal caloric intake, where this is the only method that has consistently been shown to cause an increase in organismal lifespan. Accordingly large small molecule screens were performed to identify molecular activators of the human ortholog SIRT1. This lead to the realization that resveratrol is a potent activator of SIRT1 [239]. More recently SIRT1 function has been shown to be required for CR mediated increases in mammalian lifespan [16]. Further studies revealed SIRT1 deficient mice develop an autoimmune disease [240]. Since this realization, resveratrol has been verified for its ability to increase lifespan under normal caloric conditions in yeast, C. elegans, Drosophila, a teleost, and a mouse (for a review [241]). Resveratrol is a polyphenolic molecule produced by plants in response to pathogenic infection (a phytoallexin) that is abundant in grape skin and wine. Some have suggested that it is the primary reason explaining the French paradox [242, 243].
It should be mentioned that man actually empirically discovered resveratrol's benefits in the form of many plant-based medicines over 5,000 years ago [244]. Research devoted to increasing the molecular basis of resveratrol's mechanism of action has exploded since the discovery of its tremendous promise as a cancer therapeutic in particular [241]. With this increase in research it has become evident that there are many molecular targets of action for resveratrol application, where some neuronal growth stimulatory processes have been shown to be independent of SIRT1 activation in cell culture [245].
Resveratrol clearly activates SIRT1 [239] and SIRT2 [105], while increasing the levels of SIRT3 [121] - a master regulator of cellular ATP levels [119] and mitochondrial acetylation [120]. Thus, resveratrol may be a general positive regulator of Sirtuin activities.
Interestingly, SIRT2 is enriched in oligodendrocytes and its expression correlates directly with myelination based on analysis of proteolipid protein (PLP) deficient conditions [246]. Enriched in neurons, HDAC6 may seve complementary functions as a deacetylator of alpha tubulin [247]. While SIRT2 is generally cytosolic, it does in fact deacetylate histones during M phase to regulate mitosis [248]. Much more research is needed to elucidate the potential roles of NAD-dependent SIRT2 activity in controlling neural stem cell proliferation and remyelination.
However, several resveratrol-mediated phenomena are independent of SIRT1 and in fact the mechanisms of action for resveratrol are many (for an extensive book on resveratrol [244]). Resveratrol strongly promotes neurite outgrowth in Neuro2a cells in a SIRT1 independent but AMPK-dependent fashion [245]. AMPK can also be activated by calcium signaling pathways that are independent of AMP:ATP ratios and this appears to be the mechanism by which resveratrol works. Whether this may be through o-acetyl-ADP-ribose produced from SIRT1 activation or somehow ADP cyclase dependent processes, is unknown. Several polyphenols are also now known to activate AMPK [249]. This ability of resveratrol to promote neurite outgrowth may be of particular significance in the context of recovery from MS. Resveratrol also directly inhibits fatty acid synthase [250], promotes adipocyte apoptosis [121], and has been shown to promote fat loss in vertebrates [164]. Recently, it was determined that SIRT3 functions in the mitochondria as a major regulator of ATP levels in the mitochondria [119], while resveratrol is now known to cause increases in SIRT3 expression in adipocytes [121].
Recently, SIRT1 was determined to play a critical role in the fate specification decision for neural stem cells (NSCs; [251, 252]). Under reducing conditions, SIRT1 specificies neuronal differentiation, while under oxidative conditions SIRT1 specificies glial differentiation. One rationale for such decisions would likely be that the glia, as a supportive and biochemically superior cell, can then be used to deal with the oxidative stress which itself may be more likely to prevent neurogenesis. Accordingly, this presents interesting possibilities when one considers pathways from nicotinic acid. Application of high doses nicotinic acid is known to increases NADPH, and reduced glutathione, thus leading to more reducing conditions, while also decreasing angiotensin induced inflammatory R(O/N)S production and nuclear factor- κB (NF-κB) activity [253]. Further, applying NAD precursors increases NAD levels. Collectively, this may have the effect of providing a favorable reducing environment while simultaneously activating SIRT1 (Fig. 7). Thus this has the potential to favor neuronal regeneration. Application of niacin to the pellagric mental hospitals of the 1940s resulted in recoveries that were immediately obvious in many patients that had previously been listless, semi-catatonic, apathetic, and lacking in appetite [5]. The most common pathology observed in the brains of pellagrins involves neurodegeneration described as chromatolysis [147]. The potential for using nicotinic acid or other NAD precursors for stimulating neuronal regeneration is unclear.
Fig. (7).
Pharmacological nicotinic acid administration is predicted to favor neuronal genesis over gliogenesis due to the combination of SIRT1 activation combined and increased reducing conditions. SIRT1 controls redox mediated fate specification from neural stem cells. Pharmacological application of nicotinic acid increases NAD which activates SIRT1 (1) while also promoting reducing conditions by increased NADPH and GSH (2). Reducing conditions, being less stressful, allow neuronal specification instead of support cells. Otherwise under oxidizing conditions supporting glial cells are likely to be more immediately needed in order to correct the chemical imbalance, thus potentially favoring pathogenic gliosis. As discussed in section 2.1, minimizing gliosis can help foster remyelination. Thus the dual activity that nicotinic acid exerts by providing both NAD and reducing conditions is expected to respectively increase SIRT1 activity and the neuronal fat specification from stem cells that is likely to be needed for a complete and sustained recovery from MS attacks.
Another cellular strategy commonly considered for treating MS involves attempts to minimize activation of microglia [97]. SIRT1 is the focus of strategies to halt microglial activation as well through SIRT1 mediated activation of the transcription factor Liver X Receptor (LXR) or through inhibition of NF-κB as well (for a review [254]). Thus, SIRT1 activators persist as an increasingly area of investment for pharmacological drug discovery.
8.4. PARP-1 Inhibition
8.4.1 PARP-1 Mechanism of Action in Multiple Sclerosis
Poly(ADP)ribose is formed in astrocytes, microglia, lymph nodes, and neurons in the murine EAE model of MS [9, 146], while it has not been examined in the TMEV model. Correspondingly, NAD along with ATP are both rapidly depleted in axons upon physical degeneration [37], while complimentary administration using NAD [36] or the immediate biosynthetic precursors [27] dramatically delay neurodegeneration. Removal of PARP-1 activity by genetic or pharmacological [9, 146, 255-261] significantly and repeatedly ameliorates EAE induced demyelination and leukocyte infiltration in animal models of MS (for a review [262]).
Application of the NAD precursor nicotinamide exerts a profound benefit in preventing EAE-induced demyelination [7]. Similar to the EAE resistant WldS mouse [37, 97, 98], inhibition of PARP-1 also delays axonal degeneration [36]. Collectively the sum data of all of these results support the simplest explanation for PARP-1 inhibitor protective effect as involving the sustained conservation and maintenance of NAD levels.
While the exact etiology of MS is unknown, there are immune components to the pathogenesis that involve generation of reactive oxygen species (ROS). ROS is known to play a role in disruption of the brain barrier, phagocytosis, degradation of myelin, and neurodegeneration particularly arising from microglia [263, 264]. Similarly NAD-depletion occurs after hydrogen peroxide treatment in macrophages and lymphocytes [265]. Endogenous hydrogen peroxide can be produced locally by immune system cells via a two step reactions process catalyzed first by NADPH oxidase (encoded by NOX1-5; for a review see [266]) which produces superoxide, followed by superoxide dismutase, which produces hydrogen peroxide. The hydrogen peroxide is then converted to inert molecules via reactions catalyzed by glutathione peroxidase followed by glutathione reductase. Alternatively, myeloperoxidase (MPO) can use hydrogen peroxide to produce hypochlorous acid. MPO has been observed in microglia and is now being considered as a target for neurodegenerative diseases [267]. Inhibition of NADPH oxidase or over-expression of superoxide dismutase can inhibit microglial activation [268]. Most interestingly, NAD deficiencies lead to increased activation of NADPH oxidase, decreased GSH levels, and ultimately genomic DNA damage which causes dramatic quantifiable reductions of NAD through PARP-1 activation [269]. Application of nicotinamide reverses this process. Collectively, NAD both prevents free radical production and the associated often lethal PARP-1 mediated NAD depletion. By minimizing ROS production, NAD precursors may help to attenuate MS pathogenesis.
8.4.2. Minocycline and PJ-34
Minocycline has been appreciated as an exceptionally potent bacteristatic member of the tetracycline family of drugs. Similar to niacin, minocycline has been used as an antibiotic for over 50 years. More recently however, minocycline has gained increasing recognition as a potent neuroprotectant in animal models of MS, Parkinson's Disease, stroke, Amyotrophic Lateral Sclerosis (for an excellent review [270]). Interestingly, NAD or NAD target proteins (e.g. SIRT1 through PGC1 alpha mitogenesis; for a review [271]) have been shown to provide relief in all of these models as well. This does not come without risk for adverse drug reactions including a slight risk for drug-induced Systemic Lupus Erythematosus [272, 273].
For many years the mechanism of action for minocycline neuroprotection was unknown. However, in 2006 Alano et al. revealed that minocycline is a nanomolar affinity PARP-1 inhibitor capable of protecting neurons from an otherwise lethal chemical alkylation mutagenic stress [117]. PAR formation was reduced and NAD levels were preserved by minocycline application. Previously, the cost of purchase for a water-soluble nanomolar affinity PARP-1 inhibitor such as PJ-34 was 1,000 times higher. Most importantly, the Alano study provided a long needed mechanistic explanation of the distinguishing properties of this distinctive antibiotic.
Minocycline provides protection in EAE mediated pathogenesis [256-258, 262] and is currently being tested in clinical trials of multiple sclerosis [255]. Whether or not this approach will prove to be so beneficial in the chronic inflammatory MS model provided by TMEV-IDD remains to be determined. Minocycline is not without adverse side effects. Minocycline drug-induced lupus has clearly been observed previously [274, 275]. Thus again, it is possible that the enforced prevention of NAD depletion may come with the risk for adverse immune system effects. Similar to the application of NAD itself [48, 173], minocycline provides tremendous protection from focal ischemia [276, 277]. Further, this was shown to involve a marked reduction in microglia activation [277].
Early on basic PARP-1 inhibitors were determined to be capable of delaying onset of clinical symptoms in EAE models [9, 261]. PARP-1 inhibitors were determined to reduce expression of inflammatory cytokine production in the CNS [9]. PJ-34 is a nanomolar affinity PARP-1 inhibitor with a long history. In EAE it has been shown to delay EAE onset of pathogenesis and shift the reportoire of cytokines from pro-inflammatory Th1 to more anti-inflammatory Th2 profiles [259]. TNFα, IFNγ, and iNOS expression levels were reduced in the context of PJ34 treatment.
The association between minocycline usage and drug-induced lupus would seem to underscore the importance of NAD depletion as a mechanism for limiting the autoimmune response, where the enforced inhibition of PARP-1 mediated NAD depletion with minocycline may apparently on occasion be involved in promoting hyperproliferation of the immune system with the terrible effect of promoting Systemic Lupus Erythematotosus (SLE [272, 273]). Others believe that minocycline induced SLE is actually a low risk [278]. Accordingly, it would seem that NAD depletion is a mechanism for competing for essential resources that is highly conserved in evolution. It is used in the complex metazoan immune system to limit proliferation of autoreactive T cells. This is most clearly defined with respect to IDO dependent pathways and is increasingly being appreciated for CD38 dependent pathways, both working to promote immunotolerance. The function of PARP-1 in immunotolerance is less explored to date where the vast bulk of PARP-1 research focus has been on cancer. Recently however, the role of PARP-1 and related family members in immune function are beginning to increase, where steroidal immunosuppression for example may require PARP-1 activity in order to promote T cell apoptosis [279]. Pharmacologic analysis suggests that inhibition of PARP-1 may promote the production of toleragenic dendritic cells, and thus is under consideration in host-versus graft disease and transplantation settings [280]. Another experimentally based mechanism proposed by Grader-Beck et al. suggests that PARP-1 may generate PAR antigen that promotes SLE, a disease characterized by anti-nuclear autoreactivity [281]. In this model they further suggest that accumulated PAR may promote immune induced apoptotic events. Finally inhibition or genetic deletion of PARP-1 prevents activation induced ADP ribosylation of nuclear factor of activated T cells, thus causing immunosuppression [282], where the target genes of PARP-1 in T cells are now being elucidated [283].
8.4.3. PARP-1 and Wallerian Degeneration
The ability of WldS to prevent loss of NAD while not overtly causing any obviously measurable increase in basal NAD levels is typical of what is seen when PARP-1 inhibitors are used. PARP-1 inhibitors prevent stress-induced depletion of NAD and associated loss of ATP [9, 109, 162, 265, 284]). In the original paper that elucidated the role of NAD-SIRT1 in the WldS phenotype, PARP-1 inhibitors were tested and in fact they did provide noticeable delay of Wallerian degeneration on their own [36]. However, the neuroprotective effects were more pronounced when activators of SIRT1 were applied. This included the direct application of NAD itself, while subsequent studies revealed that immediate precursors to NAD could also provide neuroprotection, where neither nicotinic acid nor nicotinamide were able to provide significant protection [27].
8.5. CD38 Inhibition with 2,2'-Dihydroxyazobenzene/Dyhydroxybenzene
Attempts are underway to consider CD38 inhibitors for their potential use in controlling inflammation or at least as molecular probes for basic research. Most recently 2,2'-dihydroxyazobenzene/2,2'hydroxybenzene (DHAB/DAB; Fig. 5) was realized to perform well as an inhibitor of the second phase calcium response in a rat model of cardiac hypertrophy (Cardiovascular Research Inhibition of ADP-ribosyl cyclase attenuates angiotensin II-induced cardiac hypertrophy Gul et al). In these experiments, angiotensin II (AII) treatment alone caused an initial release of calcium stores via IP3 signaling that was followed by a a sustained release of intracellular calcium via the CD38 catalyzed product, cADPR. Accordingly, inhibition of CD38 prevented the secondary sustained release of calcium, thus resulting in prevention of cardiac hypertrophy.
AII induced cardiomyocyte hypertrophy and cell death can be prevented by preserving NAD supplies through PARP-1 inhibition [111] or via increasing expression of NAD-dependent Sirtuins [112, 233]. Inhibition of CD38 would be expected to prevent depletion by CD38 itself as well as any other NAD depletion that may arise secondary to the sustained release of intracellular calcium stores through CD38 mediated generation of the second messengers cADPR, NAADP, and ADPR. However, NAD levels were not examined in this study. So it is unknown whether the desirable activities seen with the CD38 inhibitor DAB may in part be due to maintenance of NAD levels.
CD38 deficient mice challenged by an enforced high fat induced obesity model resistant weight gain through a pathway starting from dramatic increases in basal NAD levels that then activate SIRT1, which leads to increased mitochondrial biogenesis [155]. This strongly suggests that maintenance of NAD levels may be a major mechanism of action for DAB. Similarly, application of NAD itself has recently been proven to be effective at promoting fat loss in zebrafish larvae [164]. Therefore, one obvious application for DAB would be in the treatment of obesity. However, it is quite possible that DAB may also be most useful in the treatment of MS.
As described in greater detail above, the CD38 knockout mouse revealed that CD38 serves critical functions in both innate and acquired immune systems [152, 157]. Constitutively expressed in hematopoietic stem cells [163], CD38 is required for chemotaxis of professional APCs [152] and activation of microglia [138]. Thus, inhibition of CD38 with DAB may exert desirable effects in preventing inflammation and microglial activation. Since azo bonds are generally toxic, it will be especially necessary to examine dose dependence and potential effects on secondary infections arising from a weakened immune system.
Given that CD38 deficiency causes a persistent elevation of NAD, it will be most interesting to determine how this affects multiple sclerosis. As described in section 4, similar persistent maintenance of NAD levels seen in the WldS mouse actually exacerbates pathogenesis in TMEV-IDD models of MS while decreasing pathogenesis in EAE models of MS [98]. If the effect is persistent in a manner resembling the WldS effect, then we may see a similar delay of EAE mediated neurodegeneration but exacerbation in the TMEV-IDD model. If however, CD38 is required for the lymphocyte infiltration seen in TMEV-IDD, then under CD38 deficient TMEV-IDD conditions pathogenesis may be reduced. Finally, DAB should be examined in both of these contexts at a variety of concentrations. These kinds of experiments have the potential to dramatically help increase our understanding of the potential for developing CD38 inhibitors as a potential therapeutic approach.
8.6. Combinatorial Approaches and the Need for a Higher Throughput Animal Model
Given that EAE pathogenesis can be ameliorated by inhibition of PARP-1 mediated NAD depletion [9, 146, 255-261], activation of NAD-dependent Sirtuins [104, 230], complementation of NAD deficiencies [7], or increased IDO activity [31-34, 130] it stands to reason that we may see enhanced therapeutic benefits if combinatorial approaches are incorporated.
However, the animal models currently used for MS research for examining small molecule activities providing therapeutically beneficial activities is severely limited by the amount of time and investment needed to measure small molecule activity. Most studies only examine one or two different small molecules. Studies that making direct quantitative comparisons of different molecules for their ability to stave off otherwise progressive EAE have never been done before.
One rising animal model currently being developed involves using the most primitive vertebrate genetic model organism, the zebrafish Danio rerio. Transparent as a larva, transgenic zebrafish designed to express GFP in oligodendrocytes [285] are currently being used to identify small molecules that may stimulate myelination. Already this organism is being exploited towards making tremendous strides in identifying the genes most critical to proper developmental myelination [286]. These genes will be surely be considered some day as candidate genes potentially responsible for causing MS.
Of likely greater clinical significance however, the zebrafish model stands poised to be exploited for the identification of the most effective molecular therapeutics for providing relief against multiple sclerosis. Zebrafish possess have all of the standard Th1 derived cytokines and lymphocytes that are commonly associated with multiple sclerosis. Zebrafish are amenable to tracking large numbers of animals for quantifying EAE induced demyelination, neurodegeneration, and concomitant paralysis measured by tracking their movement [287]. Thus, adult zebrafish are currently being developed as EAE models of MS towards increasing the rate of small molecule drug discovery as well hopefully increasing the quantifiable nature of these animal model based assays (personal communication).
9. CELLULAR STRATEGIES
9.1. Interaction Between Myelin Specific Autoimmunity and Axonal Degeneration
In the CNS, regeneration of the neurons and their processes does not occur in general. Remyelination is very slow, if at all. In most clinical and experimental treatment of MS and its animal models, even though the treatments are effective to suppress clinical signs, disease exacerbation is often seen once the treatment is stopped [288]. Then, why do MS patients need early treatment? It is most important to understand that the delay of disease progression and the prolonged remission period by the drug treatment are important for the quality of life of patients and likely for enabling any possible long term recovery process. In addition, we would like to propose that early intervention can contribute to intervene with self-perpetuating vicious cycles made by interactions between autoimmune cells, microglia, axons, and myelin sheaths. We will discuss three such interactions shown in Fig. (8).
Fig. (8).
Self-perpetuating neurodegeneration cycles made by interactions between immune cells, microglia, axons and myelin sheaths. a) Anti-myelin immunity cause primary demyelination, leading to axonal degeneration (lesion development from the inside myelin to the outside axon, Outside-In model). Then, degenerated axons activates microglia and induces oligodendrocyte apoptosis, resulting in secondary demyelination (lesion development from the inside axon to the outside myelin, Inside-Out model). Myelin debris would be taken up by microglia and macrophages, which present myelin antigen to autoimmune T cells; this will lead to the second cascade reaction. Wlds mutation prevents axonal degeneration, interfering with a vicious cycle made by the Outside-In and Inside-Out models. b) Axons and myelin require each other in their development and maintenance. Myelin protein abnormality results in axonal degeneration, in which SIRT2 may play a role. Axonal degeneration leads to apoptosis of oligodendrocytes and downregulation of myelin protein mRNA expression. c) Microglia activation and axonal degeneration can make a self-perpetuating cycle. Activated microglia produce proinflammatory factors, and damage axons. Axonal degeneration can activate microglia, including upregulation of MHC class II molecules on the surface of microglia. CD200-CD200R interaction on the neuron-microglia may play a role in the cycle.
In EAE, both wild-type B6 and WldS mice were primed by MOG and developed the same levels of anti-myelin autoimmune responses. However, later on, enhancement of autoimmune responses was seen only in B6 mice. The enhanced myelin-specific immune response in B6 mice with EAE can be explained in the following scenario (Fig. 8a). After induction of EAE, anti-MOG autoimmune responses initially attack the myelin sheath, and axons are injured by the extreme inflammation secondarily (here, the lesion develops from the outside myelin to the inside axon, Outside-In model) [289]. This results in degeneration of severed axons (Wallerian degeneration), leading to microglia activation and oligodendrocyte apoptosis (secondary demyelination) along the degenerated tract, distant from the original lesion [290, 291]. This time, pathology develops from the inside axon to the outside myelin (Inside-Out model). Degenerated myelin and oligodendrocytes would then be phagocytosed by activated macrophages and microglia, leading to myelin antigen presentation in the CNS, which enhances (or induces) myelin-specific autoimmune responses. The enhanced myelin specific autoimmune responses can attack the myelin from the outside, and trigger a second cascade of inflammation. In contrast, although WldS mice mount a similar degree of anti-myelin autoimmune responses at 2 weeks p.i., a lack (or a delay) of Wallerian degeneration prevents the inflammatory cascade, resulting in no enhancement of anti-myelin immune responses or disease progression.
9.2. Axon-Myelin Interaction
Axons and oligodendrocytes can profoundly affect each other's development and maintenance (Fig. 8b). In the case of axonal influences upon oligodendrocytes, axonal loss results in degeneration of the myelin sheath. In clinical and experimental spinal cord injury and the optic nerve during the developmental period, oligodendrocyte apoptosis follows along the transected degenerated axons [84, 292-294]. Interestingly, Lassmann et al. [295] described that some ultrastructural change in myelin degradation following optic nerve transection was similar to the one seen in patients with MS. Experimental optic nerve transection has significant effects on myelin protein mRNA expression in oligodendrocytes of optic nerve; a decrease in MBP and PLP mRNA expression and an increase in myelin-associated glycoprotein mRNA at 14 and 28 days after transaction [296].
Since axotomy also causes marked changes in the other glial population, including astrocytes, of the optic nerve, the alteration in myelin gene expression could ascribe to not only axonal loss but also astrocytosis and microgliosis. Nevertheless, this provides evidence that axons do influence myelin protein genes in oligodendrocytes. Similarly, stability of PLP mRNA has been shown to be decreased after axotomy in the PNS [297]. Although a decrease in myelin RNA has been demonstrated in TMEV infection (reviewed in [82]), damage in oligodendrocyte is thought to be a primary cause of down regulation of myelin RNA. However, as discussed above, myelin RNA can be down regulated secondarily to axonal damage.
Conversely, axonal preservation has been shown to require myelin proteins [289, 298]. Mouse mutants that lack expression of either PLP or 2',3'-cyclic nucleotide phosphodiesterase (CNP) have normal myelin sheaths, despite the fact that PLP and CNP are expressed exclusively in oligodendrocytes in the CNS [299]. However, aged PLP-null and CNP-null mice develop neurological signs, such as ataxia and hind limb paralysis, resulting from axonal degeneration. Human patients with a null mutation of the PLP1 gene develop axonopathy in the absence of major myelin abnormality. Interestingly, in PLP-null mice, the NAD-dependent deacetylase SIRT2, was virtually absent, while SIRT2 is expressed in oligodendrocytes in wild-type mice [246]. This suggests that SIRT2 may play a role in support of axons by oligodendrocytes, while SIRT2 has been suggested to play a critical modulating role in controlling morphological differentiation of oligodendrocyte precursors [300].
9.3. Microglia and Axon Interaction
Axonal (Wallerian) degeneration can induce local microglia activation, including up regulation of MHC class II molecules, without recruitment of peripheral lymphocytes (Fig. 8c) [85, 299, 301]. Block et al. [302] proposed that microglia activation and neuronal damage can lead to progressive neurodegeneration, regardless of the instigating stimuli. First, activated microglia initiates neuron damage by producing neurotoxic proinflammatory factors, including prostaglandin PGE2, IL-1β, TNFα and superoxide. Second, microglia can become activated in response to neuronal damage (reactive microgliosis), which is toxic to neighboring neurons, resulting in a perpetuating cycle of neuron damage and microglia activation [302]. Interaction between CD200 on neurons and CD200R on microglia may play a role in this cycle.
When microglia activation and axonal degeneration occur in hosts who have encephalitogenic T cells in the periphery, this can lead to lymphocytes recruitment at the site of axonal degeneration in the CNS. In both EAE and TMEV infection, axonal degeneration with microglia activation can target inflammatory lesions at the site of Wallerian degeneration [290, 291].
Since the above three interactions can make a vicious cycle, intervention of any step can theoretically stop the cycle, leading to disease remission. However, many steps, including apoptosis, axonal degeneration, and microglia activation, have physiological beneficial roles. For example, axonal degeneration can prevent spread of neurotropic virus in the CNS, and activated microglia is important for innate immunity. As we discussed in this review, treatments that modulate NAD pathways can influence many different steps and cell types. Thus, the effects of such experimental treatments require careful assessment, which is necessary for clinical application of the treatment in future therapeutic trial in human patients.
9.4. NAD Targets, NAD, and Stem Cells
Stem cells have become increasingly appreciated for their potential use in treating MS and are now being considered for use in clinical trials [303]. Enzymes involved in NAD biosynthesis (IDO), degradation with calcium signaling (CD38), and epigenetic modifications (SIRT1) have all now been connected with stem cells, where IDO has been directly implicated in the mechanism controlling stem cell mediated protection from EAE pathogenesis [34]. When placed in the correct environment, neural stem cells can trans-differentiate to the neighboring cell type (reviewed [304]). Stem cells can provide relief from EAE pathogenesis [305-309]. It is reasonable to assume that endogenous stem or progenitor cells may be normally functioning in a healthy normal recovery process.
The NAD utilizing enzyme CD38 is well known as a marker of hematopoietic stem cells [157], where it serves demonstrably requisite chemotactic functions [137, 310] in neutrophil, macrophage, and dendritic cells as well as required roles in microglial activation [138, 139]. Finally, SIRT1 has entered the field of stem cell biology, where it has been realized that SIRT1 is not only expressed in stem cells, but it serves critical roles at the interface between R(O/N)S and p53-Nanog-pluripotency maintenance. Given the expanding role of both NAD and stem cells in both aging and therapeutic approaches to treating clinical MS, were briefly review and speculate on the involvement of NAD-dependent proteins in stem cell maintenance, proliferation, and chemotaxis.
The importance of IDO in this process was recently examined as a candidate mechanism of action given the well-established immunotoleragenic function. Matysiak et al. revealed that IFNγ-mediated induction of IDO with respective activities played an essential role in both prevention of demyelination and increased myelin repair [34]. This is consistent with the known roles for microglia in both pathogenesis and also repair. Lymphocyte proliferation was noticeably reduced, consistent with the known mechanism for IDO mediated stimulation of immunotolerance.
SIRT1 has newly appreciated roles in controlling the maintenance of stem cells [311-313]. Embryonic stem cells produce a small basal level of intrinsic reactive oxygen species as a byproduct of aerobic metabolism. To minimize damage that could otherwise kill this population of cells that is essential to life of a metazoan, p53 induces expression of several anti-oxidant generating enzymes including GPX, SESN1, and SESN2. In the nucleus, p53 also insures proper expression of the Nanog factors, which is required for pluripotency maintenance, and suppression of differentiation [311]. Slight elevations in R(O/N)S lead to p53-mediated repression of Nanog and associated loss of pluripotency. When R(O/N)S levels become too high however, then p53 leaves the nucleus all together to begin the apoptotic program via the mitochondria. Significantly, the NAD-dependent deacetylase, SIRT1, acts as the rheostat sensing redox levels and communicating to p53 by direct deacetylation [314]. Accordingly, SIRT1 has been shown to be intimately involved in the maintenance of stem cells.
Interestingly, when pellagrins were being rescued with niacin in the 1940s, there was a range of recovery rates [5, 187]. Some recovered overnight while others required at least a month of treatment to recover. Still others had suffered irreversible damage. It is reasonable to consider that irreversible pellagric pathology may be due to the complete loss of critical stem or progenitor cell populations due in part to a failure of NAD to activate SIRT1 towards maintaining stem cell populations. Thus, we would expect excessive R(O/N)S under these conditions, which would be expected to lead to excessive apoptosis.
Nicotinic acid is distinguished among the NAD precursors owing to the presence of a high affinity nicotinic acid G-protein coupled receptor, GPR109a/HM74a. Binding of nicotinic acid to GPR109a leads to a massive production the prostaglandins PGE2 [315] PGD2, PGF2, PGH2, and PGI2. PGE2 molecule was recently isolated in a small molecule screen by performing RNA in situ hybridization with transparent zebrafish larvae to identify activities stimulating stem cell proliferation ([316] and perspective [317]). The mechanism by which PGE2 increases stem cell proliferation is uncertain. One possibility may be that PGE2 is inducing IDO has is commonly used by immunologists to generate toleragenic monocytes [216, 318]. IDO induction can ultimately lead to increased NAD production within the respective cell. Perhaps this increases proliferation as needed. This, way thinking suggests that it is the cell with increased NAD that is ready to proliferate as needed. The idea of IDO mediated increases in proliferation is consistent with both the observations of the NAD precursor tryptophan as a lymphocyte mitogen as well as the well known observation that cancer cells are notorious in their over-expression of IDO [319-321]. Finally, as described for nicotinic acid treatment for promoting reducing conditions discouraging pathogenic gliosis in favor of neuronal specification (Fig. 7 above), we would predict that nicotinic acid mediated increases in reducing conditions would favor the maintenance of critical progenitor cells.
The ADP cyclase, CD38 is well known as a marker of stem cells [157]. It is also known to be required for chemo-taxis of neutrophils [137], dendritic cells [137], and macrophages [310]. Thus, it is likely that CD38 serves critical functions in stem cell chemotaxis. While the WldS protective effect did not decrease T cell infiltration, it did decreases microglial activation and decreased macrophage accumulation [97]. Both of the latter processes are believed to involve CD38. The connection between the nuclear NAD maintenance seen in WldS and CD38 activity is unclear. Nonetheless CD38 is constitutively expressed in the nucleus of hematopoietic cells [163], thus some kind of relationship between nuclear NMNAT1 and nuclear CD38 may be functioning in hematopoietic cells. It would be most interesting to cross WldS with the CD38 deficient mouse to increase our understanding of these relationships.
10. LACK OF DIETARY VARIETY CAUSING NAD DEFICIENCIES AND POTENTIAL CONNECTIONS TO MS ETIOLOGY TODAY
Just as we learn about gene function through phenotypic analysis of loss of expression, so we can learn about NAD function by reviewing the symptoms of NAD deficiency. Significantly, for the case of vitamin deficiency we can immediately consider pharmacological administration of NAD precursors as a treatment approach to disease given their extensive record of safe use in the clinic.
Lack of the precursors for NAD (tryptophan, nicotinic acid or nicotinamide) results in a curable dietary illness, pellagra, the symptoms for which are often generalized by the four Ds: dermatitis, diarrhea, dementia and death [322]. Pellagra occurred at an epidemic scale during the first two decades of the 20th century in the southern United States of America and is considered possibly the most devastating nutritional deficiency ever known in American history [323]. Pellagra is largely preventable by the application of nicotinic acid/niacin or nicotinamide/niacinamide [6]. Pellagra is often considered to be a disease that no longer appreciably exists in the first world other than in cases of tuberculosis drug induced pellagra [324, 325], chemotherapy induced pellagra [326, 327], or in connection with alcoholism [47, 147, 328]. However, analysis of western niacin status suggests inadequate niacin status occurs with a fairly high frequency at approximately 15% of the population [329]. The most consistent neuropathologic abnormality in pellagra is reported to be central chromatolysis of neurons [47, 330], where chromatolysis is defined as the loss of Nissl body staining as a result of neuronal death from injury, fatigue, or depletion of energy supplies
Most clinical signs result from the effects on the brain and immune systems. Further, pellagrins harbor many chronic infections; diarrhea and constipation chiefly due to gut infections. Tryptophan is also an obligatory precursor of 5-hydroxytryptamine (serotonin). Tryptophan crosses the blood-brain barrier, like tyrosine, predominantly by the carrier system for long chain neutral amino acids. Therapeutically, pure tryptophan has been administered orally as an antidepressant and a hypnotic. Small numbers of MS patients have also been treated with tryptophan [331, 332]. The 30-day daily tryptophan treatment of MS patients resulted in elevation of tryptophan and 5-HIAA levels in the cerebrospinal fluid (CSF) and a slight alleviation of clinical symptoms, including motility, bladder disturbances, and the mood of patients. In these studies, the effects were attributed to the neural transmission changes, and effects on the immune system or NAD synthesis were not considered. In addition, synthetic tryptophan metabolite, N-3,4-dimethoxycinnamoyl anthranilic acid, was shown to suppress an animal model for MS [333].
Humans are particularly vulnerable to NAD deficiency. This became particularly evident with introduction of the first rice and flour foods to the general population in the 1870's, which immediately resulted in epidemics of the NAD deficiency disease known as pellagra. Between 1900 and 1940, at least 100,000 individuals in the southern United States died of pellagra ([334] and excellent review [335]). In 1907, pellagra was the leading cause of death in mental hospitals [5]. Joseph Goldberger noticed the disease was particular to people on a limited diet while those ingesting cow's milk were spared. In the 1937 a University of Wisconsin chemist Conrad Elvehjem discovered that nicotinic acid prevented black tongue using a dog model of pellagra. [336]. The mandatory enrichment of niacin in the 1940's led to the prevention of such overt epidemics [6]. Overt pellagra is largely preventable by the application of nicotinic acid/niacin or nicotinamide/niacinamide [6].
While this was one of the greatest success stories in public health history, the concentration of optimal mandatory niacin fortification remains controversial even today and should be reexamined in light of the realization of R(O/N)S-mediated PARP-1 actively depletes NAD. Pellagra is often considered to be a disease that no longer exists in the developed countries other than in cases of tuberculosis drug-induced pellagras [324, 325], chemotherapy-induced pellagra [326, 327], or in connection with alcoholism [47, 147, 328]. However, analysis of western niacin status suggests inadequate niacin status occurs with a fairly high frequency at approximately 15% of the population [329]. The most consistent neuropathologic abnormality in pellagra is reported to be central chromatolysis of neurons [47, 330], where chromatolysis is defined as the loss of Nissl body staining as a result of neuronal death from injury, fatigue, or depletion of energy supplies.
Pellagra can result from either a passive process evoked by dietary insufficiency or even active drug induced processes including tuberculosis drugs [324, 325] and excessive alcohol consumption [47, 147]. By extension, pellagric pathology can also arise from immune system mediated NAD depletion. However, NAD deficiency can also arise from activation of NAD depleting pathways. NAD depleting pathways can be activated by poor diet or autoimmune dys-regulation. Here we briefly touch on potential examples of diet-induced NAD deficiency. The modern western diet remains disproportionately high in refined sugars and corn [337]. The annual sugar consumption per individual has increased by over 10 fold in the western world since in the early 1800s [338]. All of these dietary changes correlated directly with increased refining. How well this may historically correlate with the incidence of MS is unknown. Significantly however, glucose actively promotes NAD depletion through PARP-1 activation [339, 340]. Nicotinamide can both inhibit PARP-1 enzyme activity and supply NAD to the cell. Nicotinamide protects against diabetes in animal models and continues to warrant active consideration in clinical studies (reviewed [142]). Limitations in NAD likely persist as a major rate-limiting factor controlling age-related disease onset. Extremely high doses of nicotinic acid of over 1000 fold higher than the RDA are commonly used to correct dyslipidemia that often arises from excessive high fat diets. The minimal amount of NAD precursor needed to stave off disease is likely to depend on dietary habits and immune function. Standardized RDA levels of NAD precursors are an area of active debate. The mechanisms of immune mediated NAD depletion involving activation of PARP-1, CD38, and IDO are discussed in greater detail sections above.
Corn was one of the first and most commonly accepted suspects believed to be causing pellagra back in the 1920's because corn consumption so clearly correlated with the occurrence of the often-deadly disease [5]. Entire conferences were focused on studying corn as the cause of the pellagra epidemics. Eventually it was determined that corn was not causing pellagra at all. Interestingly the Native American Indians (who had been eating corn for thousands of years at this point) realized you had to treat corn with lye to get something important out of it (tryptophan, the only precursor for the endogenous synthesis of NAD.). New to corn, the first Americans did not process corn with lye and so they failed to obtain any niacin from corn. Amazingly, if the first Europeans arriving in the new world had chosen to prepare corn the way the Native American Indians had been doing it, then there may never have been the pellagra epidemics of Europe starting in the 17th century and dramatically expanding with the development of refined foods particularly in America itself, the original home of corn. With corn so prevalent in the diet and the often very simplistic diets that lacked variety, including mostly corn meal and molasses, people in the southern United States of America soon suffered from pellagra. In 1912 it was shown that a maize only diet caused a multitude of health problems [341]. More recently it was shown that corn only diets lead to severe defects in brain serotonin levels [342]. As briefly mentioned above, application of tryptophan to multiple sclerosis patients exerted a positive effect on the mood of patients presumably ascribed to changes in neural transmission working through serotonin receptors [331]. Deficits of tryptophan due to diet or persistent activation of IDO, may lead to serotonin deficits effecting neurodegeneration in MS. Today corn itself is disproportionately over-represented in the modern western diet more so than any other source of food [343]. A rather homogenous modern diet deficient in tryptophan may contribute more than we realize to MS pathogenesis a result of insufficient dietary NAD precursor.
Dementia results from neurodegeneration most commonly from Alzheimer's Disease followed by vascular dementia [344]. There are few known molecules that can cure a form of dementia and few known examples of genetic mutations that cause dementia. However, NAD precursors including nicotinic acid or nicotinamide are well known for their dramatic activity in reversing pellagric dementia [5] and one genetic mutation causing dementia is the mutation in the genes TYROBP/DAP12 or TREM2 has been identified as the cause of polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL) also known as Nasu-Hakola disease. PLOSL pathogenesis involves inflammatory demyelination with axonal neurodegeneration resembling MS. Inhibition of TREM-2 exacerbates EAE [345]. A soluble form of TREM-2 has now been identified as significantly elevated in the CSF of multiple sclerosis patients but not non-inflammatory neurologic disease patients [103].
Thus, it would appear as though the soluble form of TREM-2 present in MS patients may be titrating the ligand away from binding to TREM2 expressed on the membrane of professional APCs. This would be expected to inhibit TREM2 function, therefore exacerbating pathogenesis as seen in murine EAE models [345]. DAP12 has been identified as inversely expressed with respect to IDO in professional antigen presenting cells as soon as they become toleragenic [346], an activity intimately connected with increased IDO activity. Accordingly it is expected that loss of DAP12/TYROBP or TREM2 function as predicted in MS, would lead to persistent IDO increases in professional APCs that would drain local tryptophan supplies. This is expected to lead to neurodegeneration characteristic of NAD deficiency or tryptophan deficiency [142]. Homeostatic balance of IDO activity in MS is clearly important for recovery immune induced demyelination, where supplementation using nontryptophan NAD precursors is expected to control such dys-regulation of endogenous NAD precursor (tryptophan) levels based on studies performed using mouse models [7].
11. CONCLUSIONS
The contrasting effects on clinical MS pathogenesis of persistent WldS-mediated NAD maintenance in chronic viral models of MS (TMEV-IDD) versus immune-mediated models of acute MS attacks (EAE) strongly support the need to consider giving glia the control of NAD synthesis and distribution to neurons as needed. Nicotinic acid appears to be the preferred substrate for NAD biosynthesis in glia [43] and may exert a immunotoleragenic effect through GPR109a mediated induction of IDO specifically in professional APCs. More direct comparative pharmacological research comparing EAE versus TMEV-IDD using nicotinic acid and other NAD precursors is needed to quantify the therapeutic potential of individual approaches to treating MS. Consideration of both models of MS is especially important since MS etiology is unknown yet exceptionally common in the population. The undeniable and continued success of NAD precursor therapy for correcting lipodystrophy and mental health disease strongly supports the need for further exploratory investigations into the potential for high dose concentration therapeutic windows in treating multiple sclerosis.
ACKNOWLEDGEMENTS
We thank Daniel Doty, Andy Luu, and Faris Hasanovic for excellent technical assistance. This work was supported by the National Multiple Sclerosis Society (NMSS, PP1499) and the National Institutes of Health (NIH, R21NS059724). We are grateful to the critical comments and contributions of Christine Miller of Johns Hopkins University, Dharmesh D. Desai, Carmen Wheatley, and Soumya C. Chari for helping to prepare this manuscript. This provided critical firsthand insight into the mechanisms NAD mediated delay of neurodegeneration in EAE models of multiple sclerosis.
ABBREVIATIONS
- BBB
Blood-brain barrier
- CNP
2',3'-Cyclic nucleotide phosphodiesterase
- EAE
Experimental autoimmune (allergic) encephalomyelitis
- IDO
Indoleamine 2,3-dioxygenase
- IFN
Interferon
- MBP
Myelin basic protein
- MHC
Major histocompatibility complex
- MOG
Myelin oligodendrocyte glycoprotein
- NA
Nicotinic acid
- NAD
Nicotinamide adenine dinucleotide
- NAM
Nicotinamide
- NaMN
Nicotinic acid mononucleotide
- NAMR
Nicotinamide riboside
- NMN
Nicotinamide mononucleotide
- PARP-1
Poly(ADP)ribose polymerase
- PG
Prostaglandin
- PLP
Myelin proteolipid protein
- PNC
Prokaryotic nicotinamidase
- PP
Primary progressive
- PR
Progressive relapsing
- Professional APCs
Professional antigen presenting cells
- SP
Secondary progressive
- R(O/N)S
Reactive oxygen or nitrogen species
- RR
Relapsing-remitting
- TMEV-IDD
Theiler's murine encephalomyelitis virus-induced demyelinating disease
- TNF
Tumor necrosis factor
- W
Tryptophan
- WldS
Wallerian degeneration slow mouse
REFERENCES
- [1].Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD(+) precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–30. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- [2].Tang K, Sham H, Hui E, Kirkland JB. Niacin deficiency causes oxidative stress in rat bone marrow cells but not through decreased NADPH or glutathione status. J Nutr Biochem. 2008 doi: 10.1016/j.jnutbio.2007.10.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [3].Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–9. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- [4].Khan JA, Forouhar F, Tao X, Tong L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opin Ther Targets. 2007;11:695–705. doi: 10.1517/14728222.11.5.695. [DOI] [PubMed] [Google Scholar]
- [5].Etheridge EW. The butterfly caste: a social history of pellagra in the South. Greenwood Pub. Co; Westport, Conn: 1972. [Google Scholar]
- [6].Park YK, Sempos CT, Barton CN, Vanderveen JE, Yetley EA. Effectiveness of food fortification in the United States: the case of pellagra. Am J Public Health. 2000;90:727–38. doi: 10.2105/ajph.90.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kaneko S, Wang J, Kaneko M, Yiu G, Hurrell JM, Chitnis T, et al. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J Neurosci. 2006;26:9794–804. doi: 10.1523/JNEUROSCI.2116-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tempel W, Rabeh WM, Bogan KL, Belenky P, Wojcik M, Seidle HF, et al. Nicotinamide riboside kinase structures reveal new pathways to NAD+ PLoS Biol. 2007;5:e263. doi: 10.1371/journal.pbio.0050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chiarugi A. Inhibitors of poly(ADP-ribose) polymerase-1 suppress transcriptional activation in lymphocytes and ameliorate autoimmune encephalomyelitis in rats. Br J Pharmacol. 2002;137:761–70. doi: 10.1038/sj.bjp.0704934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ott M, Demisch L, Engelhardt W, Fischer PA. Interleukin-2, soluble interleukin-2-receptor, neopterin, L-tryptophan and beta 2-microglobulin levels in CSF and serum of patients with relapsing-remitting or chronic-progressive multiple sclerosis. J Neurol. 1993;241:108–14. doi: 10.1007/BF00869773. [DOI] [PubMed] [Google Scholar]
- [11].Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008 doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Esquifino AI, Cano P, Jimenez V, Cutrera RA, Cardinali DP. Experimental allergic encephalomyelitis in male Lewis rats subjected to calorie restriction. J Physiol Biochem. 2004;60:245–52. doi: 10.1007/BF03167069. [DOI] [PubMed] [Google Scholar]
- [13].Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol. 2008 doi: 10.1189/jlb.0208133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Esquifino AI, Cano P, Jimenez-Ortega V, Fernandez-Mateos MP, Cardinali DP. Immune response after experimental allergic encephalomyelitis in rats subjected to calorie restriction. J Neuroinflammation. 2007;4:6. doi: 10.1186/1742-2094-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–73. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, et al. SIRT1 regulates energy metabolism and response to caloric restriction in mice. PLoS ONE. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–63. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- [18].Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol. 2004;53:1003–9. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- [19].Wacker DA, Ruhl DD, Balagamwala EH, Hope KM, Zhang T, Kraus WL. The DNA binding and catalytic domains of poly(ADP-ribose) polymerase 1 cooperate in the regulation of chromatin structure and transcription. Mol Cell Biol. 2007;27:7475–85. doi: 10.1128/MCB.01314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol Dis. 2004;15:618–29. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- [21].Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- [22].Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27:12884–92. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Grant RS, Passey R, Matanovic G, Smythe G, Kapoor V. Evidence for increased de novo synthesis of NAD in immune-activated RAW264.7 macrophages: a self-protective mechanism? Arch Biochem Biophys. 1999;372:1–7. doi: 10.1006/abbi.1999.1381. [DOI] [PubMed] [Google Scholar]
- [24].Kujundzic RN, Lowenthal JW. The role of tryptophan metabolism in iNOS transcription and nitric oxide production by chicken macrophage cells upon treatment with interferon gamma. Immunol Lett. 2008;115:153–9. doi: 10.1016/j.imlet.2007.11.003. [DOI] [PubMed] [Google Scholar]
- [25].Grant RS, Naif H, Espinosa M, Kapoor V. IDO induction in IFN-gamma activated astroglia: a role in improving cell viability during oxidative stress. Redox Rep. 2000;5:101–4. doi: 10.1179/135100000101535357. [DOI] [PubMed] [Google Scholar]
- [26].Grant R, Kapoor V. Inhibition of indoleamine 2,3-dioxygenase activity in IFN-gamma stimulated astroglioma cells decreases intracellular NAD levels. Biochem Pharmacol. 2003;66:1033–6. doi: 10.1016/s0006-2952(03)00464-7. [DOI] [PubMed] [Google Scholar]
- [27].Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci. 2006;26:8484–91. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Suh SW, Hamby AM, Swanson RA. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia. 2007;55:1280–6. doi: 10.1002/glia.20440. [DOI] [PubMed] [Google Scholar]
- [29].Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–10. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- [31].Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;129:186–96. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- [32].Kwidzinski E, Bunse J, Kovac AD, Ullrich O, Zipp F, Nitsch R, et al. IDO (indolamine 2,3-dioxygenase) expression and function in the CNS. Adv Exp Med Biol. 2003;527:113–8. doi: 10.1007/978-1-4615-0135-0_13. [DOI] [PubMed] [Google Scholar]
- [33].Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. Faseb J. 2005;19:1347–9. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- [34].Matysiak M, Stasiolek M, Orlowski W, Jurewicz A, Janczar S, Raine CS, et al. Stem cells ameliorate EAE via an indoleamine 2,3-dioxygenase (IDO) mechanism. J Neuroimmunol. 2008;193:12–23. doi: 10.1016/j.jneuroim.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA, Lowry CA. Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med. 2006;8:1–27. doi: 10.1017/S1462399406000068. [DOI] [PubMed] [Google Scholar]
- [36].Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–3. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- [37].Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, et al. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005;170:349–55. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Stevens MJ, Li F, Drel V, Abatan O, Kim H, Burnett D, et al. Nicotinamde reverses neurological and neurovascular deficits in streptozotocin diabetic rats. J Pharmacol Exp Ther. 2006;320(1):458–64. doi: 10.1124/jpet.106.109702. [DOI] [PubMed] [Google Scholar]
- [39].Lee EJ, Wu TS, Chang GL, Li CY, Chen TY, Lee MY, et al. Delayed treatment with nicotinamide inhibits brain energy depletion, improves cerebral microperfusion, reduces brain infarct volume, but does not alter neurobehavioral outcome following permanent focal cerebral ischemia in Sprague Dawley rats. Curr Neurovasc Res. 2006;3:203–13. doi: 10.2174/156720206778018749. [DOI] [PubMed] [Google Scholar]
- [40].Ayoub IA, Maynard KI. Therapeutic window for nicotinamide following transient focal cerebral ischemia. Neuroreport. 2002;13:213–6. doi: 10.1097/00001756-200202110-00008. [DOI] [PubMed] [Google Scholar]
- [41].Sakakibara Y, Mitha AP, Ogilvy CS, Maynard KI. Post-treatment with nicotinamide (vitamin B(3)) reduces the infarct volume following permanent focal cerebral ischemia in female Sprague-Dawley and Wistar rats. Neurosci Lett. 2000;281:111–4. doi: 10.1016/s0304-3940(00)00854-5. [DOI] [PubMed] [Google Scholar]
- [42].Li F, Chong ZZ, Maiese K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front Biosci. 2004;9:2500–20. doi: 10.2741/1412. [DOI] [PubMed] [Google Scholar]
- [43].Grant RS, Kapoor V. Murine glial cells regenerate NAD, after peroxide-induced depletion, using either nicotinic acid, nicotinamide, or quinolinic acid as substrates. J Neurochem. 1998;70:1759–63. doi: 10.1046/j.1471-4159.1998.70041759.x. [DOI] [PubMed] [Google Scholar]
- [44].Lu H, Burns D, Garnier P, Wei G, Zhu K, Ying W. P2X7 receptors mediate NADH transport across the plasma membranes of astrocytes. Biochem Biophys Res Commun. 2007;362:946–50. doi: 10.1016/j.bbrc.2007.08.095. [DOI] [PubMed] [Google Scholar]
- [45].Lu H, Swanson RA, Ying W. American Society for Neurosciences Annual Meeting.2006. [Google Scholar]
- [46].Barres BA, Smith SJ. Neurobiology. Cholesterol--making or breaking the synapse. Science. 2001;294:1296–7. doi: 10.1126/science.1066724. [DOI] [PubMed] [Google Scholar]
- [47].Ishii N, Nishihara Y. Pellagra among chronic alcoholics: clinical and pathological study of 20 necropsy cases. J Neurol Neurosurg Psychiatry. 1981;44:209–15. doi: 10.1136/jnnp.44.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ying W, Wei G, Wang D, Wang Q, Tang X, Shi J, et al. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front Biosci. 2007;12:2728–34. doi: 10.2741/2267. [DOI] [PubMed] [Google Scholar]
- [49].Ying W. NAD+ and NADH in Neuronal Death. J Neuroimmune Pharmacol. 2007;2:270–5. doi: 10.1007/s11481-007-9063-5. [DOI] [PubMed] [Google Scholar]
- [50].Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costa-lat R, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–62. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- [51].Bergersen LH. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007;145:11–9. doi: 10.1016/j.neuroscience.2006.11.062. [DOI] [PubMed] [Google Scholar]
- [52].Carson MJ, Anglen CS, Ploix C. Multiple sclerosis. In: Minagar A, Alexander JS, editors. A disease of miscommunication between the immune and central nervous systems? Humana Press Inc.; Totwa, New Jersey: 2005. pp. 17–39. [Google Scholar]
- [53].Lassmann H. Models of multiple sclerosis: new insights into pathophysiology and repair. Curr Opin Neurol. 2008;21:242–7. doi: 10.1097/WCO.0b013e3282fee94a. [DOI] [PubMed] [Google Scholar]
- [54].Bitsch A, Bruck W. Differentiation of multiple sclerosis subtypes: implications for treatment. CNS Drugs. 2002;16:405–18. doi: 10.2165/00023210-200216060-00004. [DOI] [PubMed] [Google Scholar]
- [55].Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- [56].Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- [57].Peltonen L. Old suspects found guilty--the first genome profile of multiple sclerosis. N Engl J Med. 2007;357:927–9. doi: 10.1056/NEJMe078147. [DOI] [PubMed] [Google Scholar]
- [58].Brown SJ. The role of vitamin D in multiple sclerosis. Ann Pharmacother. 2006;40:1158–61. doi: 10.1345/aph.1G513. [DOI] [PubMed] [Google Scholar]
- [59].Kira J, Tobimatsu S, Goto I. Vitamin B12 metabolism and massive-dose methyl vitamin B12 therapy in Japanese patients with multiple sclerosis. Intern Med. 1994;33:82–6. doi: 10.2169/internalmedicine.33.82. [DOI] [PubMed] [Google Scholar]
- [60].Miller A, Korem M, Almog R, Galboiz Y. Vitamin B12, demyelination, remyelination and repair in multiple sclerosis. J Neurol Sci. 2005;233:93–7. doi: 10.1016/j.jns.2005.03.009. [DOI] [PubMed] [Google Scholar]
- [61].Reynolds EH, Linnell JC. Vitamin B12 deficiency, demyelination, and multiple sclerosis. Lancet. 1987;2:920. doi: 10.1016/s0140-6736(87)91411-5. [DOI] [PubMed] [Google Scholar]
- [62].Baethmann M, Wendel U, Hoffmann GF, Gohlich-Ratmann G, Kleinlein B, Seiffert P, et al. Hydrocephalus internus in two patients with 5,10-methylenetetrahydrofolate reductase deficiency. Neuropediatrics. 2000;31:314–7. doi: 10.1055/s-2000-12947. [DOI] [PubMed] [Google Scholar]
- [63].Kishi T, Tanaka Y, Ueda K. Evidence for hypomethylation in two children with acute lymphoblastic leukemia and leukoencephalopathy. Cancer. 2000;89:925–31. doi: 10.1002/1097-0142(20000815)89:4<925::aid-cncr28>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [64].Simmons A. Herpesvirus and multiple sclerosis. Herpes. 2001;8:60–3. [PubMed] [Google Scholar]
- [65].Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–5. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- [66].Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- [67].Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med. 2000;343:898–904. doi: 10.1056/NEJM200009283431301. [DOI] [PubMed] [Google Scholar]
- [68].Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet. 2007;370:389–97. doi: 10.1016/S0140-6736(07)61194-5. [DOI] [PubMed] [Google Scholar]
- [69].Rodriguez M, Zoecklein LJ, Howe CL, Pavelko KD, Gamez JD, Nakane S, et al. Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler's murine encephalomyelitis virus infection. J Virol. 2003;77:12252–65. doi: 10.1128/JVI.77.22.12252-12265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Adams O, Besken K, Oberdorfer C, MacKenzie CR, Russing D, Daubener W. Inhibition of human herpes simplex virus type 2 by interferon gamma and tumor necrosis factor alpha is mediated by indoleamine 2,3-dioxygenase. Microbes Infect. 2004;6:806–12. doi: 10.1016/j.micinf.2004.04.007. [DOI] [PubMed] [Google Scholar]
- [71].Adams O, Besken K, Oberdorfer C, MacKenzie CR, Takikawa O, Daubener W. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J Virol. 2004;78:2632–6. doi: 10.1128/JVI.78.5.2632-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bodaghi B, Goureau O, Zipeto D, Laurent L, Virelizier JL, Michelson S. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J Immunol. 1999;162:957–64. [PubMed] [Google Scholar]
- [73].Sanni LA, Thomas SR, Tattam BN, Moore DE, Chaudhri G, Stocker R, et al. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. Am J Pathol. 1998;152:611–9. [PMC free article] [PubMed] [Google Scholar]
- [74].MacKenzie CR, Hadding U, Daubener W. Interferon-gamma-induced activation of indoleamine 2,3-dioxygenase in cord blood monocyte-derived macrophages inhibits the growth of group B streptococci. J Infect Dis. 1998;178:875–8. doi: 10.1086/515347. [DOI] [PubMed] [Google Scholar]
- [75].Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–12. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Silva NM, Rodrigues CV, Santoro MM, Reis LF, Alvarez-Leite JI, Gazzinelli RT. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect Immun. 2002;70:859–68. doi: 10.1128/iai.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Byrne GI, Lehmann LK, Landry GJ. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986;53:347–51. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Owens T. Animal models for multiple sclerosis. Adv Neurol. 2006;98:77–89. [PubMed] [Google Scholar]
- [79].Tsunoda I, Fujinami RS. Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler's murine encephalomyelitis virus. J Neuropathol Exp Neurol. 1996;55:673–86. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- [80].Tsunoda I, Kuang LQ, Tolley ND, Whitton JL, Fujinami RS. Enhancement of experimental allergic encephalomyelitis (EAE) by DNA immunization with myelin proteolipid protein (PLP) plasmid DNA. J Neuropathol Exp Neurol. 1998;57:758–67. doi: 10.1097/00005072-199808000-00005. [DOI] [PubMed] [Google Scholar]
- [81].Tsunoda I, Kuang LQ, Theil DJ, Fujinami RS. Antibody association with a novel model for primary progressive multiple sclerosis: induction of relapsing-remitting and progressive forms of EAE in H2s mouse strains. Brain Pathol. 2000;10:402–18. doi: 10.1111/j.1750-3639.2000.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tsunoda I, Fujinami RS. Experimental Models of Multiple Sclerosis. In: Lavi E, Constantinescu CS, editors. TMEV and neuroantigens: Myelin genes and proteins, molecular mimicry, epitope spreading and autoantibody-mediated remyelination. New York, N.Y.: 2005. pp. 593–616. [Google Scholar]
- [83].Tsunoda I, Lane TE, Blackett J, Fujinami RS. Distinct roles for IP-10/CXCL10 in three animal models, Theiler's virus infection, EAE, and MHV infection, for multiple sclerosis: implication of differing roles for IP-10. Mult Scler. 2004;10:26–34. doi: 10.1191/1352458504ms982oa. [DOI] [PubMed] [Google Scholar]
- [84].Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- [85].Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- [86].Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–7. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- [87].Gillingwater TH, Wishart TM, Chen PE, Haley JE, Robertson K, MacDonald SH, et al. The neuroprotective WldS gene regulates expression of PTTG1 and erythroid differentiation regulator 1-like gene in mice and human cells. Hum Mol Genet. 2006;15:625–35. doi: 10.1093/hmg/ddi478. [DOI] [PubMed] [Google Scholar]
- [88].Ludwin SK, Bisby MA. Delayed Wallerian degeneration in the central nervous system of Ola mice: an ultrastructural study. J Neurol Sci. 1992;109:140–7. doi: 10.1016/0022-510x(92)90160-m. [DOI] [PubMed] [Google Scholar]
- [89].Perry VH, Lunn ER, Brown MC, Cahusac S, Gordon S. Evidence that the rate of wallerian degeneration is controlled by a single autosomal dominant gene. Eur J Neurosci. 1990;2:408–413. doi: 10.1111/j.1460-9568.1990.tb00433.x. [DOI] [PubMed] [Google Scholar]
- [90].Glass JD, Griffin JW. Neurofilament redistribution in transected nerves: evidence for bidirectional transport of neurofilaments. J Neurosci. 1991;11:3146–54. doi: 10.1523/JNEUROSCI.11-10-03146.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Glass JD, Griffin JW. Retrograde transport of radiolabeled cytoskeletal proteins in transected nerves. J Neurosci. 1994;14:3915–21. doi: 10.1523/JNEUROSCI.14-06-03915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Watson DF, Glass JD, Griffin JW. Redistribution of cytoskeletal proteins in mammalian axons disconnected from their cell bodies. J Neurosci. 1993;13:4354–60. doi: 10.1523/JNEUROSCI.13-10-04354.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Koike T, Yang Y, Suzuki K, Zheng X. Axon & dendrite degeneration: its mechanisms and protective experimental paradigms. Neurochem Int. 2008;52:751–60. doi: 10.1016/j.neuint.2007.09.007. [DOI] [PubMed] [Google Scholar]
- [94].Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- [95].Fainzilber M, Twiss JL. Tracking in the Wlds--the hunting of the SIRT and the luring of the Draper. Neuron. 2006;50:819–21. doi: 10.1016/j.neuron.2006.05.023. [DOI] [PubMed] [Google Scholar]
- [96].Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24:728–43. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- [97].Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, et al. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am J Pathol. 2007;170:1695–712. doi: 10.2353/ajpath.2007.060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Tsunoda I, Tanaka T, Terry EJ, Fujinami RS. Contrasting roles for axonal degeneration in an autoimmune versus viral model of multiple sclerosis: When can axonal injury be beneficial? Am J Pathol. 2007;170:214–26. doi: 10.2353/ajpath.2007.060683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Tsunoda I, Terry EJ, Marble BJ, Lazarides E, Woods C, Fujinami RS. Modulation of experimental autoimmune encephalomyelitis by VLA-2 blockade. Brain Pathol. 2007;17:45–55. doi: 10.1111/j.1750-3639.2006.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005;175:3075–83. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- [101].Kawamura H, Aswad F, Minagawa M, Malone K, Kaslow H, Koch-Nolte F, et al. P2X7 receptor-dependent and -independent T cell death is induced by nicotinamide adenine dinucleotide. J Immunol. 2005;174:1971–9. doi: 10.4049/jimmunol.174.4.1971. [DOI] [PubMed] [Google Scholar]
- [102].Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, et al. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004;173:3748–54. doi: 10.4049/jimmunol.173.6.3748. [DOI] [PubMed] [Google Scholar]
- [103].Piccio L, Buonsanti C, Cella M, Tassi I, Schmidt RE, Fenoglio C, et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain. 2008 doi: 10.1093/brain/awn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Shindler KS, Ventura E, Rex TS, Elliott P, Rostami A. SIRT1 activation confers neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007;48:3602–9. doi: 10.1167/iovs.07-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Suzuki K, Koike T. Resveratrol abolishes resistance to axonal degeneration in slow Wallerian degeneration (WldS) mice: activation of SIRT2, an NAD-dependent tubulin deacetylase. Biochem Biophys Res Commun. 2007;359:665–71. doi: 10.1016/j.bbrc.2007.05.164. [DOI] [PubMed] [Google Scholar]
- [106].Tsunoda I. Axonal degeneration as a self-destructive defense mechanism against neurotropic virus infection. Future Virol. 2008;3:579–593. doi: 10.2217/17460794.3.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Roussarie JP, Ruffie C, Edgar JM, Griffiths I, Brahic M. Axon myelin transfer of a non-enveloped virus. PLoS ONE. 2007;2:e1331. doi: 10.1371/journal.pone.0001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Taylor MP, Kirkegaard K. Potential subversion of autophagosomal pathway by picornaviruses. Autophagy. 2008;4:286–9. doi: 10.4161/auto.5377. [DOI] [PubMed] [Google Scholar]
- [109].Chiarugi A. Intrinsic mechanisms of poly(ADP-ribose) neurotoxicity: three hypotheses. Neurotoxicology. 2005;26:847–55. doi: 10.1016/j.neuro.2005.01.012. [DOI] [PubMed] [Google Scholar]
- [110].Kolthur-Seetharam U, Dantzer F, McBurney MW, de Murcia G, Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–7. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- [111].Pillai JB, Gupta M, Rajamohan SB, Lang R, Raman J, Gupta MP. Poly(ADP-ribose) polymerase-1-deficient mice are protected from angiotensin II-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:H1545–53. doi: 10.1152/ajpheart.01124.2005. [DOI] [PubMed] [Google Scholar]
- [112].Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–30. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- [113].Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- [114].Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–60. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Cai AL, Zipfel GJ, Sheline CT. Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur J Neurosci. 2006;24:2169–76. doi: 10.1111/j.1460-9568.2006.05110.x. [DOI] [PubMed] [Google Scholar]
- [116].Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem. 2004;279:18895–902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- [117].Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci USA. 2006;103:9685–90. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Shall S, de Murcia G. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res. 2000;460:1–15. doi: 10.1016/s0921-8777(00)00016-1. [DOI] [PubMed] [Google Scholar]
- [119].Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, et al. A role for the mitochondrial deacetylase SIRT3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–14. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008;22:1367–1371. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- [122].Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- [123].Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2008 doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- [124].Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–13. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- [125].Staykova MA, Linares D, Fordham SA, Paridaen JT, Willenborg DO. The innate immune response to adjuvants dictates the adaptive immune response to autoantigens. J Neuropathol Exp Neurol. 2008;67:543–54. doi: 10.1097/NEN.0b013e31817713cc. [DOI] [PubMed] [Google Scholar]
- [126].Murray MF. The human indoleamine 2,3-dioxygenase gene and related human genes. Curr Drug Metab. 2007;8:197–200. doi: 10.2174/138920007780362509. [DOI] [PubMed] [Google Scholar]
- [127].Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- [128].Miller CL, Llenos IC, Cwik M, Walkup J, Weis S. Alterations in kynurenine precursor and product levels in schizophrenia and bipolar disorder. Neurochem Int. 2008;52:1297–303. doi: 10.1016/j.neuint.2008.01.013. [DOI] [PubMed] [Google Scholar]
- [129].Osmond H, Hoffer A. Massive niacin treatment in schizophrenia. Review of a nine-year study. Lancet. 1962;1:316–9. doi: 10.1016/s0140-6736(62)91259-x. [DOI] [PubMed] [Google Scholar]
- [130].Xiao BG, Liu X, Link H. Antigen-specific T cell functions are suppressed over the estrogen-dendritic cell-indoleamine 2,3-dioxygenase axis. Steroids. 2004;69:653–9. doi: 10.1016/j.steroids.2004.05.019. [DOI] [PubMed] [Google Scholar]
- [131].Zhu WH, Lu CZ, Huang YM, Link H, Xiao BG. A putative mechanism on remission of multiple sclerosis during pregnancy: estrogen-induced indoleamine 2,3-dioxygenase by dendritic cells. Mult Scler. 2007;13:33–40. doi: 10.1177/1352458506071171. [DOI] [PubMed] [Google Scholar]
- [132].Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–86. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- [133].Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–23. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- [134].Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–18. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- [135].Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073-1074::25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- [136].Iqbal J, Zaidi M. TNF regulates cellular NAD+ metabolism in primary macrophages. Biochem Biophys Res Commun. 2006;342:1312–8. doi: 10.1016/j.bbrc.2006.02.109. [DOI] [PubMed] [Google Scholar]
- [137].Partida-Sanchez S, Gasser A, Fliegert R, Siebrands CC, Dammermann W, Shi G, et al. Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by adp-ribose, the major product generated by the CD38 enzyme reaction. J Immunol. 2007;179:7827–39. doi: 10.4049/jimmunol.179.11.7827. [DOI] [PubMed] [Google Scholar]
- [138].Mayo L, Jacob-Hirsch J, Amariglio N, Rechavi G, Moutin MJ, Lund FE, et al. Dual role of CD38 in microglial activation and activation-induced cell death. J Immunol. 2008;181:92–103. doi: 10.4049/jimmunol.181.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Franco L, Bodrato N, Moreschi I, Usai C, Bruzzone S, Scarfi S, et al. Cyclic ADP-ribose is a second messenger in the lipopolysaccharide-stimulated activation of murine N9 microglial cell line. J Neurochem. 2006;99:165–76. doi: 10.1111/j.1471-4159.2006.04031.x. [DOI] [PubMed] [Google Scholar]
- [140].Opitz CA, Wick W, Steinman L, Platten M. Tryptophan degradation in autoimmune diseases. Cell Mol Life Sci. 2007;64:2542–63. doi: 10.1007/s00018-007-7140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Kaper T, Looger LL, Takanaga H, Platten M, Steinman L, Frommer WB. Nanosensor detection of an immunoregulatory tryptophan influx/kynurenine efflux cycle. PLoS Biol. 2007;5:e257. doi: 10.1371/journal.pbio.0050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Penberthy WT. Pharmacological targeting of IDO-mediated tolerance for treating autoimmune disease. Curr Drug Metab. 2007;8:245–66. doi: 10.2174/138920007780362545. [DOI] [PubMed] [Google Scholar]
- [143].Iribarren P, Cui YH, Le Y, Wang JM. The role of dendritic cells in neurodegenerative diseases. Arch Immunol Ther Exp (Warsz) 2002;50:187–96. [PubMed] [Google Scholar]
- [144].Ifergan I, Kebir H, Bernard M, Wosik K, Dodelet-Devillers A, Cayrol R, et al. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain. 2008;131:785–99. doi: 10.1093/brain/awm295. [DOI] [PubMed] [Google Scholar]
- [145].Pashenkov M, Teleshova N, Link H. Inflammation in the central nervous system: the role for dendritic cells. Brain Pathol. 2003;13:23–33. doi: 10.1111/j.1750-3639.2003.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kauppinen TM, Suh SW, Genain CP, Swanson RA. Poly(ADP-ribose) polymerase-1 activation in a primate model of multiple sclerosis. J Neurosci Res. 2005;81:190–8. doi: 10.1002/jnr.20525. [DOI] [PubMed] [Google Scholar]
- [147].Sakai K, Nakajima T, Fukuhara N. A suspected case of alcoholic pellagra encephalopathy with marked response to niacin showing myoclonus and ataxia as chief complaints. No To Shinkei. 2006;58:141–4. [PubMed] [Google Scholar]
- [148].Piercecchi-Marti MD, Pelissier-Alicot AL, Leonetti G, Terve JP, Cianfarani F, Pellissier JF. Pellagra: a rare disease observed in a victim of mental and physical abuse. Am J Forensic Med Pathol. 2004;25:342–4. doi: 10.1097/01.paf.0000136589.28903.e5. [DOI] [PubMed] [Google Scholar]
- [149].Belladonna ML, Grohmann U, Guidetti P, Volpi C, Bianchi R, Fioretti MC, et al. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol. 2006;177:130–7. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- [150].Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–86. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- [152].Partida-Sanchez S, Rivero-Nava L, Shi G, Lund FE. CD38: an ecto-enzyme at the crossroads of innate and adaptive immune responses. Adv Exp Med Biol. 2007;590:171–83. doi: 10.1007/978-0-387-34814-8_12. [DOI] [PubMed] [Google Scholar]
- [153].Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345:1386–92. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- [154].Young GS, Choleris E, Lund FE, Kirkland JB. Decreased cADPR and increased NAD+ in the Cd38-/- mouse. Biochem Biophys Res Commun. 2006;346:188–92. doi: 10.1016/j.bbrc.2006.05.100. [DOI] [PubMed] [Google Scholar]
- [155].Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–39. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- [156].Mallone R, Perin PC. Anti-CD38 autoantibodies in type? diabetes. Diabetes Metab Res Rev. 2006;22:284–94. doi: 10.1002/dmrr.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Cockayne DA, Muchamuel T, Grimaldi JC, Muller-Steffner H, Randall TD, Lund FE, et al. Mice deficient for the ectonicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood. 1998;92:1324–33. [PubMed] [Google Scholar]
- [158].Rutter GA, Bellomo EA. Ca2+ signalling: a new route to NAADP. Biochem J. 2008;411:e1–3. doi: 10.1042/BJ20080282. [DOI] [PubMed] [Google Scholar]
- [159].Vasudevan SR, Galione A, Churchill GC. Sperm express a Ca2+-regulated NAADP synthase. Biochem J. 2008;411:63–70. doi: 10.1042/BJ20071616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ziegler M. New functions of a long-known molecule. Emerging roles of NAD in cellular signaling. Eur J Biochem. 2000;267:1550–64. doi: 10.1046/j.1432-1327.2000.01187.x. [DOI] [PubMed] [Google Scholar]
- [161].Gul R, Park JH, Kim SY, Jang KY, Chae JK, Ko JK, et al. Inhibition of ADP-ribosyl cyclase attenuates angiotensin II-induced cardiac hypertrophy. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvn232. [DOI] [PubMed] [Google Scholar]
- [162].Sims JL, Berger SJ, Berger NA. Poly(ADP-ribose) Polymerase inhibitors preserve nicotinamide adenine dinucleotide and adenosine 5'-triphosphate pools in DNA-damaged cells: mechanism of stimulation of unscheduled DNA synthesis. Biochemistry. 1983;22:5188–94. doi: 10.1021/bi00291a019. [DOI] [PubMed] [Google Scholar]
- [163].Orciani M, Trubiani O, Guarnieri S, Ferrero E, Di Primio R. CD38 is constitutively expressed in the nucleus of human hematopoietic cells. J Cell Biochem. 2008 doi: 10.1002/jcb.21887. [DOI] [PubMed] [Google Scholar]
- [164].Jones KS, Alimov AP, Rilo HL, Jandacek RP, Woollett LA, Penberthy WT. A High Throughput Live Transparent Animal Bioassay to Identify Non-toxic Small Molecules or Genes that Regulate Vertebrate Fat Metabolism for Obesity Drug Development. Nutr Metab (Lond) 2008;5:23. doi: 10.1186/1743-7075-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Iqbal J, Zaidi M. Extracellular NAD+ metabolism modulates osteoclastogenesis. Biochem Biophys Res Commun. 2006;349:533–9. doi: 10.1016/j.bbrc.2006.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Roelofs-Haarhuis K, Wu X, Nowak M, Fang M, Artik S, Gleichmann E. Infectious nickel tolerance: a reciprocal interplay of tolerogenic APCs and T suppressor cells that is driven by immunization. J Immunol. 2003;171:2863–72. doi: 10.4049/jimmunol.171.6.2863. [DOI] [PubMed] [Google Scholar]
- [167].Read S, Mauze S, Asseman C, Bean A, Coffman R, Powrie F. CD38+ CD45RB(low) CD4+ T cells: a population of T cells with immune regulatory activities in vitro. Eur J Immunol. 1998;28:3435–47. doi: 10.1002/(SICI)1521-4141(199811)28:11<3435::AID-IMMU3435>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- [168].McCandless EE, Klein RS. Molecular targets for disrupting leukocyte trafficking during multiple sclerosis. Expert Rev Mol Med. 2007;9:1–19. doi: 10.1017/S1462399407000397. [DOI] [PubMed] [Google Scholar]
- [169].Correale J, Villa A. The blood-brain-barrier in multiple sclerosis: functional roles and therapeutic targeting. Autoimmunity. 2007;40:148–60. doi: 10.1080/08916930601183522. [DOI] [PubMed] [Google Scholar]
- [170].Antonelli A, Ferrannini E. CD38 autoimmunity: recent advances and relevance to human diabetes. J Endocrinol Invest. 2004;27:695–707. doi: 10.1007/BF03347507. [DOI] [PubMed] [Google Scholar]
- [171].Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- [172].Buelow B, Song Y, Scharenberg AM. The poly(ADP-ribose) polymerase PARP-1 is required for oxidative stress-induced TRPM2 activation in lymphocytes. J Biol Chem. 2008 doi: 10.1074/jbc.M802673200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–95. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- [174].Endres M, Wang ZQ, Namura S, Waeber C, Moskowitz MA. Ischemic brain injury is mediated by the activation of poly(ADP-ribose)polymerase. J Cereb Blood Flow Metab. 1997;17:1143–51. doi: 10.1097/00004647-199711000-00002. [DOI] [PubMed] [Google Scholar]
- [175].Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Hasko G, Mabley JG, Nemeth ZH, Pacher P, Deitch EA, Szabo C. Poly(ADP-ribose) polymerase is a regulator of chemokine production: relevance for the pathogenesis of shock and inflammation. Mol Med. 2002;8:283–9. [PMC free article] [PubMed] [Google Scholar]
- [177].Szabo C, Lim LH, Cuzzocrea S, Getting SJ, Zingarelli B, Flower RJ, et al. Inhibition of poly (ADP-ribose) synthetase attenuates neutrophil recruitment and exerts antiinflammatory effects. J Exp Med. 1997;186:1041–9. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Zingarelli B, Szabo C, Salzman AL. Blockade of Poly(ADP-ribose) synthetase inhibits neutrophil recruitment, oxidant generation, and mucosal injury in murine colitis. Gastroenterology. 1999;116:335–45. doi: 10.1016/s0016-5085(99)70130-7. [DOI] [PubMed] [Google Scholar]
- [179].Song Y, Buelow B, Perraud AL, Scharenberg AM. Development and validation of a cell-based high-throughput screening assay for TRPM2 channel modulators. J Biomol Screen. 2008;13:54–61. doi: 10.1177/1087057107310986. [DOI] [PubMed] [Google Scholar]
- [180].Okuno A, Fukuwatari T, Shibata K. Urinary excretory ratio of anthranilic acid/kynurenic acid as an index of the tolerable amount of tryptophan. Biosci Biotechnol Biochem. 2008;72:1667–72. doi: 10.1271/bbb.70630. [DOI] [PubMed] [Google Scholar]
- [181].Smith MJ, Garrett RH. A heretofore undisclosed crux of eosinophilia-myalgia syndrome: compromised histamine degradation. Inflamm Res. 2005;54:435–50. doi: 10.1007/s00011-005-1380-7. [DOI] [PubMed] [Google Scholar]
- [182].Silver RM, Heyes MP, Maize JC, Quearry B, Vionnet-Fuasset M, Sternberg EM. Scleroderma, fasciitis, and eosinophilia associated with the ingestion of tryptophan. N Engl J Med. 1990;322:874–81. doi: 10.1056/NEJM199003293221302. [DOI] [PubMed] [Google Scholar]
- [183].Hankes LV, Coenen HH, Rota E, Langen KJ, Herzog H, Wutz W, et al. Effect of Huntington's and Alzheimer's diseases on the transport of nicotinic acid or nicotinamide across the human blood-brain barrier. Adv Exp Med Biol. 1991;294:675–8. doi: 10.1007/978-1-4684-5952-4_91. [DOI] [PubMed] [Google Scholar]
- [184].Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24:228–32. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- [185].Spector R. Niacin and niacinamide transport in the central nervous system. In vivo studies. J Neurochem. 1979;33:895–904. doi: 10.1111/j.1471-4159.1979.tb09919.x. [DOI] [PubMed] [Google Scholar]
- [186].Hoffer A. Treatment of arthritis by nicotinic acid and nicotinamide. Can Med Assoc J. 1959;81:235–8. [PMC free article] [PubMed] [Google Scholar]
- [187].Hoffer A. Adventures in Psychiatry: The Scientific Memoirs of Dr. Abram Hoffer. KOS Publishing; 2005. [Google Scholar]
- [188].Yang J, Klaidman LK, Chang ML, Kem S, Sugawara T, Chan P, et al. Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model. Pharmacol Biochem Behav. 2002;73:901–10. doi: 10.1016/s0091-3057(02)00939-5. [DOI] [PubMed] [Google Scholar]
- [189].Sheridan C. Genentech raises stakes on PARP inhibitors. Nat Biotechnol. 2006;24:1179–80. doi: 10.1038/nbt1006-1179. [DOI] [PubMed] [Google Scholar]
- [190].Hoffer A, Osmond H, Callbeck MJ, Kahan I. Treatment of schizophrenia with nicotinic acid and nicotinamide. J Clin Exp Psychopathol. 1957;18:131–58. [PubMed] [Google Scholar]
- [191].Thompson MM, Reed SC, Cockerill GW. Therapeutic approaches to raising plasma HDL-cholesterol levels. Nat Clin Pract Cardiovasc Med. 2004;1:84–9. doi: 10.1038/ncpcardio0044. [DOI] [PubMed] [Google Scholar]
- [192].Sampietro T, Bigazzi F, Dal Pino B, Puntoni M, Bionda A. HDL: the `new' target of cardiovascular medicine. Int J Cardiol. 2006;108:143–54. doi: 10.1016/j.ijcard.2005.04.036. [DOI] [PubMed] [Google Scholar]
- [193].Vance JE, Campenot RB, Vance DE. The synthesis and transport of lipids for axonal growth and nerve regeneration. Biochim Biophys Acta. 2000;1486:84–96. doi: 10.1016/s1388-1981(00)00050-0. [DOI] [PubMed] [Google Scholar]
- [194].Karten B, Hayashi H, Campenot RB, Vance DE, Vance JE. Neuronal models for studying lipid metabolism and transport. Methods. 2005;36:117–28. doi: 10.1016/j.ymeth.2004.11.004. [DOI] [PubMed] [Google Scholar]
- [195].Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27:1933–41. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Carter JM, Demizieux L, Campenot RB, Vance DE, Vance JE. Phosphatidylcholine biosynthesis via CTP:phosphocholine cytidylyltransferase 2 facilitates neurite outgrowth and branching. J Biol Chem. 2008;283:202–12. doi: 10.1074/jbc.M706531200. [DOI] [PubMed] [Google Scholar]
- [197].Micheli V, Simmonds HA, Sestini S, Ricci C. Importance of nicotinamide as an NAD precursor in the human erythrocyte. Arch Biochem Biophys. 1990;283:40–5. doi: 10.1016/0003-9861(90)90609-3. [DOI] [PubMed] [Google Scholar]
- [198].Henderson LM, Gross CJ. Metabolism of niacin and niacinamide in perfused rat intestine. J Nutr. 1979;109:654–62. doi: 10.1093/jn/109.4.654. [DOI] [PubMed] [Google Scholar]
- [199].Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282:24574–82. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- [200].Hernandez M, Wright SD, Cai TQ. Critical role of cholesterol ester transfer protein in nicotinic acid-mediated HDL elevation in mice. Biochem Biophys Res Commun. 2007;355:1075–80. doi: 10.1016/j.bbrc.2007.02.079. [DOI] [PubMed] [Google Scholar]
- [201].van der Hoorn JW, de Haan W, Berbee JF, Havekes LM, Jukema JW, Rensen PC, et al. Niacin Increases HDL by Reducing Hepatic Expression and Plasma Levels of Cholesteryl Ester Transfer Protein in APOE*3Leiden.CETP Mice. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.171363. [DOI] [PubMed] [Google Scholar]
- [202].Rubic T, Trottmann M, Lorenz RL. Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacin. Biochem Pharmacol. 2004;67:411–9. doi: 10.1016/j.bcp.2003.09.014. [DOI] [PubMed] [Google Scholar]
- [203].Zhao SP, Yang J, Li J, Dong SZ, Wu ZH. Effect of niacin on LXRalpha and PPARgamma expression and HDL-induced cholesterol efflux in adipocytes of hypercholesterolemic rabbits. Int J Cardiol. 2008;124:172–8. doi: 10.1016/j.ijcard.2006.12.032. [DOI] [PubMed] [Google Scholar]
- [204].Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101:20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- [205].Santos-Gallego CG, Ibanez B, Badimon JJ. HDL-cholesterol: is it really good? Differences between apoA-I and HDL. Biochem Pharmacol. 2008;76:443–52. doi: 10.1016/j.bcp.2008.04.020. [DOI] [PubMed] [Google Scholar]
- [206].Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–75. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- [207].Joy T, Hegele RA. Is raising HDL a futile strategy for atheroprotection? Nat Rev Drug Discov. 2008;7:143–55. doi: 10.1038/nrd2489. [DOI] [PubMed] [Google Scholar]
- [208].Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9:352–5. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- [209].Knowles HJ, te Poele RH, Workman P, Harris AL. Niacin induces PPARgamma expression and transcriptional activation in macrophages via HM74 and HM74a-mediated induction of prostaglandin synthesis pathways. Biochem Pharmacol. 2006;71:646–56. doi: 10.1016/j.bcp.2005.11.019. [DOI] [PubMed] [Google Scholar]
- [210].Storer PD, Xu J, Chavis JA, Drew PD. Cyclopentenone prostaglandins PGA2 and 15-deoxy-delta12,14 PGJ2 suppress activation of murine microglia and astrocytes: implications for multiple sclerosis. J Neurosci Res. 2005;80:66–74. doi: 10.1002/jnr.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, et al. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–15. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- [212].Diab A, Hussain RZ, Lovett-Racke AE, Chavis JA, Drew PD, Racke MK. Ligands for the peroxisome proliferator-activated receptor-gamma and the retinoid X receptor exert additive anti-inflammatory effects on experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:116–26. doi: 10.1016/j.jneuroim.2003.11.010. [DOI] [PubMed] [Google Scholar]
- [213].Kaplan JM, Cook JA, Hake PW, O'Connor M, Burroughs TJ, Zingarelli B. 15-Deoxy-delta(12,14)-prostaglandin J(2) (15D-PGJ(2)), a peroxisome proliferator activated receptor gamma ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock. 2005;24:59–65. doi: 10.1097/01.shk.0000167108.88376.f2. [DOI] [PubMed] [Google Scholar]
- [214].Gille A, Bodor ET, Ahmed K, Offermanns S. Nicotinic acid: pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol. 2008;48:79–106. doi: 10.1146/annurev.pharmtox.48.113006.094746. [DOI] [PubMed] [Google Scholar]
- [215].Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, et al. (D)-{beta}-Hydroxybutyrate Inhibits Adipocyte Lipolysis via the Nicotinic Acid Receptor PUMA-G. J Biol Chem. 2005;280:26649–52. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- [216].Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–81. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Basu TK, Mann S. Vitamin B-6 normalizes the altered sulfur amino acid status of rats fed diets containing pharmacological levels of niacin without reducing niacin's hypolipidemic effects. J Nutr. 1997;127:117–21. doi: 10.1093/jn/127.1.117. [DOI] [PubMed] [Google Scholar]
- [218].Garg R, Malinow M, Pettinger M, Upson B, Hunninghake D. Niacin treatment increases plasma homocyst(e)ine levels. Am Heart J. 1999;138:1082–7. doi: 10.1016/s0002-8703(99)70073-6. [DOI] [PubMed] [Google Scholar]
- [219].Basu TK, Makhani N, Sedgwick G. Niacin (nicotinic acid) in non-physiological doses causes hyperhomocysteineaemia in Sprague-Dawley rats. Br J Nutr. 2002;87:115–9. doi: 10.1079/BJN2001486. [DOI] [PubMed] [Google Scholar]
- [220].Reynolds EH. Multiple sclerosis and vitamin B12 metabolism. J Neuroimmunol. 1992;40:225–30. doi: 10.1016/0165-5728(92)90137-a. [DOI] [PubMed] [Google Scholar]
- [221].Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev. 2008;66:250–5. doi: 10.1111/j.1753-4887.2008.00031.x. [DOI] [PubMed] [Google Scholar]
- [222].Aoyagi S, Archer TK. Nicotinamide uncouples hormone-dependent chromatin remodeling from transcription complex assembly. Mol Cell Biol. 2008;28:30–9. doi: 10.1128/MCB.01158-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through AKT, BAD, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2:271–85. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- [225].Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008;83:804–16. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- [226].Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–75. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–6. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- [228].Reddy P, Sun Y, Toubai T, Duran-Struuck R, Clouthier SG, Weisiger E, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–73. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Kyrylenko S, Kyrylenko O, Suuronen T, Salminen A. Differential regulation of the Sir2 histone deacetylase gene family by inhibitors of class I and II histone deacetylases. Cell Mol Life Sci. 2003;60:1990–7. doi: 10.1007/s00018-003-3090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Singh NP, Hegde V, Hofseth LJ, Nagarkatti M, Nagarkatti P. Resveratrol (trans-3,5,4'-trihydroxystilbene) Ameliorates Experimental Allergic Encephalomyelitis (EAE) Primarily via Induction of Apoptosis in T cells Involving Activation of AhR and ER. Mol Pharmacol. 2007 doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–12. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- [232].Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–35. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku-70. Mol Cell Biol. 2008 doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–32. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- [235].Nakamura Y, Ogura M, Tanaka D, Inagaki N. Localization of mouse mitochondrial SIRT proteins: shift of SIRT3 to nucleus by co-expression with SIRT5. Biochem Biophys Res Commun. 2008;366:174–9. doi: 10.1016/j.bbrc.2007.11.122. [DOI] [PubMed] [Google Scholar]
- [236].Scher MB, Vaquero A, Reinberg D. SIRT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007;21:920–8. doi: 10.1101/gad.1527307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Cooper HM, Spelbrink JN. The human SIRT3 protein deacetylase is exclusively mitochondrial. Biochem J. 2008;411:279–85. doi: 10.1042/BJ20071624. [DOI] [PubMed] [Google Scholar]
- [238].Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–8. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- [239].Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- [240].Sequeira J, Boily G, Bazinet S, Saliba S, He X, Jardine K, et al. SIRT1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008 doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- [241].Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- [242].Sun AY, Simonyi A, Sun GY. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med. 2002;32:314–8. doi: 10.1016/s0891-5849(01)00803-6. [DOI] [PubMed] [Google Scholar]
- [243].Liu BL, Zhang X, Zhang W, Zhen HN. New enlightenment of French Paradox: resveratrol's potential for cancer chemoprevention and anti-cancer therapy. Cancer Biol Ther. 2007;6:1833–6. doi: 10.4161/cbt.6.12.5161. [DOI] [PubMed] [Google Scholar]
- [244].Aggarwal BB, Shishodia S. Resveratrol in health and disease. CRC Taylor & Francis; Boca Raton: 2006. [Google Scholar]
- [245].Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–22. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Werner HB, Kuhlmann K, Shen S, Uecker M, Schardt A, Dimova K, et al. Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J Neurosci. 2007;27:7717–30. doi: 10.1523/JNEUROSCI.1254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Southwood CM, Peppi M, Dryden S, Tainsky MA, Gow A. Microtubule deacetylases, SIRT2 and HDAC6, in the nervous system. Neurochem Res. 2007;32:187–95. doi: 10.1007/s11064-006-9127-6. [DOI] [PubMed] [Google Scholar]
- [248].Vaquero A, Sternglanz R, Reinberg D. NAD+-dependent deacetylation of H4 lysine 16 by class III HDACs. Oncogene. 2007;26:5505–20. doi: 10.1038/sj.onc.1210617. [DOI] [PubMed] [Google Scholar]
- [249].Zhang F, Sun C, Wu J, He C, Ge X, Huang W, et al. Combretastatin A-4 activates AMP-activated protein kinase and improves glucose metabolism in db/db mice. Pharmacol Res. 2008;57:318–23. doi: 10.1016/j.phrs.2008.03.002. [DOI] [PubMed] [Google Scholar]
- [250].Tian WX. Inhibition of fatty acid synthase by polyphenols. Curr Med Chem. 2006;13:967–77. doi: 10.2174/092986706776361012. [DOI] [PubMed] [Google Scholar]
- [251].Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, et al. SIRT1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–94. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- [252].Libert S, Cohen D, Guarente L. Neurogenesis directed by SIRT1. Nat Cell Biol. 2008;10:373–4. doi: 10.1038/ncb0408-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.04.044. [DOI] [PubMed] [Google Scholar]
- [254].Gan L, Mucke L. Paths of convergence: sirtuins in aging and neurodegeneration. Neuron. 2008;58:10–4. doi: 10.1016/j.neuron.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Ruggieri M, Pica C, Lia A, Zimatore GB, Modesto M, Di Liddo E, et al. Combination treatment of Glatiramer Acetate and Minocycline affects phenotype expression of blood monocyte-derived dendritic cells in Multiple Sclerosis patients. J Neuroimmunol. 2008 doi: 10.1016/j.jneuroim.2008.04.032. [DOI] [PubMed] [Google Scholar]
- [256].Luccarini I, Ballerini C, Biagioli T, Biamonte F, Bellucci A, Rosi MC, et al. Combined treatment with atorvastatin and minocycline suppresses severity of EAE. Exp Neurol. 2008;211:214–26. doi: 10.1016/j.expneurol.2008.01.022. [DOI] [PubMed] [Google Scholar]
- [257].Herrmann I, Kellert M, Spreer A, Gerber J, Eiffert H, Prinz M, et al. Minocycline delays but does not attenuate the course of experimental autoimmune encephalomyelitis in Streptococcus pneumoniae-infected mice. J Antimicrob Chemother. 2007;59:74–9. doi: 10.1093/jac/dkl446. [DOI] [PubMed] [Google Scholar]
- [258].Giuliani F, Fu SA, Metz LM, Yong VW. Effective combination of minocycline and interferon-beta in a model of multiple sclerosis. J Neuroimmunol. 2005;165:83–91. doi: 10.1016/j.jneuroim.2005.04.020. [DOI] [PubMed] [Google Scholar]
- [259].Scott GS, Kean RB, Mikheeva T, Fabis MJ, Mabley JG, Szabo C, et al. The therapeutic effects of PJ34 [N-(6-oxo-5,6-dihydrophen-anthridin-2-yl)-N,N-dimethylacetamide.HCl] a selective inhibitor of poly(ADP-ribose) polymerase, in experimental allergic encephalomyelitis are associated with immunomodulation. J Pharmacol Exp Ther. 2004;310:1053–61. doi: 10.1124/jpet.103.063214. [DOI] [PubMed] [Google Scholar]
- [260].Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125:1297–308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- [261].Scott GS, Hake P, Kean RB, Virag L, Szabo C, Hooper DC. Role of poly(ADP-ribose) synthetase activation in the development of experimental allergic encephalomyelitis. J Neuroimmunol. 2001;117:78–86. doi: 10.1016/s0165-5728(01)00329-0. [DOI] [PubMed] [Google Scholar]
- [262].Kauppinen TM. Multiple roles for poly(ADP-ribose)polymerase-1 in neurological disease. Neurochem Int. 2007;50:954–8. doi: 10.1016/j.neuint.2006.11.010. [DOI] [PubMed] [Google Scholar]
- [263].Mirshafiey A, Mohsenzadegan M. Antioxidant Therapy in Multiple Sclerosis. Immunopharmacol Immunotoxicol. 2008:1–34. doi: 10.1080/08923970802331943. [DOI] [PubMed] [Google Scholar]
- [264].van Horssen J, Schreibelt G, Bo L, Montagne L, Drukarch B, van Muiswinkel FL, et al. NAD(P)H:quinone oxidoreductase 1 expression in multiple sclerosis lesions. Free Radic Biol Med. 2006;41:311–7. doi: 10.1016/j.freeradbiomed.2006.04.013. [DOI] [PubMed] [Google Scholar]
- [265].Schraufstatter IU, Hinshaw DB, Hyslop PA, Spragg RG, Cochrane CG. Oxidant injury of cells. DNA strand-breaks activate poly-adenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986;77:1312–20. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- [267].Lefkowitz DL, Lefkowitz SS. Microglia and myeloperoxidase: a deadly partnership in neurodegenerative disease. Free Radic Biol Med. 2008;45:726–31. doi: 10.1016/j.freeradbiomed.2008.05.021. [DOI] [PubMed] [Google Scholar]
- [268].Liu Y, Hao W, Letiembre M, Walter S, Kulanga M, Neumann H, et al. Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47-PHOX-mediated generation of reactive oxygen species. J Neurosci. 2006;26:12904–13. doi: 10.1523/JNEUROSCI.2531-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Benavente CA, Jacobson EL. Niacin restriction upregulates NADPH oxidase and reactive oxygen species (ROS) in human keratinocytes. Free Radic Biol Med. 2008;44:527–37. doi: 10.1016/j.freeradbiomed.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–22. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- [271].Rasouri S, Lagouge M, Auwerx J. SIRT1/PGC-1: a neuroprotective axis? Med Sci (Paris) 2007;23:840–4. doi: 10.1051/medsci/20072310840. [DOI] [PubMed] [Google Scholar]
- [272].Lawson TM, Amos N, Bulgen D, Williams BD. Minocycline-induced lupus: clinical features and response to rechallenge. Rheumatology (Oxford) 2001;40:329–35. doi: 10.1093/rheumatology/40.3.329. [DOI] [PubMed] [Google Scholar]
- [273].Hess EV. Environmental chemicals and autoimmune disease: cause and effect. Toxicology. 2002;181-182:65–70. doi: 10.1016/s0300-483x(02)00256-1. [DOI] [PubMed] [Google Scholar]
- [274].Mongey AB, Hess EV. Drug insight: autoimmune effects of medications-what's new? Nat Clin Pract Rheumatol. 2008;4:136–44. doi: 10.1038/ncprheum0708. [DOI] [PubMed] [Google Scholar]
- [275].Margolis DJ, Hoffstad O, Bilker W. Association or lack of association between tetracycline class antibiotics used for acne vulgaris and lupus erythematosus. Br J Dermatol. 2007;157:540–6. doi: 10.1111/j.1365-2133.2007.08056.x. [DOI] [PubMed] [Google Scholar]
- [276].Morimoto N, Shimazawa M, Yamashima T, Nagai H, Hara H. Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Brain Res. 2005;1044:8–15. doi: 10.1016/j.brainres.2005.02.062. [DOI] [PubMed] [Google Scholar]
- [277].Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95:15769–74. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Rubin RL. Drug-induced lupus. Toxicology. 2005;209:135–47. doi: 10.1016/j.tox.2004.12.025. [DOI] [PubMed] [Google Scholar]
- [279].Drazen DL, Bilu D, Edwards N, Nelson RJ. Disruption of polys(ADP-ribose) polymerase (PARP) protects against stress-evoked immunocompromise. Mol Med. 2001;7:761–6. [PMC free article] [PubMed] [Google Scholar]
- [280].Aldinucci A, Gerlini G, Fossati S, Cipriani G, Ballerini C, Biagioli T, et al. A key role for poly(ADP-ribose) polymerase-1 activity during human dendritic cell maturation. J Immunol. 2007;179:305–12. doi: 10.4049/jimmunol.179.1.305. [DOI] [PubMed] [Google Scholar]
- [281].Grader-Beck T, Casciola-Rosen L, Lang TJ, Puliaev R, Rosen A, Via CS. Apoptotic splenocytes drive the autoimmune response to poly(ADP-ribose) polymerase 1 in a murine model of lupus. J Immunol. 2007;178:95–102. doi: 10.4049/jimmunol.178.1.95. [DOI] [PubMed] [Google Scholar]
- [282].Olabisi OA, Soto-Nieves N, Nieves E, Yang TT, Yang X, Yu RY, et al. Regulation of transcription factor NFAT by ADP-ribosylation. Mol Cell Biol. 2008;28:2860–71. doi: 10.1128/MCB.01746-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Saenz L, Lozano JJ, Valdor R, Baroja-Mazo A, Ramirez P, Parrilla P, et al. Transcriptional regulation by poly(ADP-ribose) polymerase-1 during T cell activation. BMC Genomics. 2008;9:171. doi: 10.1186/1471-2164-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Schraufstatter IU, Hyslop PA, Jackson J, Cochrane CC. Oxidant injury of cells. Int J Tissue React. 1987;9:317–24. [PubMed] [Google Scholar]
- [285].Yoshida M, Macklin WB. Oligodendrocyte development and myelination in GFP-transgenic zebrafish. J Neurosci Res. 2005;81:1–8. doi: 10.1002/jnr.20516. [DOI] [PubMed] [Google Scholar]
- [286].Pogoda HM, Sternheim N, Lyons DA, Diamond B, Hawkins TA, Woods IG, et al. A genetic screen identifies genes essential for development of myelinated axons in zebrafish. Dev Biol. 2006;298:118–31. doi: 10.1016/j.ydbio.2006.06.021. [DOI] [PubMed] [Google Scholar]
- [287].Panula P, Sallinen V, Sundvik Y, Kolehmainen J, Torkko V, Tiittula A, et al. Modulatory Neurotransmitter Systems and Behavior: Towards Zebrafish Models of Neurodegenerative Diseases Zebrafish. 2007;3:235–247. doi: 10.1089/zeb.2006.3.235. [DOI] [PubMed] [Google Scholar]
- [288].Chan J, Ban EJ, Chun KH, Wang S, McQualter J, Bernard C, et al. Methylprednisolone induces reversible clinical and pathological remission and loss of lymphocyte reactivity to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. Autoimmunity. 2008;41:405–13. doi: 10.1080/08916930802011258. [DOI] [PubMed] [Google Scholar]
- [289].Tsunoda I, Fujinami RS. Inside-Out versus Outside-In models for virus induced demyelination: axonal damage triggering demyelination. Springer Semin Immunopathol. 2002;24:105–25. doi: 10.1007/s00281-002-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Konno H, Yamamoto T, Suzuki H, Yamamoto H, Iwasaki Y, Ohara Y, et al. Targeting of adoptively transferred experimental allergic encephalitis lesion at the sites of Wallerian degeneration. Acta Neuropathol. 1990;80:521–6. doi: 10.1007/BF00294613. [DOI] [PubMed] [Google Scholar]
- [291].Tsunoda I, Tanaka T, Saijoh Y, Fujinami RS. Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration. Am J Pathol. 2007;171:1563–75. doi: 10.2353/ajpath.2007.070147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Barres BA, Raff MC. Axonal control of oligodendrocyte development. J Cell Biol. 1999;147:1123–8. doi: 10.1083/jcb.147.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Beattie CE, Melancon E, Eisen JS. Mutations in the stumpy gene reveal intermediate targets for zebrafish motor axons. Development. 2000;127:2653–62. doi: 10.1242/dev.127.12.2653. [DOI] [PubMed] [Google Scholar]
- [294].Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–6. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- [295].Lassmann H, Ammerer HP, Kulnig W. Ultrastructural sequence of myelin degradation. I. Wallerian degeneration in the rat optic nerve. Acta Neuropathol. 1978;44:91–102. doi: 10.1007/BF00691474. [DOI] [PubMed] [Google Scholar]
- [296].McPhilemy K, Mitchell LS, Griffiths IR, Morrison S, Deary AW, Sommer I, et al. Effect of optic nerve transection upon myelin protein gene expression by oligodendrocytes: evidence for axonal influences on gene expression. J Neurocytol. 1990;19:494–503. doi: 10.1007/BF01257239. [DOI] [PubMed] [Google Scholar]
- [297].Jiang H, Duchala CS, Awatramani R, Shumas S, Carlock L, Kamholz J, et al. Proteolipid protein mRNA stability is regulated by axonal contact in the rodent peripheral nervous system. J Neurobiol. 2000;44:7–19. doi: 10.1002/1097-4695(200007)44:1<7::aid-neu2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [298].Popko B. Myelin: not just a conduit for conduction. Nat Genet. 2003;33:327–8. doi: 10.1038/ng0303-327. [DOI] [PubMed] [Google Scholar]
- [299].Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–74. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- [300].Tang BL, Chua CE. SIRT2, tubulin deacetylation, and oligodendroglia differentiation. Cell Motil Cytoskeleton. 2008;65:179–82. doi: 10.1002/cm.20253. [DOI] [PubMed] [Google Scholar]
- [301].Konno H, Yamamoto T, Iwasaki Y, Suzuki H, Saito T, Terunuma H. Wallerian degeneration induces Ia-antigen expression in the rat brain. J Neuroimmunol. 1989;25:151–9. doi: 10.1016/0165-5728(89)90132-x. [DOI] [PubMed] [Google Scholar]
- [302].Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- [303].Miller RH, Bai L. Cellular approaches for stimulating CNS remyelination. Regen Med. 2007;2:817–29. doi: 10.2217/17460751.2.5.817. [DOI] [PubMed] [Google Scholar]
- [304].Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch Neurol. 2008;65:452–6. doi: 10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- [305].Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–94. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- [306].Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–61. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- [307].Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, et al. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol. 2007;61:209–18. doi: 10.1002/ana.21033. [DOI] [PubMed] [Google Scholar]
- [308].Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–27. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- [309].Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, et al. Neurosphere-derived multipotent precursors promote neuro-protection by an immunomodulatory mechanism. Nature. 2005;436:266–71. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- [310].Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, Gao JL, Murphy PM, Oppenheimer N, et al. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol. 2004;172:1896–906. doi: 10.4049/jimmunol.172.3.1896. [DOI] [PubMed] [Google Scholar]
- [311].Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell. 2008;2:241–51. doi: 10.1016/j.stem.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Mantel CR, Wang RH, Deng C, Broxmeyer HE. SIRT1, notch and stem cell “age asymmetry”. Cell Cycle. 2008;7:2821–5. doi: 10.4161/cc.7.18.6517. [DOI] [PubMed] [Google Scholar]
- [313].Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, et al. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–59. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- [315].Benyo Z, Gille A, Kero J, Csiky M, Suchankova MC, Nusing RM, et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115:3634–40. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–11. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Broxmeyer HE. Mechanism unknown: prostaglandin E2 may improve HSC therapies. Cell Stem Cell. 2007;1:135–6. doi: 10.1016/j.stem.2007.05.008. [DOI] [PubMed] [Google Scholar]
- [318].von Bergwelt-Baildon MS, Popov A, Saric T, Chemnitz J, Classen S, Stoffel MS, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–37. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- [319].Zheng X, Koropatnick J, Li M, Zhang X, Ling F, Ren X, et al. Reinstalling antitumor immunity by inhibiting tumor-derived immunosuppressive molecule IDO through RNA interference. J Immunol. 2006;177:5639–46. doi: 10.4049/jimmunol.177.8.5639. [DOI] [PubMed] [Google Scholar]
- [320].Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–9. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- [321].Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- [322].Williams AC, Ramsden DB. Pellagra: A clue as to why energy failure causes diseases? Med Hypotheses. 2007;69:618–28. doi: 10.1016/j.mehy.2007.01.029. [DOI] [PubMed] [Google Scholar]
- [323].Lanska DJ. Stages in the recognition of epidemic pellagra in the United States: 1865-1960. Neurology. 1996;47:829–34. doi: 10.1212/wnl.47.3.829. [DOI] [PubMed] [Google Scholar]
- [324].Darvay A, Basarab T, McGregor JM, Russell-Jones R. Isoniazid induced pellagra despite pyridoxine supplementation. Clin Exp Dermatol. 1999;24:167–9. doi: 10.1046/j.1365-2230.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- [325].Jorgensen J. Pellagra probably due to pyrazinamide: development during combined chemotherapy of tuberculosis. Int J Dermatol. 1983;22:44–5. doi: 10.1111/j.1365-4362.1983.tb02114.x. [DOI] [PubMed] [Google Scholar]
- [326].Stevens HP, Ostlere LS, Begent RH, Dooley JS, Rustin MH. Pellagra secondary to 5-fluorouracil. Br J Dermatol. 1993;128:578–80. doi: 10.1111/j.1365-2133.1993.tb00240.x. [DOI] [PubMed] [Google Scholar]
- [327].Dreizen S, McCredie KB, Keating MJ, Andersson BS. Nutritional deficiencies in patients receiving cancer chemotherapy. Postgrad Med. 1990;87:163–7. 170. doi: 10.1080/00325481.1990.11704531. [DOI] [PubMed] [Google Scholar]
- [328].Monteiro JP, da Cunha DF, Filho DC, Silva-Vergara ML, dos Santos VM, da Costa JC, Jr., et al. Niacin metabolite excretion in alcoholic pellagra and AIDS patients with and without diarrhea. Nutrition. 2004;20:778–82. doi: 10.1016/j.nut.2004.05.008. [DOI] [PubMed] [Google Scholar]
- [329].Jacobson EL. Niacin deficiency and cancer in women. J Am Coll Nutr. 1993;12:412–6. doi: 10.1080/07315724.1993.10718330. [DOI] [PubMed] [Google Scholar]
- [330].Leigh D. Pellagra and the nutritional neuropathies: a neuropathological review. J Ment Sci. 1952;98:130–42. doi: 10.1192/bjp.98.410.130. [DOI] [PubMed] [Google Scholar]
- [331].Hyyppa MT, Jolma T, Riekkinen P, Rinne UK. Effects of L-tryptophan treatment on central indoleamine metabolism and short-lasting neurologic disturbances in multiple sclerosis. J Neural Transm. 1975;37:297–304. doi: 10.1007/BF01258656. [DOI] [PubMed] [Google Scholar]
- [332].Harrer G, Fischbach R. Effect of specific amino acids on the hotbathtest in multiple sclerosis patients. J Neural Transm. 1973;34:205–14. doi: 10.1007/BF01367510. [DOI] [PubMed] [Google Scholar]
- [333].Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–5. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- [334].Asimov I. Asimov's Giude to Science. Basic Books Inc., Publishers; New York: 1972. [Google Scholar]
- [335].Kirkland J. Handbook of vitamins. Taylor & Francis; Boca Raton: 2007. [Google Scholar]
- [336].Elvehjem CA, Madden RJ, Strong FM, Woolley DW. Relation of nicotinic acid and nicotinic acid amide to canine black tongue. J. Am. Chem. Soc. 1937;59:1767. [Google Scholar]
- [337].Pollan M. In Defense of Food: An Eater's Manifesto. Penguin Press; 2008. [Google Scholar]
- [338].Cleave TL. The saccharine disease : conditions caused by the taking of refined carbohydrates, such as sugar and white flour. J. Wright; Bristol: 1974. [Google Scholar]
- [339].Horvath EM, Benko R, Gero D, Kiss L, Szabo C. Treatment with insulin inhibits poly(ADP-ribose)polymerase activation in a rat model of endotoxemia. Life Sci. 2008;82:205–9. doi: 10.1016/j.lfs.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Ihnat MA, Thorpe JE, Kamat CD, Szabo C, Green DE, Warnke LA, et al. Reactive oxygen species mediate a cellular `memory' of high glucose stress signalling. Diabetologia. 2007;50:1523–31. doi: 10.1007/s00125-007-0684-2. [DOI] [PubMed] [Google Scholar]
- [341].Willcock HG, Hopkins FG. Nutrition classics from The Journal of Physiology. 19061907;35:88–102. The importance of individual amino-acids in metabolism. Observations on the effect of adding tryptophane to a dietary in which zein is the sole nitrogenous constituent. By Willcock Edith G., Hopkins F. Gowland. Nutr Rev. 1975;33:15–7. doi: 10.1111/j.1753-4887.1975.tb07079.x.
- [342].Fernstrom JD, Wurtman RJ. Effect of chronic corn consumption on serotonin content of rat brain. Nat New Biol. 1971;234:62–4. doi: 10.1038/newbio234062a0. [DOI] [PubMed] [Google Scholar]
- [343].Pollan M. The omnivore's dilemma : a natural history of four meals. Penguin Press; New York: 2006. [Google Scholar]
- [344].Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. J Clin Invest. 2005;115:1449–57. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, et al. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2007;37:1290–301. doi: 10.1002/eji.200636837. [DOI] [PubMed] [Google Scholar]
- [346].Orabona C, Puccetti P, Vacca C, Bicciato S, Luchini A, Fallarino F, et al. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood. 2006;107:2846–54. doi: 10.1182/blood-2005-10-4077. [DOI] [PubMed] [Google Scholar]