Abstract
Changes of the water permeability aqùaporin (AQP) activity of leaf cells were investigated in response to different light regimes (low versus high). Using a cell pressure probe, hydraulic properties (half-time of water exchange, T1/2 ∞ 1/water permeability) of parenchyma cells in the midrib tissue of maize (Zea mays L.) leaves have been measured. A new perfusion technique was applied to excised leaves to keep turgor constant and to modify the environment around cells by perfusing solutions using a pressure chamber. In response to low light (LL) of 200 μmol m−2 s−1, T1/2 decreased during the perfusion of a control solution of 0.5 mM CaCl2 by a factor of two. This was in line with earlier results from leaf cells of intact maize plants at a constant turgor. In contrast, high light (HL) at intensities of 800 μmol m−2 s−1 and 1800 μmol m−2 s−1 increased the T1/2 in two-thirds of cells by factors of 14 and 35, respectively. The effects of HL on T1/2 were similar to those caused by H2O2 treatment in the presence of Fe2+, which produced ·OH (Fenton reaction; reversible oxidative gating of aquaporins). Treatments with 20 mM H2O2 following Fe2+ pre-treatments increased the T1/2 by a factor of 30. Those increased T1/2 values could be partly recovered, either when the perfusion solution was changed back to the control solutuion or when LL was applied. 3mM of the antioxidant glutathione also reversed the effects of HL. The data suggest that HL could induce reactive oxygen species (ROS) such as ·OH, and they affected water relations. The results provide evidence that the varying light climate adjusts water flow at the cell level; that is, water flow is maximized at a certain light intensity and then reduced again by HL. Light effects are discussed in terms of an oxidative gating of aquaporins by ROS.
Keywords: Aquaporin, cell pressure probe, glutathione, hydraulic conductivity, hydrogen peroxide, light, oxidative gating, reactive oxygen species, Zea mays
Introduction
Plants experience various regimes of light intensity, which is an important issue during photosynthesis. In response to light, stomata open to fix carbon diffusing in as CO2, and plants lose substantial amounts of water using the same pathway. Therefore, plants developed strategies to manage the use of water efficiently in response to changes in the light regime. It has been shown that the overall hydraulic conductance of leaves is affected by light (Sack et al., 2002; Lo Gullo et al., 2005; Nardini et al., 2005; Tyree et al., 2005; Sack and Holbrook, 2006; Cochard et al., 2007). In part, this could have happened either by a change of the conductance of the vascular system (Zwieniecki et al., 2001) or by changes of water permeability of the living cells of xylem parenchyma or bundle sheath (cell Lp; Nardini et al., 2005; Tyree et al., 2005). The change in cell Lp in response to light was speculated to occur via a gating of water channel activity sitting in the cell membranes (aquaporins, AQPs). However, there has been, to date, no direct evidence of a gating of AQPs by light. According to Cochard et al. (2007), the level of AQP transcripts increased in response to light. Kim and Steudle (2007) recently started to fill the gap by measuring changes in cell Lp in response to light. In leaf cells of maize, they found that turgor as well as light affected cell Lp. To separate the effects of light from those of turgor, they kept the turgor constant and found that, when light was varied at low absolute values of light intensity, AQP activity increased as the light intensity increased, which was in agreement with earlier results obtained at the whole leaf level (see above).
There is increasing evidence that many environmental factors affect AQPs (Steudle, 2000; Maurel and Chrispeels, 2001; Tournaire-Roux et al., 2003; Lee et al., 2005). Light could be one of them. It is plausible that a gating of AQPs by light may involve an oxidative gating of AQP activity (Henzler et al., 2004; Ye and Steudle, 2006). As photosynthesis produces O2 and reduction equivalents, damage by reactive oxygen species (ROS) may be anticipated by reactions involving the partial or complete reduction of O2 and the production of ROS (such as superoxide, H2O2, and the hydroxyl radical ·OH; Foyer and Noctor, 2000; Dietz, 2008). In the Chara internode and maize roots, an oxidative gating of AQPs has been demonstrated by Henzler et al. (2004) and Ye and Steudle (2006). These authors showed that AQPs could be closed by H2O2/·OH, and that closure was reversible. According to Aroca et al. (2005), treatment with 100 μM H2O2 decreased the root hydraulic conductance in a chilling-sensitive, but not in a chilling-tolerant maize genotype. Oxidative gating may be a common response to different kinds of stresses (Pastori and Foyer, 2002; Xiong et al., 2002), and it may provide appropriate adjustments in water relations (Ye and Steudle, 2006).
In the present study, the experiments of Kim and Steudle (2007) have been extended from low to high levels of light intensity to provide further insights into the mechanism(s) of a gating of cell Lp via changes of AQP activity. Measured cells were parenchyma cells in the midrib of maize leaves (Westgate and Steudle, 1985; Wei et al., 1999). These cells are (i) located in the vicinity of photosynthetically active cells, stomata, and xylem vessels; (ii) easy to puncture due to their large size; and (iii) also suitable for long-term measurements in single cells (up to 6 h), since the midrib tissue could be well fixed, and has, therefore, been used as a model system by Kim and Steudle (2007). A cell pressure probe was applied to access cell Lp. The perfusion of excised leaves allowed infiltration of leaf tissue via cut xylem vessels to adjust apoplastic concentrations and to apply H2O2 and the antioxidant glutathione (GSH). Most importantly, turgor could be kept constant by infiltration, i.e., this variable affecting AQP activity was excluded during light treatments. Different effects of light at two light regimes were investigated [low light (LL), up to 650 μmol m−2 s−1; and high light (HL), 800 μmol m−2 s−1 and 1800 μmol m−2 s−1]. In response to LL, cell Lp increased. HL regimes, however, caused a decrease, as did the infiltration of solutions of H2O2/·OH. The data are discussed in terms of gating of AQPs by ROS.
Materials and methods
Plant material
Maize (Zea mays L. cv. symphony) plants were grown from caryopses in soil in a greenhouse of Bayreuth University as described by Kim and Steudle (2007). In the lab, where the experiments were performed, the ambient light intensity was ∼5 μmol m−2 s−1 (20–25 °C; relative humidity = 30–60%). Experiments were conducted on 4- to 8-week-old plants, which were 0.8–1.2 m tall and had about eight leaves.
The parenchyma cells used in the pressure probe experiments were located in the midrib region 100–200 mm behind the tip of leaves. Cells measured were located at a distance of 100–200 μm from the abaxial surface of the midrib, i.e. in the same range as those used by Kim and Steudle (2007). They usually contained no chlorophyll, but they were close to photosynthetically active cells (∼50 μm away; see cross-section in Fig. 1 of Kim and Steudle, 2007). Third or fourth leaves from the plants were used for experiments. Leaf blades were cut to a length of ∼0.3–0.4 m. About 40 mm of the leaf tip was cut and removed to enhance transport through xylem vessels by perfusion.
Fig. 1.
Experimental set-up used. A pressure chamber was used to perfuse a leaf tissue at constant turgor pressure during illumination and to provide a particular ionic apoplastic environment. The chamber was provided with different solutions, which were infiltrated at a pressure of 0.1–0.2 MPa above atmospheric. When pressurizing the basal cut end of the xylem, guttation droplets appeared at the leaf margin and at the cut surface of the leaf outside the pressure chamber. During perfusion, water flow across the tissue was substantial, tending to exchange the solution of the apoplast within 5–20 min (see Materials and methods). A cell pressure probe was used to measure turgor and hydraulic conductivity of a cell (cell Lp). The meniscus between the cell sap and silicone oil in the probe was controlled while observing it with a stereo microscope.
Experimental set-up using a CPP
A Leitz manipulator (Wetzler, Germany) was used to carry a cell pressure probe (CPP; Kim and Steudle, 2007). It was fixed on a thick iron plate placed on a heavy stone table to minimize vibrations during the CPP measurements. Using magnetic bars, a cut leaf was fixed upside down on a metal sledge to expose the midrib securely for measurements of cell hydraulics. The basal cut end of the leaf was encased in a pressure chamber (Fig. 1), where it was immersed in a solution of defined composition (see below). The microcapillary attached to the CPP was filled with silicone oil (oil type AS4 from Wacker, Munich, Germany). It had a fine tip of up to ∼8 μm in diameter. When midrib cells were punctured, a meniscus formed between the cell sap and oil within the tip and tended to move away from the cell. Using the metal rod of the CPP, the meniscus was re-adjusted at a position close to the surface of the midrib. This restored cell sap volume close to its original value. The pressure transducer of the CPP measured turgor pressure (P), which was recorded by a computer. To investigate the hydraulic conductivity of cell membranes (Lp), hydrostatic relaxations of turgor were induced by instantaneously moving the meniscus to another position and keeping it there. To avoid effects of large pressure pulses (‘energy injection’; Wan et al., 2004), peak sizes of hydrostatic relaxations were < 0.1 MPa. The half-times of hydrostatic relaxation, i.e. the time taken for pressure pulses to reach half of the original peak (T1/2s), were inversely proportional to Lp. Further details of the function of the CPP can be found in previous studies (e.g. Steudle, 1993; Henzler and Steudle, 1995). In this study, T1/2 was used as a direct measure of changes of Lp to reduce the effect of error propagation when calculating Lp (Wan et al., 2004; Lee et al., 2005; Ye and Steudle, 2006). This could be accepted, as the volumetric elastic modulus of the cell (ε), which is also a parameter to determine Lp, did not change significantly during measurements (Kim and Steudle, 2007). To avoid variations between cells, relative changes of T1/2 rather than absolute changes were often compared. Treatments were performed on individual cells. This required measurement of hydraulic parameters of a given cell for up to 4 h.
Perfusion of leaves with solutions of defined composition (defined apoplastic environment)
The apoplastic environment of cells was varied during treatments, for example by infiltration of AQP inhibitors. In order to perfuse a leaf tissue at constant turgor pressure during illumination and to provide a certain ionic apoplastic environment, the pressure chamber (see above) was provided with different solutions, which were infiltrated at a pressure of 0.1–0.2 MPa above atmospheric. When pressurizing the basal cut end of the xylem, guttation droplets appeared at the leaf margin and at the cut surface of the leaf outside of the pressure chamber. To test how long it took for the perfusion solution to move across the tissue, 70 mM H2O2 solution was injected in the chamber. The appearance of H2O2 in the other cut end and leaf margins was qualitatively tested with KI and starch solution. Droplets in the midrib of the cut end were collected and added to KI–starch solution. The presence of H2O2 in the droplets changed the solution to a blue colour. According to the tests, the time required for the solution to move across the tissue and to modify the apoplastic solution was 5–20 min, depending on the pressure applied to the pressure chamber and resistance in the water pathway of the leaf. During 1 h perfusion at 0.2 MPa, the amount of solution that passed through the leaf tissue was of an order of magnitude which was similar to the volume of the excised leaf. Assuming the apoplast was 10% of the leaf volume (Kosala Ranathunge, University of Bonn, personal communication), this was equivalent to 10 times the volume of the apoplast. The perfusion solution could be exchanged via a tube leading into the chamber, which could be closed by a valve, when the chamber was pressurized. This allowed exchange of the apoplastic solution quickly and also for the solution to be changed back to the original solution during measurements with an individual cell. To minimize variability between cells, it was intended to measure effects and reversibility on individual cells.
Illumination experiments
In order to run the CPP experiments, both the microcapillary and tissue near the cell punctured had to be illuminated using an Osram halogen lamp (150 W, Xenophot HLX, Munich, Germany) through glass fibre optics (Schott, Mainz, Germany). The light intensity at the tissue level was ∼50 μmol m−2 s−1. The T1/2s were continuously measured after a cell was punctured. Using the glass fibre optics, the light intensity could be increased to values as high as 200 μmol m−2 s−1 (LL). Usually, the effects of light treatment were measured following changes of T1/2. To apply light intensities which are similar to that in the field during a bright day (∼2000 μmol m−2 s−1; Nobel, 1999), a screen projector as used for powerpoint presentations (AstroBeamX211 with 200 W UHP lamp, A+K, UK) was positioned at a distance of 200–300 mm from the specimen. This was usually turned on for 15 min to produce light intensities of between 800 μmol m−2 s−1 and 1800 μmol m−2 s−1 at the tissue level [as regulated by the distance of the light source from the leaf surface (HL)]. The UV content of the light source (UVA and UVB of the spectrum from 290 nm to 390 nm) was measured by a UV light meter (PeakTech, Ahrensburg, Germany). It was verified that the light source contained less UV light (1–2 W m−2) than the sunlight measured outside on a bright day (PAR=1900 μmol m−2 s−1, UV=47 W m−2). Relaxations (T1/2s) were continuously measured during light and other treatments.
Perfusion solutions
As a control, leaves were perfused with a solution of 0.5 mM CaCl2 solution, which resulted in a constant turgor pressure for at least 4 h in a given cell, even under conditions of high rates of transpiration such as occur at HL. Effects of H2O2 were measured by changing from 0.5 mM CaCl2 to solutions that contained either 20 mM or 70 mM H2O2 in addition to the CaCl2. In the presence of natural levels of Fe2+, this should produce ·OH (Fenton reaction: H2O2+Fe2+=Fe3++OH–+·OH). In order to enhance the level of ·OH, leaves were perfused with 0.5 mM CaCl2+3 mM FeSO4 for 1–2 h, and then the perfusion solution was changed to 0.5 mM CaCl2+20 mM H2O2 to increase the oxidative stress. To see the recovery from stress treatment, solutions were changed back to 0.5 mM CaCl2. To demonstrate the effects of the antioxidant GSH, leaves were perfused by 3 mM GSH and 0.5 mM CaCl2, and the perfusion solution was then changed back to the control solution. The solution containing GSH had a pH of 3.5. Other solutions had a pH of 4.8, which was similar to the known apoplastic pH of leaves in different plant species (4.7–5.1; Felle and Hanstein, 2007).
Results
Half-times of hydrostatic relaxations, T1/2 ∞ 1/Lp
From the pressure relaxations, Lp values could be worked out, when the elastic modulus (ε) was also measured. However, in most cases, T1/2s (inversely proportional to cell Lp) were taken as a measure of cell Lp because ε did not change during treatments for a given cell (Kim and Steudle, 2007). In most cases, there was no transient effect of puncturing on T1/2 (Lp) as observed with young maize roots (Wan et al., 2004). When there was such an effect, it took 10 min, which was sufficient to achieve a constant T1/2. At the ambient light intensity of 50 μmol m−2 s−1, the T1/2s of cells from leaves infiltrated with 0.5 mM CaCl2 ranged from 0.3 s to 35 s (mean±SD, 4.8±7.7 s, n=31 cells; Fig. 2). The variability of T1/2 was also known in the same tissue of intact maize plants (Kim and Steudle, 2007). However, >75% of those cells (24 out of 31 cells with 0.5 mM CaCl2) had T1/2s of <4 s, and one-third of cells had T1/2s between 1.0 s and 2.0 s (12 out of 31 cells; Figs 2, 3A). Cells infiltrated with 0.5 mM CaCl2/3 mM FeSO4 had T1/2s similar to those infiltrated with 0.5 mM CaCl2 (range=0.8–17 s; mean±SD, 3.6±3.8 s, n=24 cells; 21 out of 24 cells had a T1/2 <4 s; Figs 2, 3B). Usually, cells probed from one leaf had similar T1/2s. For example, six cells probed from the same leaf had T1/2s ranging from 0.9 s to 2.3 s. The large T1/2s occasionally measured were observed in a few leaves and they were probably caused by a closure of AQPs, even in the absence of inhibitors or HL. Those T1/2s could be reduced by light treatment of up to 650 μmol m−2 s−1, according to the earlier results of Kim and Steudle (2007).
Fig. 2.
Frequency histogram of half-time of water exchange, T1/2 (inversely proportional to cell Lp). More than 80% of cells from excised leaves which were perfused with either 0.5 mM CaCl2 or 0.5 mM CaCl2+3 mM FeSO4 had T1/2s of <4 s. There were a few leaves showing only large T1/2s.
Fig. 3.
Representative relaxation curves used to measure half-times of water exchange, T1/2s. (A) The half-time of cells from excised leaves which were perfused with 0.5 mM CaCl2 typically ranged between 1.0 s and 2.0 s. A reversible oxidative gating of AQPs was demonstrated by following an individual cell in B to D. (B) Half-times of cells in the presence of Fe2+ were similar to those in CaCl2 and this was used as the control. (C) On the same cell as in B, addition of 20 mM H2O2 produced OH· by the Fenton reaction and caused a substantial increase in T1/2 by a factor of 27, i.e. Lp was reduced by the same factor. (D) Subsequent exchange to 0.5 mM CaCl2 to remove radicals resulted in a partial recovery of T1/2 (Lp), i.e. the effect was reversible, at least to some extent.
Responses to ·OH (Fe2+ and H2O2)
When adding 20 mM H2O2 to the reference solution (0.5 mM CaCl2) at a light intensity of 50 μmol m−2 s−1, there was no effect on T1/2 (four cells tested). Most probably, this means that Fe2+ in apoplasts was not adequate to produce a sufficiently high level of ·OH (Fenton reaction; H2O2+Fe2+=Fe3++OH–+·OH). However, when the concentration of H2O2 was raised to 70 mM, T1/2 increased by a factor of 4. When perfusing with 3 mM FeSO4+0.5 mM CaCl2, T1/2s were similar to those when using only 0.5 mM CaCl2 (see above). There was a marked increase in T1/2 when 20 mM H2O2 solutions were perfused following 2 h treatments with Fe2+ (Fig. 3C). It can be seen from the figure that T1/2 increased by a factor of as much as 14 most likley due to the action of ·OH (Fig. 3C). Changing back to 0.5 mM CaCl2 again reduced T1/2 by 50% within 30 min, to a value of 700% of the original (Fig. 3D). Recovery was observed for only up to 30 min, because long-term measurements following oxidative responses and recovery in individual cells were demanding; however, there could have been further recovery during long-term measurements. It were stressed that, during measurements, turgor pressure was kept constant. Similar experiments showing inhibition by H2O2/Fe2+ treatment and partial recovery were repeated in three different cells. Overall, T1/2 increased due to the treatment by a factor of 30, and recovered to 600% of the original value within 30 min after changing back to the control perfusion solution (Fig. 4). More data showing substantial increases in T1/2 due to H2O2/FeSO4 treatment are shown for other cells in Fig. 5A (see below).
Fig. 4.
Summary of effects of H2O2/Fe2+ on cell Lp. Values are means ±SD from three independent experiments as in Fig. 2B–D, and are shown as fold changes in T1/2 taking the value in the presence of FeSO4/CaCl2 solution as the reference. There was a significant effect of H2O2/Fe2+ (P <0.05, t-test, n=3 cells). The effects of treatments were measured following individual cells. The absolute values of T1/2 are shown in the inset.
Fig. 5.
Low light (LL) treatment of 200 μmol m−2 s−1 reduced T1/2, at constant turgor, as in the whole-plant experiments of Kim and Steudle (2007). (A) Cells having a T1/2 <2 s at the ambient light (AL) intensity of 50 μmol m−2 s−1 were manipulated to have a T1/2 >2 s by H2O2/Fe2+ treatment as shown in Fig. 3. The increased T1/2 was reduced by 30 min LL treatment by a factor of 7 (different letters indicate significant difference j t-test at P <0.05, n=3 cells). (B) Cells originally having a T1/2 >2 s at AL in CaCl2 solution showed a significant reduction in T1/2 during 30 min LL by a factor of 2 (P <0.05, t-test, n=4 cells). Values are means ±SD and are shown as fold changes. The absolute values of T1/2 are shown in the inset.
Responses to low light of 200 μmol m−2 s−1
The response to LL (200 μmol m−2 s−1) was measured in cells which originally had a low T1/2 of ∼1 s in the FeSO4/CaCl2 solution, but were then inhibited to have a large T1/2 by the addition of 20 mM H2O2. When cells exhibited long T1/2s in the presence of H2O2/FeSO4, LL of 200 μmol m−2 s−1 caused a significant reduction of T1/2 by a factor of 7 within 30 min (Fig. 5A, n=3 cells). In one experiment, T1/2 increased by a factor of 4 caused by treatment with 70 mM H2O2 could be recovered to its original value by LL of 200 μmol m−2 s−1 (data not shown). For cells which already had a large T1/2 of >2 s in the control solution (AQPs already closed), LL treatment reduced the T1/2 (Kim and Steudle, 2007). It can be seen from Fig. 5B that T1/2s were reduced by a factor of 2 within 30 min (n=4 cells). In one of those four cells, T1/2 levelled off to a small value within 30 min of light treatment, but not in the others. Longer light treatments may further reduce T1/2. It can be seen from these results that although light effects were substantial, there was a considerable variability in the LL responses.
Responses to high light of 800 μmol m−2 s−1 and 1800 μmol m−2 s−1
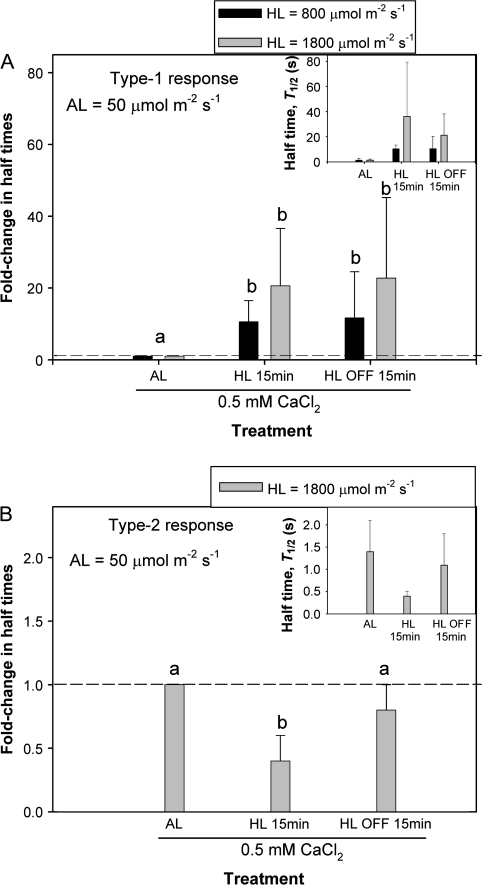
Although there was an overall trend of a reduction of T1/2 following HL treatment, this was statistically not significant. As during the LL treatment, there was a substantial variability between cells. For example, HL of 800 μmol m−2 s−1 and 1800 μmol m−2 s−1 reduced the T1/2 during the first 5–10 min of the 15 min period of illumination, but then T1/2 increased substantially by factors of 14 and 35, respectively (Fig. 6A, n=3–4 cells; type-1 response). In total, 10 cells showed an increase in T1/2 due to HL. The 15 min treatment was chosen as the maximum treatment which could be applied at constant turgor and with stable cells. Longer HL treatments caused a continuous decrease in turgor pressure, which indicated damage to the cells. In this respect, punctured cells could have been more prone to damage in the presence of light stress than other cells (see Discussion). In eight out of 10 cells, the T1/2s remained large when light was turned off for 30 min, which was the maximum period of time measured. There were, however, cells, which were hardly affected by HL, as seen in Fig. 6B (n=5 cells; type-2 response). In those cells, there was only a 37–86% reduction within 15 min. Those five cells were from two leaves, and no cells in those leaves showed a type-1 response. Maximum temperature changes on the leaf surface due to illumination with 800 μmol m−2 s−1 and 1800 μmol m−2 s−1 were 3 °C and 7 °C, respectively.
Fig. 6.
Two types of response to high light (HL) treatment. (A) HL of 800 μmol m−2 s−1 (black bars, n=4 cells) and 1800 μmol m−2 s−1 (grey bars, n=3 cells) increased the T1/2 at constant turgor in CaCl2 solution. The increase in T1/2 began ∼10 min after HL was turned on. The largest T1/2s caused by HL were significantly larger than those measured at ambient light (AL) intensity before HL treatments (P <0.05, t-test). During 15 min after the light was switched off, T1/2s remained large, i.e. not reversible within 15 min. (B) There were cells in which T1/2 did not increase but decreased due to the effect of HL of 1800 μmol m−2 s−1 (grey bars, n=5 cells). Values are means ±SD and are shown as relative changes. The absolute values of T1/2 are shown in the inset.
Responses to GSH
Cells pre-treated with the antioxidant GSH were exposed to a 15 min period of illumination at the intensity of 1800 μmol m−2 s−1. As seen in Fig. 7, a cell which had been perfused with 3 mM GSH+0.5 mM CaCl2 for 0.5–1.0 h increased its T1/2 by a factor of 46 by HL, as in the absence of GSH. However, in the presence of GSH, T1/2 recovered back to its original level within 15 min when the light was turned off (n=5 cells). This was different from treatment in the absence of GSH. Pre-treatment with 3 mM GSH of 24 h duration had pronounced ameliorative effects. In contrast to short treatments, T1/2s showed no significant increase with HL (n=4 cells). To confirm that the lack of response was due to the presence of GSH rather than a coincidence originating from the variable responses between cells (one-third of cells did not react to HL; see above), by following one cell tests were conducted to determine whether the T1/2 in control solution increased in response to HL, and then that the addition of GSH caused recovery of the T1/2 to its original value. On the same cell, light treatment in the presence of GSH caused only a temporary increase in T1/2, which eventually recovered to the original value at the ambient light intensity (data not shown). In the reverse type of experiment using 3 mM GSH, there was no response of the T1/2 by 15 min HL treatment following treatment with 3 mM GSH solution for 3 h. On the same cell, the exchange of GSH solution by the reference solution and waiting for its complete removal (∼1 h) caused a substantial increase of T1/2 by 15 min HL treatment (data not shown). Overall, the results indicate that there was a clear ameliorative effect of GSH on cell Lp (T1/2). It is unlikely that the effect of the GSH solution is due to changes in pH (from 4.8 to 3.5), since cytosolic acidification is known to reduce water permeability by a gating of AQPs (Tournaire-Roux C et al., 2003). The effects of HL may be related to oxidative stress in the presence of HL (see Discussion).
Fig. 7.
In the presence of the antioxidant glutathione (GSH), the effects of HL treatment at 1800 μmol m−2 s−1 could be reversed. Cells pre-treated with 3 mM GSH+0.5 mM CaCl2 for 0.5–1.0 h (black bars, n=4 cells) had an increased T1/2 due to HL as in the absence of GSH (as in Fig. 5). In the presence of GSH, in contrast, there was a recovery within 15 min after the light was switched off. Cells pre-treated with GSH for 24 h (grey bars, n=5 cells) did not show an increase in T1/2 by HL (P <0.05, t-test). Values are means ±SD and are shown as fold changes. The absolute values of T1/2 are shown in the inset.
Discussion
The results of this study indicate that HL intensity inhibits the AQPs in perfused leaves of maize plants. This extends earlier findings of Kim and Steudle (2007), who showed that, at constant turgor, LL intensity had an ameliorative effect on cell Lp, most probably by acting on AQPs. The treatment of the tissue with oxidants (H2O2 and ·OH as produced by the Fenton reaction; H2O2+Fe2+=Fe3++OH–+.OH·) had an effect similar to HL. Perfusion with a solution of the antioxidant GSH increased cell Lp (reduced T1/2) in cells having a long T1/2. The presence of the antioxidant tended to prevent the inhibition by HL. This may indicate that there was indeed an action of ROS on AQPs in the leaf caused by HL. Similar findings of an oxidative gating of AQPs have been shown with Chara internodes and maize roots (Henzler et al., 2004; Ye and Steudle, 2006). In Chara, H2O2 in the presence of Fe2+ caused a reversible oxidative gating and reduced cell Lp by >90%. In the presence of rapidly permeating solutes, anomalous (negative) osmosis could be observed when AQPs were closed (Henzler et al., 2004). Ye and Steudle (2006) showed that in root cells, AQP activity was reduced by a factor of 9. At the whole root level, the reduction was smaller by a factor of 3, as expected from the composite transport model of the root. In the present study, effects of HL on cell Lp in leaves were shown for the first time, suggesting that this may also be related to an oxidative gating. It is known that during light stress, ROS develop in leaves by the partial reduction of oxygen or from hydrogen peroxide produced in metabolic reactions (Foyer and Noctor, 2000).
It may be argued that the huge effects caused by HL on cell Lp (AQP activity) could be an artefact caused by the fact that cells had to be punctured to measure water relations parameters, and that these cells were more susceptible to stress. If true, the effects on the ‘intact’ system could have been different. In principle, this may be true, but is unlikely, because cells punctured by the CPP had stable turgor for up to 6 h, indicating high membrane integrity and stability. Also, punctured cells showed reversible responses during treatments (light, inhibitors, and GSH), as expected. This was true, although there was a substantial variability between cells which could have been caused by other factors (see below). It appears that there is, at present, no alternative technique for measuring light responses at the level of individual, intact tissue cells, which is completely non-invasive.
Because a molecular analysis of AQPs was not provided, the interpretation of the present data in terms of a gating of AQPs may be questioned. In the present study, what was directly measured was the water permeability of parenchyma cells in the midrib of cut leaves from maize plants (cell Lp). However, it would be difficult to find an alternative plausible interpretation different from the one offered. In maize, AQPs were classified into four different groups of proteins, as plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins, Nod26-like intrinsic proteins, and small and basic intrinsic proteins (Chaumont et al., 2001). To date, there have been no data showing the effects of light on any of these AQPs. In walnut, the transcript abundance of two AQPs (JrPIP2,1 and JrPIP 2,2) in response to light was correlated to the overall leaf hydraulic conductance (Kleaf; Cochard et al., 2007). The activation of AQPs by light has been speculated from Kleaf measurements (Nardini et al., 2005; Tyree et al., 2005). Kim and Steudle (2007) started to fill the gap between the two levels by measuring changes in cell Lp in response to light. All the currently available data attribute changes in cell Lp to a gating of AQPs. The results of this study support this interpretation, namely by involving an oxidative gating of AQPs in addition to the light effect. HL decreased cell Lp most probably by closure of AQPs, whereas LL aided the recovery of cell Lp to a high value by opening of AQPs. The closure of AQPs by HL could be tightly connected to an oxidative gating by ROS produced during HL treatment (see below).
Earlier it was found that both turgor and light affect cell Lp, hence a perfusion technique in cut leaves was applied to keep the turgor constant. Kim and Steudle (2007) showed that, as transpiration increased during light treatment, turgor decreased, and this resulted in a decrease of cell Lp. Turgor had to be kept constant to separate the effects of turgor from those of light. The perfusion technique used in the present study allowed this. Furthermore, leaves could be perfused with solutions of defined composition. For example, when using the perfusion technique, either H2O2 or the antioxidant GSH could be perfused. In the future, this technique could be used further to test other solutes such as heavy metals or solutions with different pH or pKa. It is unlikely that the perfusion of leaf tissue with solutions saturated with oxygen could have caused the cells to be deprived of oxygen and carbon dioxide, but this point needs to be clarified in further experiments.
The light intensities used in this study were up to 1800 μmol m−2 s−1, which is comparable with outdoors on a bright day (∼2000 μmol m−2 s−1; Nobel, 1999). It is known that HL produces ROS. ROS are unstable partly reduced oxygen species, produced as by-products of photosynthesis (singlet oxygen, H2O2, hydroxyl radical, superoxide; Foyer and Noctor, 2000; Jiang and Zhang, 2001). In Arabidopsis, Fryer et al. (2002) could visualize the generation of ROS in response to a light intensity of 600 μmol m−2 s−1 by digital imaging. ROS are known to cause oxidative damage such as lipid peroxidation, denaturation of proteins, and DNA mutation (Jiang and Zhang, 2001). Plants have protective mechanisms to get rid of stresses caused by ROS by an adjustment of antioxidants such as ascorbate (ASC) or GSH. In the present study, HL treatment resulted in a decrease of cell Lp, which was similar to that obtained by exogenous H2O2 (Fe2+) applied by perfusion. This supports the idea of an action of ROS during HL. In addition, the effects of HL were greater, when the Fe2+ level in the tissue was elevated. Further support for an action of ROS was derived from the fact that perfusion of the tissue with the antioxidant GSH protected AQPs from inhibition, probably by reducing ROS. Although the amounts of GSH which reached the inside of the cells were probably small (Gukasyan et al., 2002), the intracellular ratio GSHred/GSHox, which determines the redox potential, could have been high and sufficient to reduce ROS. From the findings of Henzler et al. (2004) on Chara internodes, it is known that ROS (including ·OH) react on AQPs, even when ROS are present at a very low concentration. Overall, the different circumstantial evidence suggested that the response of cell Lp to HL was caused by ROS.
Available data on overall water transport in leaves in response to light (Kleaf; see Introduction) suggest a trend for light increasing Kleaf. Light intensities used in those studies ranged from 400 μmol m−2 s−1 to 1400 μmol m−2 s−1 for different herbaceous and woody species (Sack et al., 2002, 1200 μmol m−2 s−1; Nardini et al., 2005, 400 μmol m−2 s−1; Tyree et al., 2005, 1000–1200 μmol m−2 s−1; Cochard et al., 2007, 1400 μmol m−2 s−1). At first sight this seems to contradict the present findings (i.e. a decrease in cell Lp at HL of 800 μmol m−2 s−1 and 1800 μmol m−2 s−1). However, this may not be true for the following reasons. (i) There is an increase of cell Lp at lower light intensity (Fig. 5A; Kim and Steudle, 2007). It may be that there is a maximum cell Lp in response to light intensity, which may be species dependent. This has to be determined in future studies. (ii) Kleaf may incorporate effects other than those related to membranes (Zwieniecki et al., 2001; Tyree et al., 2005). (iii) So far, increases of Kleaf have been demonstrated using the ‘high pressure flow meter’ (HPFM), where high pressures were applied during infiltration, which, in part, was different from the present measurements (Sack et al., 2002, 0.5–0.6 MPa; Nardini et al., 2005, 0.15 MPa; Tyree et al., 2005, 0.3–0.5 MPa; Cochard et al., 2007, 0.2 MPa). Using figleaf gourd, Lee et al. (2008) measured midrib cell Lp in response to a light intensity of 300 μmol m−2 s−1. Those authors observed a decrease in Lp. However, they did not control turgor, which may have decreased as transpiration increased with light.
There was a wide variability in response to light between cells. Light effects may be different not only for different species but also for tissues. For example, in Arabidopsis, singlet oxygen and superoxide production were primarily located in mesophyll tissue whereas hydrogen peroxide accumulation was localized in the vascular tissues (Fryer et al., 2002). Maize leaf cells are known to have differential intercellular partitioning of GSH metabolism (Noctor et al., 2002). Glutathione reductase was localized only in leaf mesophyll cells, but other antioxidant enzymes could be restricted to bundle sheath cells. In the present study, there was also some variability in response to HL in that one-third of cells did not show stress response to HL. Although the former possibility cannot be neglected, there is an indication that this variability in response to HL could have originated from differences of individual leaves. All the five cells measured from two leaves showed no stress response. Those leaves could have been more resistant to HL. Problems related to variability of cells were in part solved by measuring the effects of treatments in individual cells, which required measurements to be taken for at least 1–4 h. Further investigations on the response of different types of cells to light (oxidative) stress are required. Although there have been attempts to work out the contribution of vessels and of non-vascular tissue in the overall measurements, it is still necessary to work out, in greater detail, the main hydraulic resistances in leaf water transport and how they depend on light intensity (Cochard et al., 2004; Nardini et al., 2005; Sack et al., 2005).
Although the exact mechanism(s) of how ROS react with AQPs are not yet known, it has been shown that AQPs reversibly close in the presence of ROS (Henzler et al., 2004; Ye and Steudle, 2006). ROS could be present in different amounts at different light levels. At LL intensity, the level of ROS could be low and ROS could be reduced by the antioxidants present such as ASC or GSH. However, regardless of the precise mechanisms of the action of ROS, the data indicate that there are interactions between light climate and water relations, which should be of key importance during photosynthesis. To maximize photosynthesis, enough water, carbon dioxide, and light should be gained. The open/closed state of stomata is regulated not only by light but largely by the water status of leaves. Stomata can only be kept open when there is enough water uptake. Plants need to manage the resources to maximize productivity. As the present results show, the management of water resources may be a complex process involving many factors required to maximize productivity, which requires an interaction between light intensity and water flow. The present data indicate that LL may promote water flow, but that, at HL, water flows are down-regulated using ROS as messengers, which may be a common ‘alarm’ signalling system to provide defences against harmful environmental challenges (Pastori and Foyer, 2002).
In conclusion, the study presents the first evidence of an inhibition of cell Lp by HL, which was most probably caused by an action of ROS on AQPs. HL responses of parenchyma cells in the midrib of the maize leaf were similar to those caused by H2O2/·OH treatment. On the other hand, the antioxidant GSH had an ameliorative effect. Unlike HL, AQP activity increased at LL, which was in agreement with earlier results from leaves of intact plants. There should be an optimal light intensity to maximize water flow across leaf cells, but enhanced water flow could be inhibited at a certain light intensity. One may speculate that, by acting on the redox status of leaves, the light climate directly interacts with the water status of plants in a way which is different from that via stomata. It involves an action on the water supply of leaves by triggering cell Lp (AQP activity).
Acknowledgments
The authors thank Burkhard Stumpf (Department of Plant Ecology, University of Bayreuth) for his expert technical assistance. They are indebted to Dr Kosala Ranathunge (University of Bonn, Germany) for reading the manuscript. This work was supported by a grant of the Deutscher Akademischer Austauschdienst (DAAD) to YXK.
References
- Aroca R, Amodeo G, Fernandez-Illescas S, Herman EM, Chaumont F, Chrispeels MJ. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiology. 2005;137:341–353. doi: 10.1104/pp.104.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiology. 2001;125:1206–1215. doi: 10.1104/pp.125.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Nardini A, Coll L. Hydraulic architecture of leaf blades: where is the main resistance? Plant, Cell and Environment. 2004;27:1257–1267. [Google Scholar]
- Cochard H, Venisse J-S, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiology. 2007;143:122–133. doi: 10.1104/pp.106.090092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ. Redox signal integration: from stimulus to networks and genes. Physiologia Plantarum. 2008;133:459–468. doi: 10.1111/j.1399-3054.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- Felle HH, Hanstein S. Probing apoplastic ion relations in Vicia faba as influenced by nutrition and gas exchange. In: Sattelmacher B, Horst WJ, editors. The apoplast of higher plants: compartment of storage, transport and reactions. Dordrecht, The Netherlands: Springer; 2007. pp. 295–306. [Google Scholar]
- Foyer CH, Noctor G. Tansley Review No. 112. Oxygen processing in photosynthesis: regulation and signalling. New Phytologist. 2000;146:359–388. [Google Scholar]
- Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photo-oxidative stress responses in leaves. Journal of Experimental Botany. 2002;53:1249–1254. [PubMed] [Google Scholar]
- Gukasyan HJ, Lee VHL, Kim KJ, Kannan R. Net glutathione secretion across primary cultured rabbit conjunctival epithelial cell layers. Investigative Ophthalmology & Visual Science. 2002;43:1154–1161. [PubMed] [Google Scholar]
- Henzler T, Steudle E. Reversible closing of water channels in Chara internodes provides evidence for a composite transport model of the plasma membrane. Journal of Experimental Botany. 1995;46:199–209. [Google Scholar]
- Henzler T, Ye Q, Steudle E. Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals. Plant, Cell and Environment. 2004;27:1184–1195. [Google Scholar]
- Jiang MY, Zhang JH. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant and Cell Physiology. 2001;42:1265–1273. doi: 10.1093/pcp/pce162. [DOI] [PubMed] [Google Scholar]
- Kim YX, Steudle E. Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays. Journal of Experimental Botany. 2007;58:4119–4129. doi: 10.1093/jxb/erm270. [DOI] [PubMed] [Google Scholar]
- Lee SH, Chung GC, Steudle E. Low temperature and mechanical stresses differently gate aquaporins of root cortical cells of chilling-sensitive cucumber and -resistant figleaf gourd. Plant, Cell and Environment. 2005;28:1191–1202. [Google Scholar]
- Lee SH, Zwiazek JJ, Chung GC. Light-induced transpiration alters cell water relations in figleaf gourd (Cucurbita ficifolia) seedlings exposed to low root temperatures. Physiologia Plantarum. 2008;133:354–362. doi: 10.1111/j.1399-3054.2008.01082.x. [DOI] [PubMed] [Google Scholar]
- Lo Gullo MA, Nardini A, Trifilo P, Salleo S. Diurnal and seasonal variations in leaf hydraulic conductance in evergreen and deciduous trees. Tree Physiology. 2005;25:505–512. doi: 10.1093/treephys/25.4.505. [DOI] [PubMed] [Google Scholar]
- Maurel C, Chrispeels MJ. Aquaporins. A molecular entry into plant water relations. Plant Physiology. 2001;125:135–138. doi: 10.1104/pp.125.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A, Salleo S, Andri S. Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv Margot) Plant, Cell and Environment. 2005;28:750–759. [Google Scholar]
- Nobel PS. Physicochemical and environmental plant physiology. San Diego: Academic Press; 1999. [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. Journal of Experimental Botany. 2002;53:1283–1304. doi: 10.1093/jexbot/53.372.1283. [DOI] [PubMed] [Google Scholar]
- Pastori G, Foyer CH. Common components, networks, and pathways of cross-tolerance to stress: the central role of ‘redox’ and abscissic acid mediated controls. Plant Physiology. 2002;129:460–468. doi: 10.1104/pp.011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Holbrook NM. Leaf hydraulics. Annual Review of Plant Biology. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- Sack L, Melcher PJ, Zwieniecki MA, Holbrook NM. The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. Journal of Experimental Botany. 2002;53:2177–2184. doi: 10.1093/jxb/erf069. [DOI] [PubMed] [Google Scholar]
- Sack L, Tyree MT, Holbrook NM. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytologist. 2005;167:403–413. doi: 10.1111/j.1469-8137.2005.01432.x. [DOI] [PubMed] [Google Scholar]
- Steudle E. Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue and organ level. In: Smith JAC, Griffiths H, editors. Water deficits: plant responses from cell to community. Oxford: BIOS Scientific Publishers; 1993. pp. 5–36. [Google Scholar]
- Steudle E. Water uptake by roots: effects of water deficit. Journal of Experimental Botany. 2000;51:1531–1542. doi: 10.1093/jexbot/51.350.1531. [DOI] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, Bligny R, Maurel C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature. 2003;425:393–397. doi: 10.1038/nature01853. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Nardini A, Salleo S, Sack L, Omari BE. The dependence of leaf hydraulic conductance on irradiance during HPFM measurements: any role for stomatal response? Journal of Experimental Botany. 2005;56:737–744. doi: 10.1093/jxb/eri045. [DOI] [PubMed] [Google Scholar]
- Wan XC, Steudle E, Hartung W. Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. Journal of Experimental Botany. 2004;55:411–422. doi: 10.1093/jxb/erh051. [DOI] [PubMed] [Google Scholar]
- Wei C, Tyree MT, Steudle E. Direct measurement of xylem pressure in leaves of intact maize plants. A test of the cohesion–tension theory taking hydraulic architecture into consideration. Plant Physiology. 1999;121:1191–1205. doi: 10.1104/pp.121.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgate ME, Steudle E. Water transport in the midrib tissue of maize leaves. Plant Physiology. 1985;78:183–191. doi: 10.1104/pp.78.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong LM, Schumaker KS, Zhu JK. Cell signaling during cold, drought and salt stress. Plant Cell. 2002;14(suppl.):S165–S183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Steudle E. Oxidative gating of water channels (aquaporins) in corn roots. Plant, Cell and Environment. 2006;29:459–470. doi: 10.1111/j.1365-3040.2005.01423.x. [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Melcher PJ, Holbrook NM. Hydrogel control of xylem hydraulic resistance in plants. Science. 2001;291:1059–1062. doi: 10.1126/science.1057175. [DOI] [PubMed] [Google Scholar]