Abstract
A new approach is described to analyse the barrier properties of the outer part of rice (Oryza sativa L.) roots towards oxygen. By using a root-sleeving O2 electrode, radial oxygen loss at different distances from the root apex was measured and related to the corresponding root structure. In addition, internal oxygen concentrations were precisely adjusted using a newly developed perfusion technique. Thus, the oxygen permeability coefficient of the outer part of the root (OPR) could be calculated, since both (i) the oxygen flow across the OPR and (ii) the oxygen concentration gradient across the OPR from inside to outside were known. On the basis of the permeability coefficient, it can be decided whether or not different rates of oxygen loss across the OPR are due to changes in the OPR structure and/or to changes in the concentration gradient. The technique was applied to rice root segments, which enabled rapid perfusion of aerenchyma. In the present study, roots of rice grown under aerobic conditions were used which should have a higher O2 permeability compared with that of plants grown in deoxygenated solution. Both radial oxygen losses and permeability coefficients decreased along the root, reaching the lowest values at the basal positions. Values of oxygen permeability coefficients of the OPR were corrected for external unstirred layers. They decreased from (2.8±0.2)×10−6 m s−1 at 30 mm to (1.1±0.2)×10−6 m s−1 at 60 mm from the apex (n=5; ±SE). They were similar to those measured previously for cuticles. Low diffusional oxygen permeability of the OPR suggested that the barrier to radial oxygen loss was effective. This may help to retain oxygen within the root and enhance diffusion of oxygen towards the apex in the presence of a relatively high water permeability. The results are discussed in terms of the inter-relationship between the water and oxygen permeabilities as roots develop in either aerated or deoxygenated (stagnant) media.
Keywords: Aerenchyma, oxygen permeability coefficient, Oryza sativa, radial oxygen loss, rice root
Introduction
Rice (Oryza sativa L.) is often grown in waterlogged soils, which are usually anaerobic and chemically reduced (Ponnamperuma, 1984). Under these conditions, aeration of roots depends solely on supplies of oxygen from the shoot through the aerenchyma, which provides a low resistance internal pathway for the movement of gases in plants (Armstrong, 1979). During its passage to the root tips, oxygen may be either consumed by respiration or diffuse radially to the rhizosphere. Radial oxygen loss (ROL) reduces the supply of oxygen to the roots, which would decrease the ability of roots to penetrate into anaerobic soils (Armstrong, 1979; Jackson and Drew, 1984). Forming a barrier in outer cell layers of the basal root zones diminishes losses of oxygen to the rhizosphere, enhancing longitudinal oxygen diffusion towards the root apex (Armstrong, 1979; Jackson and Armstrong, 1999; Colmer, 2003b). However, a physical barrier to ROL may also restrict water and nutrient uptake by roots (Armstrong, 1979; Koncalova, 1990).
In earlier studies, it was shown that the hydraulic conductivity of rice roots is low in comparison with other species (e.g. maize; Miyamoto et al., 2001; Ranathunge et al., 2003). This may result in problems for the roots to meet transpirational demands by the shoot (Miyamoto et al., 2001). Rice may suffer from water shortage during the day, even when plants are growing under waterlogged conditions (Hirasawa et al., 1992). The problem was related to suberization/lignification of the roots, which are required to keep the oxygen within the plant. However, it was shown that, at least for roots grown under aerated conditions, the main hydraulic resistance was located in the endodermis rather than in the outer part of roots (OPR), despite the presence of a suberized exodermis with Casparian bands and an additional layer of lignified fibre cells (Ranathunge et al., 2003). The ability to retain oxygen in the aerenchyma in the presence of high water permeability may be achieved by differences in the transport mechanism — diffusional versus bulk water flow (Ranathunge et al., 2003). This view could be supported by differences between diffusional and bulk water (Lp) permeabilities. The lack of data on permeability coefficients for oxygen diffusion across outer cell layers has limited the understanding of the mechanisms controlling ROL and prevented quantitative comparison of the radial oxygen permeabilites in the outer cell layers (Colmer, 2003b; Ranathunge et al., 2004). Knowing the permeability coefficient of the OPR and, further, how to manipulate its expression may contribute to identification of the genetic regulation of the ROL (Colmer, 2003b). Subsequent genetic manipulations may help to develop new improved cultivars and rice hybrids with higher yield than existing high-yielding varieties.
There are many data in the literature reporting different aspects of ROL from plant roots. Several techniques have been developed to evaluate ROL, with the most frequently used being the root-sleeving O2 electrode (Armstrong, 1979; Visser et al., 2000; Colmer, 2003a,b). These methods provided quantitative data of ROL. However, as the forces driving ROL could not be determined unambiguously, none of them enabled the oxygen permeability coefficients of roots to be worked out.
In the present study, a new perfusion technique was developed to measure, for the first time, the oxygen permeability coefficient (PdO2) of the OPR of rice. The well-defined structure of the OPR (four cell layers) is separated from the stele by aerenchyma. Perfusion of aerenchyma of root segments with gas mixtures of known oxygen concentrations and at the same time measuring radial losses of oxygen allowed quantification of the permeability coefficients of the cell layers exterior to aerenchyma. The results indicated that absolute values of the PdO2 were relatively low and decreased towards the base as roots developed. They showed that rice roots may retain oxygen in the root in the presence of rather high water permeability, which may be important for the productivity of rice plants. It should be noted that the O2 permeability of roots grown in aerated hydroponics should be much larger than that of roots grown under stagnant (deoxygenated) conditions. However, in the present study, aerated conditions were used to (i) establish the technique and (ii) compare the O2 permeability coefficient with the water permeability of the OPR, which has already been measured for rice grown in aerated hydroponics culture (Ranathunge et al., 2003, 2004).
Materials and methods
Plant material
Seedlings of an upland rice cultivar (Oryza sativa L., cv. Azucena; International Rice Research Institute, Manila, Philippines) were grown in a climatic chamber using aerated hydroponics as detailed previously (day/night rhythm: 12/12 h, 27/22 °C, light intensity: 500 μmol m−2 s−1; Miyamoto et al., 2001; Ranathunge et al., 2003). The composition of the nutrient solution was (in mM): 0.09 (NH4)2SO4, 0.05 KH2PO4, 0.05 KNO3, 0.03 K2SO4, 0.06 Ca(NO3)2, 0.07 MgSO4, 0.11 Fe-EDTA, and the micronutrients (in μM): 4.6 H3BO3, 1.8 MnSO4, 0.3 ZnSO4, and 0.3 CuSO4. The overall osmotic concentration was 3 mM and the pH 5.5–6.0. Plants used in experiments were 30–40 d old. The overall lengths of roots used in experiments were 90–200 mm. The diameters of adventitious roots were 0.9–1.2 mm.
Measurements of ROL from roots of intact plants
Rates of ROL from adventitious roots of intact plants were measured by an amperometric mode using cylindrical platinum O2 electrodes developed by Armstrong (1967) and Armstrong and Wright (1975). Root systems were immersed in a chamber containing a deoxygenated 0.1% agar solution with 5.0 mol m−3 KCl (to ensure adequate electrical conductivity) and 0.5 mol m−3 CaSO4 (Colmer et al., 1998). The presence of 0.1% agar was sufficient to minimize convection within the solution and to provide stagnant conditions. For deoxygenation, the agar solution was boiled for 20 min and bubbled with nitrogen gas while cooling down. The shoot base was fixed to the rubber lid on the top of the chamber so that shoots were in the air and roots were in the O2-free medium. The residual oxygen left in the medium was 0.05 μM, as measured using the O2 electrode. The measurements were taken in climatic chambers where plants had previously been grown.
For each plant, one adventitious root was inserted through the cylindrical O2 electrode (i.d., 2.25 mm; height, 5 mm) fitted with guides to keep the root in the centre of the electrode. Prior to measurements, plants were acclimated for 2 h in the deoxygenated medium. ROL measurements were taken along the root by positioning the centre of the electrode at distances of 10, 20, 30, 40, 50, and 60 mm from the root apex. Above 60 mm, laterals began to emerge from the primary root, which prevented further measurements. Diameters of roots at given positions were measured using a digital calliper (700-Digital, Mitutoyo, China).
To measure ROL, the polarizing voltage applied to the platinum electrode results in a reduction of oxygen to hydroxyl ions as in a Clark electrode (O2+2H2O+4e– =4 OH–; Clark et al., 1953). The current (in μA) required to reduce the oxygen leaving the root was converted to values of oxygen flux from the root (ROL; Armstrong and Wright, 1975). This calculation assumed that all the oxygen was completely converted according to the stoichiometry given and that there were no deviations due to polarization effects (see below).
Responses of ROL to an increased O2 concentration around the shoot
The roots of intact plants were immersed in a Perspex chamber in the O2-free medium and shoots were left in the air, as described above. Then, the chamber with the plant was placed in a 12.0 l glass container flushed with humidified air (20.3% O2 equivalent to 21% O2 of dry air) at a rate of 100 l h−1. ROLs were measured at positions of 10, 30, or 60 mm from the root apex. When ROL at a given position reached a steady-state value, the O2 concentration in the container was changed to 38.7 and subsequently to 58.1, and 96.8% (this equates to 40, 60, and 100% O2 in dry gas), and the responses to ROL were measured. After each step of different O2 concentrations, humidified pure N2 gas was applied to the shoot, until a minimum value of ROL was reached. This was done to remove high levels of O2 in the plant prior to a change. Root diameters were measured using a digital calliper (700-Digital, Mitutoyo, China). The measurements were taken in the climatic chambers, where plants had previously been grown (27 °C).
Measurements of radial oxygen flow (JO2) and of the permeability coefficient for oxygen (PdO2) of rice root segments
In order to work out the PdO2s of the OPR, the concentration difference of oxygen in aerenchyma and in the medium has to be known. The latter was assumed to be zero to a good approximation. The former was provided by perfusing excised root segments with a moistened gas mixture of O2 and N2 of known oxygen concentration as shown in Fig. 1. The resulting ROL from segments was measured. Since the term ROL was always used to describe oxygen loss from intact plants, the oxygen loss from root segments will be denoted here as radial oxygen flow (JO2, also in nmol m−2 s−1 as ROL).
Fig. 1.
(A) Schematic diagram of the experimental set-up used to measure radial oxygen flow (JO2) across the outer part of rice roots (OPR). The root segment was inserted through a cylindrical platinum electrode and its ends were glued to glass capillaries. Perfusion of the aerenchyma with the moist gas mixtures at overpressures of 10, 20, or 30 kPa (reference: atmospheric pressure) was performed from the inlet side of the segment connected to tanks with different mixtures of compressed O2/N2 of known oxygen concentration. Radial oxygen flow (JO2) was measured as an electrical current. (B) Schematic diagram to show radial oxygen flow across the outer part of the root segment during perfusion.
Root segments 50 mm in length were excised at distances of 20 mm and 70 mm from the root apex, where aerenchyma was sufficiently developed, and inserted through the cylindrical O2 electrode. Using polyacrylamide glue (UHU, Bühl, Germany), the cut ends of the segments were fixed to glass capillaries (inner diameters of 1.0–1.3 mm, depending on root diameter) placed and firmly secured onto a rack within the Perspex chamber using metal bands (Fig. 1). Subsequently, the chamber was filled with deoxygenated 0.1% agar solution (containing 5.0 mol m−3 KCl and 0.5 mol m−3 CaSO4) and closed with the Perspex lid. To remove extant oxygen, the agar solution was bubbled with pure nitrogen gas for 20 min after closing the chamber. The basal end of the root segment was connected to tanks with different mixtures of compressed O2/N2 of oxygen concentrations of 21, 40, 60, and 100% (tolerance ±2%; Reissner Gase GmbH & Co KG, Lichtenfels, Germany), via a glass capillary and Teflon tubes (virtually not permeable for oxygen; i.d. 0.7 and o.d. 2.4 mm). To avoid dehydration of root segments by dry gas, O2/N2 mixtures were moistened before they entered the root segment. As shown in Fig. 1, the other end of the root segment (outlet) was open to the atmosphere via another Teflon tube. Root segments were perfused with gas mixtures at overpressures of 10, 20, and 30 kPa (reference atmospheric pressure=100 kPa), to ensure that the rate of axial flow of oxygen was higher than that of the radial loss and higher than the oxygen consumption by respiration (see Discussion).
Since the root segments immersed in deoxygenated 0.1% agar were perfused with gas of known oxygen concentrations, the corresponding JO2s in nmol m−2 s−1 could be measured. Permeability coefficients of the OPR were calculated from the slope of JO2/Ci curves where Ci is the concentration of oxygen in the liquid phase of aerenchyma at the inner surface of the OPR. Due to polarization effects at the platinum electrode, responses of JO2 to increasing O2 concentrations were non-linear (see Results and Discussion). Hence, the permeability coefficients had to be calculated only from the initial slopes of the flow/concentration curves (Ci=0–38.7% O2 in the humidified gas; see below).
To test whether or not the apparent decrease of PdO2 (non-linear relationship between JO2 and Ci) at high rates of JO2 was caused by polarization effects (see Discussion), tubes of silicone and Teflon (PTFE), which differed in their permeabilities for oxygen, were used for comparison. Dimensions of the polymer tubes were similar to those of roots: the length was 50 mm, and internal and outer diameters were 0.5 mm and 0.9 mm, respectively for silicone (Laboflex, Kronlab, Dinslaken, Germany). For PTFE, dimensions were 0.5 mm and 1.0 mm, respectively (Laboflon, Kronlab). The tube segments were glued to glass capillaries and placed in a Perspex chamber as described above. Radial oxygen flows were measured during perfusion of gas at different oxygen concentration at an overpressure of 20 kPa at the entrance of tube segments (mean of 10 kPa see Results and Discussion).
In order to calculate PdO2 of roots and polymer tubes, Ci should be given in mol m−3 rather than in % O2. The internal concentration Ci was calculated from the partial pressure of oxygen according to Henry's law:
| (1) |
where KH is Henry's law constant (74.68 kPa m3 mol−1 at 25 °C; Atkins and de Paula, 2007) and PO2 is the partial pressure of oxygen. Since the gases were humidified, PO2 was corrected for the saturation water vapour pressure (3167 Pa=3.167 kPa=31.67 mbar at 25 °C; Jones, 1992). Hence, the oxygen pressure in H2O-saturated air was 20.33 kPa and not 20.95%. The overpressure inside the system also had to be taken into account. An overpressure of 10 kPa (0.01 MPa) equates to 2.03 kPa of oxygen. Overall, the partial pressure of oxygen in air perfused across the root segments was thus 22.4 kPa. Similarly, for oxygen concentrations of 40, 60, and 100%, partial pressures were 42.6, 63.9, and 106.5 kPa, respectively. This calculation assumed nominal partial O2 pressures given by the supplier, a mean pressure along the segments, and a rapid equilibration of the thin layer of water at the inner surface of the OPR, which is reasonable. Because of the convective mixing of the gases in the aerenchyma, there were no radial gradients in PO2 (and Ci) along the interior of segments. The high rates of perfusion of N2/O2 mixtures ensured that the JO2 and respiration rates were much smaller compared with the axial flow of oxygen (see Discussion), so the concentration of oxygen along the aerenchyma remained constant.
The measurements of radial oxygen flow and permeability coefficient were taken along the root at distances of 30, 40, 50, and 60 mm from the root apex. Since the radial oxygen flow could increase the oxygen concentration outside of the root, the agar solution was always bubbled with nitrogen before each change of the concentration of perfused gas mixture. Measurements were taken at 25 °C in a temperature-controlled room.
In cases, where root segments were not properly fixed to glass capillaries or were injured during handling, the experiments were stopped. Leakages were easily detected by observing air bubbles coming out at the points where capillaries were not properly fixed to segments or at places where root segments were injured during handling. Since experiments with a given segment lasted for 3–6 h, root segments had to be tested for the viability of the cells of the OPR. Root segment were randomly selected for treatment with Evan's blue stain (Fischer et al., 1985).
Calculation of the diffusivity of oxygen across the OPR. Effects of the unstirred layer caused by the cylindrical shell of agar between root and electrode
Values of permeability coefficients obtained in the present study incorporated diffusion of O2 across the shell of agar around the root. This unstirred layer effect might have been substantial when the overall PdO2 was relatively large. The measured values of PdO2 were the inverse of a resistance (or resistivity), which consisted of two resistances in series: (i) resistance of the OPR; and (ii) the resistance of the shell of agar between the root and the electrode surface. Before calculating the PdO2 corrected for the unstirred layer (and diffusion coefficient of O2 in the OPR), those two resistances had to be separated. Since the diffusion coefficient of oxygen in 0.1% agar (Dagar) was not known for 25 °C, this was estimated from literature data. At 22 °C, the diffusion coefficient of O2 in 0.2–2% agar was 2.1×10−9 m2 s−1 (Revsbech, 1989), which relates to 2.18×10−9 m s−1 for water at the same temperature. Using the ratio of 2.1/2.18, Dagar was calculated from the diffusion coefficient of O2 in water at 25 °C, which is 2.38×10−9 m2 s−1 (Millington, 1959). This resulted in a Dagar=2.3×10−9 m2 s−1 for 0.1% agar at 25 °C. The oxygen permeability coefficient of the agar shell (Pagar) was calculated by (Ye et al., 2006):
| (2) |
where rr is the radius of the root and re is the radius of the electrode. It can be easily verified that Equation 2 reduces to Pagar=Dagar/(re–rr), when rr ≈ re. The corrected value of the permeability of the OPR (POPR) can be obtained from:
| (3) |
Estimated values of POPR allowed calculation of the diffusivity of oxygen in the OPR (DOPR) at different distances from the root apex according to (Ye et al., 2006):
| (4) |
where ra is the radius of the root minus the thickness of the OPR [the thickness of the OPR (rr–ra) was taken as 85 μm, overall root diameter: 1 mm (Ranathunge et al., 2004)]. Again, for sufficiently small differences (rr–ra), Equation 4 reduces to POPR=DOPR/(rr–ra).
Qualitative detection of zones of ROL using the oxidation indicator dye methylene blue
Using the redox indicator methylene blue, sites of oxygen release from roots of intact rice plants and root segments were identified (Conlin, 1986; see also Chabbi et al., 2000). A solution containing 0.75% agar was prepared by heating to boiling. The mixture was then cooled to below 60 °C while stirring. At 60 °C, methylene blue was added at a concentration of 2 mg l−1. The blue solution containing the oxidised dye was further cooled down to 40 °C, and then was reduced by addition of 0.75 g l−1 sodium dithionite (Na2S2O4). The solution was stirred until it was completely colourless. Healthy rice root systems were carefully placed vertically in a plastic cylinder without damaging them. Then the cylinder was filled with the methylene blue–agar solution, which was cooled to 35 °C. This solution resulted in solid agar at room temperature. The shoot base was fixed to the wall of the cylinder, so that the shoots were in the air. The open surface of agar was immediately covered with plastic wrap. The cylinder was placed in the climatic chamber, where the seedlings had been grown, and left for ∼20 h. At places where oxygen was lost from the roots, methylene blue was converted from the colourless (reduced) to the blue (oxidised) form, which was indicated by the formation of blue haloes around the roots. Photographs were taken after removing the roots from the agar medium.
The methylene blue technique was also used to visualize the sites of oxygen loss from perfused rice root segments. Both ends of root segments, excised at distances of 20–80 mm from the apex, were glued to glass capillaries with inner diameters of 1.0–1.3 mm. One of the glass capillaries was connected to the source of gas, and the other end remained open. Root segments were placed in a small box, covered with methylene blue agar, and perfused with moistened air (20.3% oxygen) at an overpressure of 20 kPa for 1 h. Photographs were taken using a digital camera (Nikon D40, Nikon Corporation).
Statistical analysis
Data on ROL, JO2, and PdO2 were analysed using one-way analysis of variance (ANOVA) to examine the effects of oxygen concentration or position along the root axis. Means are accompanied by standard errors, and were compared using the least significant difference test (LSD) at the P ≤0.05 level.
Results
ROL from adventitious roots of intact plants
Measurements of ROL were taken from adventitious roots of intact plants at different distances from the root apex, with the shoot in air and the roots in the O2-free medium. ROL was referred to 1 m2 of root surface. Although there was substantial variation between roots, the data given in Fig. 2A indicate a significant maximum of ROL at ∼30 mm from the apex (F5,42=9.7; P ≤0.05). Presumably the increase of ROL from the distance of 10 mm to 30 mm was the result of the increase of porosity as roots developed aerenchyma. At later stages of development (40–60 mm), the drop of ROL was most probably due to the suberization/lignification of the OPR (see Discussion; Armstrong and Armstrong, 1988; Colmer, 2003b). To minimize the effects of the variability between roots (Fig. 2A), which tended to obscure the maximum ROL at 30 mm, the data of each root were referred to 100% at 30 mm, i.e. relative rather than absolute values were given (Fig. 2B). As seen in Fig. 2B, ROL at 30 mm was significantly different from that at other distances, except at 40 mm (F5,42=15.2; P ≤0.05). At a distance of 60 mm, ROL was smaller by a factor of 4.3 (on average). The finding suggested that the barrier to ROL, which developed in the OPR was quite effective.
Fig. 2.
(A) Rates of radial oxygen loss (ROL) along adventitious roots of intact rice seedlings grown in aerated nutrient solution for 30–40 d. Measurements were taken in a growth chamber at 27 °C for roots of 90–120 mm in length, which were in O2-free medium, with the shoots in the air. (B) Relative values of ROL are given. To minimize variations between roots, the data for each root were referred to 100% at 30 mm. Data given are means ±SE (n=8).
Responses of ROL to increased O2 concentration around the shoot
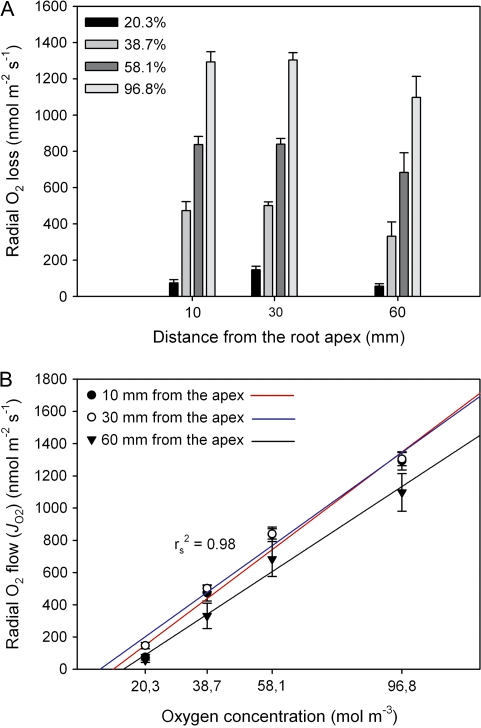
Oxygen concentrations around the shoot were manipulated to test whether or not ROL increased with increasing oxygen concentration in the atmosphere as one would expect. Responses of ROL to different O2 concentrations around the shoot (20.3–96.8% O2/N2 mixtures, when considering the small effect due to humidification of the gases) at different distances from the root apex are shown in Fig. 3 (10, 30, and 60 mm). As the O2 concentration increased, rates of ROL significantly increased at all distances (F3,17=142.8 for 10 mm, F3,21=297.2 for 30 mm, F3,14=40.1 for 60 mm; P ≤0.05; Fig. 3A). However, in contrast to the perfusion experiments with root segments (see Figs. 5, 6), rates of O2 losses were much smaller (see Discussion). According to Fig. 3B, the increases of ROL were linear, but in all cases the regression lines did not pass through the origin. This may be explained by the fact that, at low O2 concentrations in the cortex, most of the oxygen is used by respiration. Extrapolating the regressions to intercept the x-axis provides concentration values when the use of oxygen in the plant just compensates axial diffusional transport along aerenchyma. The compensation points would be at 11, 7, and 14% for 10, 30, and 60 mm from the apex, respectively. The linear extrapolation may be questioned, and more data are required in the range of low concentrations (see Discussion). However, for all oxygen concentrations, there was a trend that the highest ROL was observed at 30 mm from the root tip, but this was only significant for 20.3% (F2,18=7.9; P ≤0.05; see Fig. 2 and Discussion).
Fig. 3.
Effect of increasing oxygen concentration around the shoot of intact rice plants on radial oxygen loss (ROL) at distances of 10, 30, and 60 mm from the apex, when roots were in O2-free agar solution. Measurements were taken in a growth chamber at 27 °C for roots of 90–120 mm in length. (A) For a given position, rates of ROL significantly increased as the concentration of oxygen increased (P ≤0.05). Due to suberization and/or lignification of basal parts of the roots, the response in ROL at a distance of 60 mm was smaller than that of other distances for each concentration difference. (B) Increases of ROL with increasing oxygen concentration were linear. Extrapolating the regressions to intercept the x-axis provided concentration values when the use of oxygen in the plant just compensated axial diffusional transport along aerenchyma. Red, blue, and black lines represents regressions for 10, 30, and 60 mm, respectively. Data are means ±SE (n=5–7).
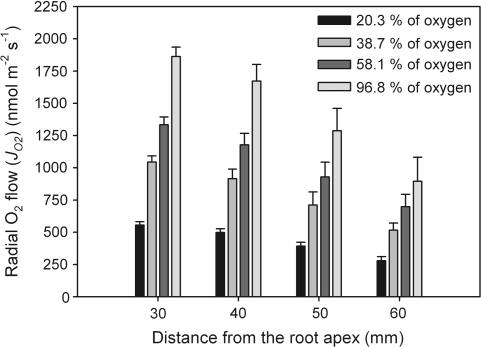
Fig. 5.
Radial O2 flow (JO2) along rice root segments when perfused with moistened O2/N2 gas mixtures at different oxygen concentrations of 20.3, 38.7, 58.1, and 96.8% (equating to 21, 40, 60, and 100% O2 in dry gas) at a mean overpressure of 10 kPa. For all oxygen concentrations, values of JO2 decreased along the root. When considering a given position, there was a clear trend for JO2 to increase as the oxygen concentration increased. Measurements were taken at 25 °C in a temperature-controlled room. Data given are means ±SE (n=5–24).
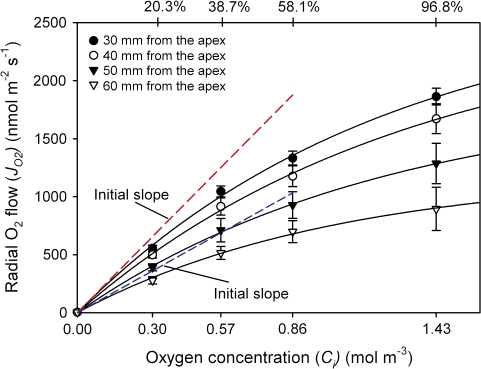
Fig. 6.
Rates of radial oxygen flow (JO2) along root segments plotted against the internal oxygen concentration (Ci). According to Henry's law, 20.3, 38.7, 58.1, and 96.8% O2 (at a mean overpressures of 10 kPa) equated to 0.3, 0.57, 0.85, and 1.43 mol m−3 at the inner surface of the OPR, respectively. The radial O2 flows (JO2) increased with increasing O2 concentrations, but increases in JO2 were not linear, which was due to limitations of the technique (see Discussion). Red and blue dashed lines indicate the initial slopes (0% O2) of JO2/Ci curves for 30 mm and 60 mm, respectively. Data given are means ±SD (n=5–24 root segments).
JO2 and PdO2 measured with root segments
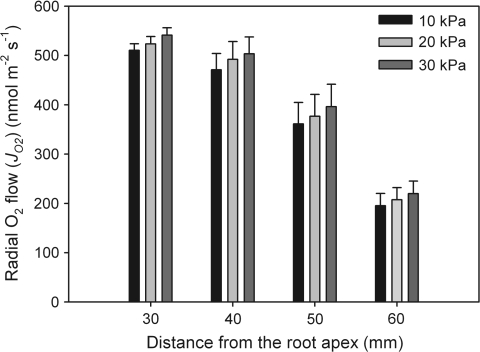
Perfusion of aerenchyma with different oxygen concentrations and different overpressures (axial flow rates) was performed with rice root segments placed in O2-free medium, and radial oxygen flows (JO2s) were measured. When root segments were perfused with humidified air (20.3% O2) at overpressures of 10, 20, or 30 kPa at the entrance of segments (Teflon tubes), there was a slight increase in JO2, but this was not significant (F2,21=1.1, 0.2, 0.2, and 0.3 for 30, 40, 50, and 60 mm, respectively; P>0.05; Fig. 4). It was also not significant when data were not pooled (as in the figure) but were plotted root-by-root (data not shown). Different overpressures were used to check whether the axial perfusion of aerenchyma with oxygen was rapid enough to provide a constant internal O2 concentration along the root interior and to compensate for losses of oxygen by respiration (see Discussion). According to gas laws, higher absolute pressures at the inlet (110, 120, and 130 kPa) would increase the partial oxygen pressure (PO2) and the actual concentration of oxygen along the tubes (segments). Assuming that the drop in pressure would be largely within the segments and would be linear for the three different pressures, average values of 105, 110, and 115 kPa were used to calculate PO2 and the actual O2 concentration (see Discussion). As seen in Fig. 4, there was a drop of JO2 along the root from 30 mm to 60 mm (see Fig. 5). It was not possible to make measurements at distances below 30 mm (see Discussion). For the rest of the experiments, an overpressure of 20 kPa (0.02 MPa) was used at the entrance of the segments, which was sufficient to keep the constant O2 concentration in the aerenchyma (see Discussion).
Fig. 4.
Rates of radial oxygen flow (JO2) along the root when perfused with 20.3% O2 at overpressures of 10, 20, and 30 kPa at the entrance of the segments (reference atmospheric pressure 100 kPa=0.1 MPa). Assuming a linear drop in pressure along the segments for the three different pressures, average values of 105, 110, and 115 kPa were used to calculate PO2 and the actual O2 concentration. For each distance from the root apex, radial oxygen flows increased with increasing overpressures, but increases were not significantly different. Measurements were taken at 25 °C in a temperature-controlled room. Data given are means ±SE (n=8).
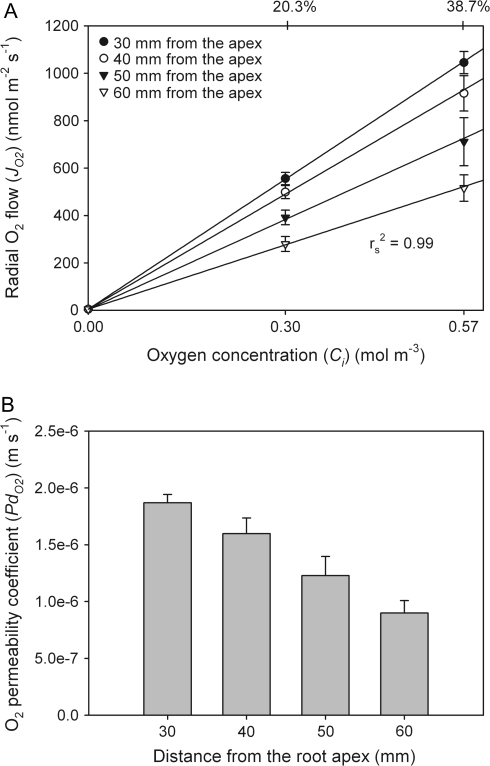
When individual root segments were perfused with O2/N2 mixtures at the oxygen concentrations of 20.3, 38.7, 58.1, and 96.8% (correction for humidification considered; for corresponding partial pressures, see above), rates of radial oxygen flow (JO2) decreased along the root for each oxygen concentration, being the highest at 30 mm (Fig. 5). For each oxygen concentration, JO2 was not significantly different between 30 mm and 40 mm (F3,92=17.6 for 20.3%, F3,16=10.2 for 38.7%, F3,20=9.2 for 58.1%; F3,20=8.5 for 96.8%; P ≤0.05). However, it was always significant between 30 mm and 50 mm, and between 40 mm and 60 mm. The marked differences between 40 mm and 50 mm from the apex and 50 mm and 60 mm were observed only, when root segments were perfused with 20.3% oxygen. The decrease of JO2 in the developing root may be explained in terms of the formation of apoplastic barriers in the OPR (see Discussion). When considering a given position, there was a clear trend for JO2 to increase as the oxygen concentration increased. With exceptions at 50 mm and 60 mm, increases were always significant (F3,37=173.6, 74.1, 27.5, and 14.5 for 30, 40, 50, and 60 mm, respectively; P ≤0.05). At greater distances from the tip, differences in absolute values of JO2 tended to become smaller in response to an increasing O2 concentration.
At all four distances, increases of JO2 with increasing concentration were not linear (Fig. 6). This may be caused by unstirred layers outside the root, where zero concentration of oxygen at the electrode was assumed (all O2 immediately reduced). Another reason may be an incomplete oxygen reduction, when two electrons are consumed per oxygen molecule rather than four, as described by Hahn et al. (1975; see Discussion). Because of the polarization effects at the platinum electrode at high JO2, only the initial slopes of the curves in Fig. 6 were used to calculate the oxygen permeability coefficient (Fig. 7A). The highest values of the oxygen permeability coefficient of the OPR (PdO2) along the rice root segments were at the positions closer to the root apex (30 mm) and decreased along the root, reaching the lowest value at 60 mm (Fig. 7B). PdO2 was significantly different between distances of 30 mm and 50 mm, and 40 mm and 60 mm (F3,16=11.1; P ≤0.05). The mean value of PdO2 at 60 mm was 0.9±0.1×10−6 m s−1, which was 2-fold smaller than that at 30 mm (1.9±0.07×10−6 m s−1).
Fig. 7.
(A) Because of limitations of the technique in the range of high O2 concentrations (see Fig. 6), only the initial slopes (0–38.7% of O2) of JO2/Ci curves were used to calculate permeability coefficients of oxygen (PdO2). (B) The highest values of PdO2 were close to the root apex and decreased along the root, which is most probably due to suberization and/or lignification of roots. Data given are means ±SE (n=5).
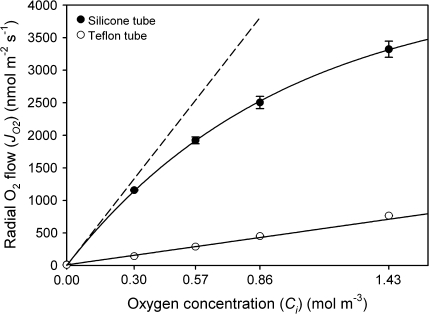
Radial oxygen flow across silicone and Teflon tubes
To tackle the problems arising from measurements at high JO2, the radial flow of oxygen across the OPR of rice roots was mimicked using silicone and Teflon tubes of diameters similar to that of rice root segments. Tubes were perfused with the same mixture of O2/N2 gases (20.3, 38.7, 58.1, and 96.8% O2), and rates of radial oxygen flow measured. The two different polymer tubes were employed, because they differed markedly in their permeability for oxygen (Zhang and Cloud, 2006). When using silicone tubes, the responses in JO2 to increased O2 concentration were similar to those obtained for rice roots. In contrast, they were substantially smaller for Teflon (Fig. 8). Also, there was a non-linear response for silicone tubes (as for rice) and a linear response for Teflon (Fig. 8). The oxygen permeability coefficient (PdO2) of silicone rubber, calculated from the initial slope of the JO2/Ci curve, was larger by a factor of 7.8 than that obtained for Teflon. Absolute values of permeability coefficients of silicone and Teflon tubes were 3.5±0.07×10−6 m s−1 and 0.50±0.006×10−6 m s−1, respectively (n=3; ±SE). Correcting for the resistance of the shell of agar between the tube and the electrode surface, the oxygen permeability coefficients of silicone and Teflon tubes were 10± 0.06×10−6 m s−1 and 0.54±0.009×10−6 m s−1, respectively. As for the root segments, the data suggest limitations of the electrode technique in the presence of high ROL/JO2 as they occurred at O2 concentrations >38.7% (see Discussion).
Fig. 8.
Rates of radial oxygen flow from silicone (i.d., 0.5 mm; o.d., 0.9 mm) and Teflon (PTFE; i.d., 0.5 mm; o.d., 1.0 mm) tubes, when perfused with moistened gas mixtures of 20.3, 38.7, 58.1, and 96.8% concentration of O2 at a mean overpressure of 10 kPa. As for the root segments, the relationship between JO2 and O2 concentration was non-linear for silicone rubber (high permeability for O2), but was linear over the entire range for Teflon (low O2 permeability). The dashed line for silicone rubber indicates the initial slope (0% O2) of the JO2/Ci curve. Data are means ±SE (n=3).
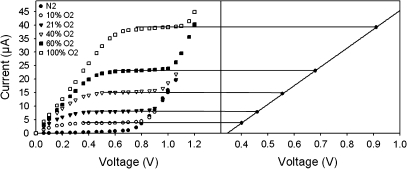
The effects of non-complete reduction of oxygen at high rates of radial oxygen flow were tested further by increasing the voltage in steps to a polarizing voltage, which was higher than that usually used during ROL/JO2 measurements (∼0.5 V). Here, the voltage was increased every 20 s by 0.02 V up to 1.2 V and the resulting diffusional current was measured at each step change. Figure 9 shows the current–voltage curves for different oxygen concentrations perfused through the silicone tube at a mean overpressure of 10 kPa (0, 9.6, 20.3, 38.7, 58.1, and 96.8%; values corrected for the humifidification effect). There was a linear relationship between the oxygen concentration and the applied voltage, i.e. higher oxygen concentrations required a higher voltage to reach the plateau, where the oxygen is completely reduced. It can be seen from Fig. 9 that saturation was reached at 0.46 V for 20.3% oxygen and at 0.92 V for 96.8% O2. However, such a high plateau potential could not have been applied for 20.3% oxygen due to sharp increases of the current, which may have been caused by other effects such as the discharge of hydrogen ions at the electrode (Armstrong, 1967). Hence, to measure JO2, the usual procedure from the literature using the technique of Armstrong (1967, 1979) had to be adapted to the situation of high oxygen flow by increasing the polarizing voltage. In the literature, this limitation had not yet been made clear. This is the case because the usual ROLs (Gibberd et al., 1999; McDonald et al., 2002; Colmer, 2003a) were substantially smaller than the JO2s measured in the present study. According to the present results, the measured maximum ROL was also much lower than the maximum JO2 (1300 nmol m−2 s−1 as compared with 1860 nmol m−2 s−1 for 96.8% O2 at 30 mm from the apex; see Discussion).
Fig. 9.
Current–voltage curves for oxygen concentrations of 0, 9.6, 20.3, 38.7, 58.1, and 96.8% perfused through the silicone tubes at the mean overpressure of 10 kPa. The polarizing voltage was increased in 0.02 V steps every 20 s up to 1.2 V, and the corresponding diffusional current was measured for each step change. Plateaus were reached when rates of oxygen diffusion to the electrode became independent of the voltage applied. It can be seen that there is a linear relationship between the oxygen concentration and the applied voltage.
Calculating the diffusivity of oxygen across the OPR
According to Equation 2, the resistivity of the shell of agar (1/Pagar) was calculated to be 1.8×105 s m−1, which was 33±1% (30 mm) and 16±2% (60 mm) of the measured 1/PdO2 (5.4±0.2×106 s m−1 and 11.9±1.6×105 s m−1 for 30 mm and 60 mm from the apex, respectively). Hence, the agar layer did not dominate the overall resistivity, but could not be neglected. Correcting for the unstirred layers (1/Pagar) in Equation 3, POPR resulted in values of 2.8±0.2×10−6 m s−1 (at a distance of 30 mm from the apex) and 1.1±0.2×10−6 m s−1 (60 mm). These values related to the overall measured PdO2s of 1.9±0.07×10−6 (30 mm) and 0.9±0.1×10−6 m s−1 (60 mm). Since the thickness of the OPR was known (85 μm, Ranathunge et al., 2004), estimated values of POPR allowed calculation of the diffusivity of oxygen across the OPR according to Equation 4 (assuming a homogenous barrier). At 25 °C, the radial oxygen diffusivity, DOPR, varied along the root, being 2.6±0.2×10−10 m2 s−1 and 1.0±0.1×10−10 m2 s−1 at 30 mm and 60 mm from the apex, respectively.
Determining zones of ROL using the redox dye methylene blue
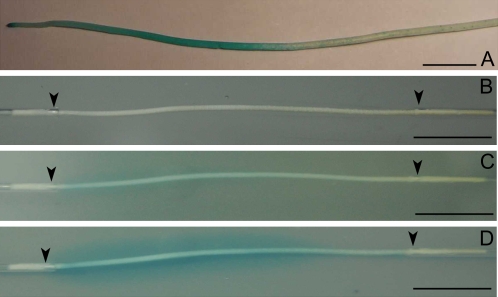
Measurements of ROL with the root-sleeving platinum electrode provided a quantitative measure of oxygen loss from the roots. With the methylene blue agar technique, it was possible to visualize the pattern of ROL from roots by formation of the blue haloes at the sites where roots lost oxygen. For rice roots of intact plants placed in the growth chamber for 20 h, ROL occurred from the root apex up to distance of 60–100 mm. At larger distances, only blue patches on the surface of the roots were observed. The photograph in Fig. 10A was taken after removing roots from the agar medium (methylene blue was attached to the root surface at the sites of oxygen loss).
Fig. 10.
(A) Development of oxidised methylene blue stains on the surface of the adventitious rice root. The rice root systems were placed for ∼20 h in 0.75% agar medium, which contained the redox indicator methylene blue in reduced form. The photographs was taken after removing roots from the agar medium. (B–D) Development of oxidised methylene blue haloes around the rice root segments. Root segments excised at a distance of 20–80 mm from the root apex were perfused with moistened air (20.3% O2) at an overpressure of 20 kPa for 0 (B), 15 (C), and 30 (D) min. Blue haloes were formed at the sites of oxygen loss from the segments. Black arrows indicate fixing points of the root segments to glass capillaries. Bar=10 mm.
When segments of rice roots were perfused with humidified air (20.3% O2), blue haloes developed along the segments with time, first appearing at positions closer to root apices. After 15 min, slight blue oxidised haloes were observed up to a distance of 50 mm from the apex (Fig. 10C). At this time, oxygen losses from more basal positions were not visible. After 30 min, blue haloes were well formed at positions closer to the apices, and started to develop at the basal parts of the root (Fig. 10D). After 45 min, the entire root segments were surrounded by oxidised blue haloes (not shown).
Discussion
A new technique to measure permeability coefficients of the diffusion of oxygen across the OPR has been introduced in this study. The technique has been applied to rice roots grown under aerobic conditions, and the oxygen permeability was measured along the roots. There are not many quantitative data regarding the permeability of oxygen in plants. Most of the data refer to the permeability of cuticles isolated from leaves of plants such as Citrus aurantium L., the pericarp of Capsicum annuum L., and Solanum lycopersicum L., or from various conifer needles, or phellems isolated from trees (Lendzian, 1982; Lendzian et al., 1986; Groh et al., 2002). Values presented for cuticles varied between 3×10−7 (C, aurantium), 1.4×10−6 (C. annuum), and 1.1×10−6 (S. lycopersicum) m s−1. The range of permeabilities of cuticular membranes to oxygen for amphibious and submerged plants was 5–143×10−6 m s−1 (Frost-Christensen et al., 2003). The oxygen permeability of phellem of Aesculus hippocastanum was 7×10−6 m s−1. As plant cuticles are usually regarded as rather impermeable, the present values of (1.9–0.9)×10−6 m s−1 obtained for the OPR of rice roots may be regarded as rather low and the OPR an effective barrier for oxygen. This conclusion does not change when the effects of unstirred layers are taken into account, which would result in values of (2.8–1.1)×10−6 m s−1 for just the OPR. However, it has to be pointed out that the permeability of the OPR (PdO2) of rice roots grown under aerated conditions should be substantially larger than the PdO2 of roots grown in stagnant solution. Garthwaite et al. (2008) assessed the oxygen permeability coefficient across the cell layers exterior to aerenchyma of stagnantly grown Hordeum marinum. The values were 2.9×10−5 at 5 mm from the apex and 1.1×10−6 m s−1 at 80 mm from the apex, which was similar to the values obtained in this study, although there were substantial differences in the anatomy of the roots (see below).
The permeability coefficients of the outer cell layers of barley roots presented by Garthwaite et al. (2008) were assessed from measurements of ROL while either varying shoot O2 partial pressure or cooling the rooting medium to cancel out any respiratory effects. By means of these manipulations and in conjunction with calculated internal resistances (OPR, pore space, agar solution between root and sleeving electrode), the authors estimated the overall resistance of the outer cell layers (O2 diffusion coefficient in the external cell layers). Another indirect method of calculating diffusion of oxygen across outer layers was presented by Armstrong et al. (2000) and applied to roots of Phragmites australis. It was based on the assumption that the drop of oxygen concentration across the epidermal–hypodermal cylinder was dependent on the diffusive resistance within the cylinder, the rate of oxygen consumption within it, and the radial oxygen transfer across it to the rhizosphere. Radial oxygen profiles at different distances from the root apex were obtained using Clark-type microelectrodes. The present approach differs from that of Armstrong et al. (2000) and Garthwaite et al. (2008) in that it did not require assumptions to be made about respiratory effects (the oxygen concentration in the aerenchyma was fixed by the rapid perfusion, see below), porosity, or root pore space resistances (e.g. the resistance to oxygen diffusion at the junction between shoot and root). The technique presented herein enabled, for the first time, PdO2 to be measured directly, since the oxygen concentration in the aerenchyma and radial flows of oxygen were known.
The experiments with rice root segments precluded measurement of JO2 at distances closer than 30 mm from the root apex, where the intercellular gas-filled spaces were not sufficiently developed for axial gas perfusion. In roots of the hydroponically grown rice, aerenchyma started to develop at 10–15 mm behind the apex (Ranathunge et al., 2003). Therefore, attempts at perfusing root segments excised at 10 mm failed, although ROL could be measured. In these experiments, the driving force for the radial oxygen flow was the difference in oxygen concentration (21, 40, 60, or 100%) between the aerenchyma and the outer O2-free agar solution. The oxygen concentration of the gas mixtures perfused through the aerenchyma resulted in an equivalent concentration (or activity) in the water film right at the inner surface of the OPR, driving the oxygen transport across the outer cell layers into the deoxygenated medium. To provide a constant oxygen concentration along the entire root segments, and to ensure that the rate of axial perfusion was much more rapid than the radial loss, perfusion had to be carried out at a certain overpressure at the entrance of segments. The overpressure of 20 kPa (average of 10 kPa=1 m water column) was tested and found to be sufficient to provide rapid perfusion, which could have been estimated from the fact that air bubbles appeared at the outlet side of segments. The overpressure of 30 kPa was rejected in order to avoid excessive pressurization of the roots. There was a slight tendency of JO2 to increase when the overpressure increased from 10 kPa to 30 kPa, but this was not significant (Fig. 4). Perhaps it was caused by the higher partial pressure of oxygen or due to a slight increase in the volume of aerenchyma and a slight expansion of the OPR.
In the perfusion experiments, the forces driving the oxygen across the OPR (partial oxygen pressure in the aerenchyma) have been worked out from the oxygen concentrations in the O2/N2 gas mixtures taking into account that moistening the gas mixtures would reduce the partial oxygen pressure by ∼3%. Also, the use of an overpressure was considered. The overpressure applied at the inlet side of the segments was assumed to drop linearly along the root. It results from the fact that the inner diameters of the tubes connecting the root segment with the moistening bottle and the air (Fig. 1) were substantially larger than those of aerenchyma, and there was no overpressure at the exit side of the segments. Hence, in all calculations of Ci, the mean value from the overpressure at the entrance and exit was used (for an overpressure of 20 kPa this was 10 kPa). As the resistance to axial gas flow was much smaller compared with the radial flow, the assumption of a linear drop is reasonable.
As the gas flow along the aerenchyma of root segments was viscous in nature, the volume flow in m3 s−1 was calculated according to a modified Hagen–Poiseuille equation (viscous flow through a concentric annulus; see Chapter 6.6 of White, 1999). The amount of oxygen supplied by the perfusion at the mean overpressure of 10 kPa was calculated to be 3.7×10−7 g s−1 at an internal oxygen concentration equivalent to 20.3% O2. On a root volume basis, Luxmoore et al. (1970) calculated the overall rate of oxygen losses caused by respiration in rice root segments to be 3.9×10−8 g O2 cm−3 of tissue s−1 (mean for segments excised at 20–60 mm from the apex). Since the volume of the 50 mm long segments used in this study was ∼3.9×10−2 cm3, the amount of oxygen used for respiration was estimated to be 1.5×10−9 g O2 s−1. This is only 0.4% of the oxygen supplied by perfusion. In addition, the mean JO2 along the root segments for 20.3% was 1.4 g cm−2 s−1. This equates to 2.2×10−9 g O2 s−1 of a radial loss of O2, or 0.58% of the oxygen supplied by perfusion. Hence, when using high rates of perfusion, a modification of the internal oxygen concentration (Ci) by either respiration or JO2 could be neglected. At maximum, both effects should be ∼1%. Overall, the effects of water vapour pressure (although considered), respiration, and radial flows of O2 were relatively small and did not have a significant impact on the final results. Another error could result from variations in the composition of the gas mixtures used (40, 60, and 100% O2), which were given by the supplier at an accuracy of ±2% (see above). Even more important, however, were variabilities between roots. Nevertheless, the data clearly indicate a decrease of PdO2 as the roots developed (see Results), which is most probably due to a suberization/lignification of the OPR.
The present data showed that the increase in oxygen concentration in the perfusing gas did not result in a linear increase in radial oxygen flow (JO2), as one may expect. Similar deviations from linearity were observed for silicone tubes of dimensions similar to that of rice roots at similar rates of radial O2 flow. However, there was a linear response, when JO2s were substantially smaller, such as with Teflon tubes. A linear relationship was also observed for intact plants, where the oxygen concentration was manipulated around the shoot, but the absolute values of ROL were relatively small, compared with those of JO2. Hence, the effect of non-linearity was related to the fact that, at high rates of oxygen flow, the cylindrical platinum electrode did not give a linear response. In the usual ROL experiments, where O2 is supplied from the shoot, the polarizing voltage of ∼0.5 V was sufficient to reduce all oxygen lost from the root (Armstrong, 1967). In these experiments, absolute values of ROL were relatively small and the OPR acted as a membrane with relatively high permeation resistance such as the membrane in the Clark electrode (usually Teflon). This also applied when root segments were perfused with oxygen concentrations of up to 40%, where the polarizing voltage of ∼0.5 V was sufficient to reduce all O2.
Non-linear effects of Clark-type electrodes were already known from other studies, but not as yet for plant roots. For example, for blood gas analysers it was shown that the response of PO2 electrodes was often not linear with oxygen concentrations at high partial pressure of oxygen (PO2; Crampton et al., 1969; Severinghaus and Bradley, 1969; Mapleson et al., 1970; Hill and Tilslay, 1973). The non-linearity was linked to an incomplete reduction of oxygen, which is reduced to hydroxyl ions in two steps (Hahn et al., 1975):
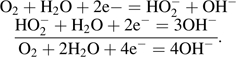 |
(5) |
In the first step, two electrons reduce oxygen to one hydrogen peroxide anion and one hydroxyl ion. In the second step of the reaction, two more electrons are required to reduce HO2– to three molecules of OH–. As discussed by Hahn et al. (1975), an incomplete reduction of oxygen, where only two electrons are consumed rather than four, may lead to a considerable non-linearity, which would underestimate the JO2 at high oxygen concentrations. According to the overall equation for the reduction of oxygen at the platinum electrode, the Nernst equation would predict that, at a 10-fold change of PO2, the electrode potential should change by only 59.2/4=14.8 mV (=RT/4F at 25 °C). As the actual voltage used to overcome the increase in PO2 was much bigger, there should have been polarizing effects due to a concentration polarization and/or a reaction polarization (Hack, 1986). This would explain the bending of JO2/Ci. It appears that the precise nature of the processes resulting in an underestimation of JO2 at high rates of oxygen flow is not yet known (such as with rice root segments used in the present study and silicone tubes). Overall, what remains is a caveat when using platinum electrodes in an amperometric way such as the ring electrode in the presence of high JO2, as done in this study. As shown, the polarizing effect can be overcome by increasing the polarizing voltage (Fig, 9). However, there may be limits to using high polarizing voltages due to reduction of hydrogen ions at the electrode, which occurs above 0.8 V (Armstrong, 1967). Nevertheless, complications in terms of polarizing effects can be excluded when the rates of radial flow of oxygen are low such as during usual ROL experiments (Colmer et al., 1998; Gibberd et al., 1999; Visser et al., 2000; McDonald et al., 2002; Colmer, 2003a).
The present data of ROL may be common with many wetland plants, which contain a constitutive barrier to oxygen losses in the basal root zones (Colmer et al., 1998; Visser et al., 2000; McDonald et al., 2002; Colmer, 2003a). The growth in stagnant conditions may induce a barrier in plants, where basal positions remain permeable to O2 when grown in aerated media (Colmer et al., 1998; McDonald et al., 2002; Colmer, 2003a). Stagnant growth may increase the barrier in plants, where the barrier is constitutively present. Thus, there may be criticism that, in the present study, roots grown in aerated solution were used, which may not reflect the natural paddy field conditions, where PdO2 of the OPR should be substantially lower. The aerated growth conditions were used to (i) develop the new technique for measuring PdO2 and (ii) compare it with already existing data on water permeability across the OPR (see below; Ranathunge et al., 2003, 2004). Presently, the perfusion technique is used to measure the permeability coefficient of roots of plants grown in stagnant conditions. Preliminary results showed that growth in deoxygenated solution significantly reduced PdO2 along entire root axes. If this is true, the generally held view that under anoxic conditions the oxygen transport to the root tips limits root growth may be questioned, when the axial diffusional resistance is low. Knowledge about the oxygen permeability across the OPR may help to model the oxygen flow in rice roots.
The anatomical basis of the barrier to ROL was not examined in the present study. However, in previous reports it was linked to the dense arrangement of the hypodermal cylinder in Halophila ovalis (Connell et al., 1999), suberization and/or lignification of outer cell layers (Armstrong and Armstrong, 1988; Jackson and Armstrong, 1999), and a combination of densely packed cells, suberin deposits, lignification, and oxygen consumption by cells exterior to aerenchyma (Armstrong et al., 2000). In rice, the barrier to ROL may be related to either densely packed sclerenchyma cells with thick lignified secondary walls or suberized exodermis with Casparian bands, or both. Exodermis formed a tight juncture with the epidermis and a layer of sclerenchyma (Clark and Harris, 1981). Exodermal suberin lamellae of hydroponically grown rice were observed at ∼30 mm from the tip and matured at ∼50 mm (Ranathunge et al., 2003, 2004). Well-developed Casparian bands were found at ∼50 mm from the apex (Ranathunge et al., 2003). Different root structures among a range of plant species may be directly related to differences in their oxygen diffusion coefficients across cell layers exterior to aerenchyma. The diffusion coefficient of the outer part of rice roots, DOPR, varied along the root, being 2.6±0.2×10−10 m2 s−1 and 1.0±0.1×10−10 m2 s−1 at 30 mm and 60 mm from the apex, respectively. These values are smaller by factors of 9 and 24 than the diffusion coefficient of oxygen in water at the same temperature. According to Armstrong et al. (2000), the oxygen diffusivity of the outer cell layers of P. australis was estimated to be 5.5×10−11 m2 s−1 at 29 mm from the apex. This is smaller by about one order of magnitude than the DOPR of rice roots, most probably due to the existence of a suberized multilayered exodermis (Soukup et al., 2006). In Hordeum marinum, grown in stagnant agar nutrient solution, the diffusion coefficient was reduced from 1.6×10−9 m2 s−1 near the root tip (5 mm), to 6.0×10−11 m2 s−1 in the basal regions (80 mm), which was also related to anatomical changes (suberization or lignificaton as concluded from epifluorescence; Garthwaite et al., 2008).
A barrier to ROL may be an adaptive feature of plants from wetland habitats, but may also have some drawbacks such as impeding water and nutrient uptake (Armstrong, 1979; Koncalova, 1990; Colmer and Bloom, 1998; Garthwaite et al., 2008). The diffusional water permeability of the OPR (PdOPR) was 3.0±1.6×10−7 m s−1 for the immature root segments (20–50 mm from the root apex) and 2.0±0.7×10−7 m s−1 for mature root segments (50–100 mm from the apex; Ranathunge et al., 2004). It was smaller by an order of magnitude than the PdO2. However, it has to be taken into account that the PdOPR was smaller by a factor of 600 (20–50 mm from apex) and 1400 (50–100 mm from apex) than the hydraulic conductivity (high LpOPR/PdOPR ratio; Ranathunge et al., 2004). The latter parameter is the one that is important during water uptake. It should be noted that the hydraulic conductivity of the OPR was larger by a factor of 30 than the overall value measured for the hydroponically grown rice roots. In other words, the water transport across the endodermis was rate limiting. It appears that, in rice, rates of water uptake can be quite high in the presence of a high capability to retain oxygen, which is favourable for the plant. It is possible to argue that rice roots have evolved an optimum balance between O2 retention and water uptake. This may be different for roots grown under stagnant conditions, and important with regard to overall productivity of rice plant.
When considering the flow of oxygen along the whole plant from the shoot to the roots, the main resistance should be located in the OPR. It was indicated by the comparison of the slopes of the response of ROL and JO2 to increased oxygen concentration. In the experiments with intact rice plants, the slopes of the response of ROL to external O2 concentrations were not significantly different at different positions, so it was possible to calculate one slope for all data. The pooled slope would represent the O2 permeability coefficient of the whole plant (including the diffusive resistance in the shoot and in the root), which equalled 1.1±0.1×10−6 m s−1. This is smaller by a factor of 1.7 than the PdO2 at 30 mm from the root apex and 1.2-fold larger than the PdO2 at 60 mm from the root apex. The latter result of a ratio close to unity may be caused by the fact that there was some uncertainty in the measurement of both PdO2s. Overall, the comparison of the data indicates that the main resistance to oxygen flow in the plant was located in the OPR. Considering the fact that, in the present experiments, the PdO2 of roots grown in aerated medium should be higher compared with roots grown in stagnant medium, the latter conclusion should also hold for stagnantly grown plants.
In conclusion, the present study provided a new technique for measuring oxygen permeability across cell layers exterior to aerenchyma and the first quantitative data of the O2 permeability at different positions of developing roots of rice. These data are of importance to understand the mechanisms controlling ROL and the ecophysiology of wetland species. Although the rice plants used in the present study were grown under aerated conditions, the absolute values of PdO2 were relatively low, and similar to plant cuticles. Preliminary results showed that rice roots grown in stagnant conditions have much lower PdO2 compared with roots of plants grown in aerated solution. Due to the resistance of the agar layer surrounding the root, the measured PdO2s were smaller by 33–16% than the actual values of POPR, which referred to just the outer cell layers. As roots matured, the resistance to radial movement of oxygen across the OPR increased from 30 mm to 60 mm from the apex by a factor of 2.5. It appears that, in rice, this is a constitutive, adaptive feature enhancing longitudinal diffusion of oxygen to the root apex. Non-linear responses of rates of radial oxygen flow (JO2) to increased oxygen concentration in the perfusing gas mixtures indicated that some care has to be taken when using root-sleeving platinum electrodes (Clark-type electrodes without membranes) in the presence of high O2 fluxes. Experiments with silicone and Teflon tubes supported that view. At high rates of JO2, there may be processes tending to underestimate the actual value of radial flow of oxygen (e.g. incomplete reduction of O2 at the electrode surface and other polarizing effects). When high oxygen concentrations were applied around the shoot of intact plant, absolute values of JO2 were much smaller, and there were no problems with the platinum electrodes, which also agrees with literature data of ROL.
Acknowledgments
We are indebted to Dr Timothy D. Colmer (University of Western Australia) for introducing us to the ROL technique. We thank Dr Kosala Ranathunge (University of Bonn) and Yangmin Kim (University of Bayreuth) for reading and discussing the manuscript. The helpful comments and suggestions of Dr Hermann Heilmeier (Technical University, Freiberg, Germany) concerning statistical analysis are greatly acknowledged, as is the technical assistance of Burkhard Stumpf (University of Bayreuth).
References
- Armstrong J, Armstrong W. Phragmites australis: a preliminary study of soil-oxidising sites and internal gas transport pathways. New Phytologist. 1988;108:373–382. [Google Scholar]
- Armstrong W. The use of polarography in the assay of oxygen diffusing from roots in anaerobic media. Physiologia Plantarum. 1967;20:540–553. [Google Scholar]
- Armstrong W. Aeration in higher plants. Advances in Botanical Research. 1979;7:225–332. [Google Scholar]
- Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Annals of Botany. 2000;86:687–703. [Google Scholar]
- Armstrong W, Wright EJ. Radial oxygen loss from roots: the theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiologia Plantarum. 1975;35:21–26. [Google Scholar]
- Atkins P, de Paula J. Elements of physical chemistry. 4th edn. Oxford: Oxford University Press; 2007. [Google Scholar]
- Chabbi A, McKee KL, Mendelssohn IA. Fate of oxygen losses from Typha domingensis (Typhaceae) and Cladium jamaicenese (Cyperaceae) and consequences for root metabolism. American Journal of Botany. 2000;87:1081–1090. [PubMed] [Google Scholar]
- Clark LC, Wolf R, Granger D, Taylor Z. Continuous recording of blood oxygen tensions by polarography. Journal of Applied Physiology. 1953;6:189–193. doi: 10.1152/jappl.1953.6.3.189. [DOI] [PubMed] [Google Scholar]
- Clark LH, Harris WH. Observations of the root anatomy of rice (Oryza sativa L.) American Journal of Botany. 1981;68:154–161. [Google Scholar]
- Colmer TD. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.) Annals of Botany. 2003a;91:301–309. doi: 10.1093/aob/mcf114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment. 2003b;26:17–36. [Google Scholar]
- Colmer TD, Bloom AJ. A comparison of NH4+ and NO3– net fluxes along roots of rice and maize. Plant, Cell and Environment. 1998;21:240–246. [Google Scholar]
- Colmer TD, Gibberd MR, Wiengweera A, Tinh TK. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany. 1998;49:1431–1436. [Google Scholar]
- Conlin TSS. Examination of rhizospheric oxidation and its relation to iron toxicity in Carex rostrata stokes. 1986. Typha latifolia L. and Phragmites australis (Cav.). MSc Thesis, Queen's University, Kingston, ON, Canada. [Google Scholar]
- Connell EL, Colmer TD, Walker DI. Radial oxygen loss from intact roots of Halophila ovalis as a function of distance behind the root tip and shoot illumination. Aquatic Botany. 1999;63:219–228. [Google Scholar]
- Crampton Smith A, Hahn CEW. Electrodes for the measurement of oxygen and carbon dioxide tensions. British Journal of Anaesthesiology. 1969;41:731. doi: 10.1093/bja/41.9.731. [DOI] [PubMed] [Google Scholar]
- Fisher JMC, Peterson CA, Bols NC. A new fluorescent test for cell vitality using Calcofluor white M2R. Stain Technology. 1985;60:69–79. doi: 10.3109/10520298509113895. [DOI] [PubMed] [Google Scholar]
- Frost-Christensen H, Bolt Jørgensen L, Floto F. Species specificity of resistance to oxygen diffusion in thin cuticular membranes from amphibious plants. Plant Cell and Environment. 2003;26:561–569. [Google Scholar]
- Garthwaite AJ, Armstrong W, Colmer TD. Assessment of O2 diffusivity across the barrier to radial O2 loss in adventitious roots of Hordeum marinum. New Phytologist. 2008;179:405–416. doi: 10.1111/j.1469-8137.2008.02467.x. [DOI] [PubMed] [Google Scholar]
- Gibberd MR, Colmer TD, Cocks PS. Root porosity and oxygen movement in waterlogging-tolerant Trifolium tomentosum and -intolerant Trifolium glomeratum. Plant, Cell and Environment. 1999;22:1161–1168. [Google Scholar]
- Groh B, Hübner C, Lendzian KJ. Water and oxygen permeance of phellems isolated from trees: the role of waxes and lenticles. Planta. 2002;215:794–801. doi: 10.1007/s00425-002-0811-8. [DOI] [PubMed] [Google Scholar]
- Hack HP. Galvanic corrosion. The symposium on galvanic corrosion at Phoenix. 1986 ISBN:0803109814. [Google Scholar]
- Hahn CEW, Davis AH, Albery WJ. Electrochemical improvement of the performance of PO2 electrodes. Respiration Physiology. 1975;25:109–133. doi: 10.1016/0034-5687(75)90055-9. [DOI] [PubMed] [Google Scholar]
- Hill DW, Tilsley C. A comparative study of the performance of five commercial blood-gas and pH electrodes analysers. British Journal of Anaesthesiology. 1973;45:647–654. doi: 10.1093/bja/45.7.647. [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Tsuchida M, Ishihara K. Relationship between resistance to water transport and exudation rate and the effect of the resistance on the midday depression of stomatal aperture in rice plants. Japan Journal of Crop Science. 1992;61:145–152. [Google Scholar]
- Jackson MB, Armstrong W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology. 1999;1:274–287. [Google Scholar]
- Jackson MB, Drew MC. Effects of flooding on growth and metabolism of herbaceous plants. In: Kozlowski TT, editor. Flooding and plant growth. New York: Academic Press; 1984. pp. 47–128. [Google Scholar]
- Jones HG. Plants and microclimate: a quantitative approach to environmental plant physiology. 2nd edn. Oxford: Oxford University Press; 1992. [Google Scholar]
- Koncalova H. Anatomical adaptations to waterlogging in roots of wetland graminoids: limitations and drawbacks. Aquatic Botany. 1990;38:127–134. [Google Scholar]
- Lendzian KJ. Gas permeability of plant cuticles: oxygen permeability. Planta. 1982;155:310–315. doi: 10.1007/BF00429457. [DOI] [PubMed] [Google Scholar]
- Lendzian KJ, Nakajima A, Ziegler H. Isolation of cuticular membranes from various conifer needles. Trees. 1986;1:47–53. [Google Scholar]
- Luxmoore RJ, Stolzy LH, Letey J. Oxygen diffusion in the soil–plant system. II Respiration rate, permeability, and porosity of consecutive excised segments of maize and rice roots. Agronomy Journal. 1970;62:322–324. [Google Scholar]
- Mapleason WW, Horton JN, Ng WS, Imrie DD. The response pattern of polarographic oxygen electrodes and its influence on linearity and hysteresis. Medical and Biological Engineering. 1970;8:725–728. doi: 10.1007/BF02478232. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Galwey NW, Colmer TD. Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dryland grass species. Plant, Cell and Environment. 2002;25:441–451. [Google Scholar]
- Millington RJ. Gas diffusion in porous media. Science. 1959;130:100–102. doi: 10.1126/science.130.3367.100-a. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Steudle E, Hirasawa T, Lafitte R. Hydraulic conductivity of rice roots. Journal of Experimental Botany. 2001;52:1835–1846. doi: 10.1093/jexbot/52.362.1835. [DOI] [PubMed] [Google Scholar]
- Ponnamperuma FN. Effects of flooding on soils. In: Kozlowski TT, editor. Flooding and plant growth. New York: Academic Press; 1984. pp. 9–45. [Google Scholar]
- Ranathunge K, Kotula L, Steudle E, Lafitte R. Water permeability and reflection coefficient of the outer part of young rice roots are differently affected by closure of water channels (aquaporins) or blockage of apoplastic pores. Journal of Experimental Botany. 2004;55:433–447. doi: 10.1093/jxb/erh041. [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Steudle E, Lafitte R. Control of water uptake by rice (Oryza sativa L.): role of the outer part of the root. Planta. 2003;217:193–205. doi: 10.1007/s00425-003-0984-9. [DOI] [PubMed] [Google Scholar]
- Revsbech NP. Diffusion characteristics of microbial communities determined by use of oxygen microsensors. Journal of Microbiological Methods. 1989;9:111–122. [Google Scholar]
- Severinghaus J, Bradley AF. Blood gas electrodes. San Francisco Medical Centre: Internal Report, USA; 1969. [Google Scholar]
- Soukup A, Armstrong W, Schreiber L, Franke R, Votrubova O. Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmatis australis and Glyceria maxima. New Phytologist. 2007;173:264–278. doi: 10.1111/j.1469-8137.2006.01907.x. [DOI] [PubMed] [Google Scholar]
- Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ. Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant, Cell and Environment. 2000;23:1237–1245. [Google Scholar]
- White FM. Fluid mechanics. 4th edn. New York: McGraw-Hill Companies; 1999. [Google Scholar]
- Ye Q, Kim Y, Steudle E. A re-examination of the minor role of unstirred layers during the measurements of transport coefficients of Chara corallina internodes with the cell pressure probe. Plant, Cell and Environment. 2006;29:964–980. doi: 10.1111/j.1365-3040.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cloud A. Global advances in materials and processes engineering. Proceedings of the SAMPE Fall Technical Conference; Coatings, and Sealants Section. 2006. The permeability characteristics of silicone rubber. November 6–9, 2006, Dallas, TX. ISBN 978-0-938994-72-5. [Google Scholar]