Abstract
The ER-resident molecular chaperone BiP (binding protein) was overexpressed in soybean. When plants growing in soil were exposed to drought (by reducing or completely withholding watering) the wild-type lines showed a large decrease in leaf water potential and leaf wilting, but the leaves in the transgenic lines did not wilt and exhibited only a small decrease in water potential. During exposure to drought the stomata of the transgenic lines did not close as much as in the wild type, and the rates of photosynthesis and transpiration became less inhibited than in the wild type. These parameters of drought resistance in the BiP overexpressing lines were not associated with a higher level of the osmolytes proline, sucrose, and glucose. It was also not associated with the typical drought-induced increase in root dry weight. Rather, at the end of the drought period, the BiP overexpressing lines had a lower level of the osmolytes and root weight than the wild type. The mRNA abundance of several typical drought-induced genes [NAC2, a seed maturation protein (SMP), a glutathione-S-transferase (GST), antiquitin, and protein disulphide isomerase 3 (PDI-3)] increased in the drought-stressed wild-type plants. Compared with the wild type, the increase in mRNA abundance of these genes was less (in some genes much less) in the BiP overexpressing lines that were exposed to drought. The effect of drought on leaf senescence was investigated in soybean and tobacco. It had previously been reported that tobacco BiP overexpression or repression reduced or accentuated the effects of drought. BiP overexpressing tobacco and soybean showed delayed leaf senescence during drought. BiP antisense tobacco plants, conversely, showed advanced leaf senescence. It is concluded that BiP overexpression confers resistance to drought, through an as yet unknown mechanism that is related to ER functioning. The delay in leaf senescence by BiP overexpression might relate to the absence of the response to drought.
Keywords: Binding protein, BiP, drought tolerance, environmental stresses, leaf senescence, soybean
Introduction
Plants under natural conditions are continually subject to environmental stresses that adversely affect growth and productivity. As a consequence, they have evolved a variety of adaptive and molecular responses. Water deficit is among the major environmental limitations to crop productivity. Engineered overexpression of a single downstream target, such as biosynthetic enzymes for osmoprotectants, enzymes of the antioxidant system, late embryogenesis abundant (LEA) proteins, and molecular chaperones, has been shown to increase water deficit tolerance in plant model systems (Gupta et al., 1993; Wehmeyer and Vierling, 2000; Rodrigues et al., 2006; Ashraf and Foolad, 2007). The endoplasmic reticulum (ER)-resident molecular chaperone BiP (binding protein) has been shown to confer drought tolerance when constitutively overexpressed in N. tabacum (Alvim et al., 2001). However, the underlying mechanism for BiP-mediated increases in water stress tolerance and the effectiveness of this strategy on other economically relevant crops are unknown. They constitute the major focus of the present investigation.
BiP has been demonstrated to act as a multifunctional protein in animal systems (for a review see Maa and Hendershot, 2004; Malhotra and Kaufman, 2007). BiP assists in the folding of proteins and also acts in the ER quality control mechanism that recognizes unfolded or abnormally folded proteins and sends them out of the organelle for degradation (Maa and Hendershot, 2004). Conditions that disrupt ER homeostasis and promote the accumulation of misfolded or unfolded proteins in the ER lumen cause ER stress. Cells respond to ER stress by activating a protective signalling cascade, designated unfolded protein response (UPR), that allows the ER processing and folding capacities to be balanced with the cell secretory activity (Malhotra and Kaufman, 2007). In animal systems, BiP plays a major role as a sensor of disturbances in protein folding. It regulates the activity of the UPR proximal transducers, PERK, URE1, and ATF6 (Malhotra and Kaufman, 2007). Overexpression of BiP in mammalian cultured cells (Morris et al., 1997) and tobacco protoplasts (Leborgne-Castel et al., 1999) increases cell tolerance to ER stress, whereby ER stress is defined as an imbalance between the cellular demand for ER function (proper protein folding) and the capacity of the ER to carry out this function. Furthermore, antisense down-regulation of BiP mRNA has been shown to increase the sensitivity of transfected cells to ionophores (Li and Lee, 1991; Li et al., 1992) and oxidative stress (Gomer et al., 1991).
The effect of BiP overexpression on a typical ER stress response was investigated previously using a germination/survival assay in the presence of tunicamycin, a potent inducer of ER stress and an activator of UPR (Alvim et al., 2001). Transgenic seeds expressing a soybean BiP gene (soyBiPD, Figueiredo et al., 1997) were better able to cope with ER stress (Alvim et al., 2001). As in mammalian cells, in plants the BiP-mediated protection against ER stressors is related to the alleviation of the reduced protein synthesis (Morris et al., 1997; Laitusis et al., 1999; Leborgne-Castel et al., 1999; Alvim et al., 2001). In addition to alleviating ER stress and being up-regulated by treatments that promote ER stress, the ER-resident molecular chaperone BiP from soybean exhibits an unusual response to drought. Four BiP genes have been identified in soybean (Kalinski et al., 1995; Figueredo et al., 1997). Overexpression of one of these, soyBiPD, has been found to confer tolerance to drought in the plant model system N. tabacum (Alvim et al., 2001). Although the mechanism of BiP-mediated water stress tolerance has yet to be elucidated, BiP overexpression prevented the increase in anti-oxidative defences found in wild-type plants exposed to drought. Some physiological and molecular changes in the response to drought introduced by the overexpression of BiPD in soybean are investigated here, including the effect of this overexpression on soybean leaf senescence. In addition, the effect of BiPD silencing and BiPD overexpression on leaf senescence in drought-stressed tobacco is reported.
Materials and methods
Soybean transformation
A plant expression cassette containing the soyBiPD gene (GeneBank accession number AF031241) was constructed by insertion of the 2.0 kb XbaI cDNA insert of pUFV42 (Alvim et al., 2001) into the pBS35SdAMVNOS2 vector. The resulting clone pBS35SdAMVNOS2-BiP contains the BiP cDNA under control of a duplicated cauliflower mosaic virus 35S promoter with an enhancer region from the alfalfa mosaic virus and the polyadenylation signal of the nos gene. The Arabidopsis thaliana ahas gene (that confers tolerance to the herbicide imazapyr) was removed from the vector pAC321 (Aragão et al., 2000) with XbaI and inserted into the vector pFACM1 to generate pFACMahas. The BiPD expression cassette was released with SalI and NotI from pBS35SdAMVNOS2-BiP and cloned into the vector pFACMahas to yield pahasBip. The vector pahasBip was used to transform soybean (cv. Conquista) as previously decribed by Aragão et al. (2000). Primary transformants were selected by PCR. Segregation analyses of independently transformed soybean lines (35S:BiP-1, 35S:BiP-2, 35S:BiP-3, 35S:BiP-4) were performed by PCR and accumulation of BiP was monitored in each subsequent generation by immunoblottings.
PCR analysis of transgenic plants
PCR was carried out on 20 ng of genomic DNA isolated from 4-week-old greenhouse-grown transgenic plants, using 200 nM each of BiP gene-specific primers and 5 U of Taq polymerase (Invitrogen) in a final volume of 25 ml. The PCR reactions were conducted for 35 cycles (60 s at 95 °C, 60 s at 50 °C, and 60 s at 72 °C) with a final extension at 72 °C, for 5 min. The soyBiPD-specific primers, bipsoy235 (5′-GAGAGACTAATTGGAGAGGCTG-3′) and bipsoy645c (5′-ATAGGCAATGGCAGCAGCAGTG-3′), amplify a 410 bp sequence from the BiPD gene coding region and cover an intron region from genomic BiPD sequence.
Immunoblot analysis
Total protein was extracted from leaves of untransformed or transformed soybean plants as previously described by Cascardo et al. (2000). SDS-PAGE was carried out and the proteins were transferred from 10% SDS-polyacrylamide gels to nitrocellulose membranes by electroblotting. Immunoblot analyses were performed using polyclonal BiP antibodies prepared against a purified protein fraction from soybean seeds (Figueiredo et al., 1997) or against an E. coli-produced BiP carboxy domain (anti-carboxy BiP; Buzeli et al., 2002), at a 1:1000 dilution and a goat anti-rabbit IgG alkaline phosphatase conjugate (Sigma) at a 1:5000 dilution. Alkaline phosphatase activity was assayed using 5-bromo-4-chloro-3-indolyl phosphate (Sigma.) and p-nitro blue tetrazolium (Sigma).
Isolation of microsomal fraction
The isolation of the microsomal fraction from soybean leaves was performed as described by Pirovani et al. (2002). Briefly, soybean leaves were homogenized with 25 mM TRIS/HCl, pH 7.0, 250 mM sucrose, 2.5 mM dithiothreitol, 10 mM EDTA, and 0.5 mM phenylmethylsulphonyl fluoride in the ratio of 1 g tissue per 5 ml buffer. The homogenate was filtered and centrifuged for 15 min at 14 000 g and 4 °C. Microsomal preparations were isolated by centrifugation at 80 000 g for 50 min at 15 °C and the pellet was washed twice with 10 mM MES-KOH pH 6.8, 2.5 mM DTT, resuspended in 100 mM K2HPO4/KH2PO4 pH 7.5 and stored at –80 °C.
Plant growth and water stress induction
Soybean seeds of wild type (Glycine max cv. Conquista) and transgenic lines were germinated in organic soil (Bioplant), transferred to 3.0 l pots containing a mixture of soil, sand and dung (3:1:1 by vol.; Pedra et al., 2000) and grown in greenhouse conditions under natural conditions of light, relative humidity (65–85%) and temperature (15–35 °C) and approximately equal day and night length. A slow soil drying experiment was imposed in 50% of wild type and transgenic lines at the V6 stage of development. Control plants were watered daily, providing about 180 ml of water per plant. In the drought treatment the daily water supply was reduced to about 40% of that of the controls. The drought treatment lasted for 18 d. After this period, the plants again received the normal water supply for 2 weeks. The remaining 50% of plants received normal water supply continuously. All the experiments were conducted with five clones from at least four independently transformed lines. For the fast soil drying experiment, after 30 d of growth with normal water supply (V3 stage of development), severe drought stress was induced by withholding irrigation for one-week from one-half of 35S:BiP-2 (T4 generation), 35S:BiP-4 (T5 generation) and wild-type plants.
Drought experiments were also conducted using the T4 generation of transgenic control (pBI121 vector alone), transgenic sense (35S-BiPS lines), and transgenic antisense (35S-BiPAS lines) tobacco plants (Alvim et al., 2001). In this case, 2-week-old seedlings were transplanted individually into pots and grown in a growth chamber with a 12 h photoperiod at a 23/18 °C day/night temperature cycle, 240 μmol m−2 s−1 irradiance, and a relative air humidity of 60%. After 4 weeks of growth with normal water supply, one-half of the transgenic plants received 40% of the normal irrigation (restricted water regime) for 35 d and the remaining transgenic plants received normal water supply continuously (control).
For the PEG-induced dehydration experiment, soybean seeds from 35S:BiP-2 and 35S:BiP-4 transgenic lines as well as untransformed soybean seeds were germinated in MS medium (Murashige and Skoog, 1962) without and with 1% (w/v) or 2% (w/v) PEG. After 30 d, the length (cm) and the dry weight (g) of roots were determined.
Real-time RT-PCR analysis
For quantitative RT-PCR, total RNA was extracted from frozen leaves with TRIzol (Invitrogen) according to the instructions from the manufacturer. The RNA was treated with 2 U of RNase-free DNase (Promega) and further purified through RNeasy Mini Kit (QIAGEN) columns. First-strand cDNA was synthesized from 4 μg of total RNA using oligo(dT)-18 and Trancriptase Reversa M-MLV (Invitrogen), according to the manufacturer's instructions.
Real-time RT-PCR reactions were performed on an ABI7500 instrument (Applied Biosystems), using SYBR® Green PCR Master Mix (Applied Biosystems). The amplification reactions were performed as follows: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 94 °C for 15 s and 60 °C for 1 min. To confirm quality and primer specificity, the size of amplification products was verified after electrophoresis through a 1.5% agarose gel, and the Tm (melting temperature) of the amplification products was analysed in a dissociation curve, performed by the ABI7500 instrument. The primers used are listed in Table 1. For quantitation of gene expression in soybean leaves, RNA helicase (Irsigler et al., 2007) was used as the endogenous control gene for data normalization in real-time RT-PCR analysis. For the quantitation of gene expression in tobacco leaves, actin was used as a control gene (Costa et al., 2008).
Table 1.
Genes analysed and primers for qRT-PCR
| Clone description | Clone accession | Forward primer | Reverse primer |
| Calnexin | AW508066 | TGATGGGGAGGAGAAGAAAAAGGC | CACTTGGGTTTGGGATCTTGGCTC |
| BiPD | AF031241 | ATCTGGAGGAGCCCCAGGCGGTGG | CTTGAAGAAGCTTCGTCGTAAAACTAAG |
| SMP (LEA) | AW397921 | GCCGAACTGAGGAAAAGACGAACC | CTTGGGCTGTTTGTTGGGTCTTC |
| BiP transgene | BIPD-nos | ATCTGGAGGAGCCCCAGGCGGTGG | CATCGCAAGACCGGCAACAGGAT |
| RNA helicase | AI736067 | TAACCCTAGCCCCTTCGCCT | GCCTTGTCGTCTTCCTCCTCG |
| GST | AACI8566 | CGGTTCTCATCCACAATGGCAAAC | CAGCCCAGAATCTAGCCTGAGC |
| NAC2 | AY974350 | TGACCTCTATGTCCCTGCGTTA | CCCCTGTGTGAAATCATTCTGA |
| Antiquitin | AY250704 | CGAAAAGGGAGAGGAGGACTTC | TCTGGGTCACCGAAAGGCAA |
| PDI3 | AW277660 | CGAAAAGGGAGAGGAGGACTTC | TCTGGGTCACCGAAAGGCAA |
| Actin | AB158612 | AGCAAGGAAATTACCGCATTAGC | ACCTGCTGGAATGTGCTGAGA |
| NTCP-23 | AB032168 | GTGGACTGTGCTGGAGCTTTTAAT | ATAAGCCATTCTTGCCAGTGTATG |
| didi A9 | AAZ23261 | AACCCCAACTTCTTGGAACAAG | AGCAATGTCAGTCACCCCAGTA |
Fold variation, which is based on the comparison of the target gene expression (normalized to the endogenous control) between experimental and control samples, was quantified using the comparative Ct method: 2–(ΔCtTreatment–ΔCtControl). Absolute gene expression was quantified using the 2–ΔCT method and values were normalized to the endogenous control.
Photosynthesis and chlorophyll fluorescence
Photosynthetic CO2 assimilation (A), transpiration rate (E), and stomatal conductance (gs) of the third fully expanded leaf were measured by IR gas analysis using an analyser (IRGA) model LI-6400 (Li-Cor, Nebraska, EUA), at 1000 μmol m−2 s−1 irradiance during the experiment. Relative water content of leaves was determined by the relative turgidity technique (Catsky, 1974). Initial fluorescence (Fo) and maximum fluorescence (Fm) were determined using a fluorometer (Plant Efficiency Analyser, Hansatech, King's Lynn, UK). All measurements were performed between 08.30 h and 11.30 h.
Proline content determination
Proline content was determined spectrophotometrically after quantitative extraction with 80% (v/v) ethanol, reaction with ninhydrin, followed by quantitative extraction with toluene, as described by Bates et al. (1973) and using L-proline (Sigma) as standard.
Determination of chlorophyll content and lipid peroxidation
Total chlorophyll content was determined spectrophotometrically at 663 nm and 646 nm after quantitative extraction from individual leaves with 80% (v/v) acetone as described by Lichtenthaler (1987). The extent of lipid peroxidation in leaves was estimated by measuring the amount of MDA, a decomposition product of the oxidation of polyunsaturated fatty acids. The malondialdehyde (MDA) content was determined by the reaction of thiobarbituric acid (TBA) as described by Hodges et al. (1993).
Statistical analysis
Statistical analysis of data was performed using a one-way analysis of variance and the least statistic difference (LSD) test for multiple pairwise comparisons at P <0.05 with the Software Scientific (SOC) package (EMBRAPA, Brazil). Means were also compared by the Tukey test at 5% probability.
Results
Generation of soybean transgenic lines
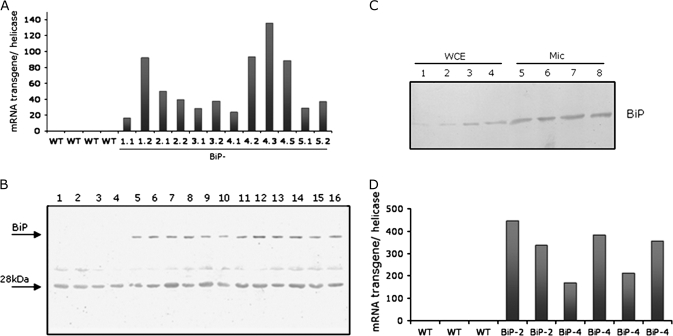
To examine the protective function of BiP against dehydration in soybean, transgenic plants were generated that expressed soyBiPD under the control of the 35S cauliflower mosaic virus promoter. Several independent transgenic lines were established; the expression level of the transgene was analysed by RT-PCR and the accumulation of BiP was monitored in each subsequent generation by immunoblotting. The independently transformed overexpressing (OE) lines constitutively accumulated higher levels of BiPD mRNA (Fig. 1A) and protein (Fig. 1B) than untransformed, wild-type controls. The antibody prepared against purified BiP from soybean seeds cross-reacted with a 28 kDa polypeptide that persisted as a contaminant in the purified BiP fraction used as antigen. To examine whether the ectopically expressed BiP protein was correctly localized in transgenic cells, microsomal fractions were prepared from soybean leaves at the V3 developmental stage and immunoblotted with an anti-BiP serum (Fig. 1C). As expected, BiP was detected in microsomal membrane-enriched fractions from wild-type leaves (lanes 5 and 6) and to a higher extent in microsomal fractions from 35S:BIP-2 and 35S:BiP-4 leaves (lanes 7 and 8). These results indicate that high levels of ectopically expressed BiP were correctly localized in the ER of soybean transgenic leaf cells. Transgene expression, analysed by RT-PCR, was also higher in roots of independently transgenic lines (Fig. 1D).
Fig. 1.
Ectopic expression of soyBiPD transgene in soybean plants. (A) mRNA abundance of soyBiPD transgene in overexpressing lines under normal growth conditions. Total RNA was isolated from leaves of wild-type (WT) plants and independently transformed soybean lines (35S:BiP-1, 35S:BiP-2, 35S:BiP-3, 35S:BiP-4, and 35S:BiP-5) and BiP transgene transcript levels were quantified by real-time PCR, using transgene-specific primers. In the nomenclature of transgenic lines, the first number indicates an independent event of transformation and the second number a different plant in a segregating population. (B) Enhanced levels of BiP in soybean transgenic lines. Equal amount of total proteins (30 μg) extracted from leaves of wild-type plants and soybean transgenic lines (as in A) were separated by SDS-PAGE and immunoblotted with anti-BiP serum. The arrows indicate the positions of BiP and a cross-reacting 28 kDa polypeptide. (C) Immunoblots of whole cell protein extracts (WCE) and microsomal fractions (Mic) of soybean leaves. Whole cell protein extracts from wild-type (lanes 1 and 2), 35S:BiP-2 (lane 3), and 35S-BiP-4 (lane 4) leaves as well as microsomal fractions from wild-type (lanes 5 and 6), 35S:BiP-2 (lane 7), and 35S-BiP-4 (lane 8) leaves were immunoblotted with anti-carboxy BiP serum. (D) Transcript accumulation of soyBiPD transgene in soybean transgenic roots. Total RNA was isolated from roots of wild-type (WT) plants and plants from two independently transformed soybean lines (BiP-2 and BiP-4) and BiP transgene transcript levels were quantified by real-time PCR, using transgene-specific primers.
Stress tolerance in 35S:BiP transgenic lines
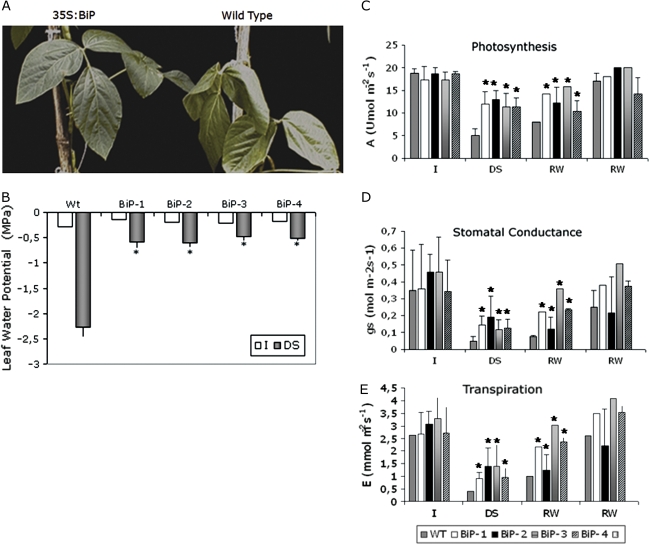
To investigate whether overexpression of BiP was correlated with water stress tolerance in soybean transgenic lines, either T3 transgenic plants at the V6 developmental stage or T4 transgenic plants at the V3 developmental stage were subjected to two distinct water deficit regimes, slow and fast soil drying treatments. In the first one, water deficit was induced by a reduction of irrigation to a 40% level for 18 d. This slow soil drying experiment was intended to mimic field conditions and to allow physiological and molecular responses to low water potential (ψw) be examined for a longer period. The soil water content of all samples was recorded as a function of time to ensure that the extent of soil drying or the severity of plant water stress was similar for all samples analysed. After 18 d of 40% irrigation the leaves of wild-type plants had completely wilted. The transgenic plants, by contrast, had normal turgid leaves (Fig. 2A). The leaf ψw of stressed wild-type plants declined to a maximum stress of –2.2 MPa, whereas the leaf ψw of transgenic plants did not decrease below –0.7 to –1.0 MPa (Fig. 2B). The leaf water status indicated that BiP overexpressing lines were protected against dehydration. However, this was not a direct result of stomatal closure because, in independent transgenic lines under stress conditions, the stomatal conductance and transpiration rate were significantly higher than in wild-type lines (Fig. 2D, E). The stomatal conductance, transpiration and photosynthetic rate did not differ in well-watered wild-type and transgenic lines (Fig. 2C, D, E). The transgenic lines also displayed normal growth and were undistinguible from the wild type (results not shown). However, under 40% irrigation the stomata did not close as much as in the wild type, and both the rate of transpiration and the rate of photosynthesis were not reduced as much as in the wild type (Fig. 2C). In the 40% irrigation experiment, the photochemical efficiency (Fv/Fm ratio) of transgenic and wild-type leaves remained unaltered and was similar to that of the well-watered counterparts (data not shown). After the 18 d period of drought, the plants were rewatered and their recovery was evaluated (Fig. 2). By contrast with the wild-type plants that required 14 d to recover (RW-14d), the transgenic lines recovered faster. Within a 5 d period of normal watering, their rate of photosynthesis and transpiration, and their stomatal conductance were similar to that of continuously normally irrigated controls. By then these parameters had significantly higher values than in the drought-stressed wild type.
Fig. 2.
Elevated levels of BiP confer drought tolerance to soybean plants under a restricted water regime. (A) Overexpression of BiP maintains leaf turgidity under drought. Drought stress was induced by reducing irrigation to 40% of the daily normal water supply. The leaves were photographed on day 18 of the experiment. (B) Leaf water potential of transgenic leaves under drought stress (reducing daily irrigation). Each value represents the mean ±SD of five replicates from three independent experiments. The asterisks indicate significant differences at P ≤0.05 as compared to the wild type. I, normally irrigated; DS, drought stress. (C, D, E) Photosynthesis and water relations in soybean transgenic lines continuously irrigated or exposed to the drought regime. Photosynthetic rate (C), stomatal conductance (D), and transpiration rate (E) of the third fully expanded leaf of WT and transgenic lines (as indicated) were measured by the LI-6400 infrared (IR) gas analyser at 1000 μmol m−2 s−1 irradiance. I, control leaves (normal irrigation). DS, leaves after 18 d of drought stress (40% of normal daily irrigation). After 18 d under the restricted water regime, the plants were rewatered with a normal water supply for 5 d (RW-5d) or 14 d (RW-5d). Each value represents the mean ±SD of five replicates from three independent experiments. The asterisks indicate significant differences at P ≤0.05 as compared to the wild type.
For the fast soil drying treatment, wild type, 35S-BiP-2, and 35S-BiP-4 lines were allowed to reach the V3 stage of development when drought was rapidly induced by withholding irrigation for 7–10 d. After the 7 d period, the leaves of wild-type lines had completely wilted, whereas the leaves of the transgenic plants were still turgid (Fig. 3A). This was associated with a significantly higher relative water content of the leaves in BiP overexpressing plants, compared with those in the wild type (Fig. 3B).
Fig. 3.
Enhanced drought tolerance in BiP-overexpressing soybean lines exposed to a 7 d suspension of watering. (A) Water stress-tolerant phenotype of transgenic lines. Water stress conditions were induced in wild type, 35S:BiP-2, and 35S:BiP-4 transgenic lines at the V3 developmental stage by withholding irrigation for 7 d when the plants were photographed. (B) Relative water content of wild-type and transgenic leaves. The relative water content of normally irrigated plants (I) and drought-stress plants (DS) was measured on the 7th day of experiment. The asterisk indicates significant differences at P ≤0.05 as compared to the wild type. (C, D, E) Physiological measurements of soybean transgenic lines continuously irrigated (I) or exposed to drought stress (DS). Photosynthetic rate (C), stomatal conductance (D), and transpiration rate (E) of the third fully expanded leaf of WT and 35S:BiP-4 lines were measured by the LI-6400 infrared (IR) gas analyser at 1000 μmol m−2 s−1 irradiance during the period of the experiment. Values represent the mean ±SD of three replicates.
Drought-induced variations on photosynthetic rate, stomatal conductance, and transpiration rate in transgenic (35S:BiP-4) and wild-type lines followed the same pattern as observed during mild water stress (see above), although, under severe drought, the differences between wild type and transgenic lines were lower but yet statistically significant (Fig. 3C, D, E; see Supplementary Fig. S1 at JXB online).
Ectopic expression of BiP in soybean does not promote typical stress avoidance responses
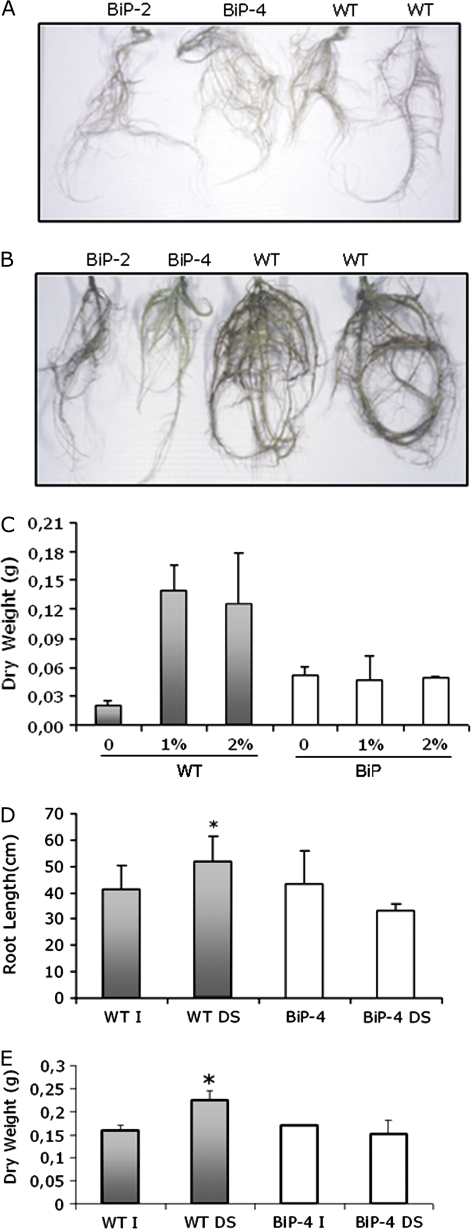
Under water stress conditions, the relative water content in BiP OE lines were maintained close to the levels in plants that were not subjected to drought stress (data not shown; Fig. 3B), suggesting that a stress avoidance mechanism could account, at least in part, for the apparent increase in water stress tolerance mediated by BiP. The reported data indicate that stomatal closure did not account for the water-stress tolerance in the BiP overexpressing lines. It is hypothesized that an adaptation in root growth might explain the drought tolerance. PEG was used for inducing a low ψw stress. In the absence of PEG, the seedlings of controls and BiP-overexpressing lines developed roots at similar growth rates (Fig. 4A). The inclusion of 1% or 2% PEG in the rooting medium resulted in a typical increase in root growth in wild-type seedlings (Fig. 4B, C). By contrast, treatment of OE seedlings with PEG did not alter significantly root morphology and root DW. Hence overexpression of BiP seemed to prevent the drought-induced increase in root growth.
Fig. 4.
Root growth of soybean seedlings exposed to a low water potential. Wild type (WT) and BiP overexpressing (BiP-2 and BiP-4 lines) soybean seedlings were grown on MS solid medium in the absence (A) and presence of 1% PEG (B) for 4 weeks when the roots were photographed. In (C), the root dry weight of 4-week-old seedlings (WT and BiP-4) grown in MS medium supplemented with 1% (PEG1) or 2% PEG (PEG2) was measured. (D) Root length of plants exposed to a drying soil. Water stress conditions were imposed in wild-type and BiP-4 transgenic lines at the V3 developmental stage by withholding irrigation for 7 d. Root length was measured on day 7. I, normally irrigated; DS, drought-stressed plants. (E) Root dry weight of plants exposed to drought. Root dry weight was measured on the 9th day of water deprivation. Asterisks indicate significant differences at P ≤0.05.
To examine whether the observed root response of soybean plants to PEG-induced osmotic stress also exists for plants growing in soil under drought stress, wild type and OE lines were subjected to a 7–9 d period of severe drought treatment when the root growth was measured (Fig. 4D, E). Drought stress treatment stimulated root growth in wild-type plants, because stressed plants had a significantly higher root length and more dry weight than the well-watered wild-type plants. By contrast, root length and root dry weight of OE lines (35S:BiP-4) under drought stress were slightly lower than those of their irrigated counterparts. Therefore, overexpression of BiP prevented the root response to water deficit.
Proline is one of the major organic osmolytes that accumulates in a variety of plant species in response to drought, as a mechanism of dehydration avoidance (Porcel and Ruiz-Lozano, 2004; Ashraf and Foolad, 2007). Under drought conditions, both wild type and transgenic (35S:BiP-4) lines accumulated free proline in leaves to a higher level than their well-watered counterparts (Fig. 5A). Nevertheless, the proline level in stressed wild-type leaves was significantly greater than in stressed transgenic leaves. The soluble sugar content in leaves was also measured from independently transgenic lines as osmoprotectants (Porcel and Ruiz-Lozano, 2004). Water stress induced the accumulation of sucrose in wild-type leaves to a higher extent than in OE leaves and there were no clear differences between glucose levels of wild type and transgenic lines (Fig. 5B, C). These results indicated that changes in soluble sugars and proline levels were not associated with the high turgidity of transgenic leaves under drought.
Fig. 5.
Drought-induced accumulation of some osmolytes in soybean transgenic lines. (A) Proline content in leaves of wild-type and 35S:BiP-4 transgenic lines under drought. Drought was induced in soybean plants at the V3 developmental stage by withholding irrigation for 7 d (DS) when the proline content of the leaves was determined. I denotes normally irrigated plants. (B, C) Soluble sugars content in leaves of wild type and independent transgenic lines (as indicated) cultivated under a restricted water regime. Drought stress (DS) conditions were imposed by reducing irrigation to a 40% level of the normal water supply for 18 d. I represents normally irrigated counterparts. After 18 d of treatment the leaf content of sucrose (B), and glucose (C) was measured. Asterisks indicate significant differences at P ≤0.05.
Overexpression of BiP inhibits induction of stress-responsive gene expression
It was hypothesized that overexpression of BiP may indirectly affect stress tolerance by inducing a higher expression of water-stress responsive genes, such as SMP (seed maturation protein P30; AW397921; Irsigler et al., 2007), GST (glutathione-S-transferase; AAC18566; Irsigler et al., 2007), NAC2 (AY974350; Irsigler et al., 2007), PDI3 (protein disulphide isomerase, isoform 3; AW277660; Irsigler et al., 2007), and antiquitin (AY250704; Rodrigues et al., 2006). SMP is a marker gene for drought responses (Irsigler et al., 2007), GST participates in anti-oxidant reactions and detoxification (Dixon et al., 1998), and PDI is involved in protein folding, just as BiP (Kamauchi et al., 2008; Urade, 2007). NAC2 encodes a nuclear transfactor and antiquitin encodes a cytosolic aldehyde dehydrogenase. A large increase of the mRNA abundance of these genes was found in leaves of wild-type plants exposed to drought. Depending on the gene a smaller or a much smaller increase in mRNA abundance was observed in leaves of the BiP overexpressing plants (Fig. 6A). These results indicate that overexpression of BiP inhibits the induction of different classes of stress-responsive genes.
Fig. 6.
Induction of drought-responsive genes in transgenic lines. (A, B) Drought was induced in soybean plants at the V3 developmental stage by withholding irrigation for 7 d. Total RNA was isolated from leaves of wild type (WT) and 35S:BiP-4 lines and the transcript levels of selected genes (as indicated) were quantified by real-time PCR, using gene-specific primers. I, normally irrigated plants; DS, drought-stressed plants. Values represent the mean ±SD of three replicates. SMP, seed maturation protein; NAC2, transcription factor from the soybean NAC family; antqtn, antiquitin; GST, glutathione-S-transferase; PDI3, protein disulphide isomerase, isoform 3; BiPD, binding protein, isoform D from soybean; CNX, calnexin.
Overexpression of BiP prevents down-regulation of BiP and calnexin gene expression by severe drought conditions
As outlined in the introduction, the accumulation of malfolded proteins activates the protective signalling cascade called UPR. The UPR results in co-ordinated transcriptional up-regulation of BiP and other genes encoding ER-resident proteins, such as calnexin (CNX). This and other proteins are involved in the proper folding and assembly of nascent proteins (reviewed by Urade, 2007). In experiments with soybean, PEG-induced dehydration has recently been shown to down-regulate genes that encode ER-resident molecular chaperones such as BiP and CNX (Irsigler et al., 2007). A similar effect of drought on these UPR-specific targets in leaves of wild-type plants (Fig. 6B) is shown here. It was also observed that under normal conditions, overexpression of BiP transgene repressed expression of endogenous BiP and CNX genes, as the OE lines accumulated lower transcript levels than in the wild type (Fig. 6B). This result is consistent with previous work in soybean (Costa et al., 2008) and in tobacco cells (Leborgne-Castel et al., 1999). However, severe drought relieved the BiP-mediated repression of UPR targets and the stressed OE lines accumulated similar levels of BiP and CNX transcripts as unstressed wild-type plants. These results indicate that overexpression of BiP suppresses the water deficit induced-down-regulation of transcripts encoding ER-resident molecular chaperones.
Expression of leaf senescence-associated markers in wild type and OE lines during drought
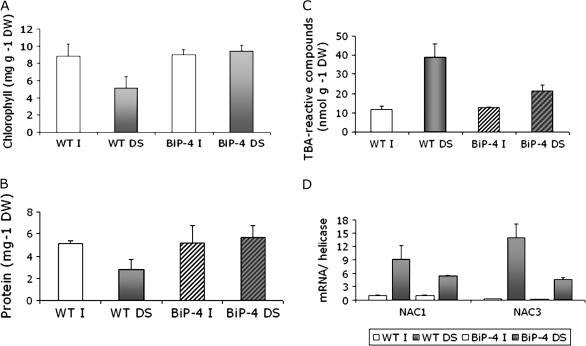
Drought is known to accelerate senescence. It was examined whether the drought-tolerant phenotype mediated by overexpression of BiP might be associated with a delay in leaf senescence. In these experiments, our transgenic tobacco plants ectopically expressing BiP in the sense or antisense orientations (Alvim et al., 2001) were also analysed. Transgenic soybeans were exposed to a fast soil-drying regime (complete stop of watering) and the leaves were assayed for hallmarks of senescence. After 7 d of progressive drought, wild-type leaves displayed more chlorophyll loss than did OE lines (Fig. 7A). Likewise, in wild-type stressed leaves, total protein content was significantly lower than in stressed OE leaves (Fig. 7B). The drought-induced senescence phenotype was examined further by measuring the accumulation of thiobarbituric acid (TBA)-reactive compounds such as malondialdehyde (MDA). These compounds are products of senescence-associated lipid peroxidation, a process resulting in the generation of reactive oxygen species (Dhindsa et al., 1981). A significantly higher concentration of TBA-reactive compounds was observed in wild-type leaves in comparison with OE lines (Fig. 7C). Expression of the senescence-associated genes, NAC1 and NAC3 (see Supplementary Fig. S2 at JXB online) was also assayed by quantitative RT-PCR (Fig. 7D). Drought induced the expression of both NAC1 and NAC3 to higher levels in wild-type leaves than in OE lines.
Fig. 7.
Ectopic expression of BiP delays drought-induced leaf senescence in soybean plants. Drought was induced in wild type and 35S:BiP-4 plants at the V3 developmental stage by withholding irrigation for 7 d. I, normally irrigated plants; DS, drought-stressed plants. Values are given as mean ±SD from three replicates. (A) Chlorophyll loss induced by drought in wild type and 35S:BiP-4 transgenic lines. (B) Total protein content of wild type and transgenic leaves exposed to a 7 d period of drought. (C) Lipid peroxidation in wild type and 35S:BiP-4 leaves. Lipid peroxidation in leaves was monitored by determining the level of TBA-reactive compounds. (D) Induction of the senescence-associated genes NAC1 and NAC3 by drought. Total RNA was isolated from irrigated and drought-stressed leaves of wild type and 35S:BiP-4 lines and gene induction was monitored by quantitative RT-PCR using gene-specific primers.
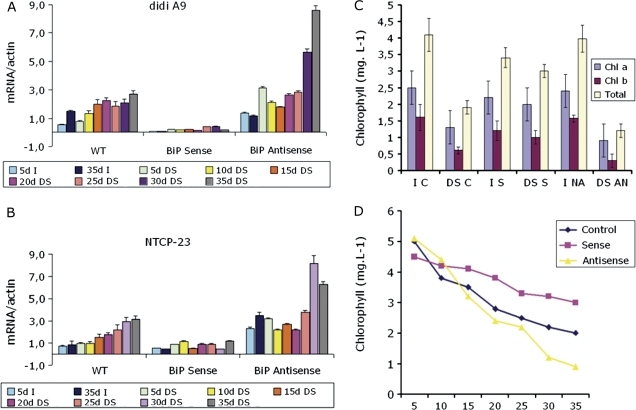
The relation between BiP mRNA abundance and leaf senescence was also studied in tobacco, using lines in which BiP was suppressed (antisense lines) and lines in which BiP was overexpressed (Alvim et al., 2001). Plants were exposed to a 40% irrigation, slow soil-drying regime for 35 d and assessed for chlorophyll loss and the expression of two genes (didi A9 and NTCP-23). didi A9 (AAZ23261) is a homologue to Arabidopsis SEN1 (Simón-Mateo et al., 2006). The Arabidopsis SEN1 (AT4G35770) is expressed during natural and dark-induced leaf senescence, as well as after pathogen infection (Schenk et al., 2005). NTCP-23 (AB032168; called CP1 in Costa et al., 2008) was shown to be up-regulated in association with tobacco leaf senescence (Ueda et al., 2000; Costa et al., 2008). The drought regime induced chlorophyll loss in OE lines although to a lesser extent than in the wild type (Fig. 8C, D). As expected, in wild-type plants, the drought treatment increased the mRNA abundance of both genes studied (Fig. 8A, B). By contrast, no such increase was found in the BiP OE lines. Conversely, in the BiP antisense lines, these senescence parameters (chlorophyll loss and expression of senescence-associated genes) were more pronounced than in wild-type plants, indicating that decreased expression of endogenous BiP gene accelerated drought-induced leaf senescence.
Fig. 8.
The onset of leaf senescence is associated with the levels of BiP expression in tobacco transgenic lines under a restricted water regime. Tobacco transgenic lines expressing BiP cDNA either in the sense or antisense orientations were exposed to progressive drought by reducing irrigation to a 40% level of the normal water supply for 35 d. I, normally irrigated plants; DS, drought-stressed plants for the indicated period of time in days. (A, B) Induction of the senescence-associated genes didi A9 and NTCP-23 under progressive drought. Total RNA was isolated from irrigated and drought-stressed leaves of wild type, sense and antisense tobacco lines at the indicated period of treatment and gene induction was monitored by quantitative RT-PCR using gene-specific primers. Values represent the mean ±SD of two replicates. (C) Chlorophyll loss induced by drought for 35 d of restricted water regime. C indicates wild-type plants; S, BIP sense; and AN, BiP antisense lines. I denotes continuously irrigated controls and DS, drought-stressed plants after 35 d of 40% of the normal daily water supply. Values represent the mean ±SD of three replicates. (D) Chlorophyll content during progressive drought. The chlorophyll content was measured in leaves of wild type (control), sense and antisense transgenic lines at the indicated period of drought in days.
Discussion
It has previously been demonstrated that overexpression of a BiP gene from soybean confers drought tolerance in tobacco. It is now shown that the same gene (soyBiPD) confers drought tolerance in soybean. The BiP-mediated drought-tolerant phenotype and the possible mode of action of BiP in conferring drought resistance is characterized further here. The role of this gene on drought-induced senescence in soybean and tobacco is also investigated. In soybean plants subjected to drought, the overexpression of BiP resulted in the maintenance of shoot turgidity. Unexpectedly, this improvement of leaf water relations was associated with less stomatal closure during drought, and less reduction of photosynthesis and transpiration. The increase in drought tolerance was also not related to some parameters of other short-term or long-term avoidance responses. For example, overexpression of BiP did not result in the accumulation of proline and sucrose, and did not result in the normal increase in root growth under drought conditions. Taken together these results show less drought stress in the drought-treated plants. This was also inferred from the lower (or very much lower) induction of typical drought-induced genes, in drought-stressed BiP overexpressing plants. It should be noted, however, that our measurement of proline and sugars did not exhaust the possibilities of osmotic adaptation. Further work on osmotic potential in the transgenic lines might still reveal an underlying osmotic effect. Interestingly, in the plants in which BiP was overexpressed, drought did also not result in the typical down-regulation of genes encoding the ER molecular chaperones BiP and CNX. The attenuation of drought-induced responses and the basal levels of ER-molecular chaperone transcripts in OE lines may reflect a lower intensity of drought-induced endogenous stress. The reason why the plants become resistant to drought when overexpressing BiP, therefore, is at present unknown. The data might suggest that changes in the ER protein-folding process may serve as an early sensing mechanism of drought stress.
PEG-induced dehydration has been demonstrated to promote a co-ordinate down-regulation of ER molecular chaperones and folding catalysts in young soybean plants (Irsigler et al., 2007). Our results showed that severe drought imposed by a drying soil also represses the expression of UPR–specific targets, such as BiP and CNX, in soybean plants. These results indicated that drought stress inversely regulates UPR-specific targets, impairing the ER folding capacity. However, overexpression of BiP prevented the drought-mediated down-regulation of UPR genes. Also, the levels of BiP and CNX transcripts in droughted OE leaves were as high as those detected in wild-type leaves under normal growth conditions. Thus, BiP overexpression may prevent the cell from sensing osmotic stress-induced variations in ER function by keeping ER basic activities to a normal level under severe drought conditions.
It was found that BiP overexpression delayed drought-induced leaf senescence. The mode of BiP action is as yet unclear. Drought stress in the plant, and thus a low leaf water potential, is known to promote leaf senescence. The absence of such a low leaf water potential in the BiP overexpressing plants might be the reason why senescence is not induced in these plants. Interestingly, decreasing BiP expression in tobacco antisense lines hastened leaf senescence, thus showing a close connection between BiP mRNA and senescence. It is not yet known if the accelerated leaf senescence in the BiP antisense plants is associated with a lower leaf water potential than in wild-type plants.
In addition to its role as molecular chaperone, in mammalian cells BiP has been shown to function as a sensor of alterations in the ER environment that activate the cytoprotective unfolded protein response (Malhotra and Kaufman, 2007). Like BiP from animal systems, plant BiP has been shown to regulate UPR negatively (Leborgne-Castel et al., 1999; Costa et al., 2008). BiP regulates UPR by controlling the activation status of the three transducers, IRE1, PERK, and ATF6 which act in concert to reduce the unfolded protein load in the ER by attenuating protein synthesis and promoting protein folding and degradation of misfolded proteins (Malhotra and Kaufman, 2007). BiP has also been demonstrated to regulate pro-apoptotic pathways that emanate from the ER when ER homeostasis cannot be restored (Reddy et al., 2003; Malhotra and Kaufman, 2007). Prolonged ER stress (accumulation of unfolded or misfolded proteins) has been shown to induce cell death, apoptotic-like responses and leaf senescence in plants (Crosti et al., 2001; Malerba et al., 2004; Zuppini et al., 2004, Wang et al., 2007; Costa et al., 2008; Watanabe and Lam, 2008). The effect of UPR on leaf senescence has recently been associated with multimeric G-protein signalling (Wang et al., 2007). Disruption of the Gβ gene impairs ER stress-induced leaf cell death and attenuates UPR in the same fashion as BiP overexpression.
BiP-mediated increases in water stress tolerance may be connected to Ca2+ signalling responses. This idea is based on several observations. Firstly, BiP has been demonstrated to play a direct role in the storage of a rapidly exchanging Ca2+ pool within the ER lumen (Lievremont et al., 1997). Thus, overexpression of BiP in sense plants may increase the ER Ca2+ storage capacity, thereby modulating Ca2+ signalling in response to drought and hence altering stress perception and response. Secondly, the cytoplasmic Ca2+ signal transduction pathway is thought to regulate turgor pressure of plant cells and to co-ordinate stomatal responses to leaf dehydration (for a review see Shinozaki and Yamaguchi-Shinozaki, 1997). Thirdly, an increase in the level of cytosolic free calcium ions is involved in signal transduction leading to one type of programmed cell death in plants, the hypersensitive response (Xu and Heath, 1998). Fourthly, in mammalian cells, the control of Ca2+ release from the ER by BiP overexpression prevented oxidative stress and cell death (Liu et al., 1997, 1998). Finally, drought promotes a rapid and excessive accumulation of reactive oxygen species (ROS) in plant cells that, in turn, causes a lipid peroxidation chain reaction resulting in chemically reactive cleavage products, largely represented by TBA-reactive compounds such as malondialdehyde (Bartels, 2001). Under drought, the level of TBA-reactive compounds was much lower in BiP OE lines as compared to wild-type lines. Thus, BiP overexpression apparently attenuated the degree of membrane degradation, which is often associated with the release of reactive oxygen species. Leaf senescence is also usually associated with an increase in membrane degradation and an increase in TBA-reactive compounds. Thus, by functional analogy with mammalian cells, the plant BiP effect on oxidative stress and leaf senescence may be associated with a capacity to control Ca2+ release from the ER. Therefore, the BiP control of ER Ca2+ homeostasis may provide a link for the apparently pleiotropic physiological effects of BiP protection under water stress. This hypothesis warrants further investigation.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Photosynthesis, stomatal conductance, and transpiration in leaves of soybean transgenic lines continuously irrigated (I) or exposed to a 7 d period of suspended irrigation (drought stress, DS).
Supplementary Fig. S2. Enhanced accumulation of GmNAC1 and GmNAC3 transcripts during leaf senescence in soybean.
Supplementary Material
Acknowledgments
We thank all the members of the Plant Molecular Biology Laboratory at BIOAGRO/UFV for technical assistance and for reading the manuscript. This research was supported by the Brazilian Government Agencies CNPq grants 50.6119/2004-1 and 470878/2006-1 (to EPBF), FAPEMIG grant EDT 523/07 and FINEP grant 01.07.610.00 (to EPBF) and Brazilian soybean genome consortium (CNPq/GENOSOJA, grant 552735/2007-8). MASV, JAQAF, and GLP were supported by a graduate fellowship from CNPq and PABR by an undergraduate scholarship from CNPq.
References
- Alvim FC, Carolino SMB, Cascardo JCM, Nunes CC, Martinez CA, Otoni WC, Fontes EPB. Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress. Plant Physiology. 2001;126:1042–1054. doi: 10.1104/pp.126.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragão FJL, Sarokin L, Vianna GR, Rech EL. Selection of transgenic meristematic cells utilizing a herbicidal molecule results in the recovery of fertile transgenic soybean (Glycine max (L.) Merrill) plants at high frequency. Theoretical and Applied Genetics. 2000;101:1–6. [Google Scholar]
- Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany. 2007;59:206–216. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant and Soil. 1973;39:205–207. [Google Scholar]
- Bartels D. Targeting detoxification pathways: an efficient approach to obtain plants with multiple stress tolerance? Trends in Plant Science. 2001;7:284–286. doi: 10.1016/s1360-1385(01)01983-5. [DOI] [PubMed] [Google Scholar]
- Buzeli RAA, Cascardo JCM, Rodrigues LAZ, Andrade MO, Loureiro ME, Otoni WC, Fontes EPB. Tissue-specific regulation of Bip/Grp78 genes: a cis-acting regulatory domain is required for BiP promoter activity in plant meristems. Plant Molecular Biology. 2002;50:757–771. doi: 10.1023/a:1019994721545. [DOI] [PubMed] [Google Scholar]
- Cascardo JCM, Almeida RS, Buzeli RAA, Carolino SMB, Otoni WC, Fontes EPB. The phosphorylation state and expression of soybean BiP isoforms are differentially regulated following abiotic stresses. Journal of Biological Chemistry. 2000;275:14494–14500. doi: 10.1074/jbc.275.19.14494. [DOI] [PubMed] [Google Scholar]
- Catsky J. Water saturation deficit (relative water content) In: Slavik N, editor. Methods of studying plant water relations. New York: Springer Verlag; 1974. pp. 136–154. [Google Scholar]
- Costa MDL, Reis PAB, Valente MAS, Irsigler AST, Carvalho CM, Loureiro ME, Aragão FJL, Boston RS, Fietto LG, Fontes EPB. A new branch of endoplasmic reticulum stress signalling and the osmotic signal converge on plant-specific asparagine-rich proteins to promote cell death. Journal of Biological Chemistry. 2008;283:20209–20219. doi: 10.1074/jbc.M802654200. [DOI] [PubMed] [Google Scholar]
- Crosti P, Malerba M, Bianchetti R. Tunicamycin and Brefeldin A induce in plant cells a programmed cell death showing apoptotic features. Protoplasma. 2001;216:31–38. doi: 10.1007/BF02680128. [DOI] [PubMed] [Google Scholar]
- Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany. 1981;32:93–101. [Google Scholar]
- Dixon DP, Cummins I, Cole DJ, Edwards R. Glutathione-mediated detoxification systems in plants. Current Opinion in Plant Biology. 1998;1:258–266. doi: 10.1016/s1369-5266(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Figueiredo JEF, Cascardo JCM, Carolino SMB, Alvim F, Fontes EPB. Water-stress regulation and molecular analysis of the soybean BiP gene family. Brazilian Journal of Plant Physiology. 1997;9:103–110. [Google Scholar]
- Gomer CJ, Ferrario A, Rucker N, Wong S, Lee AS. Glucose regulated protein induction and cellular resistance to oxidative stress mediated by porphyrin photosensitization. Cancer Research. 1991;15:6574–6579. [PubMed] [Google Scholar]
- Gupta AS, Heinen JL, Holaday AS, Burke JJ, Allen RD. Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide-dismutase. Proceedings of the National Academy of Sciences, USA. 1993;90:1629–1633. doi: 10.1073/pnas.90.4.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges DM, Delong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1993;207:604–611. doi: 10.1007/s00425-017-2699-3. [DOI] [PubMed] [Google Scholar]
- Irsigler AST, Costa MDL, Zhang P, Reis PAB, Dewey RE, Boston RS, Fontes EPB. Expression profiling on soybean leaves reveals integration of ER- and osmotic-stress pathways. BMC Genomics. 2007;8:431. doi: 10.1186/1471-2164-8-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski A, Rowley DL, Loer DS, Foley C, Buta G, Herman EM. Binding-protein expression is subject to temporal, developmental and stress-induced regulation in terminally differentiated soybean organs. Planta. 1995;195:611–621. doi: 10.1007/BF00195722. [DOI] [PubMed] [Google Scholar]
- Kamauchi S, Wadahama H, Iwasaki K, Nakamoto Y, Nishizawa K, Ishimoto M, Kawada T, Urade R. Molecular cloning and characterization of two soybean protein disulfide isomerases as molecular chaperones for seed storage proteins. FEBS Journal. 2008;275:2644–2658. doi: 10.1111/j.1742-4658.2008.06412.x. [DOI] [PubMed] [Google Scholar]
- Laitusis AL, Brostrom MA, Brostrom CO. The dynamic role of GRP78/BiP in the coordination of mRNA translation with protein processing. Journal of Biological Chemistry. 1999;274:486–493. doi: 10.1074/jbc.274.1.486. [DOI] [PubMed] [Google Scholar]
- Leborgne-Castel N, Jelitto-Van Dooren EPWM, Crofts AJ, Denecke J. Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. The Plant Cell. 1999;11:459–470. doi: 10.1105/tpc.11.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LJ, Li X, Ferrario A, Rucker N, Liu ES, Wong S, Gomer CJ, Lee AS. Establishment of a Chinese hamster ovary cell line that expresses grp78 antisense transcripts and suppresses A23187 induction of both GRP78 and GRP94. Journal of Cell Physiology. 1992;153:575–582. doi: 10.1002/jcp.1041530319. [DOI] [PubMed] [Google Scholar]
- Li XA, Lee AS. Competitive inhibition of a set of endoplasmic reticulum protein genes (GRP78, GRP94, and Erp72) retards cell growth and lowers viability after ionophore treatment. Molecular and Cellular Biology. 1991;11:3446–3453. doi: 10.1128/mcb.11.7.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomemembranes. Methods in Enzymology. 1987;148:350–382. [Google Scholar]
- Lievremont J-P, Rizzuto R, Hendeershot L, Meldolesi J. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+ Journal of Biological Chemistry. 1997;272:30873–30879. doi: 10.1074/jbc.272.49.30873. [DOI] [PubMed] [Google Scholar]
- Liu H, Bowes RC, III, van de Water B, Sillence C, Nagelkerke JF, Stevens JL. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. Journal of Biological Chemistry. 1997;272:21751–21759. doi: 10.1074/jbc.272.35.21751. [DOI] [PubMed] [Google Scholar]
- Liu H, Miller E, van de Water B, Steven JL. Endoplasmic reticulum stress proteins block oxidant-induced Ca2+ increases and cell death. Journal of Biological Chemistry. 1998;273:12858–12862. doi: 10.1074/jbc.273.21.12858. [DOI] [PubMed] [Google Scholar]
- Maa Y, Hendershot LM. ER chaperone functions during normal and stress conditions. Journal of Chemical Neuroanatomy. 2004;28:51–65. doi: 10.1016/j.jchemneu.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Malerba M, Cerana R, Crosti P. Comparison between the effects of fusicoccin, tunicamycin, and brefeldin A on programmed cell death of cultured sycamore (Acer pseudoplatanus L.) cells. Protoplasma. 2004;224:61–70. doi: 10.1007/s00709-004-0053-7. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Seminars in Cell and Developmental Biology. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Dorner AJ, Edwards CA, Hendershot LM, Kaufman J. Immunoglobulin binding protein (BiP) function is required to protect cells from endoplasmic reticulum stress but is not required for the secretion of selective proteins. Journal of Biological Chemistry. 1997;272:4327–4334. doi: 10.1074/jbc.272.7.4327. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Pedra JHF, Delú-Filho N, Pirovani CP, Contim LAS, Dewey RE, Otoni WC, Fontes EPB. Antisense and sense expression of a sucrose binding protein homologue gene from soybean in transgenic tobacco affects plant growth and carbohydrate partitioning in leaves. Plant Science. 2000;152:87–98. [Google Scholar]
- Pirovani CP, Macêdo JNA, Contim LAS, Matrangolo FSV, Loureiro ME, Fontes EPB. A sucrose binding protein homologue from soybean exhibits GTP-binding activity that functions independently of sucrose transport activity. European Journal of Biochemistry. 2002;269:3998–4008. doi: 10.1046/j.1432-1033.2002.03089.x. [DOI] [PubMed] [Google Scholar]
- Porcel R, Ruiz-Lozano JM. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. Journal of Experimental Botany. 2004;55:1743–1750. doi: 10.1093/jxb/erh188. [DOI] [PubMed] [Google Scholar]
- Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. Journal of Biological Chemistry. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Andrade MO, Gomes APS, DaMatta FM, Baracat-Pereira MC, Fontes EPB. Arabidopsis and tobacco plants ectopically expressing the soybean antiquitin-like ALDH7 gene display enhanced tolerance to drought, salinity, and oxidative stress. Journal of Biological Chemistry. 2006;57:1909–1918. doi: 10.1093/jxb/erj132. [DOI] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Rusu AG, Manners JM, Maclean DJ. The SEN1 gene of Arabidopsis is regulated by signals that link plant defence responses and senescence. Plant Physiology and Biochemistry. 2005;43:997–1005. doi: 10.1016/j.plaphy.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Simón-Mateo C, Depuydt S, de Oliveira Manes CL, Cnudde F, Holsters M, Goethals K, Vereecke D. The phytopathogen Rhodococcus fascians breaks apical dominance and activates axillary meristems by inducing plant genes involved in hormone metabolism. Molecular Plant Pathology. 2006;7:103–112. doi: 10.1111/j.1364-3703.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiology. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Seo S, Ohashi Y, Hashimoto J. Circadian and senescence-enhanced expression of a tobacco cysteine protease gene. Plant Molecular Biology. 2000;44:649–657. doi: 10.1023/a:1026546004942. [DOI] [PubMed] [Google Scholar]
- Urade R. Cellular response to unfolded proteins in the endoplasmic reticulum of plants. FEBS Journal. 2007;274:1152–1171. doi: 10.1111/j.1742-4658.2007.05664.x. [DOI] [PubMed] [Google Scholar]
- Xu HX, Heath MC. Role of calcium in signal transduction during the hypersensitive response caused by basidiospore-derived infection of the cowpea rust fungus. The Plant Cell. 1998;10:585–597. doi: 10.1105/tpc.10.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Narendra S, Fedoroff N. Heterotrimeric G protein signaling in the Arabidopsis unfolded protein response. Proceedings of the National Academy of Sciences, USA. 2007;104:3817–3822. doi: 10.1073/pnas.0611735104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Lam E. BAX Inhibitor-1 modulates endoplasmic reticulum stress-mediated programmed cell death in Arabidopsis. Journal of Biological Chemistry. 2008;283:3200–3210. doi: 10.1074/jbc.M706659200. [DOI] [PubMed] [Google Scholar]
- Wehmeyer N, Vierling E. The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiology. 2000;122:1099–1108. doi: 10.1104/pp.122.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuppini A, Navazio L, Mariani P. Endoplasmic reticulum stress-induced programmed cell death in soybean cells. Journal of Cell Science. 2004;117:2591–2598. doi: 10.1242/jcs.01126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.