Abstract
The zebrafish enteric nervous system (ENS), like those of all other vertebrate species, is principally derived from the vagal neural crest. The developmental controls that govern the specification and patterning of the ENS are not well understood. To identify genes required for the formation of the vertebrate ENS we preformed a genetic screen in zebrafish. We isolated the lessen (lsn) mutation that has a significant reduction in the number of ENS neurons as well as defects in other cranial neural crest derived structures. We show that the lsn gene encodes a zebrafish orthologue of Trap100, one of the subunits of the TRAP/mediator transcriptional regulation complex. A point mutation in Trap100 causes a premature stop codon that truncates the protein, causing a loss of function. Antisense-mediated knockdown of Trap100 causes an identical phenotype to lsn. During development Trap100 is expressed in a dynamic tissue specific expression pattern consistent with its function in ENS and jaw cartilage development. Analysis of neural crest markers revealed that the initial specification and migration of the neural crest is unaffected in lsn mutants. Phosphohistone H3 immunocytochemistry revealed that there is a significant reduction in proliferation of ENS precursors in lsn mutants. Using cell transplantation studies we demonstrate that lsn/Trap100 acts cell autonomously in the pharyngeal mesendoderm and influences the development of neural crest derived cartilages secondarily. Further we show that endoderm is essential for ENS development. These studies demonstrate that lsn/Trap100 is not required for initial steps of cranial neural crest development and migration but is essential for later proliferation of ENS precursors in the intestine.
Keywords: Neural crest, Zebrafish, craniofacial, ENS development, lessen (lsn), Trap100, proliferation
INTRODUCTION
The enteric nervous system (ENS) is derived from multipotent precursor cells that migrate from the neural crest to the intestine (Furness, 1987; Gershon, 1994). In all vertebrate species studied the majority of the ENS is derived from neural crest cells that migrate from the vagal region (Newgreen and Young, 2002b). As the ENS precursors migrate they proliferate and differentiate to form a wide variety of neuronal subtypes (Furness, 1987; Gershon, 1994). Molecules in the environment as well as lineage restrictions within the ENS precursors determine the number and specific cell fates acquired by these precursors. Failure of ENS precursors to colonize the complete gut results in the absence of enteric ganglia along varying lengths of the colon (colonic aganglionosis)(Newgreen and Young, 2002a; Newgreen and Young, 2002b). This is the most common cause of congentital intestinal obstruction in humans and is clinically referred to as Hirschsprung’s disease (HSCR)(Kapur, 1999b; Newgreen and Young, 2002a).
The molecular mechanisms that control the specification differentiation and proliferation of the neural crest have been studied extensively. Several secreted signaling molecules and their associated receptors have been identified that control directly and indirectly the morphogenesis of the ENS. These include GDNF (Cacalano et al., 1998; Enomoto et al., 1998; Moore et al., 1996; Pichel et al., 1996; Schuchardt et al., 1994), Neurturin (Heuckeroth et al., 1999; Heuckeroth et al., 1998; Rossi et al., 1999), Endothelin-3 (Baynash et al., 1994; Hosoda et al., 1994; Yanagisawa et al., 1998), BMP2/4 (Chalazonitis et al., 2004; Wu and Howard, 2002), Ihh (Indian hedgehog) (Ramalho-Santos et al., 2000), Shh (sonic hedgehog) (Fu et al., 2004; Ramalho-Santos et al., 2000; Sukegawa et al., 2000), NT-3 (Chalazonitis et al., 2001; Chalazonitis et al., 1994) and CNTF (Chalazonitis et al., 1998).
In addition to these signaling molecules, a number of transcription factors have been implicated as having a role in the specification of the ENS including Mash1 (Guillemot et al., 1993), Phox2b (Pattyn et al., 1999), SOX10 (Herbarth et al., 1998; Kapur, 1999a; Pattyn et al., 1999; Southard Smith et al., 1998), Hox11L1 (Hatano et al., 1997; Shirasawa et al., 1997), Hoxb5 (Kuratani and Wall, 1992; Pitera et al., 1999), HAND2 (Cserjesi et al., 1995; Howard et al., 1999; Srivastava et al., 1995; Wu and Howard, 2002) and AP-2alpha (Barrallo-Gimeno et al., 2004; Knight et al., 2003; O’Brien et al., 2004).
Perturbation of the function of these signaling molecules and transcription factors leads to defects in ENS. Furthermore mutations in some of these genes have been identified in patients affected with HSCR (Amiel and Lyonnet, 2001; Puri et al., 1998); however mutations in these known genes can only account for approximately 60% of familial HSCR (Amiel and Lyonnet, 2001; Puri et al., 1998).
Classical genetic studies of development have proven their utility in creating a molecular underpinning of the metazoan body plan, but the model organisms exploited to create that framework lack many of the organs found in vertebrates. To date the genes that have been identified as involved in ENS development have been identified indirectly. We therefore conducted a forward genetic screen in zebrafish to identify genes that when mutated would cause perturbations in the development of the ENS. One of the mutants we identified in this screen is lessen (lsn). lsn mutant’s have a significant reduction in the number of enteric neurons but also have defects in other neural crest and non-neural crest derived tissues including the CNS and the intestine. Here we show that the lsn mutation disrupts a zebrafish Trap100 that is required for the proliferation of enteric precursors within the embryonic intestine. Furthermore we also show that Trap100 is specifically required for the normal development of other cranial neural crest derived structures revealing that this gene has previously unappreciated tissue specific functions during embryogenesis. Transplantation of wild-type endoderm into lsn/Trap100 mutants induces posterior pharyngeal arch cartilage development, which indicates that Trap100 acts autonomously in the endoderm. Consequently this suggests that neural crest defects are secondary to endoderm defects in lsn/Trap100 mutants. This is consistent with our finding that the ENS fails to develop in zebrafish that lack intestinal endoderm.
MATERIALS AND METHODS
Zebrafish maintenance and breeding
Fish were raised and kept under standard laboratory conditions at 28.5°C (Westerfield, 1993). Embryos were staged and fixed at specific hours or days post fertilization (hpf or dpf) as described Kimmel at al (1995). To better visualize internal structures in some experiments embryos were incubated with 0.2mM 1-phenyl-2-thiourea (Sigma) to inhibited pigment formation (Westerfield, 1993).
Immunocytochemistry
Embryos were processed for immunocytochemistry as previously described (Raible and Kruse, 2000). Differentiated enteric neurons and cranial ganglia neurons were revealed with the anti-Hu mAb 16A11 (Molecular Probes) that labels differentiated neurons (Marusich et al., 1994). Serotonergic neurons were identified with an anti-5HT rabbit polyclonal antisera (Immunostar). ENS precursors were revealed with an anti-Phox-2b rabbit polyclonal antisera (Pattyn et al., 1997). Proliferating cells were identified using an anti phosphohistone –H3 mAb clone (Ser10) (Upstate) (Ajiro et al., 1996). All mAbs were visualized using an Alexa Fluor 568 anti-mouse IgG antibody (Molecular Probes) or an Alexa Fluor 488 anti-mouse IgG antibody in double-label experiments (Molecular Probes). The rabbit polyclonal Ab was visualized using an Alexa Fluor 568 anti-rabbit IgG antibody (Molecular Probes).
Mutagenesis and screening
Males of the AB line treated with ENU as described previously bred with wild-type females for at least two generations, then in-crossed to derive recessive mutations to homozygosity (Haffter et al., 1996). Clutches of in-crossed 96hpf embryos were fixed and processed for immunocytochemistry using anti Hu mAb 16A11. The number of enteric neurons was determined in a 10 somite segment of the intestine extending anteriorly from the end of the yolk extension as described (Shepherd et al., 2004).
Apoptosis Assay
Apoptotic cell death was detected in whole embryos by terminal transferase dUTP nick-end labeling (TUNEL) (In situ Cell Death Detection Kit; POD; Roche) as described (Knight et al., 2003).
Cartilage staining and histological methods
Cartilages were stained with Alcian Blue as described (Schilling et al., 1996). Embryos that were sectioned for histology were fixed and embedded in JB-4 and plastic sections were pr eformed as described previously (Pack et al., 1996).
Whole-mount in situ hybridization
Embryos were collected and processed for whole-mount in situ hybridization as previously described (Thisse et al., 1993). Digoxigenin-labeled and fluorescein-labeled riboprobes were synthesized from templates linearized with BamH1 using T7 RNA polymerase for Trap100. Other digoxigenin-labeled riboprobes used in this study were synthesized from templates linearized and transcribed as follows: crestin (Rubinstein et al., 2000), SacI and T7; phox2b (Shepherd et al., 2004), NotI and T7; dlx-2a (Akimenko et al., 1994), Foxn1 (Schorpp et al., 2002), BamH1 and T7; rag-1 (Willett et al., 1997), HindII and T7; ephA4 (Xu et al., 1995), EcoRI and T3; hoxb4 (Prince et al., 1998), KpnI and T3; ifbp (Andre et al., 2000), trypsin (Mayer and Fishman, 2003), pax9 (Nornes et al., 1996), gata6 (Pack et al., 1996), SalI and T7; foxA2 (Strahle et al., 1993), SacI and T3; ptc-1 (Concordet et al., 1996), BamHI and T3; sHH (Ekker et al., 1995), HindII and T7; ret (Bisgrove et al., 1997), NotI and T7; gfrα1a (Shepherd et al., 2001), and nNOS (Poon et al., 2003), NotI and Sp6. Digoxigenin-labeled probes were visualized with NBT/BCIP coloration reactions while fluorescein-labeled riboprobe was visualized with Fast Red (Roche). Cross-sections of whole-mount in situ hybridized embryos were cut using a Reichardt Jung cryostat. Embryos were equilibrated in 20% sucrose in PBS solution and frozen in OCT. Sections (30μm) were collected on Fisher super frost slides rehydrated in PBS and mounted in gel mount (Fisher).
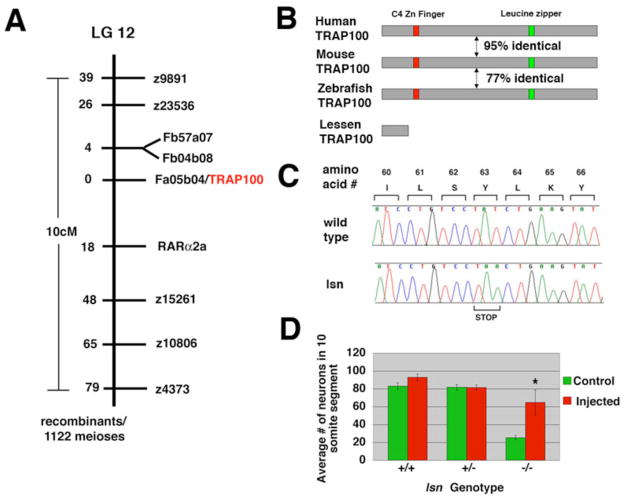
Mapping and molecular analysis of lsn
The lsnw24 (AB background) mutation was mapped by out-crossing into the polymorphic wild-type strain WIK, followed by in breeding of heterozygous progeny. We scanned the genome for linked SSLP markers by bulked segregant analysis using standard methods (Shimoda et al., 1999; Stickney et al., 2002; Talbot and Schier, 1999). This analysis placed the lsn mutation on linkage group 12 between z9891 and z4373, an interval of approximately10cM. Fine mapping using other SSLP markers and SNPs in 3′UTRs and intronic regions of ESTs that map to this region were used to further narrow the genetic interval. Using this approach we identified one EST Fa05b04 for which we had 0 recombinants out of 1122 meiosis screened. This EST encodes a zebrafish Trap100. We analyzed this candidate gene by complete sequencing of its cDNA isolated by RT-PCR from wild type and mutant embryos. The Trap100 cDNA isolated from mutants was found to have a base substitution at codon 189 of the 2970 codon open reading frame. This result was substantiated by sequencing PCR products from genomic DNA derived from 12 mutants, 12 phenotypically wild-type siblings as well as 12 unrelated wildtypes. The GenBank Accession Number for zebrafish Trap100 is XXXXXXXX.
Embryonic microinjections
Trap100 mRNA was synthesized using the mMessage mMachine kit (Ambion) and injected at a concentration of 50 ng/μl. The mRNAs were co injected with GFP mRNA, also at a concentration of 50 ng/μl, to assess expression. Approximately 1 nl of diluted mRNA was injected into 1-to 2-cell embryos using a gas-driven microinjection apparatus (Model# MMPI-2; Applied Scientific Instrumentation) through a micropipette.
Morpholino antisense oligonucleotides (Gene Tools) were designed corresponding to the start site and the with splice junction at the end of exon 3 of the genomic Trap100 sequence. The sequences were as follows:
+1/+25, CCTGTTTCAGATTCACCACCTTCAT
+199/int 3, GTGTGTTTACCTGTGAACTGATGGC
The oligos were resuspended in sterile filtered water and diluted to working concentrations in a range between 1–5 μg/μl. Approximately 1 nl of diluted morpholino was injected into 1-to 2-cell embryos using a gas-driven microinjection apparatus. Efficacy of the morpholino directed against the splice junction of exon 3 was evaluated using RT-PCR with a forward primer corresponding to the 131 bp of Trap100 (AGCTCTTCTGGAGCAGGCTA) in conjunction with a reverse primer designed to 471 bp of Trap100 (GGCCCTCAGACTGCTTTCTA). Casanova morpholino was designed to the previously described morpholino translation blocking sequence (Dickmeis et al., 2001).
Transplantation Experiments
Donor and host embryos were generated from an in cross of lsn heterozygotes. Donor embryos at the 1- to 2-cell stage were injected with 1.5pg of Tar* mRNA combined with a mixture of 5% Biotin-dextran (10K MW lysine fixable; Molecular Probes) and 5% Fluorescein-dextran (10K MW Lysine fixable; Molecular probes) to convert most of the donor cells to an endodermal cell fate (Peyrieras et al., 1998). Tar* mRNA were synthesized using the mMessage mMachine kit (Ambion) as described above. At the sphere stage, 20–40 donor cells were transplanted into unlabelled sibling host embryos. Embryos were then cultured in embryo medium with 10U/ml penicillin and 10U/ml streptomycin. Host embryo jaw cartilage development was determined on day 5 by Alcian Blue straining as described above. Donor cells from the transplant were detected in hosts using an avidin-biotinylated complex (ABC kit, Vectastain) and a DAB substrate. Genotypes of the host and donor embryos were determined by sequencing.
RESULTS
lsn mutant isolation
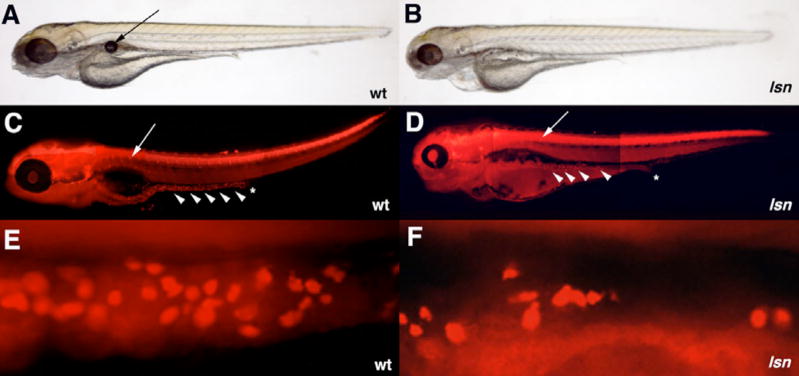
In zebrafish differentiated enteric neurons can be first detected at the anterior end of the intestine immunocytochemically at 72hpf (Bisgrove et al., 1997; Kelsh and Eisen, 2000; Shepherd et al., 2001; Shepherd et al., 2004). By 96hpf enteric neurons can be identified along the complete length of the intestine. We screened ENU-mutagenized zebrafish for recessive mutations that interfere with the neurogenesis in the ENS. Clutches of embryos were obtained from pair-wise crosses of in-crossed F2 families. The clutches of embryos were immunocytochemically stained with an anti-Hu antibody (Marusich et al., 1994) to reveal differentiated neurons and then screened under a fluorescent microscope. We identified a mutant allele (lsnw24) in which there was a significant reduction in the number of enteric neurons at 96hpf (Figure 1). lsn embryos have a 66% reduction in the number of enteric neurons over a 10 somite length segment stretching anteriorly from the cloaca (Figure 3D). This reduction in enteric neurons in lsn mutants is more pronounced in the more distal part of the intestine when compared to the anterior part. Further analysis of subtypes of ENS neurons in lsn mutants specifically serotonergic neurons by immunocytochemistry and nNOS neurons by in situ hybridization shows that there is a similar reduction in the number of these specific subtypes when compared to wild type at 96hpf (Supplemental data). These data suggest that different enteric neuron subtypes are equally affected in lsn mutants. Neuronal differentiation in other areas of the PNS appears normal in lsn mutants. The dorsal root ganglia, sympathetic neurons, and cranial ganglia are present and are comparatively normal at 96hpf based on anti-Hu immunocytochemistry (Figure 3; Supplemental Data). Further analysis of cranial ganglia development by in situ hybridization using a phox2b probe (Elworthy et al., 2005) also shows that the ganglia are normally specified at 48hpf (Supplemental data). The mutation is recessive lethal and 100% penetrant in all backgrounds tested (AB and WIK). Mutant embryos die by 7dpf. We called the mutant lessen (lsn) as the mutants had less enteric neurons as compared to wild type.
Figure 1. lsn-mutant phenotype.
(A, B) Lateral views of live larvae at 96hpf, showing the abnormal development of the jaw, eye and heart in lsn (B) as compared to wild-type (A) as well the lack of swim bladder in lsn. Arrow in (A) indicates the swim bladder. (C, D) Lateral views of 96hpf embryos stained with anti-Hu antibody to shows normal DRG development in lsn but reduced number of enteric neurons that are absent form the distal end of the gut tube. Stars (C, D) indicate the end of the gut tube. Arrows (C, D) indicate DRGs. Arrow heads (C, D) indicate enteric neurons. (E, D) Lateral views of the gut tube of 96hpf embryos stained with anti-Hu antibody showing reduced number of neurons in lsn.
Figure 3. Positional cloning of lsn.
(A) lsnw24 maps on to linkage group 12 between markers z9891 and z4373. No recombinants were found between lsnw24 and a SSCP marker in the 3′ UTR of an EST (Fa05b04) that encodes for a zebrafish Trap100. We isolated a full-length cDNA corresponding to Trap100 from homozygous-lsn mutant and wild type embryos and twelve independent clones were completely sequenced in both directions. A T to A (encoding Y to stop) mutation was identified in Trap100 cDNAs derived from homozygous-lsn mutants (B) A schematic illustrating the overall structure and percentage similarity between human, mouse and zebrafish TRAP100 and the predicted truncated TRAP100 in lsn mutants. (C) Sequence tracing of one of the 12 independent clones derived from genomic PCR of genomic DNA from mutant and wild type embryos showing TAT (Y) to TAA (stop) mutation. (D) A bar graph showing rescue of the lsn homozygous mutant enteric phenotype by injection of wild type Trap100 mRNA. Embryos derived from an incross of heetrozygote lsn fish were injected with 50pg of Trap100 mRNA, fixed and stained with anti-Hu antibody at 96hpf and genotyped. Control represents genotyped uninjected embryos from the same cross. Total number of enteric neurons in a 10-somite segment of the intestine were counted. Numbers represent the mean number of enteric neurons in this 10-somite segment ± s.e.m. for 15 embryos of each genotype for each condition. * Significantly different from control (Student’s t-test P<0.001).
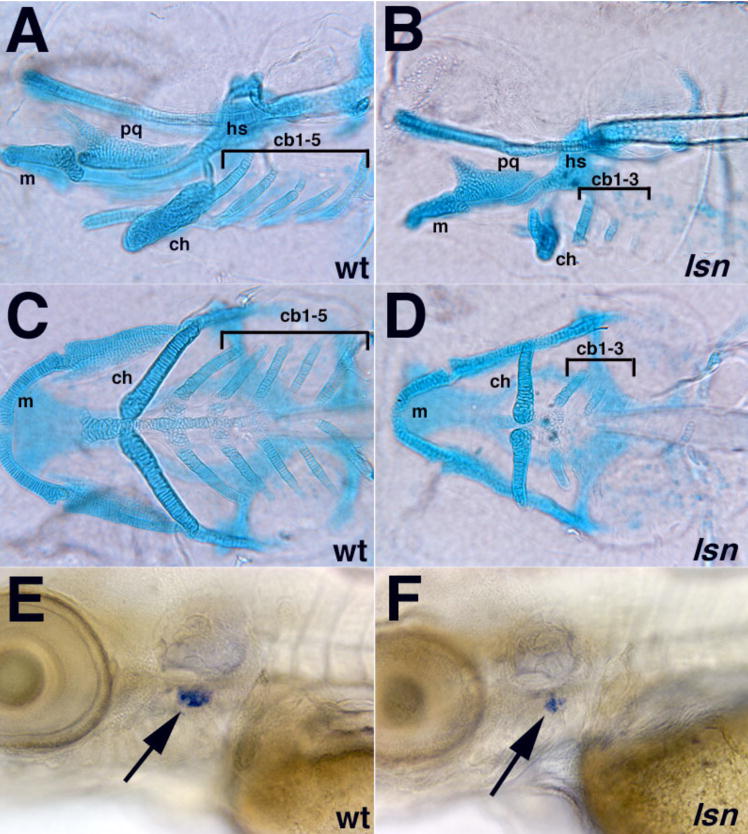
lsn mutants also have other defects in structures that are derived completely or receive cellular contributions from the neural crest. The most severely affected structures appear to be predominantly those that are derived from or receive contributions from the vagal/post-otic neural crest cells (Lam et al., 2002; Schilling and Kimmel, 1994). At 5dpf lsn mutants fail to develop the most posterior ceratobranchial cartilages (cb 4,5) while the more anterior ceratobranchial cartilages and the other anterior cartilages of the jaw are lese severely affected (Figure 2A–D). In addition there is cardiac edema apparent from 96hpf and the thymus primordium appears to not form normally based on reduced RAG-1 in situ expression (Figure 2E, F) (Willett et al., 1997). Whether the cardiac or thymus defects are due to neural crest defects has not been determined.
Figure 2. lsn mutation affects both craniofacial and thymus development.
(A–D) Alcian blue staining showing pharyngeal cartilages in120hpf wildtype (A, C) and lsn mutant (B, D) larvae shown in lateral (A, B) and ventral (C, D) views. In lsn pharyngeal cartilages develop is abnormal. Notably the ceratobranchials 4 and 5 are absent in lsn. cb 1–5, ceratobranchial cartilages 1–5; ch, ceratohyal cartilage; hs, hyosymplectic cartilage; m, Meckel’s cartilage; pq, palatoquadrate cartilage. (G and H) rag-1 expression at 96hpf in wildtype (E) and lsn mutant (F) larvae shows that lsn embryos have a significantly reduced thymic primordia (arrows).
Other neural crest derived tissues appear unaffected in lsn mutants. We detected no difference in the normal pattern of melanophores or iridophores by light microscopy at 96hpf (Figure 1). In situ hybridization studies using in situ probes for sox10 (Dutton et al., 2001) and nacre (Lister et al., 1999) at 24hpf revealed no detectable difference in the pattern of expression of these two markers suggesting that neural crest derived pigment cell development is unaffected in lsn (data not shown). We also examined the pattern of sox10 at 48 hpf as a marker of glial cells along the posterior lateral line (Kelsh and Eisen, 2000) and detected no difference in the pattern of staining in lsn as compared to wild type (data not shown). Together this suggest that the lsn mutation does not affect all neural crest derived tissues but instead affects specific axial subpopulations of neural crest cells.
The lsnw24 mutation disrupts a zebrafish Trap100
We identified the affected gene disrupted in lsn by mapping and molecular analysis of the lsnw24 mutation. Genetic mapping localized lsn to Linkage Group 12 (LG .12) between markers z9891 and z4373 (Figure 3A). By scoring 1122 meioses we established a fine map of the region using both microsatellite markers and SNPs in the 3′UTR or intronic regions of known genes or ESTs that had been mapped to this the critical interval. Using this approach we identified a zebrafish EST (fa05b04) that had no recombinants out of the 1122 meioses. Sequence analysis suggested that fa05b04 encoded a zebrafish homolog of TRAP100, a component of the TRAP/Mediator transcriptional regulation complex (Malik and Roeder, 2000; Myers and Kornberg, 2000; Rachez and Freedman, 2001). We cloned and sequenced this gene and verified by sequence comparison of the complete ORF that we had identified a zebrafish Trap100 orthologue of mouse and human TRAP100 (Figure 3B) (Yuan et al., 1998; Zhang and Fondell, 1999).
To determine if there was a mutation in this Trap100 orthologue in lsn we used RT-PCR to amplify the complete ORF of the zebrafish Trap100 gene from genotypically homozygous wild type and homozygous mutant 48hpf embryos. Sequencing of these RT-PCR products revealed that the lsn mutant Trap100 has a T to A base substitution that results in a premature stop codon at amino acid 63 in the amino terminal part of protein (Figure 3C). This base is located in the third exon of the Trap100 gene. Sequencing of this exon from genomic DNA isolated from morphologically identified 96hpf lsn mutant embryos showed that all homozygous mutant embryos have this base substitution while all homozygous wild type siblings lack this base change. As the predicted TRAP100 protein made in lsn mutants will be severely truncated (63 amino acids long as opposed to 989) and all the predicted functional domains will be missing in this truncated protein, we believe that lsn is a null allele of Trap100.
As a further demonstration that Trap100 is responsible for the lsn phenotype we performed rescue experiments using mRNA and cDNA that encoded wild type Trap100. To score the effectiveness of the rescue we counted the number of enteric neurons in a 10-somite segment of the intestine stretching anteriorly from the anus. Microinjection of either Trap100 mRNA or cDNA into embryos at the 1–2 cell stage partially rescued the number of enteric neurons in homozygous mutant embryos as compared to control injected embryos (Figure 3D; data not shown). No overexpression phenotype was observed when mRNA or cDNA was injected into heterozygous and homozygous wild type siblings (Figure 3D and data not shown).
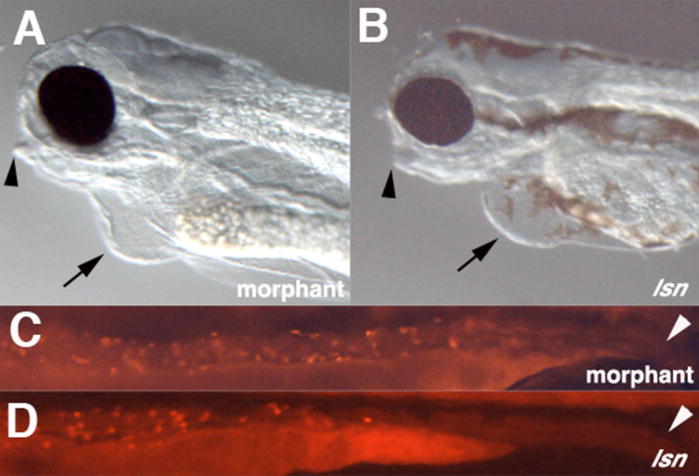
To provide additional evidence that reduction in Trap100 function causes the lsn phenotype, we injected 1–2 cell stage embryos with a morpholino antisense oligonucleotide directed against the putative translation start site of the gene or with a splice-blocking morpholino directed against the splice donor site at the end of exon 3. Injection of either morpholino resulted in jaw morphology, cardiac edema and the enteric phenotypes similar to those displayed by lsn mutants (Figure 4). The morphant phenotypes were the same for both morpholinos. In the case of the splice-blocking morpholino, the morphant phenotype correlated with a dramatic change in Trap100 transcripts in which adjoining introns are not spliced correctly as detected by RT-PCR using primers that amplify over these splice sites (data not shown). Notably, morpholino injections did not increase the severity of phenotype when injected into homozygous lsnw24 mutants, supporting the notion that lsnw24 is a complete loss-of-function mutation.
Figure 4. Effect of Trap100 antisense morpholino oligonucleotide injection on jaw, heart and enteric neuron development.
(A, B) Lateral views of the heads of embryos 96 hpf Trap 100 morphant embryos (A) and lsn mutant embryos. (C, D) Lateral views of the intestines of 96hpf embryos stained with anti-Hu antibody. Arrowheads in (A) and (B) indicate the recessed jaw in morphant and mutant embryos. Arrows in (A) and (B) indicate cardiac edema in morphant and mutant embryos. Arrow heads in (C) and (D) indicate the end of the intestine. Lack of melanophores in morphant embryos is not due the injected embryos were embryos obtained from an incross of nacre embryos (Lister et al., 1999).
Trap100 expression
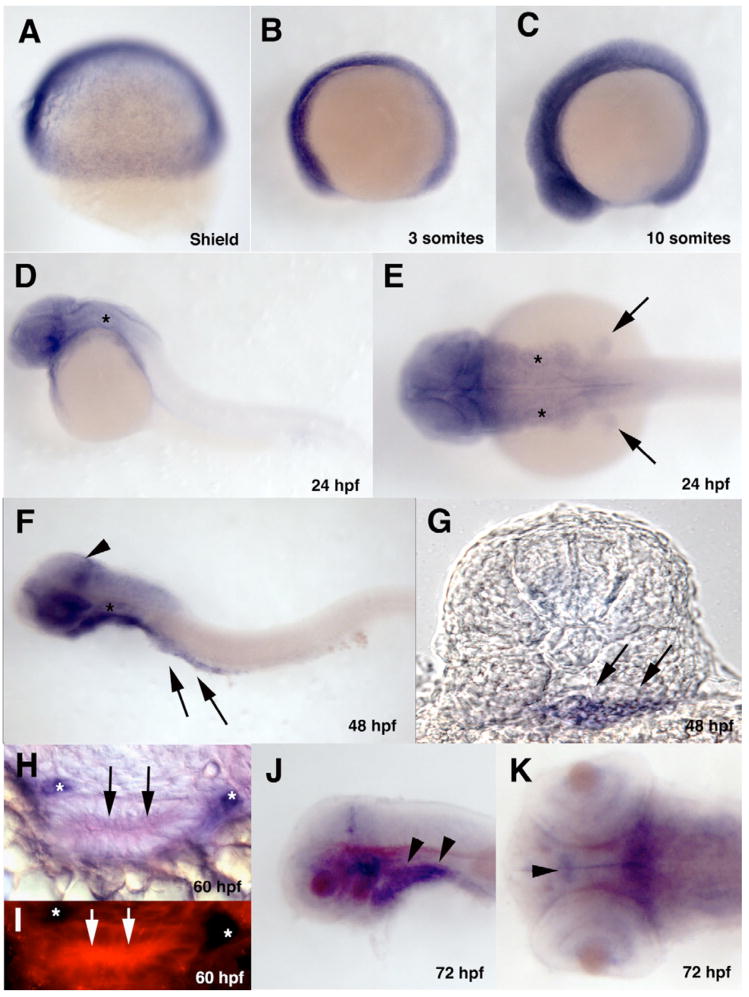
Zebrafish Trap100 is maternally expressed as determined by RT-PCR and in situ hybridization. Through the 10 somite stage, expression is ubiquitous (Figure 5A, B, C). Subsequently Trap100 expression starts to become more restricted with expression becoming reduced in the trunk and tail tissue while remaining high in more anterior parts of the embryo. By 24 hpf Trap100 is no longer detected in the trunk and tail (Figure 5D, E). Widespread expression is maintained in anterior parts of the embryo in both neural and non-neural tissue extending the length of the hindbrain including through out the pharyngeal arches. Expression is also present in the anterior parts of the gut tube. The pattern of expression at 48 hpf is essentially the same as that seen at 24 hpf but expression now extends along nearly the complete length of the gut (Figure 5F). Cross sections taken at the level of somite 4 show that the expression in the gut is throughout the mesendoderm (Figure 5G). While expression remains widespread throughout the neural and non-neural tissue in anterior parts of the embryo, three areas of higher expression of Trap100 can be seen in the CNS: in the dorsal forebrain, in the ventral midbrain and in a stripe of expressing cells in the dorsal posterior part of the midbrain (Figure 5F). At 60hpf, cross sections through the anterior part of the gut show that Trap100 expression is restricted to intestinal epithelial cells (Figure 5H, I). Double label in situ hybridization studies using a Trap100 probe in conjunction with a phox2b probe show that Trap100 is not expressed in the ENS precursors (Figure 5H, I). By 72hpf expression of Trap100 becomes much more restricted (Figure 5J, K). Expression in the CNS is restricted to discrete groups of cells in the diencephalon, in the ventral midbrain and in a stripe of cells in the dorsal posterior part of the midbrain. Weak expression can also be detected in the retina predominantly restricted to the margin. Outside of the CNS expression becomes restricted to the pharyngeal endoderm. This expression extends into the most anterior part of the gut endoderm but does not extend to more posterior parts of the gut. Pharyngeal endoderm expression is maintained through 96hpf, the latest age we examined in the present study (data not shown).
Figure 5. Developmental expression pattern of Trap100.
(A–F, J, K) Wholemount in situ hybridized embryos hybridized with a Trap100 antisense probe at the indicated developmental stages. (A, B, C, D, F, J) Lateral views. (E) Dorsal view of 24hpf embryo. (G) A transverse section taken through 48hpf embryo at the level of somite 4 showing expression through the intestinal mesendoderm. (H) A transverse section taken trough the gut tube at 60hpf after double in situ hybridization with a Trap100 fluorescein antisense probe (red) and a phox2b digoxigenin antisense probe (purple) showing intestinal epithelia expression of Trap100. (I) Same section as (H) showing Trap100 expression using fluorescence in the intestinal epithelia cells. (K) Dorsal view of the head of 72hpf embryo. (F, K, J) yolk has been removed. Arrows in (E) indicate fin buds. Arrowhead in (F) indicates increased expression of Trap100 in the posterior mesencephalon. Arrows in (F, G, H, I) indicate intestinal mesendodermal expression of Trap100. Arrowheads (J) indicate pharyngeal arch mesendodermal expression. Arrowhead (K) indicates ventral diencephalon cells expressing neurons expressing Trap100. * (D, E F) indicates the otic vesicle. * (H, I) indicates phox2b positive ENS precursors. In all wholemounts (A–F, J, K) anterior is to the left.
Trap100 is not required for endoderm-intestinal transition
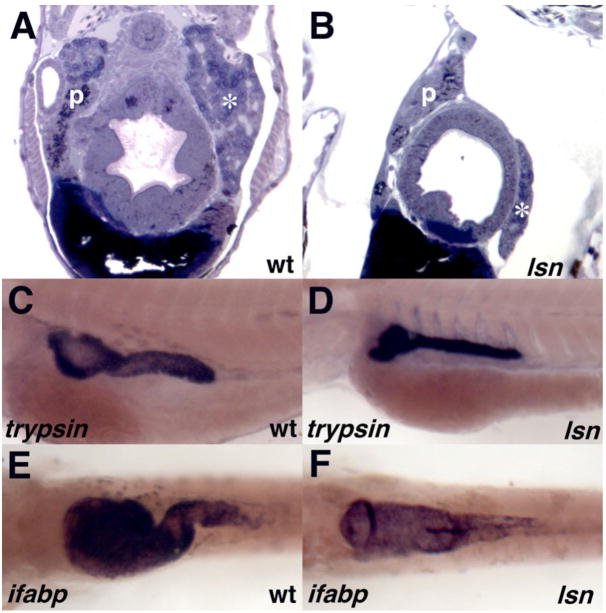
To determine if the enteric defect was also associated with other defects in intestinal development we examined cross sections of mutant embryos (Figure 6A, B). At 96hpf the gut tubes of the mutant embryos appear smaller and less well organized as compared to wild type and heterozygous siblings. Similarly the liver and pancreas are present in the mutant embryos but they are reduced in size as compared to their wild type siblings. We examined the expression pattern of several early markers of intestinal development by in situ hybridization including foxA2 (Odenthal and Nusslein-Volhard, 1998; Strahle et al., 1993), gata6 (Pack et al., 1996), sHH (Ekker et al., 1995; Roy et al., 2001) and ptc-1 (Concordet et al., 1996; Lewis et al., 1999) in genotyped wild-type and lsn homozygous mutant embryos. No difference was detected in the expression pattern of these genes (data not shown). To determine if the reduction in size of the gut tube and the pancreas seen at 96hpf was associated with a failure of these organs to functionally differentiate we carried out an in situ analysis using probes to trypsin and intestinal fatty acid binding protein (ifbp) (Figure 6 C–F) (Andre et al., 2000; Mayer and Fishman, 2003). Both genes were expressed in lsn/Trap100 mutants in comparatively normal expression patterns suggesting that the gut tube and the pancreas have undergone cytodifferentiation into more mature organs unlike the recently identified npo mutant (Mayer and Fishman, 2003). While differentiation has occurred normally, the normal looping of the gut has failed to occur, leading to an apparent straight gut tube (Figure 6F). Together these results suggest that while gut and intestinal organ development is less advanced in the lsn mutants, there has been no arrest in intestinal development and that lsn/Trap100 is not essential for the differentiation of intestinal endoderm derivatives.
Figure 6. Trap100 is required for normal intestinal development but is not required for endoderm-intestine transition.
(A, B) Cross sections of 120hpf wild type (A) and lsn mutant (B) embryos stained with Toluidine Blue. (C, D) trypsin expression at 96hpf in wholemount in situ hybridized wild type (C) and lsn mutant (D) embryos. (E, F) ifabp expression at 96hpf in wholemount in situ hybridized wild type (E) and lsn mutant (F) embryos. p, pancreas; * liver.
Trap100 is not required for neural crest specification or initial migration and patterning
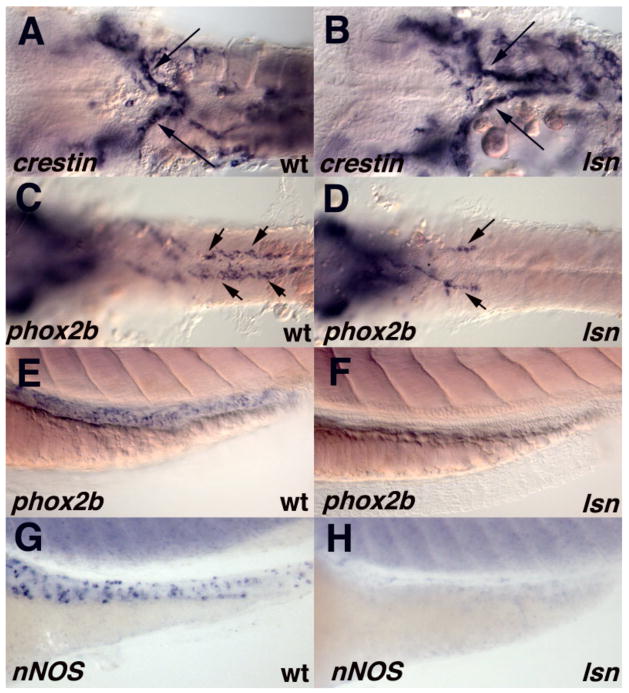
To determine the point at which neural crest development is disrupted in lsn we analyzed the expression of molecular markers of the premigratory and migrating neural crest. By gross morphology lsn mutants appear normal until about 84hpf. There is no difference in the specification or the initial migration of the cranial neural crest in lsn mutants as compared to wild type siblings as determined by in situ using the pan-crest specific marker crestin (Luo et al., 2001; Rubinstein et al., 2000) or by using other neural crest markers Sox10 and FoxD3 (Supplemental Data; data not shown). Furthermore no difference is detected in the patterning of the hindbrain from which the cranial crest neural crest arises as detected by in situ hybridization using ephA4 and hoxb4 (Prince et al., 1998; Schilling and Kimmel, 1994; Xu et al., 1995) (Supplemental Data).
We subsequently analyzed the migration and initial patterning of the jaw cartilages and the patterning of the thymus in genotyped embryos to determine if these structures had early defects in crest precursor migration or specification. No difference is observed in the migration of neural crest precursors to the jaw using a dlx2a (Akimenko et al., 1994) in situ probe or in their initial differentiation as determined by the expression of pax9 (Nornes et al., 1996) (Supplemental data). Furthermore no difference is observed in the initial patterning and specification of the thymus at 48 hpf as determined using an in situ probe for the early thymus marker Foxn1 (Schorpp et al., 2002) (Supplemental data).
We next examined the initial migration of the enteric precursors to the gut tube from the vagal region by in situ hybridization using the pan-crest specific marker crestin (Luo et al., 2001; Rubinstein et al., 2000) (Figure 7A, B) or using more specific enteric precursor markers gfrα1a and c-ret (data not shown) (Bisgrove et al., 1997; Shepherd et al., 2004). No difference in the expression of these markers is detected prior to 48hpf. However at 48hpf a difference in ENS development can be detected. In wild type embryos ENS precursors can be seen along the entire length of the intestine at 48 hpf (Figure 7C) (Shepherd et al., 2004). By contrast in lsn homozygous mutants enteric precursors have only migrated to the anterior part of the intestine by 48 hpf (Figure 7D). The lack of enteric precursor cells in the more posterior parts of the intestine is maintained at 60 and 72hpf as determined both by in situ using probes for phox2b (Elworthy et al., 2005; Shepherd et al., 2004), gfrα1a (Shepherd et al., 2001), c-ret (Bisgrove et al., 1997) and nNOS (Poon et al., 2003), and by immunocytochemistry using an anti–phox2b antibody (Pattyn et al., 1997) (Figure 7E–H & 8A, C). These results are consistent with the Hu immunocytochemistry that was used to identify the lsn mutants (Figure 1D, F).
Figure 7. lsn mutation causes a failure of enteric precursors to populate the entire length of the intestine but does not perturb the initial migration of vagal neural crest to the anterior gut.
(A, C, E, G) wild-type embryos and (B, D, F, H) lsn mutants embryos. (A, B) Ventral view of the vagal region of 36hpf embryos that have been hybridized with riboprobes for crestin. (C, D) Ventral view of the vagal region to somite 10 of 48hpf embryos that have been hybridized with riboprobes for phox2b showing a failure to of phox2b expressing cells populate the entire length of the intestine. (E, H) lateral views of the intestine 72hpf wild type (E, G) and lsn mutant (F, H) embryos that have been hybridized with riboprobes for phox2b (E, F) and nNOS (G, H). Arrows indicate the migrating enteric precursors. The yolk has been removed the embryos (A–D). Anterior is to the left.
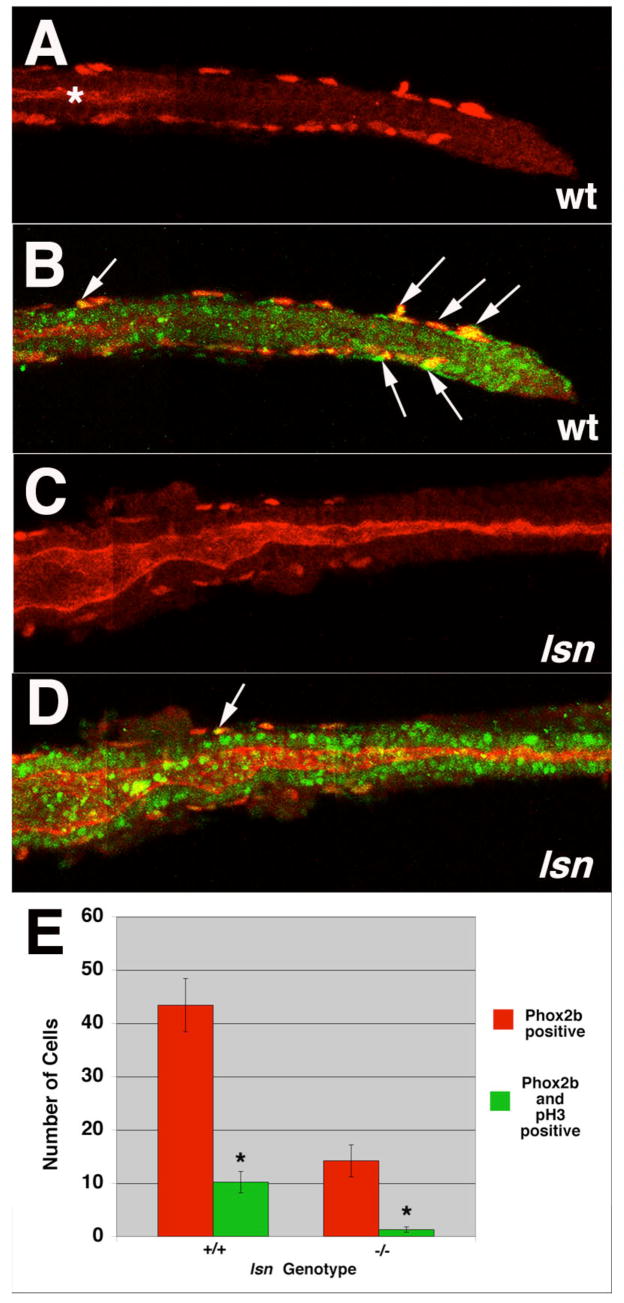
Figure 8. lsn mutants have reduced ENS precursor proliferation.
(A–D) Confocal images of dissected intestines from 48 hpf wild-type (A, B) and lsn (C, D) embryos that have been immunocytochemically stained with anti-Phox2b antibody and anti-phosphohistoneH3 antibody. (A, C) anti-Phox2b immunoreactivity in wild type (A) and lsn (C) showing the most posterior point along the gut tube that Phox2b positive cells can be identified. (B, D) Merged images of the same intestines in (A) and (C) showing anti-Phox2b and anti-phosphohistone H3 immunoreactivity in wild type (B) and lsn (D). * in (A) indicates the end of the gut lumen which has not opened all the way to the distal end of the gut tube at this developmental age. Arrows in (B) and (D) indicate double labeled cells. (E) A bar graph showing the mean number of Phox2b immunoreactive cells (red) and double-labeled Phox2b/phosphohistone H3 positive cells (green) present along the entire length of the intestine 48 hpf wild type and homozygous mutant lsn embryos. Embryos derived from an incross of heterozygote lsn fish were fixed and stained with anti-Phox2b and anti-phosphohistone H3 antibodies at 96hpf and genotyped. The intestines were then dissected from these genotyped embryos, imaged and the number of Phox2b positive cells and Phox2b + phosphohistone H3 double labeled cells were counted. Numbers represent the mean number of immunopositive cells ± s.e.m. for 10 embryos of each genotype. The difference between * was statistically significant (Student’s t-test P< 0.001).
Proliferation of migrating ENS precursors in the intestine is reduced in lsn mutants
Our results imply that the initial formation and specification of ENS precursors from the premigratory vagal neural crest as well as the initial migration of ENS precursors to the anterior end of the intestine is normal in lsn embryos. Similarly these data suggest that the initial specification and patterning of the other neural crest derived structures is not overtly perturbed in the lsn mutant.
To determine why the ENS precursors subsequently fail to populate the entire length of the intestine we examined if this phenotype resulted from an absence or reduction in gdnf expression along the intestinal mesendoderm. Previously we have shown GDNF is critically required for ENS precursor migration along the intestine (Shepherd et al., 2001). gdnf expression is unchanged in lsn mutants as compared to wild type (Supplemental data). This result combined with the observation that lsn ENS precursors have a normal expression of GDNF receptor components suggests the lsn ENS phenotype does not result from a perturbation of this signaling pathway.
To determine if the lsn ENS phenotype resulted from an increase in cell death of ENS precursors we examined whether there was an increase in the number of cells undergoing apoptosis in the intestine of 48 and 72hpf lsn embryos as compared to wild type siblings using TUNEL assay (Gavrieli et al., 1992). At both ages we observed very few cells undergoing apoptosis in the intestines of either wild type or lsn mutant embryos consistent with the recent study by Ng et al. (Ng et al., 2005)(Supplemental data). We determined that there is no difference in the number of cells undergoing apoptosis in the intestine of mutant embryos as compared to wild type siblings at either age.
Given the absence of an increase in the number of cells undergoing apoptosis in the intestine of lsn mutants we examined if the phenotype resulted from a decrease or lack of in proliferation of ENS precursors once they reached the intestine. As previously noted lsn mutants have a significant reduction in the number of ENS precursors in the intestine at 48 hpf as detected by phox2b immunocytochemistry (Figure 8) and by other ENS precursors specific markers using in situ hybridization (Figure 7 and data not shown). By undertaking double label immunoctyochemistry with the phox2b antibody and an antibody for phosphohistone H3, a marker of proliferating cells (Ajiro et al., 1996), we determined that there is a 88% reduction in the number of proliferating ENS precursors at 48hpf (Figure 8E). This result suggests that the decrease in the number of ENS precursors seen at 96hpf is caused at least in part the by the reduction in ENS precursor proliferation.
lsn/Trap100 is required non-cell autonomously for pharyngeal arch neural crest cells
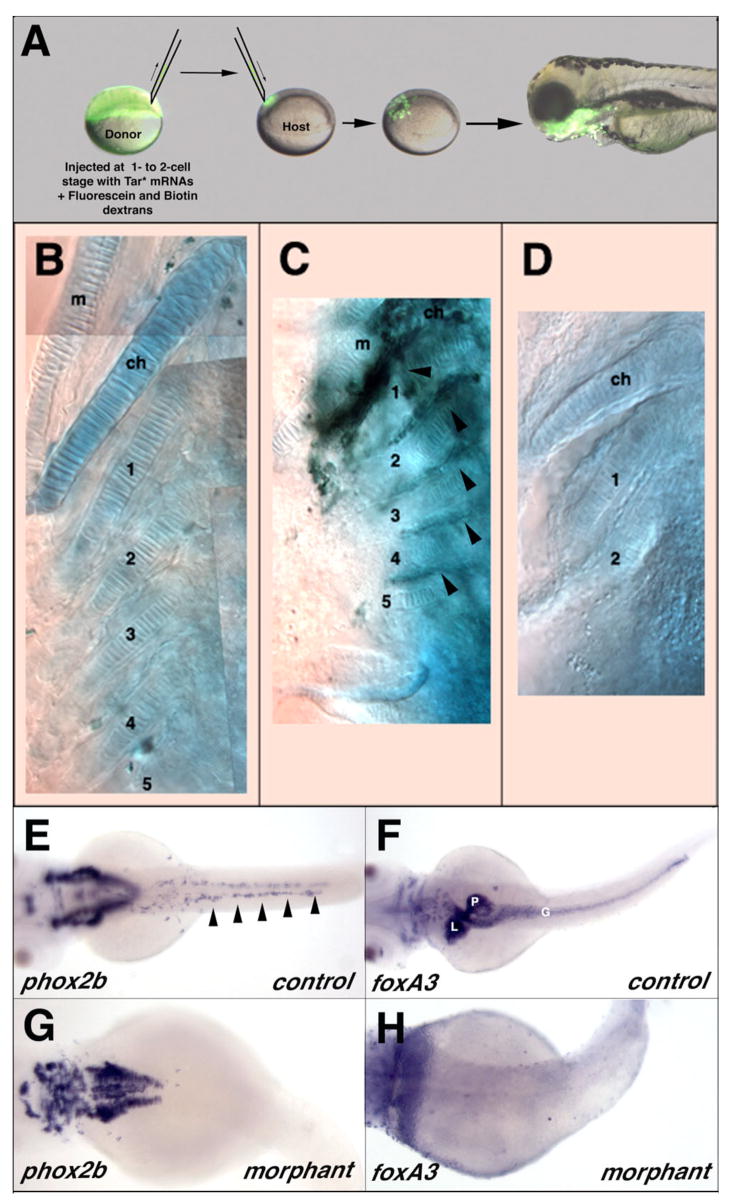
As lsn/Trap100 mutants exhibit defects in the neural crest derived head skeleton and Trap100 is widely expressed through out the pharyngeal arch mesendoderm we tested whether lsn/Trap100 is required only in the mesendoderm. We attempted to rescue lsn/Trap100 mutant cartilages by placing wild type cells into the pharyngeal mesendoderm. Transplantation of unmanipulated wild type cells usually results in small mesendedormal clones. To achieve larger clones in endodermal pouches we injected wild type donors prior to transplantation with Tar*, an activated version of the type1 TGFβ-related receptor Taram A (Tar) to convert blastomeres to an endodermal fate (David and Rosa, 2001; David et al., 2002; Peyrieras et al., 1998) (Figure 9A). As expected donor cells in the mosaic embryos contribute largely to the pharynx, endodermal pouches of the pharyngeal arches and only very rarely to the entire length of the digestive tract. In 7 out of 9 lsn larvae (78%) in which transplanted wild type cells contributed to the mesendoderm of the pharyngeal arches partial rescue of the most posterior ceratobranchial cartilages (3, 4, 5) was observed (Figure 9 C). Rescue corresponded only to regions where transplanted cells contributed to the pharyngeal pouch endoderm. These results suggest that the cartilage defects in lsn/Trap100 result from defect signaling from the endoderm.
Figure 9. Transplants suggest that lsnw24 functions cell autonomously in the endoderm and endoderm is required for ENS development.
(A–D) Transplantation of wild type endodermal cells partially rescues posterior arch cartilage formation in lsn mutants. (A) At blastula stage fluorescein-dextran labeled wild type cells expressing Tar* were transplanted into host and allowed to develop. The lateral view of the head region at 72 hpf shows grafted cells (green) have differentiated into pharyngeal endoderm derivatives. (B–D) Ventral views of 5dpf larvae stained with Alcian Blue to show cartilages. (B) Wild type control. All lower jaw cartilages are clearly identifiable. (C) lsn mutant larvae transplanted with wild type cells showing rescue of posterior ceratobranchial (3–5) in the vicinity of transplanted Tar*-expressing wild type cells (black staining) in the pharyngeal arch endoderm (arrowheads). (D) lsn mutant that lacks posterior ceratobranchials. (E–H) Casanova morphant embryos have no intestinal endoderm and no migrating ENS precursors. (E–H) Dorsal views of 48 hpf control embryos (E, F) and cas morphant embryos (G, H) that have been hybridized with riboprobes for phox2b (E, G) or foxA3 (F, H) showing a complete lack of phox2b expressing cells in the region of the intestine of morphant embryos (G) that also completely lack intestinal endoderm (H). Arrowheads (E) indicate migrating ENS precursors. m, Meckel’s cartilage of the mandibular arch; ch, ceratohyal cartilage; 1–5 ceratobranchial cartilages; G, intestine; L, liver, P, pancreas.
Endoderm is required for the normal development of the ENS
To determine whether endoderm is necessary for the development of the ENS we injected zebrafish embryos with a specific MO directed against casanova (cas) mRNA (Dickmeis et al., 2001). Cas is a Sox-related factor that is required for endoderm formation (Alexander et al., 1999; Dickmeis et al., 2001; Kikuchi et al., 2001). All Cas-MO injected embryos exhibited cardiabifida and completely lacked intestinal endoderm as determined by foxA3 expression (Figure 9F, H). When compared to uninjected controls, cas morphants completely lacked phox2b expression in the ENS precursors while expression in the hindbrain remained though the pattern of expression was altered. To confirm that this was not just a developmental delay we stained 96hpf Cas-MO embryos with anti-Hu antibodies. No Hu immunoreactivity was seen in the ventral region of the embryo where the ENS neurons normally are located (data not shown). These experiments demonstrate that endoderm is required for the development of the ENS.
DISCUSSION
In this study we show that lessen (lsn) is a mutation in a zebrafish Trap100 gene that is orthologous to genes identified in mouse and human (Yuan et al., 1998; Zhang and Fondell, 1999). TRAP100 is required for the normal development of specific neural crest derived structures in zebrafish. The lesion in lsn is a point mutation in Trap100 that introduces a stop codon in the gene’s third exon and is predicted to severely truncate the mutant protein. We predict that the lsn mutation results in a Trap100 null phenotype as the lsn TRAP100 protein lacks all its predicted functional domains. Consistent with this, injection of two different morpholinos, a translation blocking morpholino or a splice-blocking morpholino, into lsn mutants does not result in a more severe phenotype, indicating that lsn is a complete loss of function mutant. We show that TRAP100 is required for the normal proliferation of ENS precursors but not for the initial specification and migration of the ENS precursors to the anterior end of the intestine. In mice, disruption of Trap100 results in an early embryonic lethal phenotype at E9–10 primarily thought to be due to placental development defects that results in anemia and poor nutritional supply but also due to defects in overall cell growth through out the embryo (Ito et al., 2002). Because of this early embryonic lethality in the Trap100 −/− mouse, a critical role for TRAP100 in ENS development as well as the normal development of other vagal neural crest derived structures was previously unrecognized.
Trap100 has tissue specific functions during embryogenesis
Previous genetic studies of murine TRAP/Mediator complex components TRAP220 (Ito et al., 2000; Zhu et al., 2000), SRB7 (Tudor et al., 1999) and TRAP100 (Ito et al., 2002) have shown that the complex is essential for embryogenesis but the genetic null mutant mice have different degrees of phenotypic severity with Srb7 −/− mice having the most severe phenotype with embryos only reaching the blastocyst stage while Trap220 −/− mice are viable up to E11. This variation has led to the proposal that while the TRAP/Mediator complex is essential for normal cell viability, cells that express TRAP/Mediator complexes with different subunit compositions may have more specific roles regulating tissue specific transcription for those cell types with that subunit composition (Ito et al., 2002).
Our finding that the lsn mutant is viable until larval stages of zebrafish development (7dpf) is surprising given the early TRAP 100 null mouse lethality (Ito et al., 2002). Furthermore the zebrafish null phenotype does not exhibit similar defects in the spatiotemporal organization and proliferation in the neural tube and cardiovascular system as observed in mouse (Ito et al., 2002). By contrast the lsn mutant phenotype clearly shows a tissue specific role for TRAP100 in both ENS/intestinal development as well as in jaw development. The jaw development phenotype is not unique for mutations that affect TRAP/Mediator components. Haploinsufficiency in the TRAP/mediator subunit PAQ/ARC105 in DiGeorge syndrome indicates a potential specialized role in development of organs derived from the first and second branchial arches though the critical role of Tbx1 also deleted in this deficiency is thought to be the critical gene that causes the branchial arch phenotype in DiGeorge (Berti et al., 2001; Jerome and Papaioannou, 2001; Lindsay et al., 2001; Merscher et al., 2001). The lsn mutant phenotype potentially has revealed a role for TRAP100 in the development of posterior branchial arch derived cartilages. The differences between the mouse and zebrafish TRAP100 null phenotypes probably have arisen due to the critical role of TRAP100 in placental development in amniotes (Ito et al., 2002). Clarification as to whether this phenotypic difference is due to the placental defect could be revealed by mouse chimera studies. It is also possible that this difference occurs due to maternally deposited TRAP100 protein rescuing any early defects in lsn mutants.
Trap100 function in endoderm is required for normal development of neural crest derived pharyngeal structures
In this study we show that the lsn posterior jaw cartilage mutant phenotype can be rescued by transplantation of wild-type endodermal cells. This result suggests that signals from the pharyngeal mesendoderm required for the normal development of the neural crest derived cartilages are either reduced or missing. Pharyngeal cartilage development is a complex process requiring inductive signals from the surrounding endoderm and ectoderm (Couly et al., 2002; Crump et al., 2004a; Crump et al., 2004b; Hall, 1980). A large number of genes are expressed in pharyngeal arches during embryogenesis underscoring the genetic complexity of their development. A number of these genes are expressed in an evolutionarily conserved manner in zebrafish (Yelick and Schilling, 2002) and several of these genes have been shown to affect pharyngeal pouch segmentation and jaw cartilage development including fgf3 (Crump et al., 2004a; David et al., 2002), fgf8 (Crump et al., 2004a) and tbx1 (Piotrowski et al., 2003). The migration of the neural crest into the branchial arches is unaffected in lsn mutants suggesting that pharyngeal pouch segmentation is normal and that expression of these genes is unaffected in lsn. Consistent with this supposition is our data showing that the development of the epibranchial placodes is normal in lsn (Supplemental data). This is a process that has recently been show to be endoderm dependent (Holzschuh et al., 2005; Nechiporuk et al., 2005). Further investigation will be required to determine if other signaling molecules, such as Endothelin-1, that are known to play an essential role in pharyngeal cartilage specification and differentiation are affected in lsn (Kimmel et al., 2003; Miller et al., 2000; Piotrowski et al., 2003).
Trap100 is required for proliferation of vagal neural crest derived ENS precursors
In this study we show that the lsn ENS phenotype is due to a reduced level of proliferation of the ENS precursors. The findings that there is a reduced number of ENS precursors from the onset of migration along the gut and that fewer of these precursors are proliferating are consistent with the later reduction in the total number of enteric neurons seen at 96hpf. Interestingly we observe that in wildtype embryos the majority of phosphohistone H3-positive ENS precursors are the most caudal migrating ones. This observation raises the possibility that the migration of ENS precursors along the gut is a proliferative migration, i.e. it is a combination of cells actively moving distally along the intestine combined with an increased number of new ENS precursors being generated specifically at the leading edge of the migration. This is not apparent in the mouse where there appears to be no significant increase in proliferation in precursors at the leading edge of the wave of migration (Young et al., 2004; Young and Newgreen, 2001). This difference may have arisen due to the very different pattern of migration of ENS precursors along the zebrafish gut as compared to mouse and avian precursors. In zebrafish ENS precursors initially migrate along the length of the gut tube as two symmetric streams either side of the gut mesendoderm (Elworthy et al., 2005; Shepherd et al., 2004) rather than as a rostro-caudal cork screw wave of migration within in the gut mesoderm as seen in mouse (Kapur et al., 1992; Young et al., 2004) and avian embryos (Burns and Douarin, 1998; Epstein et al., 1991; Newgreen et al., 1996).
An important unresolved issue is what cellular mechanisms cause the ENS proliferation defect in lsn. One possibility is that TRAP100 is required for transcription of genes that are directly involved in the cell cycle within the ENS precursors. Consistent with this idea, one of the other components of the TRAP/Mediator complex is Cyclin C (Akoulitchev et al., 2000; Gu et al., 1999; Hengartner et al., 1998) and thus a mutation in TRAP100 that perturbed the normal association of Cyclin C with the TRAP/Mediator complex might potentially alter Cyclin C’s effects on the cell cycle (Ren and Rollins, 2004). However this proposed mechanism assumes that the mutation acts cell autonomously within the ENS precursors. As TRAP100 is not expressed in ENS precursors in the intestine (Figure 7 H, I) this cell autonomous function appears unlikely. Instead Trap100’s expression within the intestinal endoderm suggests that the ENS defect is a secondary affect of the mutation and that the lsn mutation acts cell autonomously in the intestinal endoderm. Potentially the lsn ENS phenotype could result from reduced levels of endoderm derived mitogenic factors required by the ENS precursors. Consistent with this hypothesis is our data that shows that a lack of endoderm results in a complete loss of the ENS. Further indirect support is provided by the transplantation studies that show that the lsn mutation acts cell autonomously with respect to the endoderm in the pharyngeal arches. We have not been able to test this hypothesis directly as we have been unable to generate chimeric mutant embryos that have intestinal endoderm derived principally from wild type cells. While transplanted blastomeres from tar* injected donor embryos do contribute to pharyngeal pouch endoderm these transplanted cells rarely contribute to the intestinal endoderm as has been previously reported (Piotrowski et al., 2003).
A further question that also remains unresolved relates to whether the lsn mutant phenotype results from perturbation of nuclear hormone signaling. Previous in vitro studies showed that TRAP100 is involved in enhancing transactivation of thyroid hormone receptor (TR) and vitamin D receptor (VDR) signaling in a ligand dependent manner (Zhang and Fondell, 1999), and that in TRAP100-deficient cells TRalpha, VDR, PPAR gamma and androgen receptors have attenuated transactivation function (Ito et al., 2002). Pharmacological block of TR signaling in zebrafish embryos between 3dpf and 5dpf results in a phenotype that is very similar to that seen in lsn mutant embryos with regard to the jaw, eye and intestinal epithelium phenotype (Liu and Chan, 2002). This study also showed by RT-PCR that both TRalpha and beta are expressed in a developmentally regulated fashion during this period, however the tissue-specific expression pattern of the receptors at this embryonic stage of development has not been described. Detailed studies investigating the precise spatial and temporal expression pattern of the thyroid hormone receptors and other nuclear hormone receptors will help determine which nuclear hormone activity is potentially perturbed in the lsn mutants.
In summary the identification of Trap100 as the gene responsible for the lsn mutation has revealed a previously unappreciated tissue specific function of this gene during development. This finding is of significant interest for studies relating to the function of TRAP/Mediator complexes with different subunit compositions. Furthermore the identification of TRAP100 as having an important role in ENS development has potentially identified a new gene that may be associated with HSCR.
Supplementary Material
Acknowledgments
We would like to thank: Michael Pack for foxA2 and gata6 in situ probes; Cecilia Moens for the rag-1, ephA4 and hoxb4 in situ probe; Nancy Manley for the foxN1 in situ probe; Didier Stainer for the foxA3 in situ probe. We would like to thank Karen Guillemin for information about the 5-HT antibody. We would like to thank Nancy L’Hernault for her technical assistance with the histological sectioning and Peter Kelly for technical assistance with mapping. We would also like to thank Thomas Schilling for his advice with the chimeric analysis. Research was supported by grants from NIH (DK067285) and March of Dimes (5-FY02-270) to ITS, NIH to DWR, and NIH (RR12349) to WST.
References
- Ajiro K, Yoda K, Utsumi K, Nishikawa Y. Alteration of cell cycle-dependent histone phosphorylations by okadaic acid. Induction of mitosis-specific H3 phosphorylation and chromatin condensation in mammalian interphase cells. J Biol Chem. 1996;271:13197–201. doi: 10.1074/jbc.271.22.13197. [DOI] [PubMed] [Google Scholar]
- Akimenko MA, Ekker M, Wegner J, Lin W, Westerfield M. Combinatorial expression of three zebrafish genes related to distal- less: part of a homeobox gene code for the head. J Neurosci. 1994;14:3475–86. doi: 10.1523/JNEUROSCI.14-06-03475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoulitchev S, Chuikov S, Reinberg D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature. 2000;407:102–6. doi: 10.1038/35024111. [DOI] [PubMed] [Google Scholar]
- Alexander J, Rothenberg M, Henry GL, Stainier DY. casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol. 1999;215:343–57. doi: 10.1006/dbio.1999.9441. [DOI] [PubMed] [Google Scholar]
- Amiel J, Lyonnet S. Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet. 2001;38:729–39. doi: 10.1136/jmg.38.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre M, Ando S, Ballagny C, Durliat M, Poupard G, Briancon C, Babin PJ. Intestinal fatty acid binding protein gene expression reveals the cephalocaudal patterning during zebrafish gut morphogenesis. Int J Dev Biol. 2000;44:249–52. [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Holzschuh J, Driever W, Knapik EW. Neural crest survival and differentiation in zebrafish depends on mont blanc/tfap2a gene function. Development. 2004;131:1463–77. doi: 10.1242/dev.01033. [DOI] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–85. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Berti L, Mittler G, Przemeck GK, Stelzer G, Gunzler B, Amati F, Conti E, Dallapiccola B, Hrabe de Angelis M, Novelli G, et al. Isolation and characterization of a novel gene from the DiGeorge chromosomal region that encodes for a mediator subunit. Genomics. 2001;74:320–32. doi: 10.1006/geno.2001.6566. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Raible DW, Walter V, Eisen JS, Grunwald DJ. Expression of c-ret in the zebrafish embryo: potential roles in motoneuronal development. J Neurobiol. 1997;33:749–68. [PubMed] [Google Scholar]
- Burns AJ, Douarin NM. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–47. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, D’Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA, et al. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24:4266–82. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Pham TD, Rothman TP, DiStefano PS, Bothwell M, Blair-Flynn J, Tessarollo L, Gershon MD. Neurotrophin-3 is required for the survival-differentiation of subsets of developing enteric neurons. J Neurosci. 2001;21:5620–36. doi: 10.1523/JNEUROSCI.21-15-05620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Lamballe F, Barbacid M, Gershon MD. Neurotrophin-3 induces neural crest-derived cells from fetal rat gut to develop in vitro as neurons or glia. J Neurosci. 1994;14:6571–84. doi: 10.1523/JNEUROSCI.14-11-06571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Vinson EN, MacLennan AJ, Gershon MD. Promotion of the development of enteric neurons and glia by neuropoietic cytokines: interactions with neurotrophin-3. Dev Biol. 1998;198:343–65. [PubMed] [Google Scholar]
- Concordet JP, Lewis KE, Moore JW, Goodrich LV, Johnson RL, Scott MP, Ingham PW. Spatial regulation of a zebrafish patched homologue reflects the roles of sonic hedgehog and protein kinase A in neural tube and somite patterning. Development. 1996;122:2835–46. doi: 10.1242/dev.122.9.2835. [DOI] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–73. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Crump JG, Maves L, Lawson ND, Weinstein BM, Kimmel CB. An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development. 2004a;131:5703–16. doi: 10.1242/dev.01444. [DOI] [PubMed] [Google Scholar]
- Crump JG, Swartz ME, Kimmel CB. An integrin-dependent role of pouch endoderm in hyoid cartilage development. PLoS Biol. 2004b;2:E244. doi: 10.1371/journal.pbio.0020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- David NB, Rosa FM. Cell autonomous commitment to an endodermal fate and behaviour by activation of Nodal signalling. Development. 2001;128:3937–47. doi: 10.1242/dev.128.20.3937. [DOI] [PubMed] [Google Scholar]
- David NB, Saint-Etienne L, Tsang M, Schilling TF, Rosa FM. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development. 2002;129:4457–68. doi: 10.1242/dev.129.19.4457. [DOI] [PubMed] [Google Scholar]
- Dickmeis T, Mourrain P, Saint-Etienne L, Fischer N, Aanstad P, Clark M, Strahle U, Rosa F. A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev. 2001;15:1487–92. doi: 10.1101/gad.196901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development. 2001;128:4113–25. doi: 10.1242/dev.128.21.4113. [DOI] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–55. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Elworthy S, Pinto JP, Pettifer A, Cancela ML, Kelsh RN. Phox2b function in the enteric nervous system is conserved in zebrafish and is sox10-dependent. Mech Dev. 2005;122:659–69. doi: 10.1016/j.mod.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr, Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–24. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Epstein ML, Poulsen KT, Thiboldeaux R. Formation of ganglia in the gut of the chick embryo. J Comp Neurol. 1991;307:189–99. doi: 10.1002/cne.903070203. [DOI] [PubMed] [Google Scholar]
- Fu M, Lui VC, Sham MH, Pachnis V, Tam PK. Sonic hedgehog regulates the proliferation, differentiation, and migration of enteric neural crest cells in gut. J Cell Biol. 2004;166:673–84. doi: 10.1083/jcb.200401077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JBaCM. The enteric nervous system. Glasgow: Churchill Livingstone; 1987. [Google Scholar]
- Gavrieli Y, Sherman Y, Ben Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Kirchgessner AL, Wade PR. Functional anatomy of the enteric nervous system. In: Johnson LR, editor. Physiology of the gastrointestinal tract. Vol. 1. New York: Raven Press; 1994. [Google Scholar]
- Gu W, Malik S, Ito M, Yuan CX, Fondell JD, Zhang X, Martinez E, Qin J, Roeder RG. A novel human SRB/MED-containing cofactor complex, SMCC, involved in transcription regulation. Mol Cell. 1999;3:97–108. doi: 10.1016/s1097-2765(00)80178-1. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–76. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Hall BK. Tissue interactions and the initiation of osteogenesis and chondrogenesis in the neural crest-derived mandibular skeleton of the embryonic mouse as seen in isolated murine tissues and in recombinations of murine and avian tissues. J Embryol Exp Morphol. 1980;58:251–64. [PubMed] [Google Scholar]
- Hatano M, Aoki T, Dezawa M, Yusa S, Iitsuka Y, Koseki H, Taniguchi M, Tokuhisa T. A novel pathogenesis of megacolon in Ncx/Hox11L.1 deficient mice. J Clin Invest. 1997;100:795–801. doi: 10.1172/JCI119593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner CJ, Myer VE, Liao SM, Wilson CJ, Koh SS, Young RA. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol Cell. 1998;2:43–53. doi: 10.1016/s1097-2765(00)80112-4. [DOI] [PubMed] [Google Scholar]
- Herbarth B, Pingault V, Bondurand N, Kuhlbrodt K, Hermans Borgmeyer I, Puliti A, Lemort N, Goossens M, Wegner M. Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease. Proc Natl Acad Sci USA. 1998;95:5161–5. doi: 10.1073/pnas.95.9.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM, Jr, et al. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons [see comments] Neuron. 1999;22:253–63. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev Biol. 1998;200:116–29. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuss A, Bally-Cuif L, Schilling TF. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–42. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–76. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Howard M, Foster DN, Cserjesi P. Expression of HAND gene products may be sufficient for the differentiation of avian neural crest-derived cells into catecholaminergic neurons in culture. Dev Biol. 1999;215:62–77. doi: 10.1006/dbio.1999.9450. [DOI] [PubMed] [Google Scholar]
- Ito M, Okano HJ, Darnell RB, Roeder RG. The TRAP100 component of the TRAP/Mediator complex is essential in broad transcriptional events and development. Embo J. 2002;21:3464–75. doi: 10.1093/emboj/cdf348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yuan CX, Okano HJ, Darnell RB, Roeder RG. Involvement of the TRAP220 component of the TRAP/SMCC coactivator complex in embryonic development and thyroid hormone action. Mol Cell. 2000;5:683–93. doi: 10.1016/s1097-2765(00)80247-6. [DOI] [PubMed] [Google Scholar]
- Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27:286–91. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- Kapur RP. Early death of neural crest cells is responsible for total enteric aganglionosis in Sox10(Dom)/Sox10(Dom) mouse embryos. Pediatr Dev Pathol. 1999a;2:559–69. doi: 10.1007/s100249900162. [DOI] [PubMed] [Google Scholar]
- Kapur RP. Hirschsprung disease and other enteric dysganglionoses. Crit Rev Clin Lab Sci. 1999b;36:225–73. doi: 10.1080/10408369991239204. [DOI] [PubMed] [Google Scholar]
- Kapur RP, Yost C, Palmiter RD. A transgenic model for studying development of the enteric nervous system in normal and aganglionic mice. Development. 1992;116:167–75. doi: 10.1242/dev.116.Supplement.167. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Eisen JS. The zebrafish colourless gene regulates development of non-ectomesenchymal neural crest derivatives. Development. 2000;127:515–25. doi: 10.1242/dev.127.3.515. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Agathon A, Alexander J, Thisse C, Waldron S, Yelon D, Thisse B, Stainier DY. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001;15:1493–505. doi: 10.1101/gad.892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ullmann B, Walker M, Miller CT, Crump JG. Endothelin 1-mediated regulation of pharyngeal bone development in zebrafish. Development. 2003;130:1339–51. doi: 10.1242/dev.00338. [DOI] [PubMed] [Google Scholar]
- Knight RD, Nair S, Nelson SS, Afshar A, Javidan Y, Geisler R, Rauch GJ, Schilling TF. lockjaw encodes a zebrafish tfap2a required for early neural crest development. Development. 2003;130:5755–68. doi: 10.1242/dev.00575. [DOI] [PubMed] [Google Scholar]
- Kuratani SC, Wall NA. Expression of Hox 2.1 protein in restricted populations of neural crest cells and pharyngeal ectoderm. Dev Dyn. 1992;195:15–28. doi: 10.1002/aja.1001950103. [DOI] [PubMed] [Google Scholar]
- Lam SH, Chua HL, Gong Z, Wen Z, Lam TJ, Sin YM. Morphologic transformation of the thymus in developing zebrafish. Dev Dyn. 2002;225:87–94. doi: 10.1002/dvdy.10127. [DOI] [PubMed] [Google Scholar]
- Lewis KE, Concordet JP, Ingham PW. Characterisation of a second patched gene in the zebrafish Danio rerio and the differential response of patched genes to Hedgehog signalling. Dev Biol. 1999;208:14–29. doi: 10.1006/dbio.1998.9169. [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, et al. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- Lister JA, Robertson CP, Lepage T, Johnson SL, Raible DW. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–67. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- Liu YW, Chan WK. Thyroid hormones are important for embryonic to larval transitory phase in zebrafish. Differentiation. 2002;70:36–45. doi: 10.1046/j.1432-0436.2002.700104.x. [DOI] [PubMed] [Google Scholar]
- Luo R, An M, Arduini BL, Henion PD. Specific pan-neural crest expression of zebrafish Crestin throughout embryonic development. Dev Dyn. 2001;220:169–74. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1097>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Malik S, Roeder RG. Transcriptional regulation through Mediator-like coactivators in yeast and metazoan cells. Trends Biochem Sci. 2000;25:277–83. doi: 10.1016/s0968-0004(00)01596-6. [DOI] [PubMed] [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–55. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- Mayer AN, Fishman MC. Nil per os encodes a conserved RNA recognition motif protein required for morphogenesis and cytodifferentiation of digestive organs in zebrafish. Development. 2003;130:3917–28. doi: 10.1242/dev.00600. [DOI] [PubMed] [Google Scholar]
- Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S, et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–29. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Miller CT, Schilling TF, Lee K, Parker J, Kimmel CB. sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development. Development. 2000;127:3815–28. doi: 10.1242/dev.127.17.3815. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–9. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Myers LC, Kornberg RD. Mediator of transcriptional regulation. Annu Rev Biochem. 2000;69:729–49. doi: 10.1146/annurev.biochem.69.1.729. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Raible DW. Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development. 2005;132:3717–30. doi: 10.1242/dev.01876. [DOI] [PubMed] [Google Scholar]
- Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 1. Pediatr Dev Pathol. 2002a;5:224–47. doi: 10.1007/s10024-001-0142-y. [DOI] [PubMed] [Google Scholar]
- Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 2. Pediatr Dev Pathol. 2002b;5:329–49. doi: 10.1007/s10024-001-0142-y. [DOI] [PubMed] [Google Scholar]
- Newgreen DF, Southwell B, Hartley L, Allan IJ. Migration of enteric neural crest cells in relation to growth of the gut in avian embryos. Acta Anat (Basel) 1996;157:105–15. doi: 10.1159/000147871. [DOI] [PubMed] [Google Scholar]
- Ng AN, de Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, Appel B, Dong PD, Stainier DY, Heath JK. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol. 2005;286:114–35. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Nornes S, Mikkola I, Krauss S, Delghandi M, Perander M, Johansen T. Zebrafish Pax9 encodes two proteins with distinct C-terminal transactivating domains of different potency negatively regulated by adjacent N-terminal sequences. J Biol Chem. 1996;271:26914–23. doi: 10.1074/jbc.271.43.26914. [DOI] [PubMed] [Google Scholar]
- O’Brien EK, d’Alencon C, Bonde G, Li W, Schoenebeck J, Allende ML, Gelb BD, Yelon D, Eisen JS, Cornell RA. Transcription factor Ap-2alpha is necessary for development of embryonic melanophores, autonomic neurons and pharyngeal skeleton in zebrafish. Dev Biol. 2004;265:246–61. doi: 10.1016/j.ydbio.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Nusslein-Volhard C. fork head domain genes in zebrafish. Dev Genes Evol. 1998;208:245–58. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- Pack M, Solnica-Krezel L, Malicki J, Neuhauss SC, Schier AF, Stemple DL, Driever W, Fishman MC. Mutations affecting development of zebrafish digestive organs. Development. 1996;123:321–8. doi: 10.1242/dev.123.1.321. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–75. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–70. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- Peyrieras N, Strahle U, Rosa F. Conversion of zebrafish blastomeres to an endodermal fate by TGF-beta-related signaling. Curr Biol. 1998;8:783–6. doi: 10.1016/s0960-9822(98)70303-3. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–6. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Piotrowski T, Ahn DG, Schilling TF, Nair S, Ruvinsky I, Geisler R, Rauch GJ, Haffter P, Zon LI, Zhou Y, et al. The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development. 2003;130:5043–52. doi: 10.1242/dev.00704. [DOI] [PubMed] [Google Scholar]
- Pitera JE, Smith VV, Thorogood P, Milla PJ. Coordinated expression of 3′ hox genes during murine embryonal gut development: an enteric Hox code. Gastroenterology. 1999;117:1339–51. doi: 10.1016/s0016-5085(99)70284-2. [DOI] [PubMed] [Google Scholar]
- Poon KL, Richardson M, Lam CS, Khoo HE, Korzh V. Expression pattern of neuronal nitric oxide synthase in embryonic zebrafish. Gene Expr Patterns. 2003;3:463–6. doi: 10.1016/s1567-133x(03)00063-2. [DOI] [PubMed] [Google Scholar]
- Prince VE, Moens CB, Kimmel CB, Ho RK. Zebrafish hox genes: expression in the hindbrain region of wild-type and mutants of the segmentation gene, valentino. Development. 1998;125:393–406. doi: 10.1242/dev.125.3.393. [DOI] [PubMed] [Google Scholar]
- Puri P, Ohshiro K, Wester T. Hirschsprung’s disease: a search for etiology. Semin Pediatr Surg. 1998;7:140–7. doi: 10.1016/s1055-8586(98)70009-5. [DOI] [PubMed] [Google Scholar]
- Rachez C, Freedman LP. Mediator complexes and transcription. Curr Opin Cell Biol. 2001;13:274–80. doi: 10.1016/s0955-0674(00)00209-x. [DOI] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–98. [PubMed] [Google Scholar]
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–72. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- Ren S, Rollins BJ. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell. 2004;117:239–51. doi: 10.1016/s0092-8674(04)00300-9. [DOI] [PubMed] [Google Scholar]
- Rossi J, Luukko K, Poteryaev D, Laurikainen A, Sun YF, Laakso T, Eerikainen S, Tuominen R, Lakso M, Rauvala H, et al. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR alpha2, a functional neurturin receptor. Neuron. 1999;22:243–52. doi: 10.1016/s0896-6273(00)81086-7. [DOI] [PubMed] [Google Scholar]
- Roy S, Qiao T, Wolff C, Ingham PW. Hedgehog signaling pathway is essential for pancreas specification in the zebrafish embryo. Curr Biol. 2001;11:1358–63. doi: 10.1016/s0960-9822(01)00402-x. [DOI] [PubMed] [Google Scholar]
- Rubinstein AL, Lee D, Luo R, Henion PD, Halpern ME. Genes dependent on zebrafish cyclops function identified by AFLP differential gene expression screen. Genesis. 2000;26:86–97. doi: 10.1002/(sici)1526-968x(200001)26:1<86::aid-gene11>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development. 1994;120:483–494. doi: 10.1242/dev.120.3.483. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Piotrowski T, Grandel H, Brand M, Heisenberg CP, Jiang YJ, Beuchle D, Hammerschmidt M, Kane DA, Mullins MC, et al. Jaw and branchial arch mutants in zebrafish I: branchial arches. Development. 1996;123:329–44. doi: 10.1242/dev.123.1.329. [DOI] [PubMed] [Google Scholar]
- Schorpp M, Leicht M, Nold E, Hammerschmidt M, Haas-Assenbaum A, Wiest W, Boehm T. A zebrafish orthologue (whnb) of the mouse nude gene is expressed in the epithelial compartment of the embryonic thymic rudiment. Mech Dev. 2002;118:179–85. doi: 10.1016/s0925-4773(02)00241-1. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Shepherd IT, Beattie CE, Raible DW. Functional analysis of zebrafish GDNF. Dev Biol. 2001;231:420–35. doi: 10.1006/dbio.2000.0145. [DOI] [PubMed] [Google Scholar]
- Shepherd IT, Pietsch J, Elworthy S, Kelsh RN, Raible DW. Roles for GFR{alpha}1 receptors in zebrafish enteric nervous system development. Development. 2004;131:241–249. doi: 10.1242/dev.00912. [DOI] [PubMed] [Google Scholar]
- Shimoda N, Knapik EW, Ziniti J, Sim C, Yamada E, Kaplan S, Jackson D, de Sauvage F, Jacob H, Fishman MC. Zebrafish genetic map with 2000 microsatellite markers. Genomics. 1999;58:219–32. doi: 10.1006/geno.1999.5824. [DOI] [PubMed] [Google Scholar]
- Shirasawa S, Yunker AM, Roth KA, Brown GA, Horning S, Korsmeyer SJ. Enx (Hox11L1)-deficient mice develop myenteric neuronal hyperplasia and megacolon. Nat Med. 1997;3:646–50. doi: 10.1038/nm0697-646. [DOI] [PubMed] [Google Scholar]
- Southard Smith EM, Kos L, Pavan WJ. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet. 1998;18:60–4. doi: 10.1038/ng0198-60. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Cserjesi P, Olson EN. A subclass of bHLH proteins required for cardiac morphogenesis. Science. 1995;270:1995–9. doi: 10.1126/science.270.5244.1995. [DOI] [PubMed] [Google Scholar]
- Stickney HL, Schmutz J, Woods IG, Holtzer CC, Dickson MC, Kelly PD, Myers RM, Talbot WS. Rapid mapping of zebrafish mutations with SNPs and oligonucleotide microarrays. Genome Res. 2002;12:1929–34. doi: 10.1101/gr.777302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahle U, Blader P, Henrique D, Ingham PW. Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 1993;7:1436–46. doi: 10.1101/gad.7.7b.1436. [DOI] [PubMed] [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–80. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- Talbot WS, Schier AF. Positional cloning of mutated zebrafish genes. Methods Cell Biol. 1999;60:259–86. doi: 10.1016/s0091-679x(08)61905-6. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–15. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- Tudor M, Murray PJ, Onufryk C, Jaenisch R, Young RA. Ubiquitous expression and embryonic requirement for RNA polymerase II coactivator subunit Srb7 in mice. Genes Dev. 1999;13:2365–8. doi: 10.1101/gad.13.18.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene, OR: University of Oregon Press; 1993. [Google Scholar]
- Willett CE, Zapata AG, Hopkins N, Steiner LA. Expression of zebrafish rag genes during early development identifies the thymus. Dev Biol. 1997;182:331–41. doi: 10.1006/dbio.1996.8446. [DOI] [PubMed] [Google Scholar]
- Wu X, Howard MJ. Transcripts encoding HAND genes are differentially expressed and regulated by BMP4 and GDNF in developing avian gut. Gene Expr. 2002;10:279–93. doi: 10.3727/000000002783992361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Alldus G, Holder N, Wilkinson DG. Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hindbrain. Development. 1995;121:4005–16. doi: 10.1242/dev.121.12.4005. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Yanagisawa M, Kapur RP, Richardson JA, Williams SC, Clouthier DE, de Wit D, Emoto N, Hammer RE. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125:825–36. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]
- Yelick PC, Schilling TF. Molecular dissection of craniofacial development using zebrafish. Crit Rev Oral Biol Med. 2002;13:308–22. doi: 10.1177/154411130201300402. [DOI] [PubMed] [Google Scholar]
- Young HM, Bergner AJ, Anderson RB, Enomoto H, Milbrandt J, Newgreen DF, Whitington PM. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol. 2004;270:455–73. doi: 10.1016/j.ydbio.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Young HM, Newgreen D. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. Anat Rec. 2001;262:1–15. doi: 10.1002/1097-0185(20010101)262:1<1::AID-AR1006>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yuan CX, Ito M, Fondell JD, Fu ZY, Roeder RG. The TRAP220 component of a thyroid hormone receptor- associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc Natl Acad Sci U S A. 1998;95:7939–44. doi: 10.1073/pnas.95.14.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fondell JD. Identification of mouse TRAP100: a transcriptional coregulatory factor for thyroid hormone and vitamin D receptors. Mol Endocrinol. 1999;13:1130–40. doi: 10.1210/mend.13.7.0295. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Qi C, Jia Y, Nye JS, Rao MS, Reddy JK. Deletion of PBP/PPARBP, the gene for nuclear receptor coactivator peroxisome proliferator-activated receptor-binding protein, results in embryonic lethality. J Biol Chem. 2000;275:14779–82. doi: 10.1074/jbc.C000121200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.