Abstract
We have shown previously that the inhibitory control functions of the orbitofrontal cortex (OFC) are disrupted by serotonin, but not dopamine depletions. However, both dopamine and serotonin terminals and receptors are present within the OFC and thus the aim of the present study was to determine the differential contributions of these neurotransmitters to orbitofrontal function. OFC and dopamine are involved in the process by which neutral stimuli take on reinforcing properties, by virtue of their prior association with reward, and guide behavior. Thus, we compared the performance of marmosets with dopaminergic or serotoninergic OFC depletions on a test of conditioned reinforcement. To further our understanding of serotonin in behavioral flexibility, the effect of these depletions was also compared on the extinction of a visual discrimination. Monkeys with serotonin depletions of the OFC displayed stimulus-bound responding on both tests of conditioned reinforcement and discrimination extinction suggesting that orbitofrontal serotonin plays a specific role in preventing competing, task irrelevant, salient stimuli from biasing responding. In contrast, monkeys with dopamine depletion were insensitive to conditioned reinforcers and displayed persistent responding in the absence of reward in extinction, a pattern of deficits that may reflect basic deficits in the associative processing of reward.
Keywords: behavioral flexibility, compulsions, conditioned reinforcement, extinction, reward
Introduction
The monoamines play an important modulatory role in the cognitive functions of the prefrontal cortex (PFC). Goldman-Rakic and colleagues were the first to demonstrate the importance of dopamine (DA) in the spatial working memory functions of the dorsolateral PFC (Brozoski et al. 1979). Subsequently, work from our laboratory has specifically implicated DA in higher-order attentional selection in marmosets (Crofts et al. 2001), functions that are associated with ventrolateral regions of primate PFC (Dias et al. 1996). However, the sensitivity of functions of the lateral prefrontal regions to dopaminergic manipulation is in marked contrast to the insensitivity of orbitofrontal function to dopamine depletion, as measured by visual discrimination reversal performance (Clarke et al. 2007).
The converse pattern of deficits has been reported following serotonin (5-hydroxytryptamine [5-HT]) depletions of PFC. Such depletions cause a marked perseverative deficit in reversal learning (Clarke et al. 2005) and also in another test of orbitofrontal function (Wallis et al. 2001), detour reaching (Walker et al. 2006); but they have no effect on attentional set-shifting (Clarke et al. 2005). Thus, together, these findings highlight the differential contributions of dopamine and serotonin to the cognitive functions of the PFC.
However, dopaminergic terminals and receptors are found within orbitofrontal cortex (OFC) (Berger et al. 1990, 1991; Goldman-Rakic et al. 1990; Gebhard et al. 1995) and blockade of DA receptors within the OFC reduces the break point of rats responding on a progressive ratio schedule of reinforcement, a classic test of incentive motivation (Cetin et al. 2004). In addition, 6-hydroxydopamine-induced depletions of DA within the OFC impair responding for delayed reward (Kheramin et al. 2004). These findings accord well with the known role of the mesolimbic DA system in reward processing (Robbins and Everitt 1996 for review) and the importance of the OFC in representing stimulus–reward value (Izquierdo et al. 2004; Schoenbaum and Roesch 2005; McDannald et al. 2005). Therefore, the aim of the present study was to directly compare the roles of DA and 5-HT in the reward and inhibitory control functions of the OFC.
Previously, we have shown that OFC lesions disrupt the performance of marmosets on a test of conditioned reinforcement (Pears et al. 2003). Conditioned reinforcers are stimuli in the environment that, through their predictive association with primary reward, come to guide new learning in the absence of the primary reward (Robbins 1976; Taylor and Robbins 1984). Not only are processes of conditioned reinforcement dependent upon the OFC but they are also modulated by mesolimbic dopamine, both at the level of the nucleus accumbens (Taylor and Robbins 1984) as well as the amygdala (Di Ciano and Everitt 2004). Because successful performance on acquisition of a new response for conditioned reinforcement does not depend on inhibition of established response-reward associations, we hypothesized that DA, rather than 5-HT depletions of the OFC would disrupt responding for conditioned reinforcement.
To investigate further the involvement of 5-HT in the behavioral flexibility functions of the OFC we also studied the effects of DA and 5-HT depletions of the OFC on extinction of a visual discrimination. We have argued that the perseverative deficit following 5-HT depletion of the OFC on discrimination reversal learning may reflect insensitivity to reward loss in which case such lesions would also prolong responding during extinction (Clarke et al. 2005, 2007). Alternatively, it may reflect a specific loss of inhibitory control at the level of concrete stimuli, that is, “stimulus-bound behavior.” A “one-stimulus” extinction test would not be able to distinguish these 2 hypotheses very easily but they could be distinguished using a 2-stimulus discrimination extinction test. Thus, we hypothesized that if monkeys with 5-HT depletion of the OFC are insensitive to reward loss, they would take longer than controls to extinguish their responding overall. However, if their responding becomes “stimulus bound” but is still sensitive to reward loss, then, responding to the previously rewarded stimulus would be greater than controls, but the overall rate of response extinction would be equivalent to controls.
Materials and Methods
Subjects
Twelve experimentally naïve common marmosets (Callithrix jacchus), 7 males and 5 females, housed in pairs. Five days per week they were fed 20 g of MP.E1 primate diet (Special Diet Services, Essex, UK) plus 2 pieces of carrot, and had simultaneous access to water for 2 h. At the weekend they received fruit, egg sandwiches, Marmoset Jelly, malt loaf and Rusk and had ad libitum access to water. All procedures were performed in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986.
Surgical Procedures
Selective serotonergic depletions of OFC were made using 5,7-dihydroxytryptamin (5,7-DHT) (9.92 mM; Fluka BioChemika, Sigma, Poole, UK) in saline/0.1% L-ascorbic acid. To protect noradrenaline (NA) and dopamine innervations, the solution also contained the noradrenaline uptake blocker nisoxetine hydrochloride (50 mM; Sigma) and dopamine uptake blocker GBR-12909 dihydrochloride (Sigma; 2.0 mM). The selective dopaminergic depletion of OFC was made using the neurotoxin-hydroxydopamine (6-OHDA) (4 μg/μL) in saline/0.01% ascorbic acid. To enhance the efficacy of the toxin, monoamine oxidase inhibitor Pargyline (Sigma; 50 mg/kg intraperitoneally) was administered peripherally twenty minutes prior to anesthesia.
Monkeys were premedicated with ketamine hydrochloride (0.05 mL of a 100 mg/mL solution, i.m.; Pfizer, Kent, UK), anesthetized with Saffan (aplphaxalone 0.9% w/v and alphadolone acetate 0.3% w/v, 0.4 mL, i.m.; Schering-Plough, Kenilworth, NJ), and given a 24-h prophylactic analgesic (Rimadyl; 0.03 mL of 50 mg/mL carprofen, s.c.; Pfizer), before being placed in a stereotaxic frame especially modified for the marmoset (David Kopf Instruments, Tujunga, CA). Toxin solution was injected at a rate of 0.04 μL/20 s to 10 sites bilaterally using a 30-gauge cannula attached to a 2-μL syringe (Hamilton Bonaduz AG, Bonaduz, Switzerland). See Table 1. For surgical details see Roberts et al. (1994) and Clarke et al. (2004).
Table 1.
Lesion parameters including the stereotaxic coordinates of each injection (based on the interaural plane) and injection volumes
| Coordinates (mm) |
Volume injected (μL) | ||
| AP | LM | V | |
| 16.75 | ±2.5 | 0.7a | 0.40 |
| ±3.5 | 0.7a | 0.40 | |
| 17.75 | ±2.0 | 0.7a | 0.40 |
| ±3.0 | 0.7a | 0.40 | |
| 18.50 | ±2.0 | 0.7a | 0.60 |
Indicates mm up from base of brain.
Apparatus
Behavioral testing took place in a specially designed automated test apparatus situated in a sound attenuated chamber. A detailed account has been given previously (Roberts et al. 1988). Briefly, the marmoset is positioned in front of a touch sensitive video display unit (VDU). Banana milkshake, which serves as a reward in the experiment, could be delivered through a licking spout located immediately in front of the VDU. Initially marmosets were trained to respond to visual stimuli on the touch screen. A detailed account of preliminary training has been given previously (Roberts et al. 1988, 1992). Training differed here to the extent that no explicit auditory conditioned stimulus (CS; i.e. tone) was associated with the delivery of banana milkshake.
Behavioral Procedures
All tests were written in Arachnid control language (Paul Fray, Ltd, Cambridge, UK).
Visual Discrimination Training
Marmosets were trained on a series of 2 novel simple discriminations. Discriminanda consisted of 2 white shapes (32 mm wide × 50 mm high) presented simultaneously on a touch screen). A response to the correct stimulus resulted in disappearance of both stimuli and delivery of 10-s reward, followed by a 3-s inter-trial interval (ITI) (see Fig. 1a). No explicit CS accompanied reinforcement. A response to the incorrect stimulus led to disappearance of both stimuli and a 13-s ITI. A session terminated after 30 trials or 20 min, whichever occurred sooner. Training on each simple discrimination continued until criterion of at least 90% correct (27 of 30) was achieved.
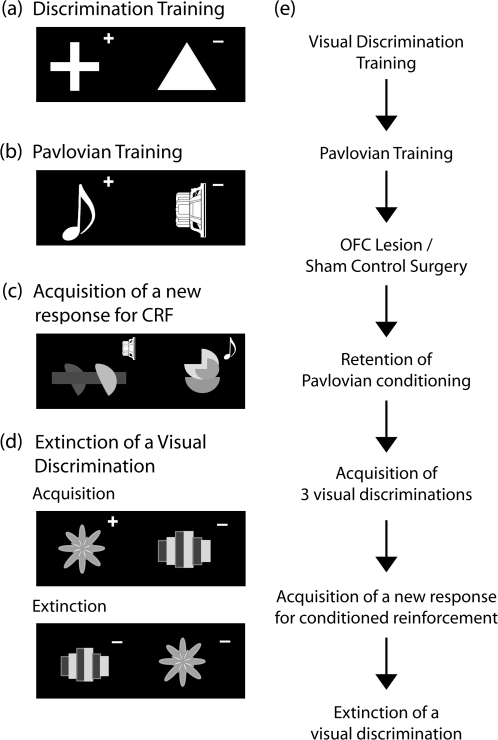
Figure 1.
Task design. (a–d) Examples of the stimulus exemplars and the reward contingencies used for the various stages of the experiment. The real stimuli for the conditioned reinforcement (CRF) task and extinction were multicolored. The rewarded and unrewarded stimuli on each discrimination is indicated by the “+” and “−,” respectively. In (c) the “speaker” and “musical quaver” symbols represent, respectively, the white noise and tone (Pavlovian conditioned stimuli: CS+/CS−) that are presented upon response to the visual stimuli. The animals receive no primary reinforcement for responding to either visual stimulus in the CRF task. (e) A flow diagram depicting the stages of training both pre- and postsurgery.
Pavlovian Training
Having achieved criterion performance on 2 simple visual discriminations marmosets underwent Pavlovian discrimination training (Fig. 1b). Two sounds, a tone and white noise, were presented for 10 s on a double alternation schedule. In a simple Pavlovian procedure one sound (CS+) signaled availability of banana milkshake whereas the other sound was never paired with reward (CS−). The allocation of sounds to CS+ and CS− was counterbalanced across subjects. Sessions consisted of 40 trials, 20 of each stimulus, on a variable time (VT) 24-s schedule. Marmosets were maintained on this schedule until they attained a relatively stringent level of criterion performance; approaching the licker on >80% of CS+ presentations and <20% of CS− presentations on 2 consecutive sessions. A stringent criterion was used to ensure a strong association had developed between the CS and reward.
Having achieved criterion performance subjects then underwent surgery. Four animals were assigned to each lesion group. Postsurgery, they were re-tested on the Pavlovian task, received a further 3 novel visual discriminations and were then tested on the conditioned reinforcement task.
Acquisition of a New Response for Conditioned Reinforcement
Two multicolored stimuli (A vs. B) were presented on the left and right of the touch screen. A response to one of the stimuli (designated S+) resulted in a 1-s presentation of the CS+, whereas a response to the other stimulus (designated S−) resulted in a 1-s presentation of the CS− (Fig. 1c). This was always followed by a 3-s ITI. Designation of S+ and S− was counterbalanced across groups. A schedule of Pavlovian pairings was superimposed on the session with a VT of 40 s. Once this VT period had elapsed, the visual stimuli were cleared from the touch screen, and after a 5-s delay, the CS+ and CS− was presented for 10 s. The CS+ presentation was concurrent with delivery of banana milkshake, whereas the CS− was never accompanied by reward. These pairings were presented according to a double alternation schedule as described for Pavlovian training. If a response was in progress when the VT elapsed, the onset of the Pavlovian pairing was delayed until the beginning of the ITI that followed the response. A 5-s ITI was always imposed immediately before a Pavlovian pairing to reduce the likelihood of reward being erroneously attributed to a response. This superimposed schedule leading to delivery of primary reward was used to prevent the marmosets from extinguishing responding before they showed discrimination of the 2 stimuli. A session ended after 20 min or 50 responses, whichever occurred first. All animals received 3 sessions.
Extinction of a Visual Discrimination
In the final stage of the experiment monkeys were presented with 2 novel multicolored stimuli. Response to S+ resulted in disappearance of the S− and 5-s presentation of the CS+ (the CS was the same as in the previous experiment, a tone for half the subjects and white noise for the others) accompanied by simultaneous delivery of banana milkshake. Responding to S− resulted in both stimuli disappearing from the screen and the house lights extinguishing for 5 s (see Fig. 1d). The ITI was 3 s. Designation of stimuli to S+ and S− was counterbalanced across subjects. Following attainment of criterion performance, that is, 90% correct on a single session, on the following session the response was extinguished. Thus, responding to either stimulus resulted in both stimuli disappearing and the house light going out for 5 s. This was repeated across sessions, with a session ending after 30 responses, when 20 min had elapsed or when no responses had been made for 5 consecutive minutes. An animal was judged to have extinguished responding (extinction criterion) when they made fewer than 10 responses on 2 sessions.
For summary of the details of the experimental design see Figure 1e.
Behavioral Measures
Pavlovian Training
The number of sessions required before attaining criterion performance pre- and postoperatively was calculated for each subject.
Visual Discrimination Training
The total number of errors made before reaching criterion performance was calculated for each subject, for each discrimination. Errors committed on the criterion day were not included in this measure.
Acquisition of a New Response for Conditioned Reinforcement
The proportion of responses made to S+ versus S− and to the individual stimuli (“A” vs. “B”) was calculated for each subject on each of the 3 test days.
Extinction
The total number of trials performed before responding was extinguished was calculated for each subject, excluding the 2 extinction criterion days. In the case of 3 animals (from the DA-depleted group) that failed to extinguish their responding after 40 sessions, their testing was discontinued and the total number of trials performed up until that point was included in the subsequent analyses. In addition, the type of responses that were made during extinction were classified as perseverative (where responding to the previously rewarded stimulus was significantly above chance) or chance (where responding was equally distributed to the 2 stimuli) using signal detection theory based on a discriminability or d′ value that was significant at α = 0.05 each tail or 0.1 2-tailed (for details see Clarke et al. 2005).
Lesion Assessment
Post mortem tissue analysis using reversed phase high-performance liquid chromatography (HPLC) assessed the specificity and extent of the selective 5-HT and DA depletions of the OFC. The exact methods used have been described in detail previously (Clarke et al. 2005). Briefly, 8- to 13-months postsurgery monkeys were euthanized, their brains removed and dissected on ice. Tissue samples were homogenized in 200 mL of 0.2 M perchloric acid and centrifuged at 4620 g for 20 min at 4 °C. The supernatant was analyzed using reversed phase HPLC and electrochemical detection. The signal was integrated using Chromeleon software (version 6.20; Dionex, Sunnyvale, CA). The system was calibrated using standards containing known amounts of 5-HT, NA and DA.
Statistics
Data were analyzed using SPSS version 12. One-way and 2-way ANOVAs were used and described in detail in the results section. Where raw data did not display heterogeneity of variance, it was transformed appropriately (see Howell 1997). Post hoc comparisons were made using paired samples t-tests.
Results
Lesion Assessment
Post Mortem Monoamine Depletions following 5,7-DHT and 6-OHDA Lesions of the OFC
Tissue analysis 8- to 13-months postsurgery, indicated substantial depletions of 5-HT in the OFC of 5,7-DHT lesioned monkeys, and of DA in the OFC of 6-OHDA lesioned monkeys (Table 2). Noradrenaline levels were unchanged. Statistical analysis, using a one-way ANOVA, with Sidak correction for multiple comparisons, revealed significant main effects of group for 5-HT (F2,9 = 23.17, P < 0.002) and DA (F2,9 = 28.3; P < 0.001) levels in the OFC. These differences were further analyzed by performing 3 uncorrected t-tests. These revealed 5-HT levels to be significantly lower in the OFC of 5,7-DHT lesioned monkeys than in 6-OHDA lesioned (t6 = 4.49; P < 0.004) or sham lesioned controls (t6 = 6.68; P < 0.001). Levels in the latter 2 groups did not differ significantly from each other (t6 = 2.35; P = 0.06 NS). DA levels were significantly lower in the OFC of 6-OHDA lesioned monkeys than either 5,7-DHT lesioned (t6 = 10.2; P < 0.0001) or sham lesioned control monkeys (t6 = 4.93; P < 0.003). Levels in the latter 2 groups did not differ significantly from each other (t6 = 1.4; P = 0.2 NS).
Table 2.
Percentage depletions of dopamine, serotonin, and noradrenaline in a range of cortical and subcortical brain regions in monkeys that had received either a dopamine lesion or a serotonin lesion of the OFC
| Region | 5-HT lesion |
Dopamine lesion |
||||
| % Depletions |
% Depletions |
|||||
| 5-HT | Dopamine | Noradrenaline | Dopamine | 5-HT | Noradrenaline | |
| OFC | 54.7a (4.1) | −15.4 (3.7) | 3.9 (4.4) | 55.7a (4.3) | 18.6 (5.2) | 35.8 (4.5) |
| Lateral PFC | 53.8 (3.5) | −4.9 (5.8) | −9.4 (19) | 17.6 (24.4) | −15.3 (29.8) | 14.5 (20.9) |
| Dorsal PFC | 7.6 (13.1) | −1.5 (10.3) | 0.006 (8.2) | 5.3 (8.7) | 2.4 (7) | 5 (8.6) |
| PM/M | 6.6 (10.5) | −9.7 (5.2) | −0.6 (7.2) | 20.7 (12.8) | 15.7 (5.8) | 13 (7.5) |
| Medial PFC | 19.8 (11.1) | 9.7 (6.9) | −0.6 (10.3) | 50.5 (3.4) | 3.8 (8.7) | 25.6 (5.8) |
| Anterior cingulate | 23.8 (7.5) | −13.3 (18.6) | 0.07 (10.4) | −2.9 (11.9) | 20.6 (6.9) | 11.2 (6.2) |
| Mid-cingulate | 12.9 (8.8) | −18.5 (10.1) | −1.9 (7.9) | 5.6 (8.1) | −4.6 (10.6) | 3.4 (6.1) |
| FR2 | 15.8 (7.5) | −74.7 (28.3) | −8 (9.9) | −19.8 (13.7) | 7.6 (7.8) | −4.4 (6.5) |
| D.L. head of caudate | 19.3 (6.9) | 3.7(7.1) | −3.4 (11.4) | 24.8 (9.7) | 35.7 (9.4) | −10.7 (29.4) |
| V.M. head of caudate | −24.7 (11.7) | 16.2 (8) | −92.3 (71.6) | 13 (3.5) | −32 (11.6) | −4.1 (20) |
| Mid-caudate | −30.2 (13.4) | 22.7 (12.9) | −35 (40.7) | 9.1 (5.1) | −23 (11.7) | 29.1 (13.6) |
| Anterior putamen | −3.5 (10.9) | 6.7 (4.1) | −16.6 (16.3) | 11.3 (5.7) | 4.6 (5.1) | −25.5 (24.7) |
| Mid-putamen | 12.9 (5.9) | 18.9 (23.6) | −14.2 (30.4) | 15.8 (17.3) | 9.7 (10.4) | −3.4 (27.8) |
| Nucleus accumbens | −2.7 (18.2) | −88 (61.6) | 21 (13.8) | −11.2 (55.7) | 6.7 (11.7) | 5.8 (12.2) |
| Amygdala | −10.2 (20.8) | 15.5 (7.3) | −27.8 (34.5) | 18.7 (11.9) | −25.5 (20.1) | −35 (29.6) |
Numbers in brackets are standard errors of the mean. Levels significantly lower than controls, 5-HT, P < 0.01; dopamine, P < 0.0001.
There were no significant depletions of any neurotransmitter in adjacent cortical or underlying subcortical regions (see Table 2). In the 5,7-DHT lesioned monkeys there was a mean 53.8% depletion in the lateral PFC, an area in which we have previously reported significant depletions using this lesion methodology (Walker et al. 2006; Clarke et al. 2007). However in the present study this depletion was not significant due to large variation between animals (F2,9 = 3.3; P = 0.7). Similarly, in the 6-OHDA lesioned group the 50.5% depletion in the medial PFC was not statistically significant (F2,9 = 1.4; P = 1), due to variability between individuals.
Thus we successfully produced chemically and anatomically specific lesions of the OFC. The levels of depletion observed here are consistent with the levels reported in previous studies at this postsurgery period (mean 10-months postsurgery) (Clarke et al. 2007). There is likely to have been some recovery by this time point because depletions at 3-months postsurgery are approximately 70–80% in the OFC for both 5-HT and DA lesions, declining to between 60% and 70% at 6-months postsurgery (Walker et al. 2006; Clarke et al. 2007). On average, behavioral testing for the conditioned reinforcement test reported here was completed 4.5-months postsurgery and that for the extinction test commenced 8-months postsurgery.
Behavioral Assessment
Preoperative Performance
Preoperatively there was no difference between groups in number of errors made before attaining criterion performance on either visual discrimination (D1: F = 1.139, D2: F < 1, see Table 3). Additionally there was no difference between groups in number of sessions required to attain criterion performance on the Pavlovian discrimination (F < 1). The total number of Pavlovian sessions for each group were 55 ± 8.7 (controls), 64.8 ± 3.8 (OFC DA depletions) and 47 ± 12.8 (OFC 5-HT depletions).
Table 3.
Mean number of errors (±SEM) made by each of the 3 lesion groups during acquisition of 2 presurgery and 3 postsurgery simple visual discriminations
| Group | Errors (±SEM) |
||||
| Preoperative |
Postoperative |
||||
| D1 | D2 | D3 | D4 | D5 | |
| Controls | 87.8 (29.4) | 59.8 (29.3) | 138.4 (37.8) | 56 (30.2) | 8.75 (3.6) |
| OFC DA | 65.3 (17.2) | 59.3 (11.3) | 150 (23) | 49.3 (8.7) | 18.3 (9.2) |
| OFC 5-HT | 51.8 (2.2) | 31.5 (17.4) | 115 (20.7) | 50.3 (22.9) | 16.5 (6.5) |
Note: There was no significant differences between groups.
Postoperative Performance
Postoperatively there was no significant difference in the number of sessions required to reattain criterion performance of the Pavlovian auditory discrimination (controls: 10.5 ± 3.0; OFC DA depletions: 9.8 ± 3.1; OFC 5-HT depletions: 6.8 ± 1.5; F < 1). In addition there were no differences in ability to acquire 3 novel visual discriminations (F values < 1, see Table 3).
Acquisition of a New Response for Conditioned Reinforcement
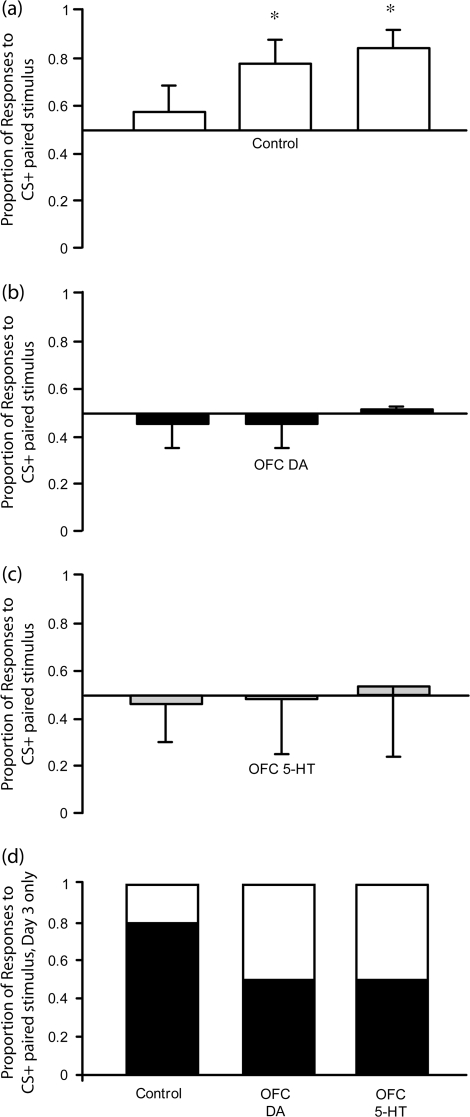
Figure 2a indicates that, as hypothesized, control monkeys showed increasingly biased responding to the visual stimulus paired with the CS+ over the 3 test sessions. In contrast, neither DA nor 5-HT depleted monkeys showed this same response bias, and on average, responded equally to both stimuli (Figs 2b and 2c). Statistical analysis using a repeated measures ANOVA on the arcsine transformed data, group (3) x test days (3), bears out this observation. There was a significant group by day interaction (F2,9 = 4.95, P < 0.04). Simple main effects analysis revealed that the proportion of responses to the stimulus paired with the CS+ (S+) in controls differed significantly across days (F1,3 = 13.27, P < 0.04). However, the responses of the 2 lesion groups did not (DA: F1,3 = 0.35, P = 0.6, 5-HT: F1,3 = 0.12, P = 0.8). Post hoc analysis by paired samples t-tests showed that the control monkeys responded significantly more to the stimulus paired with the CS+ on days 2 and 3 than they did on the first test day (t = 6.49, P < 0.01 and t = 3.64, P < 0.05). Figure 2d shows the overall proportion of responses to the stimulus paired with the CS+ versus CS− on the third test day. Although about 80% of control responses were to the stimulus paired with the CS+, the 2 lesion groups responded equally to both stimuli.
Figure 2.
Mean proportion of responses made to the stimulus paired with the CS+ on each of the 3 test days (±SEM) for (a) control (n = 4), (b) DA-depleted (n = 4), and (c) 5-HT-depleted (n = 4) groups. Mean distribution of responses to stimulus paired with CS+ (black portion) and CS− (white portion) on the final test day is shown in (d). *Control monkeys responded significantly more to the stimulus paired with the CS+ on days 2 and 3 compared with day 1, P < 0.05 whilst 5-HT and DA-depleted monkeys did not.
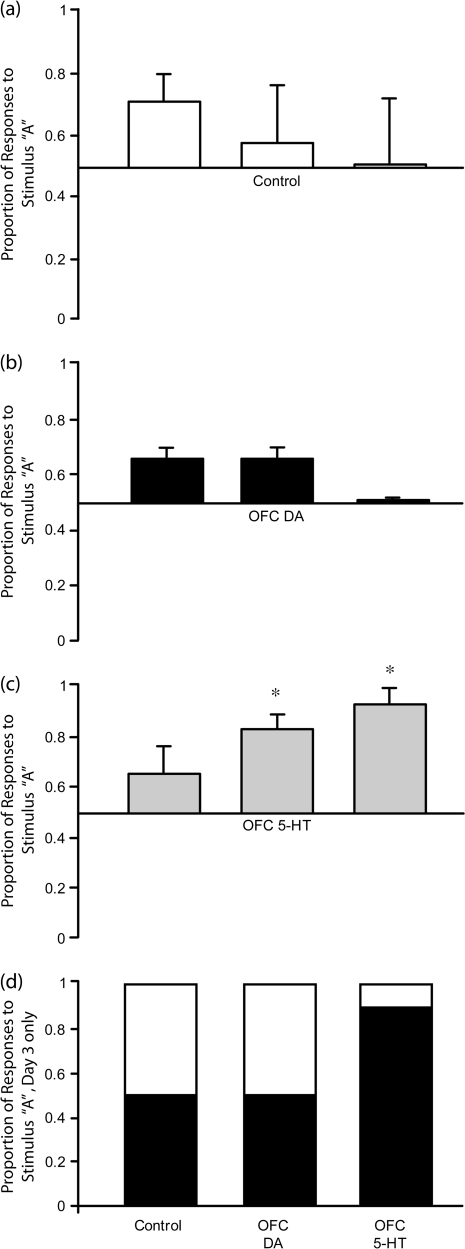
Although the responding of 5-HT depleted monkeys was not biased towards the stimulus paired with the CS+, it was not random. Instead, they chose to respond to a particular stimulus, for example, “A,” independent of its association with the CS+. In Figure 3 it can be seen that on the first test day, all groups showed an initial bias of responding towards stimulus “A,” regardless of whether it was paired with the CS+ or not. However, whereas controls (Fig. 3a) and DA-depleted animals (Fig. 3b) lost this bias over the subsequent 2 days, 5-HT depleted animals did not. Instead, this initial unconditioned stimulus bias was enhanced progressively across the 3 test days (Fig. 3c). A repeated measures 2-way ANOVA of the arcsine transformed data, group (3) x test days (3), revealed a significant group by day interaction (F2,9 = 6.1, P < 0.02). Simple main effects analysis revealed that the proportion of responses made by controls to stimulus “A” did not differ significantly across days (F < 1). However the responses of the 2 lesion groups did [dopamine F1,3 = 25.81, P < 0.05; 5-HT F1,3 = 33.98, P = 0.01]. Post hoc analysis by paired samples t-tests showed that the DA depleted monkeys responded significantly more to stimulus A on days 1 and 2 than day 3 (t = 5.08, P < 0.05 and t = 5.08, P < 0.05), indicating that these monkeys overcame their initial stimulus bias by the third day. 5-HT depleted monkeys in contrast, responded significantly more to stimulus “A” on days 2 and 3 than day 1 (t = 5.2, P < 0.05 and t = 5.8, P = 0.01), indicating that this group became increasingly more stimulus biased over days. Figure 3d shows the proportion of responses to stimulus “A” versus stimulus “B” on the third test day. Unlike control and DA-depleted groups, the 5-HT depleted group made approximately 90% of their responses to stimulus “A,” despite stimulus “A” only being paired with CS+ for half the group and not for the other half. In the control group, half the animals responded to stimulus “A” because it was associated with the CS+ and the other half responded to stimulus “B” because it was associated with the CS+. In the DA-depleted group all monkeys responded fairly equally to both stimuli despite their CS associations.
Figure 3.
Mean proportion of responses made to stimulus “A” versus stimulus “B” on each of the 3 test days (±SEM) for (a) control (n = 4), (b) DA-depleted (n = 4), and (c) 5-HT-depleted (n = 4) groups. Mean distribution of responses to stimulus “A” (black portion) and stimulus “B” (white portion) on the final test day is shown in (d). 5-HT depleted monkeys responded more to stimulus “A” than stimulus “B,” DA-depleted and control groups did not. *5-HT depleted monkeys responded more to stimulus “A” on days 2 and 3 than day 1, P = 0.01.
Thus, an arbitrary stimulus bias, to what is apparently the most visually salient stimulus was overcome in the control and DA-depleted groups, but came to dominate responding in the 5-HT depleted group.
Motoric and Motivational Factors
A comparison of the total numbers of responses that each group made on the 3 test days (Mean data; Table 4), revealed no significant differences between groups (ANOVA: F2,9 = 0.55, P = 0.6), indicating that there were no apparent motivational or motoric effects of the lesions. In addition, there was no difference in their overall responding to the licker upon presentation of the CS+ and CS− in the overlying Pavlovian schedule (Table 5). A repeated measures ANOVA on the proportion of trials in which all 3 groups approached the licker revealed no significant differences between groups (F2,9 = 0.8, P = 0.48) or across days (F1,9 = 1.3, P = 0.28) though there was a significant effect of CS (F1,9 = 66.9, P < 0.01), thus showing depleted monkeys were able to discriminate the auditory stimuli as well as control monkeys. There were no significant interactions.
Table 4.
Mean number of responses (±SEM) made by each of the 3 lesion groups to the stimuli paired with CS+ and CS− during each of the 3 test sessions
| Group | Responses (±SEM) |
|||||
| Session 1 |
Session 2 |
Session 3 |
||||
| CS+ | CS− | CS+ | CS− | CS+ | CS− | |
| Controls | 25 (3.7) | 16.8 (8) | 27.5 (1.2) | 7.8 (4) | 20.3 (4.7) | 4.3 (2.1) |
| OFC DA | 16.5 (2.7) | 20 (6.5) | 16.5 (2.9) | 22.3 (6.5) | 16.3 (6.4) | 16.3 (6.4) |
| OFC 5-HT | 16.5 (6.8) | 16.3 (2.2) | 19 (9.1) | 16.8 (5.9) | 12.3 (7.5) | 7.5 (3.9) |
Table 5.
Mean % of Pavlovian trials (±SEM) on which each of the 3 lesion groups approached the licker on presentation of CS+ and CS− during each of the 3 test sessions
| Group | % Responses (±SEM) |
|||||
| Session 1 |
Session 2 |
Session 3 |
||||
| CS+ | CS− | CS+ | CS− | CS+ | CS− | |
| Controls | 81 (19.4) | 29.6 (3.1) | 90.4 (11.1) | 19.9 (9.1) | 88.9 (7.4) | 34.8 (11.8) |
| OFC DA | 90.9 (6.3) | 20 (6.5) | 93.9 (4.6) | 17.9 (15.6) | 98 (2.3) | 18.1 (10.7) |
| OFC 5-HT | 96.2 (2.6) | 22.1 (13.1) | 87.3 (11.8) | 27.3 (12.1) | 98.1 (2.2) | 35.2 (19.1) |
Extinction of a Visual Discrimination
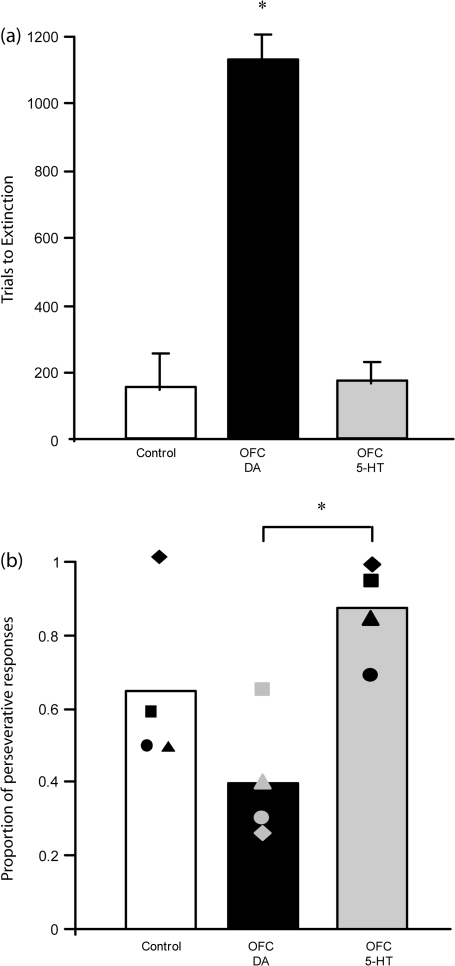
Figure 4a shows that DA-depleted monkeys continued to respond for significantly longer than control or 5-HT depleted monkeys following removal of the primary reward. One-way ANOVA revealed that there was a significant difference between groups in the number of trials before responding was extinguished (F2,9 = 47.98, P < 0.01). Post hoc analysis using Fisher's Least Significance Difference (LSD) showed that DA-depleted monkeys responded for significantly more trials than either control or 5-HT depleted monkeys (P's < 0.01), with controls and 5-HT depleted monkeys not differing from each other. However, although 5-HT depleted monkeys extinguished as quickly as controls, their overall pattern of responding differed. Figure 4b shows the mean proportion of trials completed by each group in which responding had been perseverative. 5-HT depleted monkeys tended to show a far higher proportion of perseverative responses than DA depleted or control monkeys. A one-way ANOVA revealed that there was a significant difference between groups (F2,9 = 6.29, P < 0.02). Post hoc analysis using Tukey's LSD showed that the difference between 5-HT and DA-depleted groups was significantly different (P < 0.01) but the difference between 5-HT depleted and control monkeys was not (P = 0.1). The latter was due primarily to one control monkey extinguishing responding very rapidly, before sampling the previously unrewarded stimulus.
Figure 4.
(a) Mean trials to extinction (±SEM) for control (n = 4), DA-depleted (n = 4), and 5-HT (n = 4) depleted monkeys. *DA-depleted monkeys took significantly more trials to extinguish responding than control or 5-HT depleted monkeys, P < 0.01. (b) Proportion of responses in extinction which were classified as perseverative (individual data represented by the filled shapes). *5-HT depleted monkeys made a significantly higher proportion of perseverative responses to the previously rewarded stimulus than DA-depleted monkeys, P < 0.01.
Discussion
Depletions of 5-HT and DA in the OFC produced dissociable effects on reward guided behavior. Monkeys with 5-HT depletions of the OFC displayed stimulus-bound responding on both tests of conditioned reinforcement and discrimination extinction. In the test of conditioned reinforcement, 5-HT depleted animals appeared unable to overcome initial visual stimulus preferences and thus, unlike controls, failed to develop a response bias to the visual stimulus paired with the conditioned reinforcer. Similarly, unlike controls, the responding of 5-HT depleted monkeys remained biased towards the previously rewarded stimulus on extinction of a visual discrimination, despite extinguishing as rapidly as controls overall. In contrast, DA-depleted monkeys did not display such stimulus-bound behavior. They did take considerably more trials than both controls and 5-HT depleted monkeys to extinguish their responding, with 3 out of 4 DA-depleted animals failing to extinguish in 40 sessions. However, unlike 5-HT depleted monkeys, their responding was not biased towards the previously rewarded stimulus. Moreover, they were also able to overcome initial stimulus preferences in the conditioned reinforcement study but, unlike controls, failed to develop a response bias towards the visual stimulus paired with the conditioned reinforcer.
Dopamine
An important feature of PFC function is the ability to manipulate items held in STM and use them to guide future actions. Goldman-Rakic and colleagues have shown that DA in the PFC is critical for this process (Goldman-Rakic 1987). Dopamine D1 receptor stimulation is required to maintain representations in delay-related firing in dorsolateral prefrontal neurons of monkeys performing a spatial delayed response task (Sawaguchi and Goldman-Rakic 1991; Williams and Goldman-Rakic 1995). Such findings, combined with the known actions of dopamine on synaptic transmission, have led computational models of PFC function to suggest that DA is important for stabilizing these representations and thus ensuring attention is focused on task relevant information and less susceptible to distraction (Durstewitz et al. 2000). Such models predict that hypofunction of PFC DA will result in unstable representations open to interference by distractors (Seamans and Yang 2004) consistent with recent electrophysiological findings from (Vijayraghavan et al. 2007). Moreover, behavioral studies from our own group have shown that DA depletion in the PFC disrupts attentional selection and makes performance more vulnerable to distraction (Roberts et al. 1994; Crofts et al. 2001).
The above findings relate to cognitive functions associated with lateral regions of PFC. In contrast, little is known about the specific contribution of DA to OFC function (Cetin et al. 2004; Kheramin et al. 2004). It has been suggested that the anatomical subregions of the PFC are distinguished by the type of information that they process (Goldman-Rakic 1995) and the OFC is thought to be critical for representing reward value (Tremblay and Schultz 1999; Izquierdo et al. 2004; McDannald et al. 2005; Schoenbaum and Roesch 2005). Neurones within the OFC not only signal predicted outcome value but compute the relative values of selected and unselected outcomes (Montague and Berns 2002; Wallis 2007). Animals with damage to the OFC are impaired at altering responding following changes in outcome value (Gallagher et al. 1999; Izquierdo and Murray 2005). Furthermore, Ostlund and Balleine (2007) argue that the OFC is necessary for an animal to learn and update information about the predictive relationship between environmental cues and their outcomes. In our conditioned reinforcement paradigm, performance depends on the monkey updating their existing knowledge about the association between a CS and a rewarding outcome with newly acquired knowledge about the association of a visual stimulus with the CS, in order to guide responding. The failure of DA-depleted monkeys to do this successfully may reflect the instability of this internal representation of the CS-outcome relationship in the absence of DA. An explanation of these findings in terms of DA's proposed role in reward prediction error (Schultz 2001; Daw 2007) is less plausible as there is no evidence to date that phasic dopamine, which is hypothesized to carry this signal, can be processed sufficiently rapidly by the PFC (Seamans and Yang 2004).
The importance of DA within the OFC in behavior guided by conditioned reinforcers mirrors its importance in conditioned reinforcement-driven behavior at the level of the amygdala. Like the OFC, the amygdala is important for conditioned reinforcement-driven behavior (Cador et al. 1989; Burns et al. 1993; Parkinson et al. 2001) and recently, infusions of a DA receptor antagonist (alpha-fluphenthixol) into the BLA was shown, dose dependently, to decrease responding for cocaine under a second order schedule of reinforcement, in which responding is under the control of a CS or conditioned reinforcer (Di Ciano and Everitt 2004). Although the mechanism by which conditioned reinforcers come to guide responding may differ between the BLA and OFC (personal communication, G. Schoenbaum) DA would appear to be important in both structures.
Besides abolishing discriminative behavior controlled by conditioned reinforcers, DA depletions of the OFC markedly prolonged instrumental responding in the absence of reward. A number of studies have shown that both monkeys and humans with OFC damage take longer to extinguish an instrumental response than intact controls (Butter et al. 1963; Rolls et al. 1994; Izquierdo and Murray 2005). The findings from the present study suggest that DA within the OFC is critical for this process. In extinction of a visual discrimination, the contingent relationship between stimulus and reward has been degraded, and responding should be adjusted accordingly. However, following DA depletions of the OFC monkeys failed to show such adjustment and continued to respond to the visual stimuli in the absence of reward. Indeed, 3 of the 4 DA-depleted monkeys showed no evidence of a decline in responding after 40 sessions. This persistent responding was not due to a failure to inhibit a previously rewarded response as DA-depleted animals stopped displaying perseverative responding to the previously rewarded stimulus as rapidly as controls. Instead, they continued to respond randomly to one or other of the visual stimuli (including the stimulus that had never been associated with reward), session upon session. Such overresponding is most likely to be under contextual, rather than specific stimulus, control.
A similar increase in responding during extinction of an instrumental response has been shown in rats following the peripheral administration of the D2 receptor agonist, quinpirole (Kurylo and Tanguay 2003). Thus, both an enhancement and reduction in DA function apparently prolongs unrewarded responding. Such effects may be explained by consideration of the interaction between mesocortical and mesostriatal DA. Studies have shown that blockade or depletion of DA in the PFC in both rats and marmosets result in increased DA function in the striatum (Mitchell and Gratton 1992; Roberts et al. 1994; King et al. 1997); although the effects of selective orbitofrontal DA depletions on striatal DA are as yet, unknown. This upregulation is hypothesized to result from the removal of the inhibitory influence of PFC DA on the glutamate containing projection neurons of the PFC. The resulting excitatory glutamatergic effect on the striatum is thought to lead to DA release (Pycock et al. 1980), accounting for the behavioral disinhibition observed. In addition, activation of D2 receptors by tonic increases in DA at the level of the striatum has been shown to attenuate prefrontal inputs (Grace et al. 2007). Thus, a loss of orbitofrontal DA may result not only in weakened representations of expected reward within the OFC but also a loss of prefrontal control at the level of the striatum, as a result of increased tonic DA stimulation of D2 receptors, one consequence of which may be a potentiation of habitual responding. The transition between goal directed to habitual responding has been proposed to reflect a transfer from prefrontal to striatal control of behavior and lesions of the dorsolateral striatum disrupt habit formation (Yin et al. 2004) as do lesions of the nigrostriatal DA pathway (Faure et al. 2005). Thus disruption of OFC function may expedite the transition from goal directed to habitual behavior, specifically modulated by DA, accounting for the overresponding we report here in OFC DA-depleted monkeys following extinction of a visual discrimination.
5-Hydroxytryptamine
Previous work in our laboratory has identified the importance of 5-HT in the capacity of the OFC to promote behavioral flexibility. 5-HT depletions of the OFC produce perseverative responding during serial reversal learning, acquisition of a detour-reaching task (Clarke et al. 2004, 2005, 2007; Walker et al. 2006) and during an incongruent incentive discrimination task (unpublished findings from our laboratory; Man et al.). In reversal learning, 5-HT depleted animals continue to respond to the previously rewarded stimulus, despite the change in the contingent relationship between the visual stimuli and reward. In acquisition of a detour-reaching task 5-HT depleted animals continue to exhibit a prepotent response tendency to reach directly along the animal's line of sight to the food reward, rather than make a detour reach. Finally, in the incongruent incentive discrimination task animals persist in selecting the high incentive food reward despite the response resulting in the receipt of the low incentive food reward. In all examples, the lesioned animals display stimulus-bound behavior, that is, responding directed towards specific salient stimuli, a description that also characterizes the deficits seen in the conditioned reinforcement and discrimination extinction tasks of the present study.
Although there were no competing stimulus–reward associations in the acquisition of the conditioned reinforcement task, several other factors, such as object preferences, spatial preferences and object novelty could bias responding. Indeed, it was found that a perceptual bias towards the more visually salient of the 2 stimuli determined responding initially in all subjects. However, whereas control animals were able to overcome this bias and use new learning about the association between visual stimuli and a conditioned reinforcer to guide responding, 5-HT depleted monkeys were not. In extinction too, although 5-HT depleted monkeys took no more trials to extinguish responding than controls, the responses they did make were nearly all directed towards the previously rewarded stimulus. This finding demonstrates that the perseverative impairment seen in 5-HT, OFC depleted animals on the serial reversal task does not reflect a deficit in response extinction per se. Instead it highlights the importance of 5-HT within the OFC in overcoming competing response biases to sensory stimuli and either directly, or indirectly, in promoting exploratory behavior.
Previous studies have implicated the OFC in overcoming stimulus preferences. Brush et al. (1961) found that monkeys with depletions of OFC were only impaired in acquiring a visual discrimination if they had to overcome initial object preferences. In human imaging studies, Paulus and Frank (2003) report ventromedial PFC activation when making perceptual preference judgments. In addition, the attentional processing biases that have been reported in depressed patients performing an emotional go/no go task (Murphy et al. 1999) are associated with an increased blood level oxygenation dependent response in the OFC (Elliott et al. 2002). Consistent with the present findings, 5-HT too has been linked with biases in attentional salience. For example, Hitsman et al. (2007) report that acute tryptophan depletion increased the attentional salience of smoking related cue words in smokers, independent of any change in mood.
However caution is necessary when comparing the effects of different serotonin reducing manipulations such as 5,7-DHT induced, chronic and regionally selective, terminal lesions and dietary-induced, acute, but global, tryptophan depletion. Such manipulations may have markedly different effects on tonic and phasic serotonin signalling (Cools et al. 2008) which have been hypothesized to report long-run average reward rates and punishment prediction errors, respectively (Daw et al. 2002). In addition they may have variable, if not opposing effects on punishment processing and inhibitory control, depending on their cortical or subcortical site of action (see Cools et al. 2008, for further discussion).
The results of this study indicate that 5-HT and DA modulate different functions within the OFC. The specific role of orbitofrontal 5-HT in preventing competing, task irrelevant, salient stimuli from biasing responding may be of particular relevance to our understanding of the etiology and treatment of symptoms in neuropsychiatric disorders involving marked attentional biases, including the negative biases in depression (Murphy et al. 1999) and the obsessions and compulsions in Obsessive Compulsive Disorder (OCD). Both depression and OCD are associated with structural and functional abnormalities in the OFC (Saxena and Rauch 2000; Drevets 2001) and these conditions are treated successfully with drugs that target the serotonin system such as the selective serotonin reuptake inhibitors (Brody et al. 1999; Goddard et al. 2008). In addition, the persistent responding in the absence of reward in extinction, in animals with DA depletions of the OFC, may resemble the compulsive responding of drug addicts in drug-related contexts. Not only have addicts been shown to have changes in striatal raclopride binding but these are correlated with changes in the cerebral metabolism of the OFC (Volkow and Fowler 2000).
Funding
Wellcome Trust program grant (076274/Z/04/Z) to T.W.R., B.J. Everitt, A.C.R., and B.J. Sahakian; and a Medical Research program grant (G0401411) to A.C.R.
Acknowledgments
Conducted within the University of Cambridge Behavioural and Clinical Neuroscience Institute, supported by a joint award from the Medical Research Council and the Wellcome Trust. We thank Adrian Newman for help with preparation of figures. Conflict of Interest: None declared.
References
- Berger B, Febvret A, Greengard P, Goldman-Rakic PS. DARPP-32, a phosphoprotein enriched in dopaminoceptive neurons bearing dopamine D1 receptors: distribution in the cerebral cortex of the newborn and adult rhesus monkey. J Comp Neurol. 1990;299:327–348. doi: 10.1002/cne.902990306. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, Huang SC, Wu HM, Maidment K, Baxter LR., Jr Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Brozoski T, Brown R, Rosvold H, Goldman P. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Brush ES, Mishkin M, Rosvold HE. Effects of object preferences and aversions on discrimination learning in monkeys with frontal lesions. J Comp Physiol Psychol. 1961;54:319–325. [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behav Brain Res. 1993;55:167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Butter CM, Mishkin M, Rosvold HE. Conditioning and extinction of a food-rewarded response after selective ablations of frontal cortex in rhesus monkeys. Exp Neurol. 1963;7:65–75. doi: 10.1016/0014-4886(63)90094-3. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Cetin T, Freudenberg F, Füchtemeier M, Koch M. Dopamine in the orbitofrontal cortex regulates operant responding under a progressive ratio of reinforcement in rats. Neurosci Lett. 2004;370:114–117. doi: 10.1016/j.neulet.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H, Walker S, Dalley J, Robbins T, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JCM, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Daw ND. Dopamine: at the intersection of reward and action. Nat Neurosci. 2007;10:1505–1507. doi: 10.1038/nn1207-1505. [DOI] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, Massioui NE. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard R, Zilles K, Schleicher A, Everitt BJ, Robbins TW, Divac I. Parcellation of the frontal cortex of the New World monkey Callithrix jacchus by eight neurotransmitter-binding sites. Anat Embryol (Berl). 1995;191:509–517. doi: 10.1007/BF00186741. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Shekhar A, Whiteman AF, McDougle CJ. Serotoninergic mechanisms in the treatment of obsessive-compulsive disorder. Drug Discov Today. 2008;13:325–332. doi: 10.1016/j.drudis.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Handbook of physiology. Bethesda (MD): American Physiology Society; 1987. Circuitry of the primate prefrontal cortex and the regulation of behavior by representational memory; pp. 373–416. [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallagher DW. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Spring B, Pingitore R, Munafò M, Hedeker D. Effect of tryptophan depletion on the attentional salience of smoking cues. Psychopharmacology. 2007;192:317–324. doi: 10.1007/s00213-007-0722-2. [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical methods for psychology. Belmont (CA): Wadsworth; 1997. [Google Scholar]
- Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheramin S, Body S, Ho MY, Velázquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin J, Anderson IM. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2004;175:206–214. doi: 10.1007/s00213-004-1813-y. [DOI] [PubMed] [Google Scholar]
- King D, Zigmond MJ, Finlay JM. Effects of dopamine depletion in the medial prefrontal cortex on the stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell. Neuroscience. 1997;77:141–153. doi: 10.1016/s0306-4522(96)00421-6. [DOI] [PubMed] [Google Scholar]
- Kurylo DD, Tanguay S. Effects of quinpirole on behavioral extinction. Physiol Behav. 2003;80:1–7. doi: 10.1016/s0031-9384(03)00218-x. [DOI] [PubMed] [Google Scholar]
- McDannald MA, Saddoris MP, Gallagher M, Holland PC. Lesions of orbitofrontal cortex impair rats’ differential outcome expectancy learning but not conditioned stimulus-potentiated feeding. J Neurosci. 2005;25:4626–4632. doi: 10.1523/JNEUROSCI.5301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Gratton A. Partial dopamine depletion of the prefrontal cortex leads to enhanced mesolimbic dopamine release elicited by repeated exposure to naturally reinforcing stimuli. J Neurosci. 1992;12:3609–3618. doi: 10.1523/JNEUROSCI.12-09-03609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. The contribution of orbitofrontal cortex to action selection. Ann N Y Acad Sci. 2007;1121:174–192. doi: 10.1196/annals.1401.033. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Crofts HS, McGuigan M, Tomic DL, Everitt BJ, Roberts AC. The role of the primate amygdala in conditioned reinforcement. J Neurosci. 2001;21:7770–7780. doi: 10.1523/JNEUROSCI.21-19-07770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Frank LR. Ventromedial prefrontal cortex activation is critical for preference judgments. Neuroreport. 2003;18:1311–1315. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. J Neurosci. 2003;23:11189–11201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–76. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;4:57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Q J Exp Psychol B. 1988;40:321–341. [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ, Muir JL. A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience. 1992;47:251–264. doi: 10.1016/0306-4522(92)90241-s. [DOI] [PubMed] [Google Scholar]
- Roberts A, De Salvia M, Wilkinson L, Collins P, Muir J, Everitt B, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology. 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Walker SC, Mikheenko YP, Argyle LD, Robbins TW, Roberts AC. Selective prefrontal serotonin depletion impairs acquisition of a detour-reaching task. Eur J Neurosci. 2006;23:3119–3123. doi: 10.1111/j.1460-9568.2006.04826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD. Orbitoforntal cortex and its contribution to decision making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Dias R, Robbins TW, Roberts AC. Dissociable contributions of the orbitofrontal and lateral prefrontal cortex of the marmoset to performance on a detour reaching task. Eur J Neurosci. 2001;13:1797–1808. doi: 10.1046/j.0953-816x.2001.01546.x. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]