Abstract
The normal formation and function of the mammalian cerebral cortex depend on the positioning of its neurones, which occurs in a highly organized, layer-specific manner. The correct morphology and movement of neurones rely on synchronized regulation of their actin filaments and microtubules. The p21-activated kinase (Pak1), a key cytoskeletal regulator, controls neuronal polarization, elaboration of axons and dendrites, and the formation of dendritic spines. However, its in vivo role in the developing nervous system is unclear. We have utilized in utero electroporation into mouse embryo cortices to reveal that both loss and gain of Pak1 function affect radial migration of projection neurones. Overexpression of hyperactivated Pak1 predominantly caused neurones to arrest in the intermediate zone (IZ) with apparently misoriented and disorganized leading projections. Loss of Pak1 disrupted the morphology of migrating neurones, which accumulated in the IZ and deep cortical layers. Unexpectedly, a significant number of neurones with reduced Pak1 expression aberrantly entered into the normally cell-sparse marginal zone, suggesting their inability to cease migrating that may be due to their impaired dissociation from radial glia. Our findings reveal the in vivo importance of temporal and spatial regulation of the Pak1 kinase during key stages of cortical development.
Keywords: cortical development, in utero electroporation, neuronal morphology, Pak1 kinase, radial migration
Introduction
In the cerebral cortex, glutamatergic, projection neurones arise in the germinal layers of the dorsal telencephalon and migrate radially toward the pial surface. The 6-layered structure of the neocortex is formed in an inside-out manner, where neurones born early populate deep layers and neurones born late reside more superficially (Angevine and Sidman 1961; Rakic 1974). Most γ-aminobutyric acidergic (GABAergic) interneurones arise from the ventral telencephalon and migrate tangentially toward and radially within the cerebral cortex (Anderson et al. 1997; Ang et al. 2003). Interneurones enter the cortex initially through the preplate (PP), subsequently through the marginal zone (MZ), and finally, in later embryonic stages, through the subplate (SP) and intermediate zone/subventricular zone (IZ/SVZ) (Metin et al. 2006). Remarkably, projection neurones and interneurones born at the same time share the same laminar fate, indicating that the former influence the position of the latter (Nakajima 2007).
Most projection neurones migrate toward the pial surface by climbing up radial glia (Rakic 1972). It is thought that negative factors within the MZ promote detachment of neurones from radial glia at the base of the MZ, thus preventing their entry into this area (Anton et al. 1996; Rakic 2003). The presence of an inhibitory mechanism close to the pial surface would also ensure the inverted layering of the cortex because neurones stop migrating, having passed previously formed layers. The molecular mechanisms that account for the inhibitory effects of the MZ are not fully understood. To date, the most extensively studied factor is reelin, a large glycoprotein secreted by Cajal–Retzius neurones, which are located in the upper portion of the MZ (D'Arcangelo 2006). At high concentrations, reelin can cause detachment of migrating neurones from radial fibers in an α3β1 integrin–dependent manner, supporting its inhibitory role (Dulabon et al. 2000; Schmid et al. 2005). However, at lower concentrations, reelin can also stimulate neuronal migration, challenging the hypothesis that it acts as a stop signal (D'Arcangelo 2006). The detachment of neurones at the MZ also involves signaling from the α6 integrin subunit and integrin-linked kinase (Ilk), the downstream targets of which are currently unknown (Georges-Labouesse et al. 1998; Niewmierzycka et al. 2005).
It is becoming clear that normal morphology and polarity are required for correct movement and final positioning of neurones in the cerebral cortex. Furthermore, all these processes depend on the dynamic and synergistic regulation of filamentous actin (F-actin) and microtubules. Several molecules that affect both cytoskeletal elements have been shown to control neuronal migration in the cerebral cortex, including disabled 1 (Dab1), cyclin-dependent kinase 5 (Cdk5), c-Jun N-terminal kinase (JNK), glycogen synthase kinase 3β, and microtubule-associated proteins Map1b, Lis1, and doublecortin (Ayala et al. 2007). Importantly, the p21-activated kinase 1 (Pak1) is a major target of the GTPases Rac1 and Cdc42 that control F-actin and microtubule organization (Bokoch 2003). Pak1 displays highest expression levels in the nervous system where it controls neuronal morphology and the formation of dendritic spines (Banerjee et al. 2002; Bokoch 2003; Zhong et al. 2003). We recently demonstrated that Pak1 regulates axon specification and outgrowth in differentiating hippocampal neurones (Jacobs et al. 2007). We now reveal that Pak1 controls the correct morphology, orientation, and radial migration of neurones in the cerebral cortex. Unexpectedly, Pak1 is also required to prevent neuronal entry into the cell-sparse MZ. Our findings provide a novel insight into the molecular mechanisms responsible for the correct laminar organization of the cerebral cortex and emphasize the importance of tightly regulated signaling to the cytoskeleton of differentiating neurones.
Materials and Methods
DNA Expression Vectors
Pak1Caax, Pak1T423E, and Pak1R299Caax were expressed from the pCAG-IRES-EGFP vector, whereas short hairpin RNA (shRNA) were expressed from the mU6pro vector, as described previously (Jacobs et al. 2007).
Antibodies
The following antibodies were used in this study. Anti-Pak1 (N-20), anti-CDP (M-222), and anti-NeuroD (N-19) (Santa Cruz Biotechnology, Santa Cruz, CA); anti-phospho Ser199/204 Pak1/Pak2 (New England Biolabs, Ipswich, MA); anti-β-III-tubulin (TUJ1) (Babco, Richmond, CA); anti-Map2 (AP20) and anti-vinculin (hVIN1) (Sigma-Aldrich, St Louis, MO); anti-RC2 (Hybridoma Bank, University of Iowa, Iowa City, IA); anti-nestin and anti-Ctip2 (abcam, Cambridge, UK); anti-brain lipid-binding protein (BLBP) (Millipore, Hertfordshire, UK); anti-pericentrin and anti-GM130 (BD Transduction Laboratories, Oxford, UK); anti-laminin γ1 (Alexis, AXXORA, Nottingham, UK); anti-actin (Chemicon, Southampton, UK); anti-myc (9e10 hybridomas), anti-green fluorescent protein (GFP), anti-mouse Alexa 488 or 568, and anti-rabbit Alexa 488, 568, or 633 (Invitrogen, Paisley, UK); anti-Reelin (E4 and G10; a kind gift from Sonja Rakić and André Goffinet); and anti-Tbr1 and anti-Tbr2 (a kind gift from Robert Hevner).
Immunohistochemistry, Western Blotting, and Kinase Assays
Immunohistochemistry was carried out as previously described (Causeret et al. 2007; Jacobs et al. 2007). Briefly, 20-μm tissue frozen sections were blocked in phosphate-buffered saline (PBS) containing 0.2% Triton X-100 and 0.2% fish skin gelatine for 1 h at room temperature, followed by an appropriate dilution of primary antibody in blocking solution which was left on the sections between 3 h and overnight. Subsequent to washing in PBS containing 0.2% Tween 20, the sections were incubated for 1 h with secondary antibody, rinsed in 0.01 mg/mL 4′,6-diamidino-2-phenylindole, and mounted in Prolong Gold antifade (Invitrogen). For immunohistochemistry performed using anti-Tbr1 or anti-Tbr2 antibodies, the tissues were initially briefly boiled in 0.01-M sodium citrate pH 6.0, followed by the protocol outlined above. Western blotting and kinase assays were performed as described (Causeret et al. 2007; Jacobs et al. 2007). Briefly, cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), transferred onto polyvinylidene difluoride membrane (Immobilon; Millipore, Billerica, MA), and blocked in 5% skimmed milk, followed by primary and secondary antibodies. For kinase assays, immunoprecipitations were carried out using anti-Pak1 N20 antibody and protein A sepharose. Reactions were performed on washed beads in the presence of 50-μM ATP, 1 μCi [32Pγ-ATP] (GE Healthcare, Slough, UK), 2-μg histone H4 (Roche, Welwyn, UK), 1 mM dithiothreitol, and kinase buffer (50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.5, 10 mM MgCl2) for 20 min at 30 °C. The reactions were stopped with 2× sample buffer, resolved by SDS–PAGE, dried, and exposed to autoradiography.
Cell Culture and Transfection
Cos7 cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (Invitrogen) and transfected using the calcium phosphate precipitation method as described previously (Rashid et al. 2001). Primary cortical neurones were dissociated from E17 to E18 rat embryos and cultured in neurobasal media supplemented with factor B27 (Invitrogen) as previously described (Jacobs et al. 2007). Transfections were performed using the Amaxa Nucleofector approach following the manufacturer's protocols (Amaxa Biosystems, Cologne, Germany).
In Utero Electroporation
In utero electroporation was carried out as previously described (Kawauchi et al. 2003). Briefly, DNA was electroporated into the VZ of E14.5 mouse embryo neocortices and the embryos allowed to develop in utero until birth. At postnatal day 0 (P0), the brains were fixed in 4% paraformaldehyde, cryopreserved in 30% followed by 10% sucrose in 0.12-M phosphate buffer, pH 7.4, embedded in 7.5% gelatine, 10% sucrose in 0.12-M phosphate buffer, pH 7.4, frozen in isopentane cooled to −55 °C, and 20-μm sections made using a Leica cryostat. They were subjected to immunohistochemistry as described above. Images were made using a Leica TCS SP/UV confocal microscope and visualized as Max projections through multiple stacks. Neuronal migration was scored using a Nikon TE2000 microscope, Hamamatsu Orca camera, and Openlabs software (Improvision, Coventry, UK). For each DNA construct, at least 2400 cells were counted from a minimum of 3 separate embryos.
Results
Pak1 Expression and Activation Are High in Migrating Neurones
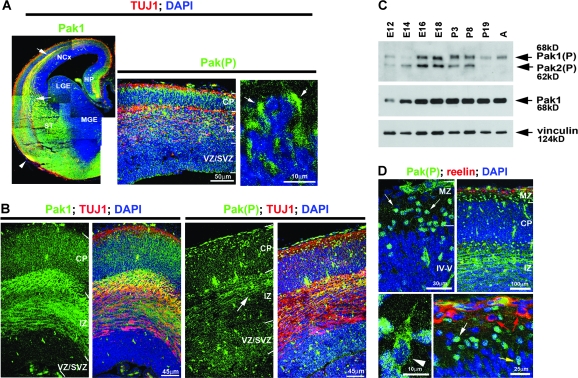
To evaluate the importance of Pak1 in the developing forebrain, we examined its expression by immunohistochemistry. At mouse E14, highest levels were evident in postmitotic neurones of the cerebral cortex, amygdala, striatum, and pyriform cortex, as well as axonal tracts of the IZ, striatum, and lateral olfactory tracts (Fig. 1A). In contrast, the proliferating neuroepithelium lining the lateral ventricle had comparatively low expression of Pak1 (Banerjee et al. 2002). A similar pattern of expression remained evident at E16 (Fig. 1B). Western blotting of lysates obtained from mouse cortices showed upregulation of Pak1 at E14 and E16, which was maintained to adulthood (Fig. 1C). Pak1 activation is accompanied by phosphorylation on S199/204 and can consequently be evaluated using a phosphospecific antibody, as previously described (Chong et al. 2001; Zhao et al. 2005; Jacobs et al. 2007). In mouse cortices, maximal levels of activated Pak1 were seen between E16 and P3 (Fig. 1C). A further decrease between P8 and P19 coincided with the completion of neuronal migration in the cerebral cortex. Pak1 is highly related to Pak2 and Pak3 (together they are referred to as Group I Pak), with which it shares conserved phosphorylation sites (Bokoch 2003). The antibody that detects Pak1 phosphorylated on S199/204 also recognizes activated Pak2 phosphorylated on S192/197 which, unlike Pak1, was downregulated in adulthood (Fig. 1C). This may reflect the reported synaptic role of Pak1 in regulating dendritic spines and its involvement in memory consolidation and extinction of contextual fear, which occur in adulthood (Penzes et al. 2003; Hayashi et al. 2004; Zhang et al. 2005; Sananbenesi et al. 2007). Because by immunohistochemistry we cannot differentiate between phosphorylated Pak1 and Pak2, we subsequently refer to positive immunostaining results using the phosphospecific antibody as Pak(P).
Figure 1.
Pak1 expression and activation in the developing forebrain. The distribution of Pak1 and the phosphorylated, activated form, Pak(P) were examined by immunohistochemistry in fixed brains (A, B, and D) and western blotting of brain lysates (C). (A) At E14, Pak1 predominates in the striatum, cerebral cortex, and dorsomedial pallium. White arrows point to the IZ axonal tracts, and an arrowhead marks the lateral olfactory tract (left panel). In the neocortex, Pak(P) is seen in cytoplasmic clusters throughout the IZ and CP (arrows in right panel). (B) At E16, Pak1 was highest in the CP and IZ. Pak(P) was seen in axonal fibers of the IZ (arrow) and cell bodies of neurones located throughout the cortex. (C) Highest levels of activated Pak1 and Pak2 were observed in mouse cortices between E16 and P8. Vinculin expression was used as a control for equal loading. (D) At E16, many cells with nuclear Pak(P) were tangentially oriented in the MZ (white arrows) and did not express reelin, as seen in Cajal–Retzius neurones (red arrows). Neurones with nuclear Pak(P) were also observed radially oriented in the CP (yellow arrow). Radially migrating neurones with cytoplasmic Pak(P) were evident in the CP (arrowhead). NCx, neocortex; LGE, lateral ganglionic eminence; HP, developing hippocampus; ST, striatum.
At E14, cytoplasmic enrichment of Pak(P) was evident in radially oriented neurones, the positions of which suggested that they were migrating toward the pial surface (Fig. 1A). At E16, Pak(P) accumulated in axonal tracts of the IZ and in the cytoplasm of radially oriented neurones (Fig. 1B,D). A number of neurones with nuclear Pak(P) were present in the MZ and IZ/SVZ where they predominantly oriented tangentially, as well as the cortical plate (CP) where they were positioned radially (Fig. 1D). In the MZ, the absence of reelin coexpression indicated that they were not Cajal–Retzius neurones (Fig. 1D). Neuronal identity was confirmed by coexpression of β-III-tubulin (TUJ1). Because the position of neurones with nuclear Pak(P) suggested an interneuronal identity, we examined E17 and P0 forebrains obtained from mice expressing GFP from a GAD67 promoter, which marked GABA-producing interneurones (Tamamaki et al. 2003). GFP-labeled interneurones were observed migrating tangentially from the medial ganglionic eminence (MGE) into the MZ and IZ/SVZ and had nuclear enrichment of Pak(P), which persisted during their subsequent radial migration through the CP (Supplementary Fig. 1). Interestingly, the levels of nuclear Pak(P) differed between individual interneurones, although no clear correlation with their positioning in the cerebral cortex was discernable. Together, these results reveal that Group I Pak kinases are expressed and activated in migrating neurones during key stages of cortical development.
Plasma Membrane Localization and Activation of Pak1 Control Radial Migration
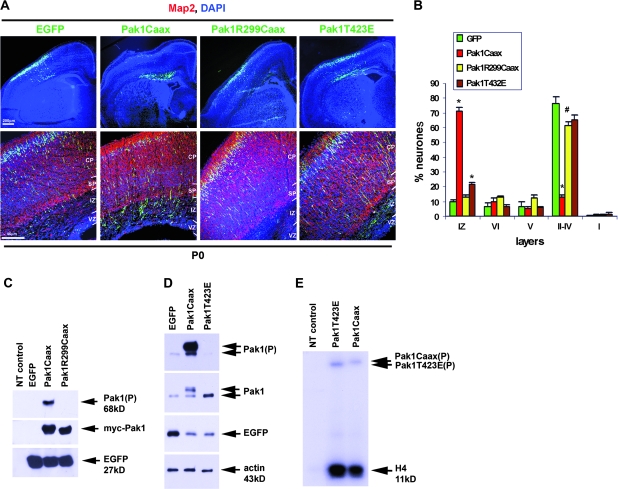
To investigate the importance of Pakl activation during cortical development, we utilized in utero electroporation into E14.5 mouse embryos, thus targeting neuronal precursors from layers II, III, and IV. This powerful technique has previously revealed the requirement for a number of proteins including p27Kip1, doublecortin, Stef/Tiam1, Map1b, P-REX1, JNK, Dab1, MAM domain containing glycosylphosphatidinositol anchor-1, microtubule affinity-regulating kinase 2 (MARK2), and Arx during radial locomotion of cortical projection neurones (Bai et al. 2003; Kawauchi et al. 2003, 2005, 2006; Yoshizawa et al. 2005; Nguyen et al. 2006; Olson et al. 2006; Takeuchi et al. 2007; Friocourt et al. 2008; Sapir et al. 2008). Pak1 normally exists in a homodimerized, inactive state in the cytoplasm and can be activated by recruitment to the membrane (Bokoch 2003). Consequently, fusion of a Ras prenylation sequence (Caax box) to the C-terminus of Pak1 renders it constitutively active (Manser et al. 1997; Daniels et al. 1998). We have previously used Pak1Caax expression to demonstrate that membrane enrichment of active Pak1 affects the ability of cultured hippocampal neurones to specify and extend an axon (Jacobs et al. 2007). Interestingly, we revealed that both plasma membrane localization and kinase activity were required for the effects of Pak1 on neuronal polarization. Thus, expression of a membrane-targeted catalytically inactive mutant, Pak1R299Caax, or a constitutively active mutant that is predominantly cytoplasmic because its plasma membrane localization depends on intracellular signaling, Pak1T423E, had no consequences on neuronal polarity. To determine the function of Pak1 activation in vivo, we compared the consequences of Pak1Caax expression in migrating cortical neurones with Pak1R299Caax and Pak1T423E. In all cases, coexpression of enhanced green fluorescent protein (EGFP) from an internal ribosome entry site allowed identification of targeted neurones, whereas EGFP expression alone was used as a control. Electroporated embryos were allowed to develop in utero until birth (P0) when their forebrains were examined for the position of EGFP-expressing neurones. As previously observed, 90 ± 1.0% of control neurones had successfully migrated into the CP with 76.3 ± 4.6% reaching its periphery, contributing to layers II–IV (Fig. 2A,B) (Kawauchi et al. 2003). In contrast, overexpression of Pak1Caax caused an overall inhibition of migration with the majority of cells residing in the IZ (71.3 ± 2.4%), only 28.7 ± 2.4% entering the CP and 12.8 ± 1.4% reaching the outer layers. This inhibition was largely dependent on Pak1 activity as 87.2 ± 1.4% of neurones expressing Pak1R299Caax were in the CP and 61.1 ± 2.8% had reached the periphery (Fig. 2A,B). Interestingly, 78.5 ± 1.4% of Pak1T423E-expressing neurones entered the CP and 65 ± 3.3% reached the periphery. However, 21.4 ± 1.4% accumulated in the IZ, suggesting that migration was affected in a relatively small proportion of targeted neurones. These results reveal that in vivo the effects of Pak1 on neuronal migration are most pronounced when it is catalytically active at the membrane, in accordance with our observations previously made in vitro (Jacobs et al. 2007).
Figure 2.
Membrane localization of active Pak1 interferes with neuronal migration in the neocortex. (A) Mouse cortices electroporated in utero with EGFP, Pak1Caax, Pak1R299Caax, or Pak1T423E. At P0, most EGFP-, Pak1R299Caax-, and Pak1T423E-expressing neurones had migrated to peripheral layers of the CP. In contrast, neurones expressing Pak1Caax predominantly remained in the IZ. (B) Percentage of distribution of electroporated neurones. n = 3, error bars (standard deviation); *P < 0.001, #P < 0.01 using Student's t-test. (C) Western blots of lysates obtained from transfected Cos7 cells expressing as shown, confirming the phosphorylation of Pak1Caax but not Pak1R299Caax. Exogenous Pak1 was detected by virtue of its myc tag. Because Cos7 cells have very low levels of endogenous Pak1, lysates from nontransfected (NT) cells were used as controls to demonstrate antibody specificity. (D) Western blots of lysates from transfected cortical neurones demonstrating lack of phosphorylation of Pak1T423E in contrast to Pak1Caax, despite the similar expression levels of both mutants. (E) Constitutive activation of the Pak1Caax and Pak1T423E mutants was confirmed by radioactive phosphorylation of histone H4. Pak1 immunoprecipitations were obtained from transfected cortical neurones, as shown. The Pak1 mutants also efficiently autophosphorylated (top arrows). At this exposure, the activity of endogenous Pak1 is only weakly detectable in the NT lane.
To directly verify expression of constitutively active or inactive Pak1 proteins, we coimmunostained electroporated brains with anti-GFP and anti-Pak1 or anti-Pak(P) antibodies. Neurones expressing Pak1Caax or Pak1R299Caax had significantly upregulated Pak1 levels, only the former of which was phosphorylated (Fig. 3B). This was confirmed by western blotting of Cos7 cells transfected with EGFP alone or together with Pak1Caax or Pak1R299Caax (Fig. 2C). Phosphorylation of Pak1 on S199/204 was evident only in lysates expressing Pak1Caax, confirming the inability of Pak1R299 to be activated. The T423E mutation on Pak1 abolishes the requirement for phosphorylation on S199/204, in contrast to Pak1Caax, as demonstrated in transfected cortical neurones (Fig. 2D). We therefore confirmed the enhanced activity of both Pak1Caax and Pak1T423E in transfected cortical neurones by Pak1-specific immunoprecipitations and subsequent phosphorylation of histone H4, a commonly used in vitro substrate of Pak1 (Fig. 2E) (Rashid et al. 2001; Jacobs et al. 2007). The results also verified the ability of both Pak1Caax and Pak1T423E proteins to autophosphorylate.
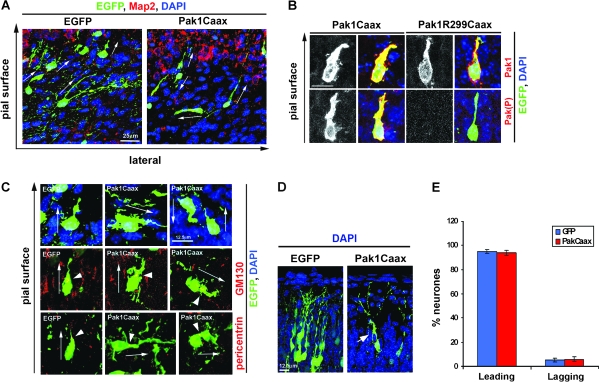
Figure 3.
Pak1Caax expression affects neuronal morphology. (A) Following expression of Pak1Caax, neurones in the IZ displayed altered morphologies, often orientating in an apparently random manner, in contrast to EGFP controls which oriented toward the pial surface (arrows point to the direction of the leading process). EGFP (green); 4′,6-diamidino-2-phenylindole (blue); Map2 (red). (B) Pak1Caax-expressing neurones migrating through the CP exhibited broader, lamellipodia-rich leading processes when compared with Pak1R299Caax-expressing controls. Enhanced activation of Pak1 is confirmed by immunostaining for Pak(P). (C) Pak1Caax-expressing neurones with curved, branched, ruffled, or misoriented leading extensions. In all examined cases, the centrosome and Golgi localized to the base of the leading process as determined by pericentrin or GM130 immunostaining, respectively (red). Arrowheads point to the centrosomes and Golgi, arrows show neuronal orientation. (D) Examples of EGFP and Pak1Caax electroporated neurones reaching peripheral layers of the cortex. Arrow points to abnormal lamellipodia protrusions in the latter. (E) Percentage of electroporated neurones in the CP and IZ with centrosomes localized to the leading or lagging migratory process. n = 3, error bars (standard deviation). No significant differences were observed.
Pak1 Activation Affects Neuronal Orientation and Morphology
Pak1Caax-expressing neurones that had failed to migrate out of the IZ displayed varied orientations, a phenotype not observed in EGFP controls (Fig. 3A). Morphologically, the Pak1Caax-expressing neurones often elaborated short processes that curved or branched and from which multiple small protrusions emerged (Fig. 3A–C). In the CP, migrating neurones expressing Pak1Caax appeared to have broader leading processes with extensive lamellipodia, which were not observed in Pak1R299Caax- or EGFP-expressing controls (Fig. 3B, Supplementary Fig. 2). The low percentage of Pak1Caax neurones that had reached superficial layers of the CP also displayed aberrant protrusions (Fig. 3D). We previously demonstrated that in vitro Pak1Caax expression affects axonal specification (Jacobs et al. 2007); in addition, projection neurones can specify their lagging process as the future axon at the time of migration through the IZ (Noctor et al. 2004). We therefore wanted to investigate whether accumulation of Pak1Caax-expressing neurones in the IZ was due to defects in polarization. In a number of cell types, the position of the centrosome marks the direction of movement (Higginbotham and Gleeson 2007). Thus, we visualized the centrosomes of EGFP-expressing neurones by immunodetection of pericentrin, a known centrosomal marker (Fig. 3C). The results revealed no significant differences in the localization of the centrosome in relation to the position of the leading migratory process between control and Pak1Caax-expressing neurones located either in the CP or in the IZ (95 ± 3.6% of controls vs. 94 ± 4.4% of Pak1Caax neurones; mean ± standard deviation, P > 0.05; Student's t-test) (Fig. 3E). The Golgi complex is also commonly used as a marker of cellular polarization and together with the centrosome defines the leading process of tangentially migrating cortical interneurones and radially migrating cerebellar granule cells (Fukata et al. 2003; Mellor 2004; Solecki et al. 2004; Metin et al. 2006). Localization of the Golgi apparatus in electroporated neurones using the GM130 antibody confirmed the findings we made by visualization of the centrosomes. Thus, the Golgi localized to the base of the leading process of all examined migrating neurones regardless of their apparent orientation (tangential, radial toward the pial surface or the VZ) (Fig. 3E). We conclude that membrane localization of catalytically active Pak1 may alter neuronal migration by misorienting their leading edge, rather than affecting the alignment of their centrosome and Golgi apparatus relative to the direction of movement. Further examination will be required to determine whether the identity of the leading process undergoes dynamic changes following altered Pak1 expression. Together, our observations suggest that in vivo the formation of polarized lamellipodia in migrating neurones depends on localized Pak1 kinase activity.
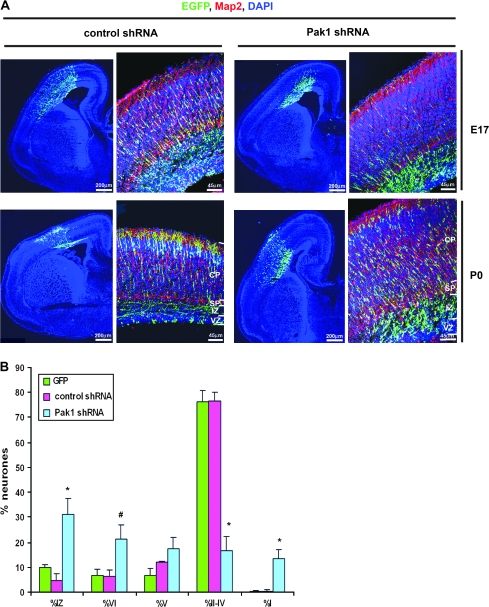
Pak1 Expression Is Required for Neuronal Migration
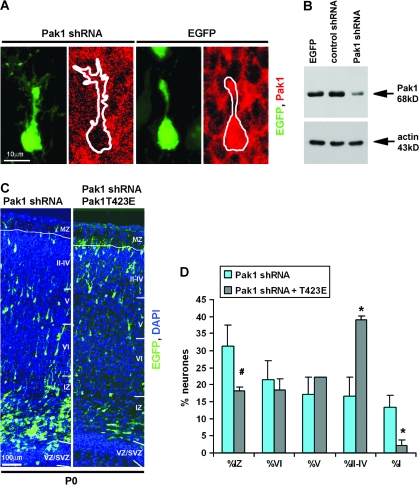
We have previously utilized an shRNA-targeting strategy to specifically reduce Pak1 expression in primary neurones. We also revealed that the shRNA constructs had no consequences on the levels or activity of Pak2 and Pak3 (Jacobs et al. 2007). To determine the function of endogenous Pak1 in the cerebral cortex, we examined the consequences of its downregulation by in utero electroporation of mouse E14.5 embryos. Targeted neurones were identified by the coexpression of EGFP, and a nonspecific shRNA was used as a control. At E17, most control shRNA–expressing neurones were seen migrating through the IZ and CP and 76.4 ± 3.5% reached layers II–IV by P0 (Fig. 4A,B). In contrast, at P0, only 16.7 ± 5.7% of Pak1 shRNA–expressing neurones had reached layers II–IV, 38.6 ± 3.3% were in the deep cortical layers V and VI, and 31.2 ± 6.2% remained in the IZ. Unexpectedly, 13.4 ± 3.4% of neurones that had migrated into the CP were observed within the MZ (Fig. 4B).
Figure 4.
Pak1 is essential for the correct laminar organization of the neocortex. (A) Mouse embryos electroporated in utero with control or Pak1-specific shRNA. Downregulation of Pak1 caused an increase in the number of electroporated neurones residing in the IZ evident at E17 and P0. (B) At P0, Pak1 shRNA caused a broad distribution of neurones throughout all layers of the neocortex including the normally cell-sparse MZ. n = 4, error bars (standard deviation); *P < 0.001, #P < 0.01, using Student's t-test. EGFP (green); Map2 (red); 4′,6-diamidino-2-phenylindole (blue).
To confirm the specificity of the shRNA constructs in vivo, we examined the levels of Pak1 in EGFP- and Pak1 shRNA–coexpressing neurones by immunohistochemistry, revealing a clear downregulation, in contrast to EGFP-expressing controls (Fig. 5A). This was further verified biochemically by western blotting of lysates obtained from transfected cortical neurones in culture (Fig. 5B) and as shown previously (Jacobs et al. 2007). To evaluate whether the migratory defects displayed by electroporated neurones were indeed due to the downregulation of Pak1, we attempted to rescue the phenotype by coexpression of Pak1 shRNAs and the constitutively active Pak1T423E mutant. The Pak1 shRNAs used in this study were designed against a highly conserved region of Pak1 present in rat, mouse, and human orthologues, thus reducing the effectiveness of potential rescue by coexpression of wild-type Pak1 from any of these species. Our attempts to make a mutant that is not recognized by the shRNA were not successful due to the inherent nucleotide instability of Pak1, which can compromise its normal catalytic function (Manser et al. 1997). The Pak1T423E mutant is constitutively active but did not cause a severe phenotype in vivo. It was therefore selected as the most appropriate reagent to counteract the effects of Pak1 shRNAs, particularly because its untargeted mRNAs would generate activated Pak1 proteins, which can be transported to the plasma membrane by endogenous signals. Our results revealed a clear rescue of the aberrant neuronal entry into the MZ with only 2.2 ± 1.4% of co-electroporated neurones entering this normally cell-sparse zone in contrast to 13.4 ± 3.4% of Pak1 shRNA–expressing neurones (Fig. 5C,D). Furthermore, we also observed a significant increase of neurones residing in layers II–IV (39.2 ± 1.1% of Pak1 shRNA + Pak1T423E vs. 16.7 ± 5.7% of Pak1 shRNA) and a reduction in the percentage arrested in the IZ (18.1 ± 1.3% of Pak1 shRNA + Pak1T423E vs. 31.2 ± 6.2% of Pak1 shRNA). Together, our results confirm the requirement for Pak1 during normal radial migration in the developing cerebral cortex.
Figure 5.
Reduction of Pak1 expression specifically affects neuronal migration. (A) Representative examples of neurones expressing Pak1 shRNA or EGFP exhibiting reduced or normal levels of Pak1, respectively. (B) Western blot of cortical lysates following transfection with EGFP, control, or Pak1 shRNA, as shown. Note significantly reduced levels of Pak1 expression. Actin was used as a control for equal loading. (C) Mouse embryos were electroporated at E14.5 with Pak1 shRNA alone or together with Pak1T423E at an equal (1:1) ratio of DNA. Note at P0, a reduced number of EGFP-labeled neurones in the IZ and a corresponding increase in cortical layers II–IV, following coexpression of Pak1T423E. EGFP (green); 4′,6-diamidino-2-phenylindole (blue). CP layers VI–II. (D) Percentage of distribution of electroporated neurones at P0 showing significant rescue by Pak1T423E. n = 3, error bars (standard deviation); *P < 0.001, #P < 0.01 using Student's t-test.
Loss of Pak1 Has Minor Consequences on Neuronal Fate
In the cerebral cortex, neuronal differentiation is distinguishable by the sequential expression pattern of particular transcriptional regulators. Thus, Pax6 is expressed in the VZ, Tbr2 in the upper VZ and SVZ, NeuroD in the upper SVZ and lower IZ, and Tbr1 in the IZ and throughout the CP (Hevner et al. 2006; Hevner 2007). Individual layers of the CP are also identifiable by the enrichment of specific markers (Molyneaux et al. 2007). Thus, layer VI is distinguished by highest levels of Tbr1, layers V and VI are marked by Ctip2, a transcription regulator, whereas layers II–IV are distinguishable by the enrichment of Cdp/Cux1 and 2, two homeobox transcription factors (Leid et al. 2004; Nieto et al. 2004; Zimmer et al. 2004; Ferrere et al. 2006; Hevner 2007; Molyneaux et al. 2007). Reduction of Pak1 levels caused neurones to accumulate in the IZ and deeper layers of the cerebral cortex, which could have resulted from their altered fate, or alternatively, to the occurrence of major morphological abnormalities. To distinguish between these 2 possibilities, we examined P0 mouse brains that had been electroporated at E14.5 for specific cortical markers. In all cases, neurones were counted in sections obtained from at least 3 embryos and presented as mean percentage ± standard error of the mean (SEM). In accordance with published reports, the marker of cortical progenitors, Tbr2, revealed a clear band of positive nuclei in the upper VZ and SVZ; a few positive cells were also observed in the IZ (Supplementary Fig. 3) (Hevner et al. 2006). Because expression of Pak1 shRNA caused neurones to arrest in the IZ, we examined EGFP-expressing neurones in this area for the presence of Tbr2. Only a few control neurones were seen in IZ, of which none expressed Tbr2. However, 6.9 ± 0.4% of Pak1 shRNA targeted neurones evident in the IZ were distinguished by this marker. Because most of the Pak1 shRNA–expressing neurones that resided in the IZ were devoid of Tbr2, these data suggested that changes in Pak1 expression do not dramatically alter the fate of progenitors. In confirmation, no significant changes were observed in the number of EGFP- and NeuroD-copositive neurones following the reduction of Pak1 expression (on average, 14.5 ± 2.8% controls and 9.2 ±4.3% Pak1 shRNA–expressing neurones). In all examined brains, NeuroD labeled cells in the SVZ and lower IZ, as predicted (Lee et al. 2000; Hevner et al. 2006). We therefore conclude that reduction in Pak1 expression does not greatly affect the differentiation of neuronal progenitors.
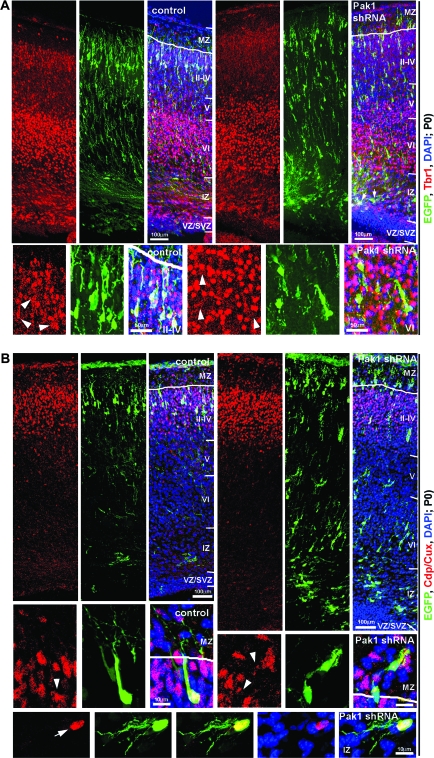
To investigate the formation of the CP, we examined the distribution of Tbr1-labeled neurones. Highest levels of Tbr1 were evident in the deep cortical layer VI, whereas neurones bordering the SVZ and IZ, throughout the IZ and in cortical layers V and II—IV, were more weakly immunopositive for this marker, as shown previously (Hevner 2007). All control shRNA–expressing neurones that had exited the proliferative zones were positive for Tbr1, with a majority (98.1 ± 0.2%) weakly and a minority (1.9 ± 0.2%) strongly labeled (Fig. 6A). Similarly, all Pak1 shRNA–expressing neurones that had migrated out of the SVZ were Tbr1 immunopositive; however, a small but significant change was observed in the proportion of those weakly and strongly stained. Thus, 94.9 ± 1.1% exhibited lower and 5.1 ± 1.1% high levels of Tbr1 (0.01 < P < 0.05, Student's t-test). These data are consistent with the conclusion that decreasing Pak1 expression has only minor consequences on neuronal differentiation. However, because low levels of Tbr1 are evident throughout the CP, these data only partially addressed the potential ability of Pak1 to affect the laminar identity of cortical neurones. Interestingly, most neurones expressing Pak1 shRNA evident in cortical layers V and VI did not exhibit the high levels of Tbr1 that distinguished their nontargeted neighbors, suggesting that they were not typical deep layer neurones. To confirm these findings, we examined the distribution of Ctip2, which is highly expressed by layer V neurones, whereas lower levels are evident in neurones of layer VI (Leid et al. 2004; Arlotta et al. 2005). Most of the controls (91.5 ± 0.6%) showed no expression of Ctip2, whereas 7.9 ± 0.7% and 0.5 ± 0.3% were weakly or strongly positive, respectively. No significant differences were evident following the reduction of Pak1 expression, where 89.4 ± 2.8% of targeted neurones were devoid of Ctip2, whereas 9.3 ± 2.7% and 1.1 ± 0.8% were weakly and strongly positive, respectively (P > 0.05; Student's t-test) (Supplementary Fig. 4).
Figure 6.
Reduced expression of Pak1 has minor consequences on neuronal fate. Electroporated brains were examined for the localization of the cortical markers Tbr1 (A) and Cdp/Cux (B) (red). (A) In control and Pak1 shRNA electroporated brains, neurones from layer VI showed highest expression of Tbr1, whereas CP and IZ neurones had lower levels of Tbr1. In the IZ, Pak1 shRNA–expressing neurones were copositive for lower levels of Tbr1 (arrows). Note that EGFP-labeled neurones located in deep cortical layers did not have high levels of Tbr1, which is characteristic of their nontargeted neighbors. White arrowheads reveal the position of the EGFP-expressing neurones that are weakly positive for Tbr1. (B) Neurones in layers II–IV expressed Cdp/Cux. High levels were detected in the majority of controls, whereas fewer Pak1 shRNA–expressing neurones were strongly positive for Cdp/Cux. In the IZ, only 7.6 ± 3% Pak1 shRNA–expressing neurones had high levels of Cdp/Cux, suggesting a change or delay in fate specification. EGFP (green); 4′,6-diamidino-2-phenylindole (blue); CP layers VI–II.
In utero electroporation of E14.5 mouse embryos should primarily target progenitors of layers II–IV. In confirmation, all EGFP-expressing controls were positive for Cdp/Cux, a marker that accumulates in layer IV and is present at lower levels in layers II and III (Nieto et al. 2004; Zimmer et al. 2004; Ferrere et al. 2006) (Fig. 6B). In our experimental setup, on average, 64.1 ± 7.4% of neurones electroporated at E14.5 had high levels of Cdp/Cux and thus exhibited layer IV characteristics, whereas 35.9 ± 7.4% had lower Cdp/Cux expression and thus likely to assume a layer II or III identity. Unexpectedly, a significant reduction was observed in the number of layer IV neurones following the downregulation of Pak1 (P < 0.001; Student's t-test). Thus, 16.5 ± 2.4% had high, 56.1 ± 3.1% low, and 27.4 ± 2.1% undetectable levels of Cdp/Cux. For greater clarification, we separately examined Pak1 shRNA–expressing neurones that had arrested in the IZ from those that had migrated into the CP. Thus, 50.2 ± 5.5% of targeted neurones that had accumulated in the IZ had no detectable Cdp/Cux, whereas 42.2 ± 3.9% had low and 7.6 ± 1.5% high levels. In the CP and MZ, only 4.6 ± 1.3% had no apparent expression of Cdp/Cux, whereas 70.05 ± 4.1% were weakly and 25.4 ± 4.7% strongly immunopositive for this marker. Together, these data suggest that the majority of neurones with reduced Pak1 expression that were able to migrate into the CP adopted a layer II–IV fate, although a significant reduction was evident in the proportion of layer IV neurones (P < 0.01; Student's t-test). Importantly, only approximately half of the neurones that had arrested in the IZ displayed a layer II–IV specification, whereas the fate of the remainder was not clarified. Overall, our results suggest that changes in Pak1 expression cause aberrant neuronal migration primarily by inducing morphological defects. The fact that only minor changes were evident in the specific expression of cortical markers suggests that altered neuronal differentiation is a secondary consequence of their misplacement.
Pak1 Is Required for Normal Neuronal Morphology
Morphologically, all neurones expressing Pak1 shRNA displayed abnormalities that were not observed in the EGFP and shRNA controls. In the IZ, Pak1 reduction caused the elaboration of disorganized extensions and broad lamellipodia protrusions that surrounded the soma (Fig. 7A). This multipolar neuronal morphology was similar in manner to our previous observations made in vitro (Jacobs et al. 2007). In electroporated neurones that resided in superficial layers of the cortex, loss of Pak1 caused a markedly reduced length of apical extensions when compared with EGFP and shRNA controls (Fig. 7A). A similar reduction in the length of leading processes was also evident in electroporated neurones that were migrating through the CP (Supplementary Fig. 2). These data confirm in vivo our observations previously made in vitro, where loss of Pak1 expression severely reduced the outgrowth of all neurites in transfected hippocampal neurones (Jacobs et al. 2007). We therefore conclude that a major function of Pak1 is to promote the organized extension of neurites.
Figure 7.
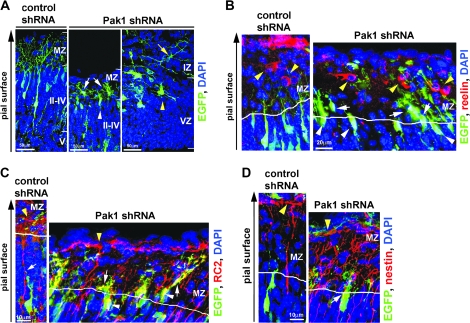
Pak1 is required for normal neuronal morphology and may affect attachment to radial glia. (A) Examples of Pak1 shRNA–expressing neurones that had migrated into the MZ (white arrows) were entering the MZ (white arrowhead) or remained in the IZ (yellow arrow). Increased ruffling was evident around the neuronal soma (yellow arrowhead). (B) The position of reelin (red)-positive Cajal–Retzius neurones (yellow arrowheads) and control or Pak1 shRNA–expressing neurones was compared by immunohistochemistry at P0. All EGFP-expressing neurones were positioned beneath Cajal–Retzius cells, although Pak1 shRNA–expressing neurones were closer. (C) RC2 (red)-expressing radial glia were visualized in electroporated brains revealing their lack of association with control shRNA–expressing neurones that had migrated to layers II–IV. In contrast, Pak1 shRNA–targeted neurones were seen in close proximity of the glial fibers, which appeared adjacent to the soma and apposed the apical neurites. (D) Immunodetection of nestin (red) confirmed the close apposition of radial progenitors and neurones expressing Pak1 shRNA that are entering the MZ. In (C) and (D), yellow arrowheads point to the glial end feet; white arrows mark overlapping radial glia and EGFP-positive neurones. EGFP (green), 4′,6-diamidino-2-phenylindole (blue); CP layers II–VI.
To further understand the mechanisms responsible for the accumulation of Pak1 shRNA–expressing neurones in the MZ, we examined their positions in relation to those of reelin-secreting Cajal–Retzius neurones. None of the EGFP-expressing neurones were copositive for reelin, confirming their aberrant presence in the MZ (Fig. 7B). Interestingly, Cajal–Retzius neurones were always located in more superficial positions than Pak1 shRNA–expressing neurones, although their proximity to the targeted neurones was greater than that observed in control brains (Fig. 7B). It is therefore likely that neurones with reduced Pak1 levels may still be able to respond, at least in part, to negative migration cues. However, their response may be compromised due to morphological defects such as the reduced outgrowth of leading processes.
A major factor in controlling the laminar position of cortical neurones is their ability to dissociate from radial glial fibers as they approach the MZ. This process has been reported to require signaling from integrin receptors and other as yet undefined junctional domain proteins (Anton et al. 1996). Interestingly, in nonneuronal cells, Pak1 is a known downstream effector from integrin signaling where its activity is regulated by cell adhesion (del Pozo et al. 2000; Zhou and Kramer 2005). It is therefore possible that loss of Pak1 caused entry of neurones into the MZ because of changes in their attachment to radial glia. To investigate this hypothesis, we compared the relationship between electroporated neurones and radial glia, the latter of which were identified by the expression of progenitor-specific markers, RC2, BLBP, and nestin (Kurtz et al. 1994; Chanas-Sacre et al. 2000; Hartfuss et al. 2001; Kalman and Ajtai 2001). The data for each marker were similar and were thus pooled together, revealing that on average 55 ± 3.2% (mean ± SEM) of control shRNA–expressing neurones in layers II–IV were in close apposition to radial fibers. In contrast, 91.3 ± 2.5% of the Pak1 shRNA–expressing neurones that were present in the MZ or at its border with cortical layers II–IV appeared to remain closely associated with the radial fibers (P < 0.001, Student's t-test). Our observations therefore suggest that Pak1 may be functionally required to induce the detachment of migrating neurones from radial glia at the basal border of the MZ and CP. We did not detect any major differences in radial glia morphology between control and Pak1 shRNA–targeted brains where RC2, BLBP, and nestin-stained fibers oriented perpendicularly to the pial surface and terminated in evident end feet (Fig. 7C,D). Close examination revealed some changes in the morphology of nestin-immunopositive radial fibers in Pak1 shRNA electroporated brains, with an apparent increase in complexity. These changes were restricted to the MZ and were not apparent in other parts of the cerebral cortex (Fig. 7D). The use of glial fibrillary acidic protein (GFAP) as a marker of mature glia revealed no differences in their overall number or morphology. No differences were observed in the organization of the laminin-enriched basement membrane (Supplementary Fig. 5). Together, our data suggest that changes in Pak1 levels primarily affect neuronal morphology and may influence their adhesiveness to radial glia. We found no evidence of altered differentiation profiles of radial glia and only minor consequences on their morphology. The fact that these changes are restricted to the MZ may reflect the increased presence of neurons in this normally cell-sparse layer.
Discussion
Pak1 is the most extensively studied member of the Pak family of kinases; however, despite its highest expression in the brain, to date, little is known of its role in the developing nervous system. Inhibition of all Group I Pak kinases has been examined in transgenic mice engineered to express the Pak autoinhibitory domain postnatally, at maximal levels after the cerebral cortex is fully formed (Hayashi et al. 2004). A reduction in the length and density of dendritic spines in layer II/III cortical neurones was observed, causing decreased memory consolidation. Interestingly, this inhibition of Pak rescued a mouse model of fragile X syndrome, which is characterized by a greater density and elongation of dendritic spines (Hayashi 07). Together, these results suggest that Group I Pak are responsible for balanced signaling to the neuronal cytoskeleton. Deletion of the mouse pak3 gene has been reported to cause severe abnormalities in synaptic plasticity; however, no developmental defects were observed which prompted the authors to suggest that Group I Pak kinases primarily control central nervous system (CNS) function rather than development (Meng et al. 2005). Significantly, these studies did not thoroughly explore the possibilities of compensatory mechanisms by Pak1 and/or Pak2 in pak3−/− mice. Furthermore, the consequences of specifically altering neuronal Pak1 or Pak2 in vivo in the developing CNS remained unexplored.
In this study, we have made the intriguing observation that migrating projection neurones and interneurones differ in their predominant enrichment of phosphorylated (activated) Pak. This finding was unexpected and significant particularly when contrasting the specific origins and modes of migration of these 2 types of neurones with their shared cortical laminar fate. Importantly, the homeobox transcription factors Dlx1/2 were recently shown to promote migration of interneurones from the ventral telencephalon to the neocortex by inhibiting the expression of Pak3 (Cobos et al. 2007). Premature appearance of Pak3 promoted extensive neurite outgrowth and consequently inhibited effective movement of interneurones from the MGE. We observed nuclear enrichment of Pak(P) in interneurones of the ventral telencephalon (data not shown), suggesting that its localized activity does not inhibit tangential movement of interneurones. Interestingly, Pak1 has been reported to regulate the function of a number of nuclear proteins, particularly during oncogenic transformation of cells (Kumar et al. 2006). Thus, Pak1 can phosphorylate transcriptional regulators NFκB, FKHR, ESR1, and SNAI1, all of which are expressed in the nervous system. Further investigations are required to establish whether nuclear activation of Pak influences the migration of interneurones from the ventral telencephalon into the cerebral cortex.
We have revealed that in vivo Pak1Caax expression causes a large proportion of cortical neurones to accumulate in the IZ, with apparently misoriented leading processes and altered morphologies. In contrast, the majority of Pak1T423E-expressing neurones successfully migrated to the CP, suggesting that activation of Pak1 at the plasma membrane has the most pronounced consequence on neuronal morphology and movement. The Pak1T423E mutant is primarily cytoplasmic due to the absence of engineered plasma membrane–targeting sequences such as the Caax box. However, recruitment of Pak1T423E to the plasma membrane by intracellular molecular pathways is possible (Nikolic M, unpublished data). It is therefore likely that neurones expressing Pak1T423E that remained in the IZ accumulated sufficient levels of plasma membrane Pak1T423E to exhibit a similar phenotype to the one seen following Pak1Caax expression. Their relatively small number may reflect the tight regulation of the molecular machinery, which acts to recruit Pak1 to the neuronal periphery. Significantly, increased accumulation of Pak1T423E- or Pak1Caax-expressing neurones was not observed in deep cortical layers V and VI, suggesting their uncompromised movement through the CP. Expression of Pak1R299Caax appeared to slightly delay migration through the deep cortical layers causing a small, but significant, reduction of neurones in layers II–IV. However, these results also confirmed that the catalytic activity of Pak1 is required to elicit a strong effect on neuronal migration. Interestingly, neurones expressing Pak1Caax that had accumulated in the IZ commonly exhibited incorrect orientations. In the vast majority of cases, the centrosome and Golgi localized to the base of the leading process, suggesting that uniform plasma membrane hyperactivation of Pak1 induces the aberrant orientation of leading lamellipodia. Significantly, some similarities exist between the Pak1 gain-of-function phenotype and the recently observed consequences of inhibited LKB1 or MARK2 kinase expression (Asada et al. 2007; Barnes et al. 2007; Sapir et al. 2008). Both LKB1 and MARK2 were shown to be required for normal polarization and migration of neurones with loss-of-function causing their accumulation in the IZ, which was attributed to functional alterations in centrosome–nuclear coupling or motility, respectively. In cultured hippocampal neurones, expression of LKB1 or MARK2 shRNA resulted in a multipolar phenotype which was highly similar to our previously observed effects of Pak1Caax expression (Chen et al. 2006; Asada et al. 2007; Jacobs et al. 2007). Downregulation of other kinases belonging to the MARK family, SADA and SADB, also affected axonal specification (Kishi et al. 2005). Together, our findings suggest that the subcellular localization of activated Pak1 plays an important role in the effectiveness of its function. Further investigations are required to establish the relationship between Pak1 and other signaling molecules such as the MARK family of kinases, which control neuronal polarity and morphology. In support, LKB1 was recently shown to positively influence the polarized activation of Pak1 by Cdc42 in nonsmall cell lung cancer tumors (Zhang et al. 2008).
Loss-of-expression studies revealed that in vivo Pak1 has a unique, nonredundant function. Proportionally, most neurones expressing Pak1 shRNA accumulated in the IZ with apparent multipolar morphologies, indicating their inability to enter the CP. These results are consistent with a number of previous reports where in utero electroporation was utilized to demonstrate that altered signaling to the cytoskeleton frequently inhibits neuronal entry into the CP. Often these effects are caused by aberrant transitions of migrating neurones between bipolar and multipolar morphologies (examples include JNK, Stef/Tiam, Map1b, Rac1, LIS1, Ndel1, doublecortin, Dclk, and MARK2) (Bai et al. 2003; Kawauchi et al. 2003, 2005, 2006; Shu et al. 2004; Tsai et al. 2005; Koizumi et al. 2006; Sapir et al. 2008). Closer examination of Pak1 shRNA–expressing neurones revealed a number of defects including shorter, misoriented neurites and increased somal lamellipodia, similar to our observations previously made in vitro (Jacobs et al. 2007). These results suggest that Pak1 is required for the correct formation and orientation of polarized protrusions, such as the leading and lagging migratory processes. Interestingly, reduction of Pak1 expression also caused the distribution of a number of neurones throughout all layers of the CP. This may reflect the nature of the shRNA approach where the severity of the phenotype depends on the levels and timing of shRNA expression. Thus, neurones arrested in the IZ may have lower levels of endogenous Pak1 than those that have migrated into the CP. Interestingly, our attempt to rescue the consequences of Pak1 loss was most effective in preventing neuronal entry into the MZ and least efficient in abolishing accumulation of neurones in the IZ, although in both cases significant improvements were evident. A likely explanation for these results is that expression of Pak1T423E was more effective at rescuing neurones already in the CP due to their higher levels of endogenous Pak1. They also confirmed that specifically Pak1, rather than Pak2 or Pak3, is required for radial migration of projection neurones in the cerebral cortex. Mechanistically, it is probable that Pak1T423E mRNAs acted to increase the pool of Pak1 RNAi targets, thus allowing sufficient levels of endogenous Pak1 mRNA to escape inhibition. However, our results do not exclude the possibility that all Group I Pak control neuronal migration, and it is quite possible that a more severe phenotype would have been evident following additional downregulation of Pak2 and/or Pak3. Alternatively, and as suggested for migrating interneurones, these 3 kinases may have nonoverlapping functions in controlling different aspects of neuronal motility.
Interestingly, to date, there have been no reports directly linking the function of Pak1 with cellular differentiation. In confirmation, no changes were evident in the pattern and distribution of progenitor and glial markers, nestin, RC2, BLBP, and GFAP following the reduction in Pak1 expression. However, we did observe a small but significant increase in Tbr2- and Tbr1-positive neurones. Our control samples revealed that in utero electroporation of mouse embryos at E14.5 primarily targets neurones destined for layers II–IV, as demonstrated by their peripheral localization and expression of Cdp/Cux. It is therefore interesting to note that only approximately half of the Pak1 shRNA–expressing neurones that had accumulated in the IZ had detectable Cdp/Cux expression, of which a minority exhibited high levels. These results suggest that a proportion of displaced neurones were still able to at least in part specify layers II–IV fate. Further studies will be required to determine whether the accumulation of Cdp/Cux-positive neurones in the IZ reflects a temporary or permanent migratory delay. The fate of IZ neurones with undetectable expression of Cdp/Cux is less clear. The presence of low levels of Tbr1 and absence of IZ marker NeuroD in most suggests that partial differentiation had occurred. Furthermore, the absence of Ctip2 or high levels of Tbr1 indicated the lack of fate switching to deep layer neurones, which was also confirmed in Pak1 shRNA–expressing neurones that had migrated to layers V and VI, respectively. One explanation for our findings is that expression of laminar-specific markers may be dependent on the correct positioning of a neurone. Therefore, the absence of Pak1 activity arrests neurones in the IZ and consequently delays or prevents neuronal differentiation. The examination of other layer II–IV markers such as Svet1 or Brn1 would further address this question (Tarabykin et al. 2001; Sugitani et al. 2002).
The unexpected consequence of Pak1 loss is the presence of targeted neurones in the MZ. Their migration into the MZ was most certainly aberrant because all the in utero electroporations were carried out using E14.5 mouse embryos and thus only labeled late-migrating neurones. Furthermore, none of the EGFP-expressing neurones were reelin positive, confirming that they are distinct from Cajal–Retzius cells. The mechanisms that stop migration at the interface between the CP and MZ involve a complex relationship between the migrating neurones, supporting glial precursors, the environment of the MZ, and basement membrane organization. Thus, the loss of extracellular (reelin), cell surface (α3β1 integrins, α6 integrin subunit, or neuron–glial junctional domain proteins), and signaling (Ilk) proteins have been associated with neuronal entry into the MZ (Anton et al. 1996; Georges-Labouesse et al. 1998; Anton et al. 1999; Sanada et al. 2004; Niewmierzycka et al. 2005). Interestingly, α3β1 integrin promotes the migration of oral squamous cell carcinoma cells by activation of Pak1 (Zhou and Kramer 2005). Furthermore, Ilk, which functions as a scaffold protein linking β1 integrin to the F-actin cytoskeleton, may induce Pak1 by activating Rac1 and Cdc42 or the Pak1-interactive exchange factor-α (αPIX) (Hannigan et al. 2005). However, the neuronal positioning defects observed in mice lacking β1 integrin or Ilk were attributed to a severely disrupted MZ or highly aberrant radial glia, respectively, putting to question the possibility that Pak1 may be their neuronal downstream target, at least during migration (Schmid et al. 2004; Niewmierzycka et al. 2005).
Interestingly, a recent report revealed a key role for the heteromeric G proteins G12 and G13 in preventing neuronal overmigration into the MZ (Moers et al. 2008). Thus, mice lacking expression of Gα12 and Gα13 were characterized by progressive invasion of neurones into the MZ, phenotypically evident from E15.5. The defects were proposed to be largely autonomous to neurones due to the absence of morphological abnormalities in radial glia and the late onset of basement membrane disruptions. Furthermore, neurones lacking Gα12 and Gα13 were nonresponsive to the repelling effects of lysophosphatidic acid (LPA), suggesting that RhoA was a likely downstream target. There have been indication that RhoA and Pak1 have functionally opposing roles (Royal et al. 2000; Alberts et al. 2005). However, Pak1 can also positively mediate LPA signaling as well as RhoA effectors (Schmitz et al. 2002). Recently published examples include a key role for Pak1 in LPA-dependent morphological changes for increased motility of human melanoma cells and fibroblast contractility on collagen matrices (Jung et al. 2004; Rhee and Grinnell 2006). It is therefore important to investigate the functional relationship among Gα12, Gα13, and Pak1 in the developing cerebral cortex. A further significant breakthrough was the recent suggestion that cullin 5 (Cul5), an important mediator of Dab1 degradation, is required for the repulsive effects of reelin in the MZ (Feng et al. 2007). Decreased expression of Cul5 induced a superficial position of targeted neurones, which predominantly localized at the boundary between the CP and the MZ. Importantly, loss of either Gα12/Gα13 or Cul5 had no consequence on the position of Cajal–Retzius neurones, which retained their superficial localization in relation to all cortical neurones, regardless of whether they resided in the CP or the MZ. Significantly, our data show that neurones with reduced Pak1 expression that had entered the MZ also located beneath Cajal–Retzius cell, suggesting a phenotypic similarity between these mutants. Further striking similarities were evident in the morphologies of neurones with reduced Cul5 or Pak1 expression, including short, disorganized/branched ascending processes and cell bodies that resided closer to Cajal–Retzius neurones. The evident phenotypic overlap between these loss-of-function models may suggest a functional link between the signaling pathways or the existence of regulatory factors that work in parallel to ensure the correct positioning of cortical neurones. Further studies will be required to evaluate the possible relationship among Cul5, Gα12/Gα13, and Pak1 as well as the response of neurones with reduced levels of Pak1 to the inhibitory effects of reelin.
It has been shown that later born neurones convert from a glial-dependent radial locomotion to somal translocation during the final stages of migration in the neocortex (Nadarajah et al. 2003). Therefore, a likely consequence of a shortened leading migratory process, caused by loss of Pak1 or Cul5, would be the inevitable presence of the soma in the MZ by the time the leading process contacts the basal lamina. Furthermore, our results suggest that neurones with reduced Pak1 remained in closer apposition to radial glial fibers than controls. Minor changes were evident in the morphology of radial glia; however, they were restricted to the MZ and may reflect the consequences of abnormal neuronal entry into this area. In confirmation, we did not detect any EGFP-expressing radial glia, indicating the non-cell autonomous nature of their morphological alterations. The possibility that changes in MZ organization occur in a secondary manner to neuronal defects was also previously observed in mice lacking Gα12 and Gα13 expression, where changes in the basement membrane occurred as a consequence of abnormal neuronal accumulation in the MZ (Moers et al. 2008).
Together, our results suggest that Pak1 is a key signaling protein that regulates the cytoskeletal changes that are responsible for the normal morphologies, movement, and positioning of neurones in the developing cerebral cortex.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
European Molecular Biology Organization (EMBO) long term fellowship to FC; Wellcome Trust project grant to MN (069441); Grant-in-Aids for Scientific Research, MEXT to YY.
Supplementary Material
Acknowledgments
We thank John Parnavelas and Sonja Rakić for their generous help and use of confocal microscopes, Nic Wells for all his support, David Turner for the mU6pro vector, Takeshi Kawauchi for technical advice, Junichi Miyazaki and Takeshi Kawauchi for pCAG-IRES-EGFP, André Goffinet for anti-reelin antibodies, Robert Hevner for anti-Tbr1 and anti-Tbr2 antibodies, and Sue Brown for laminin γ1 antibody. Conflict of Interest: None declared.
References
- Alberts AS, Qin H, Carr HS, Frost JA. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J Biol Chem. 2005;280:12152–12161. doi: 10.1074/jbc.M405073200. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Ang ES, Jr, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Anton ES, Cameron RS, Rakic P. Role of neuron-glial junctional domain proteins in the maintenance and termination of neuronal migration across the embryonic cerebral wall. J Neurosci. 1996;16:2283–2293. doi: 10.1523/JNEUROSCI.16-07-02283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Asada N, Sanada K, Fukada Y. LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J Neurosci. 2007;27:11769–11775. doi: 10.1523/JNEUROSCI.1938-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Worth D, Prowse DM, Nikolic M. Pak1 phosphorylation on t212 affects microtubules in cells undergoing mitosis. Curr Biol. 2002;12:1233–1239. doi: 10.1016/s0960-9822(02)00956-9. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Causeret F, Jacobs T, Terao M, Heath O, Hoshino M, Nikolic M. Neurabin-I is phosphorylated by Cdk5: implications for neuronal morphogenesis and cortical migration. Mol Biol Cell. 2007;18:4327–4342. doi: 10.1091/mbc.E07-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanas-Sacre G, Thiry M, Pirard S, Rogister B, Moonen G, Mbebi C, Verdiere-Sahuque M, Leprince P. A 295-kDA intermediate filament-associated protein in radial glia and developing muscle cells in vivo and in vitro. Dev Dyn. 2000;219:514–525. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1078>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Chen YM, Wang QJ, Hu HS, Yu PC, Zhu J, Drewes G, Piwnica-Worms H, Luo ZG. Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proc Natl Acad Sci USA. 2006;103:8534–8539. doi: 10.1073/pnas.0509955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C, Tan L, Lim L, Manser E. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem. 2001;276:17347–17353. doi: 10.1074/jbc.M009316200. [DOI] [PubMed] [Google Scholar]
- Cobos I, Borello U, Rubenstein JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54:873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcangelo G. Reelin mouse mutants as models of cortical development disorders. Epilepsy Behav. 2006;8:81–90. doi: 10.1016/j.yebeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Price LS, Alderson NB, Ren XD, Schwartz MA. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 2000;19:2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, Kreidberg JA, Anton ES. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- Feng L, Allen NS, Simo S, Cooper JA. Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev. 2007;21:2717–2730. doi: 10.1101/gad.1604207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrere A, Vitalis T, Gingras H, Gaspar P, Cases O. Expression of Cux-1 and Cux-2 in the developing somatosensory cortex of normal and barrel-defective mice. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:158–165. doi: 10.1002/ar.a.20284. [DOI] [PubMed] [Google Scholar]
- Friocourt G, Kanatani S, Tabata H, Yozu M, Takahashi T, Antypa M, Raguenes O, Chelly J, Ferec C, Nakajima K, et al. Cell-autonomous roles of ARX in cell proliferation and neuronal migration during corticogenesis. J Neurosci. 2008;28:5794–5805. doi: 10.1523/JNEUROSCI.1067-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, Chattarji S, Kirkwood A, Tonegawa S. Altered cortical synaptic morphology and impaired memory consolidation in forebrain-specific dominant-negative PAK transgenic mice. Neuron. 2004;42:773–787. doi: 10.1016/j.neuron.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Hevner RF. Layer-specific markers as probes for neuron type identity in human neocortex and malformations of cortical development. J Neuropathol Exp Neurol. 2007;66:101–109. doi: 10.1097/nen.0b013e3180301c06. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Higginbotham HR, Gleeson JG. The centrosome in neuronal development. Trends Neurosci. 2007;30:276–283. doi: 10.1016/j.tins.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Jacobs T, Causeret F, Nishimura YV, Terao M, Norman A, Hoshino M, Nikolic M. Localized activation of p21-activated kinase controls neuronal polarity and morphology. J Neurosci. 2007;27:8604–8615. doi: 10.1523/JNEUROSCI.0765-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ID, Lee J, Lee KB, Park CG, Kim YK, Seo DW, Park D, Lee HW, Han JW, Lee HY. Activation of p21-activated kinase 1 is required for lysophosphatidic acid-induced focal adhesion kinase phosphorylation and cell motility in human melanoma A2058 cells. Eur J Biochem. 2004;271:1557–1565. doi: 10.1111/j.1432-1033.2004.04066.x. [DOI] [PubMed] [Google Scholar]
- Kalman M, Ajtai BM. A comparison of intermediate filament markers for presumptive astroglia in the developing rat neocortex: immunostaining against nestin reveals more detail, than GFAP or vimentin. Int J Dev Neurosci. 2001;19:101–108. doi: 10.1016/s0736-5748(00)00058-7. [DOI] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration. EMBO J. 2003;22:4190–4201. doi: 10.1093/emboj/cdg413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nishimura YV, Nabeshima Y, Hoshino M. MAP1B phosphorylation is differentially regulated by Cdk5/p35, Cdk5/p25, and JNK. Biochem Biophys Res Commun. 2005;331:50–55. doi: 10.1016/j.bbrc.2005.03.132. [DOI] [PubMed] [Google Scholar]
- Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Tanaka T, Gleeson JG. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006;49:55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- Lee JK, Cho JH, Hwang WS, Lee YD, Reu DS, Suh-Kim H. Expression of neuroD/BETA2 in mitotic and postmitotic neuronal cells during the development of nervous system. Dev Dyn. 2000;217:361–367. doi: 10.1002/(SICI)1097-0177(200004)217:4<361::AID-DVDY3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Leid M, Ishmael JE, Avram D, Shepherd D, Fraulob V, Dolle P. CTIP1 and CTIP2 are differentially expressed during mouse embryogenesis. Gene Expr Patterns. 2004;4:733–739. doi: 10.1016/j.modgep.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H. Cell motility: Golgi signalling shapes up to ship out. Curr Biol. 2004;14:R434–R435. doi: 10.1016/j.cub.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Meng J, Meng Y, Hanna A, Janus C, Jia Z. Abnormal long-lasting synaptic plasticity and cognition in mice lacking the mental retardation gene Pak3. J Neurosci. 2005;25:6641–6650. doi: 10.1523/JNEUROSCI.0028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metin C, Baudoin JP, Rakic S, Parnavelas JG. Cell and molecular mechanisms involved in the migration of cortical interneurons. Eur J Neurosci. 2006;23:894–900. doi: 10.1111/j.1460-9568.2006.04630.x. [DOI] [PubMed] [Google Scholar]
- Moers A, Nurnberg A, Goebbels S, Wettschureck N, Offermanns S. Galpha12/Galpha13 deficiency causes localized overmigration of neurons in the developing cerebral and cerebellar cortices. Mol Cell Biol. 2008;28:1480–1488. doi: 10.1128/MCB.00651-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Neuronal migration in the developing cerebral cortex: observations based on real-time imaging. Cereb Cortex. 2003;13:607–611. doi: 10.1093/cercor/13.6.607. [DOI] [PubMed] [Google Scholar]
- Nakajima K. Control of tangential/non-radial migration of neurons in the developing cerebral cortex. Neurochem Int. 2007;51:121–131. doi: 10.1016/j.neuint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, Philpott A, Roberts JM, Guillemot F. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- Niewmierzycka A, Mills J, St-Arnaud R, Dedhar S, Reichardt LF. Integrin-linked kinase deletion from mouse cortex results in cortical lamination defects resembling cobblestone lissencephaly. J Neurosci. 2005;25:7022–7031. doi: 10.1523/JNEUROSCI.1695-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Olson EC, Kim S, Walsh CA. Impaired neuronal positioning and dendritogenesis in the neocortex after cell-autonomous Dab1 suppression. J Neurosci. 2006;26:1767–1775. doi: 10.1523/JNEUROSCI.3000-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- Rashid T, Banerjee M, Nikolic M. Phosphorylation of Pak1 by the p35/Cdk5 kinase affects neuronal morphology. J Biol Chem. 2001;276:49043–49052. doi: 10.1074/jbc.M105599200. [DOI] [PubMed] [Google Scholar]
- Rhee S, Grinnell F. P21-activated kinase 1: convergence point in PDGF- and LPA-stimulated collagen matrix contraction by human fibroblasts. J Cell Biol. 2006;172:423–432. doi: 10.1083/jcb.200505175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11:1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada K, Gupta A, Tsai LH. Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron. 2004;42:197–211. doi: 10.1016/s0896-6273(04)00222-3. [DOI] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Wang X, Schrick C, Neve R, Radulovic J, Tsai LH. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci. 2007;10:1012–1019. doi: 10.1038/nn1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir T, Sapoznik S, Levy T, Finkelshtein D, Shmueli A, Timm T, Mandelkow EM, Reiner O. Accurate balance of the polarity kinase MARK2/Par-1 is required for proper cortical neuronal migration. J Neurosci. 2008;28:5710–5720. doi: 10.1523/JNEUROSCI.0911-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid RS, Jo R, Shelton S, Kreidberg JA, Anton ES. Reelin, integrin and DAB1 interactions during embryonic cerebral cortical development. Cereb Cortex. 2005;15:1632–1636. doi: 10.1093/cercor/bhi041. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Shelton S, Stanco A, Yokota Y, Kreidberg JA, Anton ES. Alpha3beta1 integrin modulates neuronal migration and placement during early stages of cerebral cortical development. Development. 2004;131:6023–6031. doi: 10.1242/dev.01532. [DOI] [PubMed] [Google Scholar]
- Schmitz U, Thommes K, Beier I, Vetter H. Lysophosphatidic acid stimulates p21-activated kinase in vascular smooth muscle cells. Biochem Biophys Res Commun. 2002;291:687–691. doi: 10.1006/bbrc.2002.6493. [DOI] [PubMed] [Google Scholar]
- Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 2004;44:263–277. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- Sugitani Y, Nakai S, Minowa O, Nishi M, Jishage K, Kawano H, Mori K, Ogawa M, Noda T. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 2002;16:1760–1765. doi: 10.1101/gad.978002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A, Hamasaki T, Litwack ED, O'Leary DD. Novel IgCAM, MDGA1, expressed in unique cortical area- and layer-specific patterns and transiently by distinct forebrain populations of Cajal-Retzius neurons. Cereb Cortex. 2007;17:1531–1541. doi: 10.1093/cercor/bhl064. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170:935–945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Kawauchi T, Sone M, Nishimura YV, Terao M, Chihama K, Nabeshima Y, Hoshino M. Involvement of a Rac activator,P-Rex1, in neurotrophin-derived signaling and neuronal migration. J Neurosci. 2005;25:4406–4419. doi: 10.1523/JNEUROSCI.4955-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci. 2005;25:3379–3388. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Schafer-Hales K, Khuri FR, Zhou W, Vertino PM, Marcus AI. The tumor suppressor LKB1 regulates lung cancer cell polarity by mediating cdc42 recruitment and activity. Cancer Res. 2008;68:740–748. doi: 10.1158/0008-5472.CAN-07-2989. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–249. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Zhong JL, Banerjee MD, Nikolic M. Pak1 and its T212 phosphorylated form accumulate in neurones and epithelial cells of the developing rodent. Dev Dyn. 2003;228:121–127. doi: 10.1002/dvdy.10351. [DOI] [PubMed] [Google Scholar]
- Zhou H, Kramer RH. Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J Biol Chem. 2005;280:10624–10635. doi: 10.1074/jbc.M411900200. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Tiveron MC, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–1420. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.