Abstract
In the embryonic telencephalon, the pallial–subpallial boundary (PSB) separates the dorsal Pax6+ pallium from the ventral Gsh2+ subpallium. Previous studies have revealed that this region is a source of cells that will populate both the olfactory bulb and basal telencephalic limbic system. However, the level of progenitor cell heterogeneity and developmental genetic regulation of this progenitor region remains to be fully elucidated. In this study we carried out a comprehensive analysis of gene expression patterns at the PSB, in addition to an examination of the combinatorial function of Pax6 and Gsh2 in the specification of the PSB. First, we reveal that the PSB is comprised of a complex mix of molecularly distinct progenitor pools. In addition, by analysis of single Sey, Gsh2, and Sey/Gsh2 double mutant mice, we demonstrate that both Pax6 and Gsh2 are directly required for major aspects of PSB progenitor specification. Our analysis also reveals that the establishment of the epidermal growth factor receptor positive lateral cortical stream migratory route to the basal telencephalon is Pax6 dependent. Thus, in addition to their well-characterized cross-repressive roles in dorsal/ventral patterning our analyses reveal important novel functions of Gsh2 and Pax6 in the regulation of PSB progenitor pool specification and patterning.
Keywords: cortico-striatal border, development, gene expression, lateral cortical stream, mutant, small eye (Sey)
Introduction
The embryonic telencephalon can be parcellated into 2 broad progenitor zones, the pallium and subpallium, which are responsible for generating, in a highly precise and complex manner, telencephalic excitatory and inhibitory neurons, respectively (reviewed in Corbin et al. 2001; Marín and Rubenstein 2001; Wonders and Anderson 2006). The differential and temporally regulated expression patterns of pallial (e.g., Pax6, Emx1, Ngn2) and subpallial (e.g., Gsh2, Mash1, Dlx family members) genes define discrete progenitor domains that give rise to neuronal diversity in several telencephalic regions such as the cerebral cortex (Nery et al. 2002; López-Bendito et al. 2004; Xu et al. 2004; Butt et al. 2005; Flames et al. 2007; Fogarty et al. 2007; Miyoshi et al. 2007), olfactory bulb (OB) (Wichterle et al. 1999; Stenman, Toresson, et al. 2003; Kohwi et al. 2005; Vergaño-Vera et al. 2006; Waclaw et al. 2006; Kohwi et al. 2007), amygdala (Wichterle et al. 2001; Gorski et al. 2002; Nery et al. 2002; Remedios et al. 2007), and adult subventricular zone (SVZ) (De Marchis et al. 2007; Kohwi et al. 2007; Young et al. 2007). Emerging evidence also interestingly indicates that in a manner similar to the spinal cord, combinatorial codes of regionally expressed transcription factors endow telencephalic progenitor populations with the intrinsic potential to generate distinct neuronal cell types (reviewed in Wonders and Anderson 2006; Molyneaux et al. 2007).
From early stages of telencephalic development (by approximately embryonic day 10.0 [E10.0]), the pallium and subpallium are identified by the expression of the homeodomain containing genes Pax6 and Gsh2, respectively. The pallial–subpallial boundary (PSB, also known as the cortico-striatal border) is the region where high Pax6 expression (Pax6 is also expressed at a low level in the dorsal-most aspect of the lateral ganglion eminence (LGE) [dLGE] ventricular zone [VZ]) abuts Gsh2 expression near the cortico-striatal angle. The PSB is comprised of neural progenitor cells from both the ventral-most aspect of the pallium (VP) and dLGE (Puelles et al. 1999; Puelles et al. 2000; Yun et al. 2001; Stenman, Toresson, et al. 2003). In addition, previous studies have also revealed that progenitor cells of the PSB transiently express Sfrp2, Dbx1 (VP) and Sp8, mTsh1 (dLGE) that collectively mark this region as molecularly distinct from the rest of the telencephalon (Puelles et al. 2000; Kim et al. 2001; Caubit et al. 2005; Waclaw et al. 2006). Emerging evidence from our work (Carney et al. 2006) and that of others (Fernandez et al. 1998; Puelles et al. 1999; Puelles et al. 2000; Hirata et al. 2002; Stenman, Yu, et al. 2003; Bai et al. 2008) has revealed that a major target of lateral cortical stream (LCS) migrating PSB neurons (VP and dLGE) is the basal telencephalic limbic system, most prominently the amygdala and piriform cortex. Moreover, progenitors from the PSB generate one of two main subgroups of Cajal–Retzius neurons in the pallium (Bielle et al. 2005), as well as OB interneurons derived from the rostral migratory stream (RMS) (Waclaw et al. 2006; Kohwi et al. 2007). Thus, this region is an important source of telencephalic cell populations.
Previous studies have revealed that Pax6 and Gsh2 are required for proper positioning of the PSB by functioning to genetically cross repress each other (Toresson et al. 2000; Yun et al. 2001; Corbin et al. 2003; Stenman, Yu, et al. 2003; Yun et al. 2003). As such, the lack of Pax6 in Sey (Small eye; Hill et al. 1991) mutant mice results in an expansion of subpallial markers such as Dlx1, Dlx2, Gsh2, Mash1, Vax1 into the pallium (Stoykova et al. 1996; Corbin et al. 2000; Stoykova et al. 2000; Toresson et al. 2000; Yun et al. 2001; Kroll and O'Leary 2005). Conversely, in Gsh2 mutant mice (Szucsik et al. 1997) pallial markers such as Pax6, Ngn2, Math2, Tbr1 extend ventrally into the LGE (Corbin et al. 2000; Toresson et al. 2000; Toresson and Campbell 2001; Yun et al. 2001). Thus, in Sey mutant mice VP and lateral pallial (LP) progenitors acquire subpallial properties, whereas in Gsh2 mutant mice the dLGE is respecified to a pallial identity. Loss of VP markers, Sfrp2, Dbx1, and Tgfα in Sey mutant mice has suggested a direct requirement of Pax6 for their expression (Kim et al. 2001; Yun et al. 2001; Assimacopoulos et al. 2003; Stenman, Yu, et al. 2003). Similarly, dLGE development and gene expression is severely affected in Gsh2 mutants (Corbin et al. 2001; Yun et al. 2001; Waclaw et al. 2006). However, the interpretation of the genetic dependence of Pax6 for PSB gene expression in Sey mutants in confounded by ectopic Gsh2 expression in the VP and LP. Conversely, in Gsh2 mutants, a direct function of Gsh2 cannot be dissociated from ectopic Pax6 expression in the dLGE.
The purpose of this study was 2-fold: First, we sought to systematically analyze the expression patterns of PSB-specific genes during key developmental time points of neurogenesis. Second, we wanted to comprehensively dissect the individual and combinatorial roles of Pax6 and Gsh2 in PSB patterning, thereby providing an in-depth understanding of the genetic requirement for the establishment of neural diversity in PSB-derived structures. Our expression studies reveal that the PSB is a highly complex and dynamic telencephalic progenitor zone, comprised of multiple molecularly distinct progenitor pools. Furthermore, gene expression analysis in single Sey and Gsh2 mutant mice and Sey/Gsh2 double mutant mice, reveals that in the absence of both Pax6 and Gsh2 all examined aspects of patterning of the PSB remain abnormal. This indicates that these genes, in combination, are required for correct positioning and expression of PSB-specific genes. Moreover, analysis of markers that individually label the VP and dLGE further reveals a complex and novel differential regulation of PSB progenitor pool specification by Gsh2 and Pax6, which changes over time during development. Furthermore, we show that epidermal growth factor receptor positive (EGFR+) cells, that mark the LCS migratory population from the PSB, are also differentially regulated by Pax6 and Gsh2. Thus, our study provides important insights into the genetic requirement for the generation of the PSB, a major progenitor region for the generation of OB and limbic system cell diversity.
Materials and Methods
Animal Use
All animals used in the study were maintained according to protocols approved by the Animal Welfare and use committee at Children's National Medical Center, Washington, DC, and adhering to all animal welfare laws. For staging of the embryos, midday of the day of vaginal plug detection was considered as E0.5. Previously published Gsh2 (Szucsik et al. 1997) and Pax6 (Sey) mutants (Hill et al. 1991) were used in this study. Adult Gsh2+/− and Sey/+ heterozygotes were maintained by crosses to Swiss Webster mice (Taconic, Albany, NY). In all cases, single Gsh2−/−, Sey/Sey (referred to as Sey mutant), or Sey/Sey;Gsh2−/− double homozygous mutant (referred to as Sey/Gsh2 double mutant) embryos were generated by heterozygous crosses as previously described (Corbin et al. 2000; Toresson et al. 2000; Yun et al. 2001; Waclaw et al. 2004, 2006). In some crosses, mice also carried the Dlx2+/tauLacZ allele, and in these cases embryos and brains were stained for X-gal to visualize Dlx2 (Corbin et al. 2000). For the in situ hybridization analyses, the Gsh2−/−, Sey/Sey, and Sey/Gsh2 double mutant genotypes were each processed independently with littermate heterozygote and wild-type mice used as controls to ensure correct RNA probe labeling. A minimum of 3 mutants and controls were used for all genotypes, n numbers are given in the figure legends.
Genotyping
Genomic DNA was isolated by phenol:chloroform extraction. Genotyping of Gsh2 adult mice and embryos was carried out by PCR using previously described methods (Stenman, Wang, et al. 2003). PCR was performed using 2 primers (for adult genotyping) or 3 primers (for embryo genotyping) using a GC rich kit (Roche, Indianapolis, IN). Sey mutant embryos were identified by lack of eyes and abnormal cranio-facial features (Hill et al. 1991). Additional confirmation of genotypes was carried out on sections by analysis of known, for example, Dlx2 (RNA or X-gal staining of Dlx2+/tauLacZ sections) (Corbin et al. 2000) expression changes in Gsh2 and Sey mutant embryos. Sey/Gsh2 double mutant sections were confirmed by the lack of Pax6 and Gsh2 protein in addition to the lack of eyes and PCR for Gsh2 mutant and wild-type alleles. Male heterozygous Dlx5/6−cre-iresEGFP (Stenman, Toresson, et al. 2003, herein referred to as Dlx5/6-GFP) mice were crossed with Swiss Webster females to generate Dlx5/6-GFP+ embryos. The adults were genotyped by PCR for Cre (CRE-F: GCGGTCTGGCAGTAAAAACTATC; CRE-R: GTGAAACAGCATTGCTGTCACTT) and the embryos were identified by GFP fluorescence using a dissecting microscope.
Nonradioactive Dioxygenin-Labeled RNA In Situ Hybridization
Whole heads (E12.5, E13.5) or isolated brains (E15.5) were fixed at 4 °C in 4% paraformaldehyde (PFA) (in 0.1 M phosphate buffer, pH 7.4) (4% PFA) overnight then cryoprotected in graded sucrose concentrations (10%, 20% then 30% overnight) before embedding in Tissue-Tek OCT compound (Sakura Finetek USA, Inc., Torrance, CA). Coronal sections at a thickness of 20 (E12.5, E13.5) or 30 μm (E15.5) were prepared using a cryostat (Microm HM505E, GMI, Inc., Ramsey, MN).
Section RNA in situ hybridization was carried out by slight modifications of previously described protocols (Schaeren-Wiemers and Gerfin-Moser 1993; Wilkinson and Nieto 1993). RNA probes were prepared using dioxygenin (DIG) RNA labeling kits (Roche). Air-dried cryostat sections were postfixed in 4% PFA for 10 min followed by 2 × 5 min rinses in phosphate-buffered saline (PBS). Proteinase K (Roche) digestion (20 μg/mL in PBS) was carried out for 6 min followed by 1 × 5 min rinse in PBS, refixing for 5 min in 4% PFA and another PBS rinse. The sections were acetylated for 10 min (2.2 g triethanolamine hydrochloride [Acros Organics, Geel, Belgium], 540 μL of 10 N NaOH [Fisher Scientific, Pittsburgh, PA], 300 μL of acetic anhydride [Sigma, St Louis, MO] in 60 mL of molecular grade water [Cellgro, Herndon, VA], prior to 3 × 5 min rinses in PBS). RNA probes, prepared at a dilution of 2 μL/mL of hybridization solution (50% formamide [Invitrogen, Carlsbad, CA], 10% dextran sulfate, 1% 100× Denhart's, 250 μg/mL yeast tRNA, 0.3 M NaCl, 20 mM Tris–HCl, pH8, 5 mM ethylene diaminetetraacetic acid [EDTA], 10 mM NaPO4, 1% sarcosyl [all from Sigma except where indicated] in diethylpyrocarbonate-treated H2O [Invitrogen]), were incubated at 80 °C for 2 min. Thereafter, 250 μL of the probe mix was applied to each slide, coverslipped with Hybri-slips (Sigma) and placed in a sealed box humidified with 50% formamide and H2O and incubated at 55 °C overnight. The next day, the Hybri-slips were floated off by placing the slides in 5× saline-sodium citrate buffer (Cellgro), prior to a 30-min high stringency wash in prewarmed 50% formamide, 2× SSC at 65 °C. Next, the sections were rinsed in 3 × 10 min rinses in RNase buffer (0.5 M NaCl, 10 mM Tris–HCl, pH 7.5, 5 mM EDTA), followed by RNaseA (Roche) treatment (20 μg/mL in RNase buffer) for 30 min and one 15 min rinse in RNase buffer, all at 37 °C. The high stringency washes were repeated twice for 20 min each at 65 °C, followed by a 15 min rinse in 2× SSC, then 0.1× SSC, both at 37 °C and a PBT (PBS + 0.1% Tween 20; Sigma) rinse for 15 min at RT. The sections were blocked with 10% goat serum in PBT for 1 h at RT, prior to a 3-h incubation with an alkaline phosphatase-coupled anti-DIG antibody (1:5000 in 1% goat serum in PBT; Roche) in a humidified chamber at RT. Then, the sections were rinsed extensively in PBT at RT (4 × 15 min rinses) and then underwent 2 × 10 min rinses in freshly prepared NTMT buffer (100 mM NaCl, 100 mM Tris–HCl, pH 9.5, 50 mM MgCl2, 0.1% Tween 20). The sections were then placed in a light-protected humidified chamber with approximately 400 μL of BM-purple AP substrate (Roche) containing ∼0.25 mg/mL levamisol (Sigma) until satisfactory staining was achieved, typically overnight. Finally, the sections were rinsed twice in PBS, coverslipped using Crystal mount aqueous mounting media (Sigma) and photographed immediately.
The following probes were used in this study: Dbx1 (Lu et al. 1992; Yun et al. 2001), Dlx2 (Porteus et al. 1991), Dlx5 (Eisenstat et al. 1999), Er81 (Stenman, Toresson, et al. 2003), Gsh1 (Toresson and Campbell, 2001), Sfrp2 (Kim et al. 2001), Sp8 (Bell et al. 2003; Waclaw et al. 2006), Tgfα (Vaughan et al. 1992; Assimacopoulos et al. 2003), and mTsh1 (Caubit et al. 2005).
Immunohistochemistry
Sections prepared as described above for in situ hybridization analysis were air-dried and underwent microwave heat antigen retrieval (except for Dlx5/6-GFP+ brains which were fixed for 4 h and did not require antigen retrieval) in 10 mM sodium citrate (Fisher Scientific) pH 6.0 prior to several rinses in PBS. Subsequently, blocking of nonspecific binding sites was achieved using 10% normal serum diluted in PBS with 0.3% Triton-X (PBST; Sigma) to aid permeabilization. Primary antibodies, goat anti-β-galactosidase (β-gal) (1:200, Biogen, Cambridge, MA), sheep anti-EGFR (1:20; Millipore, Bedford, MA), rabbit anti-Gsh2 (1:2000; Toresson et al. 2000; kind gift of K. Campbell), mouse anti-Pax6 (1:2000; Developmental Studies Hybridoma Bank [DSHB], University of Iowa, Iowa City, IA), rabbit anti-Pax6 (1:1000; Covance Research Products, Berkeley, CA), mouse anti-RC2 (1:20; DSHB), rabbit anti-Tbr1 (1:1000; Hevner et al. 2001; kind gift of R. Hevner) were diluted in 1% normal serum in PBST and incubated overnight at 4 °C. Following 3 × 10 min rinses in PBS, the sections were incubated with the appropriate secondary Cy3- (1:200) or fluorescein isothiocyanate- (1:50) conjugated antibodies (serum and secondary antibodies were from Jackson Immunoresearch, West Grove, PA), diluted as for the primary antibodies, in the dark for 2 h at RT. After further rinses in PBS, nuclear counterstaining was performed by incubation in To-Pro-3 iodide (To-Pro-3; 1:100, Invitrogen) for 10 min at RT. Final rinses in PBS were carried out prior to coverslipping using Gel Mount aqueous mounting media (Sigma).
Data Analysis
Analysis of in situ hybridization experiments was performed using brightfield microscopy (Olympus BX51, Olympus, Center Valley, PA) and high-resolution digital images were captured under a ×4 objective using an Olympus D570 camera. For fluorescence, digital photographs were obtained from epifluorescence microscopy (Olympus BX61) and selected for further analysis using a Zeiss (Thornwood, NY) LSM 510 META confocal microscope. For confocal analysis, each fluorophore was scanned sequentially and Z-stacks of the images obtained were collapsed into a single projection image, or presented as individual optical sections using the LSM 510 software (Zeiss). To assess immuno-colocalization, 3 sections from at least n = 2 brains (values given in figure legends) were examined. Figures were prepared using Adobe Photoshop CS software (Adobe Systems, San Jose, CA). Adjustments to contrast were applied across each image as a whole and equally to control and mutant brains.
Results
The PSB is defined as the location of the border between progenitor cells of pallial (Pax6+) and subpallial (Gsh2+) identity (Yun et al. 2001; Corbin et al. 2003; Stenman, Yu, et al. 2003; Yun et al. 2003). To examine the regulation of PSB patterning by Gsh2 and Pax6, we used a series of well-characterized in situ hybridization probes that mark the VZ and/or SVZ progenitor cell compartments of either both the pallial and subpallial aspects of the PSB (Er81), dLGE (Sp8, mTsh1), or the VP (Sfrp2, Dbx1, Tgfα). These analyses were carried out at 2 developmental time points, E13.5 and E15.5, which represent the peak of severity of patterning defects in Gsh2 and Sey mutants (Corbin et al. 2000; Toresson et al. 2000; Yun et al. 2001; and data shown here) and partial recovery of the striatal phenotype in Gsh2 mutants (Corbin et al. 2000; Toresson and Campbell 2001; Yun et al. 2001; Yun et al. 2003), respectively. As the expression of Pax6 occurs in a high-rostral to low-caudal gradient (Walther and Gruss 1991; Stoykova and Gruss 1994), we carried out this analysis at 2 rostro-caudal levels for each marker.
Pax6 and Gsh2 are Required for the Correct Expression of Er81 at the PSB
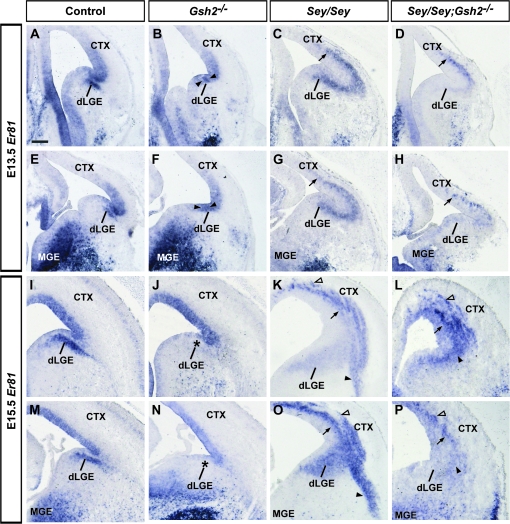
Er81 is a member of the ETS transcription factor family and is highly expressed in the dLGE SVZ, VP VZ, and other pallial and subpallial regions (Fig. 1A,E,I,M) (Stenman, Wang, et al. 2003; Flames et al. 2007). In Gsh2 mutants at both E13.5 and E15.5, Er81 expression in the dLGE SVZ is missing and there is an expansion of the domain of VZ expression at E13.5 (Fig. 1B,F,J,N). In Sey mutants at both ages, Er81 expression is absent in the VZ of the VP that is combined with an expanded expression in the SVZ in both the mutant dLGE and VP domains (Fig. 1C,G,K,O). In Sey/Gsh2 double mutants, Er81 expression remains abnormal and, although slightly less severe, closely resembles that observed in single Sey mutants at both E13.5 and E15.5 (Fig. 1D,H,L,P). Interestingly, Er81 expression reveals nascent paraventricular ectopia as previously described in Sey mutant pallium (Kroll and O'Leary 2005), which are also observed in the double mutant.
Figure 1.
Expression of Ets transcription factor Er81 at E13.5 and E15.5. Er81 is expressed in the SVZ of the dLGE and VZ of the VP as shown at E13.5 in controls (A, E). VZ expression expands ventrally in Gsh2 mutant mice (B, F, arrowheads). In Sey mutants, SVZ expression extends ectopically into the cerebral cortex (CTX, also termed the pallium) (C, G, arrows). In Sey/Gsh2 double mutants, ectopic pallial expression of Er81 resembles the Sey mutant phenotype, but is weaker (D, H, arrows). At E15.5, the dLGE SVZ expression of Er81 in controls (I, M) is missing in the Gsh2 mutant (J, N, asterisks). Conversely, in Sey single mutants (K, O) and Sey /Gsh2 double mutants (L, P), Er81 is ectopically expressed in the pallium (arrows) and forms nascent paraventricular ectopia (K, L, O, P, empty arrowhead). In the Sey mutant, a basal stream of Er81 expression is also observed (K, O, arrowheads), which is not present in the double mutant (L, P, arrowhead). n numbers are as follows: controls, n = 5 [E13.5], n = 5 [E15.5]; Gsh2−/−, n = 6 [E13.5], n = 5 [E15.5]; Sey /Sey, n = 5 [E13.5], n = 5 [E15.5]; Sey /Sey ;Gsh2−/−, n = 5 [E13.5], n = 5 [E15.5]. Scale bar: 200 μm.
Differential Regulation of dLGE Sp8 and mTsh1 Expression by Gsh2 and Pax6
Next, we examined 2 zinc finger transcription factors, Sp8 and mTsh1, which are expressed in the SVZ of the dLGE aspect of the PSB. Recent studies have revealed that Sp8 is required for the specification, migration and survival of OB interneurons (Waclaw et al. 2006) and plays a role in cortical arealization (Sahara et al. 2007; Zembrzycki et al. 2007). The mouse teashirt family of genes (mTsh1-3) encode zinc finger DNA binding proteins that are also expressed in the developing brain (Caubit et al. 2005).
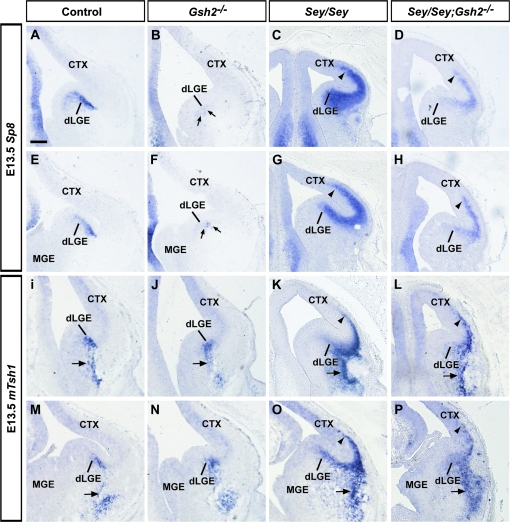
At E13.5, Sp8 is expressed at very high levels in the SVZ of the dLGE and at lower levels in the progenitor zones of the dorsolateral and dorsomedial cortex (Fig. 2A,E) (Waclaw et al. 2006). It has previously been shown that in Gsh2 mutant mice Sp8 protein is missing at E12.5, with a partial recovery of this expression by E16.5 (Waclaw et al. 2006). Consistent with this observation, we find a striking reduction in Sp8 expression at E13.5 in Gsh2 mutants (Fig. 2B,F). In contrast, in Sey mutant mice, there is an expansion of Sp8 mRNA into the Sey mutant pallium at E13.5 (Fig. 2C,G). In Sey/Gsh2 double mutants, although there appears to be some improvement of ectopic Sp8 expression, in general Sp8 expression remains abnormal (Fig. 2D,H).
Figure 2.
Expression of dLGE markers Sp8 and mTsh1 at E13.5. Sp8 is strongly expressed in the dLGE SVZ in controls at E13.5 (A, E). The level of expression is markedly diminished in Gsh2 mutants (B, F, arrows). The loss of Pax6 in Sey mutants results in ectopic pallial expression of Sp8 (C, G, arrowheads). In Sey/Gsh2 double mutant mice, this ectopic expression, although improved over the Sey single mutant phenotype, persists (D, H, arrowheads). At E13.5, mTsh1 is expressed in the dLGE SVZ and also the putative LCS migratory route (I, M, arrows). This expression is generally unaffected in Gsh2 mutants (J, N, arrows). Conversely, in the Sey mutant, ectopic mTsh1 expression is observed in both the pallium (K, O, arrowheads) and basally (K, O, arrows).This mispatterning is generally similar in the Sey/Gsh2 double mutants (L, P, arrows, arrowheads). Abbreviation: n numbers for each probe are as follows: controls, n = 4 [Sp8], n = 6 [mTsh1]; Gsh2−/−, n = 4 [Sp8], n = 4 [mTsh1]; Sey/Sey, n = 4 [Sp8], n= 5 [mTsh1]; Sey/Sey;Gsh2−/−, n= 5 [Sp8], n = 5 [mTsh1]. Scale bar: 200 μm.
Similar to Sp8, mTsh1 expression marks the dLGE SVZ. However, in contrast to Sp8, mTsh1 expression persists in presumptive migrating cells that emanate from the dLGE and form the LCS migratory route to the basal telencephalon (Fig. 2I,M). The expression of mTsh1 is maintained in the dLGE and cells of the presumptive LCS of Gsh2 mutants at E13.5 (Fig. 2J,N), which is in surprising contrast to the loss of Sp8 expression (Fig. 2B,F) thereby indicating a differential regulation of Sp8 and mTsh1 by Gsh2. In the Sey mutant telencephalon at E13.5, mTsh1 expression expands into the pallium (Fig. 2K,O). In addition, there also appears to be ectopic expression along the outer ventral telencephalon in the area of the presumptive piriform cortex. Similar to Sp8 expression in Sey/Gsh2 double mutant mice, although partially recovered, mTsh1 expression remains abnormal (Fig. 2L,P).
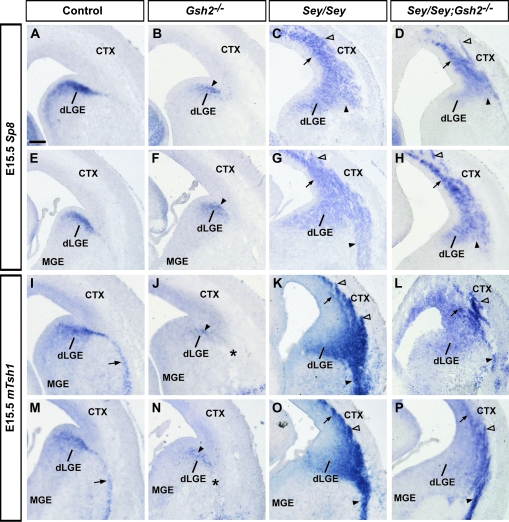
At E15.5, Sp8 is still strongly expressed in the dLGE SVZ (Fig. 3A,E), which shows partial recovery of Sp8 expression in the dLGE of Gsh2 mutants (Fig. 3B,F) as compared with E13.5 (Fig. 2B,F), similar to that observed at E16.5 (Waclaw et al. 2006). In contrast, the Sey mutant telencephalon continues to exhibit robust ectopic Sp8 expression in the pallium (Fig. 3C,G), and at medial telencephalic levels an ectopic basal stream of Sp8 expression is observed (Fig. 3G). This domain of ectopic pallial Sp8 expression, although less severe, persists in Sey/Gsh2 mutants at E15.5 (Fig. 3D,H). Sp8 expression reveals paraventricular ectopia in Sey/Gsh2 double mutants (Fig. 3D,H) as in the case of the Sey single mutant (Kroll and O'Leary, 2005; this study).
Figure 3.
Expression of dLGE markers Sp8 and mTsh1 at E15.5. At E15.5, Sp8 strongly marks the dLGE SVZ (A, E). In Gsh2 mutants, this expression pattern is largely normal (B, F, arrowheads).Sey mutants display significant ectopic expression of Sp8 in the pallium (C, G, arrows), with an enlarged basal stream at more medial levels (G, arrowhead) that is not observed more rostrally (C, arrowhead). Sey/Gsh2 double mutants display ectopic pallial expression, though at diminished levels from the Sey mutants (D, H, arrows), with nascent paraventricular ectopia (D, H, empty arrowheads). Sey/Gsh2 double mutants also do not display a basal stream of Sp8 expression (D, H, arrowheads). mTsh1 is expressed in the dLGE SVZ and putative LCS (arrows) (I, M). In Gsh2 mutants, there is diminished mTsh1 in the dLGE SVZ (arrows) with a lack of expression in the putative LCS (J, N, asterisk). In the Sey mutant, ectopic mTsh1 expression is found in the pallium (arrows) and forms paraventricular ectopia (empty arrowheads) (K, O). Basal-directed putative migration is observed at rostral (K, arrowheads) and medial (O, arrowheads) levels. Sey/Gsh2 double mutants also exhibit pallial ectopic mTsh1 expression (arrows) with paraventricular ectopia (empty arrowheads) and putative basal-directed migration (arrowheads) (L, P). n numbers for each probe are as follows: controls, n= 6 [Sp8], n= 6 [mTsh1]; Gsh2−/−, n= 5 [Sp8], n= 5 [mTsh1]; Sey/Sey, n= 5 [Sp8], n= 6 [mTsh1]; Sey/Sey;Gsh2−/−, n= 4 [Sp8], n= 5 [mTsh1]. Scale bar: 200 μm.
At E15.5, similar to E13.5, mTsh1 is expressed in the dLGE SVZ and in the LCS migratory route (Fig. 3I,M). Interestingly, although mTsh1 expression in Gsh2 mutants was normal at E13.5, at E15.5 expression in the dLGE and LCS is markedly reduced (Fig. 3J,N), revealing that mTsh1 expression is temporally regulated by Gsh2 (Fig. 3J,N).
Ectopic pallial and basal expression of mTsh1 persists at E15.5 in the Sey mutant (Fig. 3K,O). Moreover, double mutant analysis shows that the loss of ectopic Gsh2 in Sey mutants does not significantly rescue the abnormal phenotype (Fig. 3L,P). The formation of mTsh1+ paraventricular ectopia shows that dorsal telencephalic progenitors in the Sey mutant pallium are respecified to not only an Sp8 lineage (Kroll and O'Leary 2005; this study), but to mTsh1 and Er81 lineages. Furthermore, removal of Gsh2 from the Sey mutant pallium is not sufficient to rescue the respecification of dorsal telencephalic precursors to these dLGE identities.
In summary, this analysis reveals that 1) Sp8 and mTsh1 appear to be differentially regulated by Gsh2 in a temporally dependent manner and 2) altered expression of Sp8 and mTsh1 in Sey/Gsh2 double mutants broadly resembles that of single Sey mutants.
Differential Regulation of Ventral Pallial Markers Sfrp2 and Dbx1 by Pax6 and Gsh2
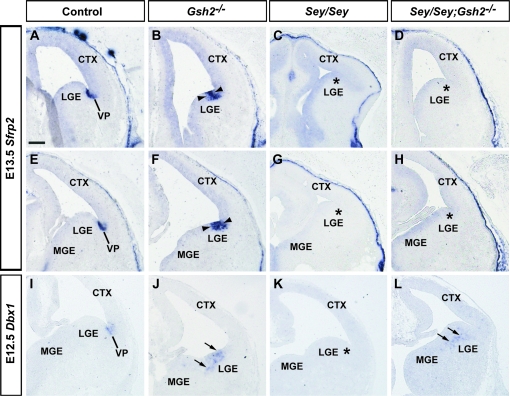
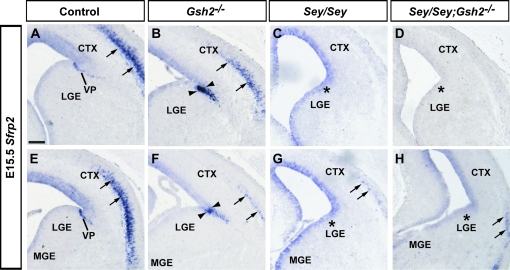
Sfrp2, an inhibitor of the WNT secreted growth factor protein is expressed in the VZ of the VP, as shown at E13.5 and E15.5 (Figs 4 and 5A,E). In Gsh2 mutants at E13.5, the expression domain of Sfrp2 expands ventrally (Fig. 4B,F). This expansion remains present at E15.5 (Fig. 5B,F). In agreement with previous findings at E13.5 (Kim et al. 2001; Muzio et al. 2002), we observe that the telencephalic expression of Sfrp2 at the PSB is missing in Sey mutant mice at E13.5 and E15.5 (Figs 4 and 5C,G). At E13.5 and E15.5, lack of both Pax6 and Gsh2 in Sey/Gsh2 double mutant mice does not restore Sfrp2 expression at the PSB at rostral or medial levels (Figs 4 and 5D,H). Thus, we reveal that the Pax6-regulated expression of Sfrp2 is independent of Gsh2.
Figure 4.
Expression of VP markers Sfrp2 and Dbx1 at E13.5 and E12.5. Sfrp2 is expressed in the VP as shown at E13.5 (A, E). This expression domain is expanded ventrally in Gsh2 mutants (B, F, arrowheads). Sfrp2 expression is absent at the PSB (asterisk) in the Sey mutant (C, G, asterisks). In Sey/Gsh2 double mutants expression is not restored (D, H, asterisks) Dbx1 is expressed in the VP at E12.5 (I). Loss of Gsh2 results in ectopic Dbx1 expression in the LGE (J, arrows). In Sey mutants, Dbx1 is missing in the VP (K, asterisk). In Sey/Gsh2 double mutants Dbx1 expression is re-expressed, but mislocalized in the dLGE SVZ (L, arrows). n numbers for each probe are as follows: controls, n= 6 [Sfrp2], n= 3 [Dbx1]; Gsh2−/−, n= 4 [Sfrp2], n= 3 [Dbx1]; Sey/Sey, n= 4 [Sfrp2], n= 2 [Dbx1]; Sey/Sey;Gsh2−/−, n= 4 [Sfrp2], n= 2 [Dbx1]. Scale bar: 200 μm.
Figure 5.
Expression of Sfrp2 at E15.5. Sfrp2 is expressed in the VP and layer V neurons of the cortex (A, E, arrows). In Gsh2 mutants, the VP expression domain is expanded (B, F , arrowheads) but reduced in the lateral cortex (B, F, arrows). In Sey mutants, VP expression of Sfrp2 is missing (asterisks) and layer V expression is drastically reduced (arrows) (C, G). In Sey/Gsh2 double mutants, Sfrp2 expression is not restored in the VP (asterisks), and is severely reduced in the lateral cortex (arrows) (D, H). n numbers are as follows: controls, n= 6; Gsh2−/−, n= 4; Sey/Sey, n= 4; Sey/Sey;Gsh2−/−, n= 4. Scale bar: 200 μm.
Dbx1 is a homeodomain gene that, similar to Sfrp2, is expressed in the VZ of the VP aspect of the PSB (Yun et al. 2001; Medina et al. 2004; Bielle et al. 2005). The VP generates cells destined for the pallium (Bielle et al. 2005) and the basolateral and lateral amygdala (Stenman, Yu, et al. 2003; Carney et al. 2006). We analyzed the expression of Dbx1 at E12.5, after which VP expression is downregulated (Fig. 4I). In Gsh2 mutants, Dbx1, similar to Sfrp2 at E13.5, is ectopically expressed in the dLGE (Fig. 4J). We further observe that Sey mutants lack Dbx1 expression at the PSB (Fig. 4K). Interestingly, in contrast to Sfrp2, removal of ectopic Gsh2 in the Sey mutant pallium re-initiated expression of Dbx1 in the LGE of Sey/Gsh2 double mutants (Fig. 4L). However, this expression appears to be ectopically localized to the SVZ. This reveals that Pax6 is required for Dbx1 expression at the VP and Gsh2 functions to repress ectopic Dbx1 expression in the LGE. Thus, similar to the above differential regulation of mTsh1 and Sp8 by Gsh2 and Pax6, Sfrp2, and Dbx1 are also regulated in a complex manner by Gsh2 and Pax6.
Regulation of Tgfα Expression by Gsh2 and Pax6 at E13.5 and E15.5
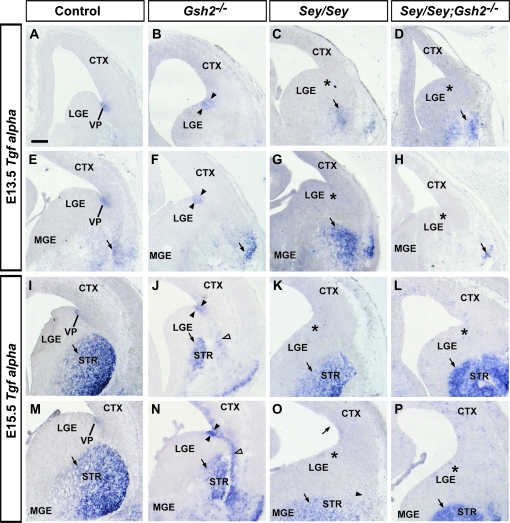
Next, we examined expression of the secreted factor Tgfα, which is a ligand for EGFR in the developing forebrain (Kornblum et al. 1997). At E13.5 and E15.5, Tgfα is strongly expressed in the VP aspect of the PSB (Fig. 6A,E,I,M) (Assimacopoulos et al. 2003). In Gsh2 mutants at E13.5, Tgfα expression ectopically extends into the LGE (Fig. 6B,F), similar to other VP markers, Sfrp2 and Dbx1 (Fig. 4B,F,J). At E15.5 in Gsh2 mutants, Tgfα expression remains slightly expanded (Fig. 6J,N) along with ectopic expression along the route of the putative LCS (Fig. 6N). In agreement with previous findings (Assimacopoulos et al. 2003), Tgfα expression is absent in the VP in Sey mutants at both ages (Fig. 6C,G,K,O). Similar to Sfrp2, but in contrast to the VP marker Dbx1, expression of Tgfα is not restored at the PSB in the Sey/Gsh2 double mutants at either age (Fig. 6D,H,L,P).
Figure 6.
Expression of secreted factor Tgfα at E13.5 and E15.5. Tgfα is expressed in the VP and basal telencephalon at E13.5 (A, E, arrow). In Gsh2 mutants, VP expression is expanded ventrally (arrowheads) and basal expression is maintained (arrow) (B, F). In Sey mutants, VP expression is missing (asterisks) whereas basal expression is unaffected (arrows, C, G). VP expression remains absent in Sey/Gsh2 double mutants (asterisks), though basal expression is present (arrows) (D, H). At E15.5, expression is observed in the VP and differentiating striatum (STR, arrows) (I, M). In Gsh2 mutants, VP expression is expanded (arrowheads), and a putative ectopic basal migration is observed (empty arrowheads). A smaller domain of expression is observed in the striatum (J, N, arrows). In Sey mutants, VP expression is missing (asterisks), although striatal expression remains (arrows) (K, O). In Sey/Gsh2 double mutants, expression at the VP is not restored (asterisks), and remains present in the striatum (arrows) (L, P). n numbers are as follows: controls, n= 4 [E13.5], n= 4[E15.5]; Gsh2−/−, n= 3 [E13.5], n= 3 [E15.5]; Sey/Sey, n= 3 [E13.5], n= 4 [E15.5]; Sey/Sey;Gsh2−/−, n= 3 [E13.5], n= 3 [E15.5]. Scale bar: 200 μm.
Interneuron markers Dlx2 and Dlx5
The Dlx family of homeobox transcription factors comprises 6 genes, 4 of which are expressed in the developing forebrain (Bulfone, Puelles, et al. 1993; Simeone et al. 1994; Anderson et al. 1997; Liu et al. 1997). Dlx1 and Dlx2 are expressed in the subpallial VZ and SVZ, whereas Dlx5 and Dlx6 are strongly expressed in the subpallial SVZ and postmitotic regions (Eisenstat et al. 1999). We have previously shown that in the absence of Gsh2, there is a lack of Dlx2+ cells along the LCS migratory route to the basal telencephalon (Carney et al. 2006). Therefore, the presence of mTsh1-positive cells along the putative LCS in the Gsh2 mutant in this study is surprising (Fig. 2J,N). To this end, we sought to examine the expression patterns of Dlx2 and Dlx5, which in addition to marking subpallial cells, demonstrate migrating cells of the LCS. At E13.5, Dlx mRNA and protein expression is confined to the subpallial progenitor zones and postmitotic cells undergoing tangential migration (Supplemental Figs 1 and 2A,E) (Porteus et al. 1991; Bulfone, Kim, et al. 1993; Porteus et al. 1994; Puelles et al. 2000; Nery et al. 2003; Carney et al. 2007; Flames et al. 2007). At E15.5, Dlx2 and Dlx5 streams are clearly observed along the LCS (Supplemental Figs 1 and 2I,M). In Gsh2 mutant mice, Dlx2 and Dlx5 expression is truncated along the emanating LCS (Supplemental Figs 1 and 2J,N) (Szucsik et al. 1997; Corbin et al. 2000; Nery et al. 2002; Carney et al. 2006). In the Sey mutant, there appears to be a large ectopic stream of LCS cells destined for the basal telencephalon that is also consistent with other findings (Supplemental Figs 1 and 2K,O) (Caubit et al. 2005). This abnormal putative migratory stream appears to persist in Sey/Gsh2 double mutants (Supplemental Fig. 1L and Supplemental Fig. 2L,P).
In addition to putative migratory defects along the LCS as defined by Dlx2 and Dlx5 expression in both Sey and Sey/Gsh2 double mutants, we also observe alterations in cortical patterning that is generally consistent with previous observations. At both E13.5 and E15.5, in the Sey mutants, there is a large ectopic expression of Dlx2 and Dlx5 in the cerebral cortex indicative of a broad ventralization of the dorsal telencephalon (Supplemental Figs 1 and 2C,G,K,O). In Sey/Gsh2 double mutants this phenotype is slightly improved (Supplemental Figs 1 and 2D,H) (Toresson et al. 2000).
Alterations in LCS cell migration in Gsh2, Sey, and Sey/Gsh2 double mutants at E15.5
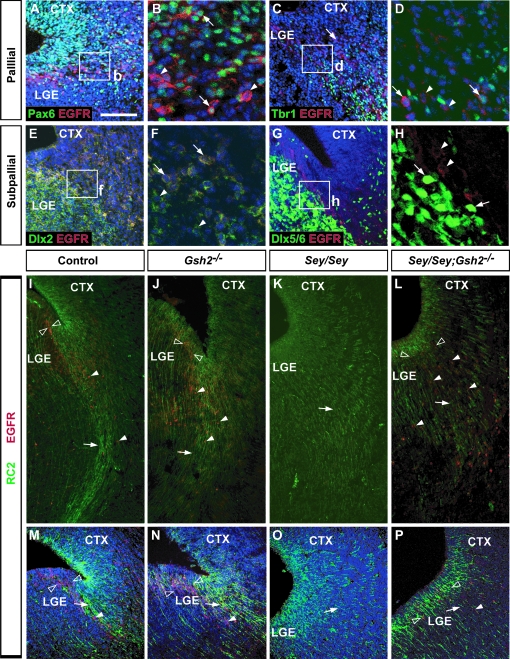
To examine defects in cell migration along the LCS in Gsh2, Sey, and Sey/Gsh2 double mutant mice, we carried out immunohistochemical analysis for EGFR, which is expressed at the PSB and in migrating cells of the LCS (Caric et al. 2001). First, we further characterized the cell types of the PSB that express EGFR. To this end, we performed dual immunolabeling experiments to label VZ and SVZ/mantle cells that are pallial-derived (Pax6+ and Tbr1+) and subpallial-derived (Dlx2+ and Dlx5/6+) at E15.5, which represents the time point of robust LCS cell migration of both populations (Carney et al. 2006). We observe that many EGFR+ cells at the PSB also express Pax6 (Fig. 7A,B) and a minority colocalize with the pallial mantle marker Tbr1 (Fig. 7C,D). To analyze whether the subpallial progenitors also coexpress EGFR, EGFR immunolabeling was combined with β-gal immunohistochemistry in sections from Dlx2+/tauLacZ mice or colocalization with endogenous GFP from Dlx5/6-GFP mice. We found that many EGFR+ cells at the PSB are also β-gal+, indicating that these cells are Dlx2+ (Fig. 7E,F). In contrast, Dlx5/6-GFP+ cells do not express EGFR (Fig. 7G,H). Although this was somewhat surprising, it has been previously shown that not all Dlx2+ cells also express Dlx5 (Eisenstat et al. 1999), thus the Dlx2+/EGFR+ population may be a subset of this subpallial Dlx2+/Dlx5/6- population. Alternatively, this EGFR+ population may also be part of the subset of Dlx2+ cells at the PSB that are also Pax6+ (Carney et al. 2006). Taken together, these data suggest that EGFR+ cells of the LCS arise from progenitors of both the pallial and subpallial aspect of the PSB.
Figure 7.
Alterations in LCS cell migration in Gsh2, Sey, and Sey/Gsh2 double mutants at E15.5. Low magnification of dual immunolabeling for Pax6 (green, A), Tbr1 (green, C), Dlx2 (green, E) and EGFR (red) shows coexpression of a subset of cells at the PSB (A, C, E). Higher magnification of boxed regions in (A, C, E) show individual double EGFR+ cells (arrows) colocalized with Pax6 (B, arrows), Tbr1 (D, arrows), or Dlx2 (F, arrows) intermingled with EGFR+ cells not expressing any of these markers (arrowheads). In contrast, there is no colocalization of endogenous Dlx5/6-GFP labeling (green, arrows) and EGFR immunolabeling (red, arrowheads) (G, H). RC2 (green) and EGFR (red) dual immunolabeling reveals the LCS radial glial scaffold (arrows) and EGFR+ migratory cells (arrowheads) emanating from the PSB (empty arrowheads) (I, M). In Gsh2 mutants, the VZ EGFR+ domain appears expanded (empty arrowheads), with a more diffuse RC2 immunolabeling (arrows) (J, N). EGFR+ cells (arrowheads), although disorganized, remain present along the LCS. In Sey mutants, EGFR+ cells are completely absent, and the RC2+ radial glia along the LCS migratory route is missing (K, O, arrows). In Sey/Gsh2 double mutants, RC2+ radial glia (arrows) resemble Sey mutants, but EGFR+ cells are now present along the LCS (arrowheads) and occupy an expanded domain at the PSB (empty arrowheads) (L, P). To-Pro-3 counterstaining (blue) is shown in the majority of panels. n numbers are as follows: EGFR and Pax6 or Dlx2 or Tbr1, n= 2; EGFR/Dlx5/6-GFP, n= 2; EGFR/RC2: controls, n= 3; Gsh2−/−, n= 3; Sey/Sey, n= 4; Sey/Sey;Gsh2−/−, n= 3. Scale bar: A, C, E, G, M–P: 100 μm, I–L: 40 μm, B, D, F, H: 30 μm.
We next examined the status of the EGFR+ LCS population and the radial glial scaffold in Gsh2, Pax6, and Sey/Gsh2 mutants by dual immunolabeling for EGFR and RC2 at E15.5. In controls, strong RC2 expression defines the radial glial scaffold of the LCS migratory route to the basal telencephalon, and EGFR+ cells are observed at the PSB and along the LCS (Fig. 7I,M). In Gsh2 mutant mice, the RC2+ radial glia scaffold appears to occupy a larger domain at the PSB, concomitant with a wider domain of EGFR+ cells in the LGE and the LCS (Fig. 7J,N). Conversely, in Sey mutants, the fasciculated RC2+ processes of the LCS radial glia scaffold are missing, and RC2 labeling appears diffuse (Fig. 7K,O) (Stoykova et al. 1997). Also, there is a striking absence of EGFR expression both at the PSB and in the LCS in Sey mutants (Fig. 7K). In Sey/Gsh2 double mutants, the RC2+ immunolabeling also appears diffuse, similar to the Sey mutant. Interestingly, EGFR expression is restored in the double mutant, although EGFR+ cells appear scattered in the LGE and LCS (Fig. 7L,P). This observation is similar to the restoration of Dbx1 expression in the LGE in Sey/Gsh2 mutants (Fig. 4), and reveals that the loss of EGFR expression at the PSB and LCS in Sey mutant mice is a consequence of ectopic Gsh2 expression.
Gsh1 expression in single Sey, Gsh2, and Sey/Gsh2 double mutants
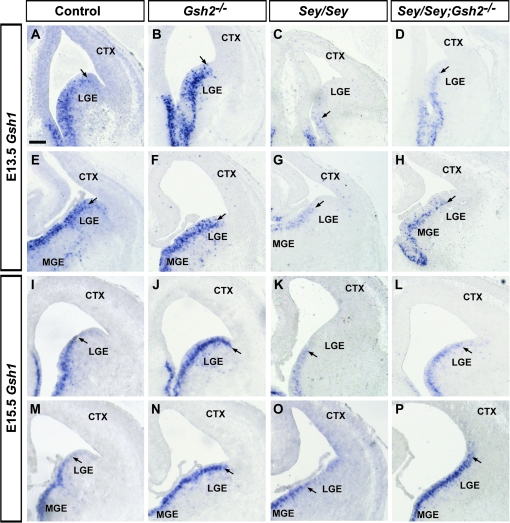
Previously it has been shown that Gsh1 can rescue many, but not all, of the subpallial patterning defects in found in Gsh2 mutants starting at around E14 (Toresson and Campbell 2001; Yun et al. 2001, 2003). As described previously (Toresson and Campbell 2001), and shown here at E13.5 and E15.5, Gsh1 is normally expressed at high levels in the medial ganglionic eminence (MGE) and ventral LGE, with intense expression in the VZ and in scattered cells in the SVZ (Fig. 8A,E,I,M). In Gsh2 mutant mice at E13.5, high levels of expression extend to the PSB, most prominent at rostral levels (Fig. 8B,F). At E15.5 in Gsh2 mutant mice, high levels of Gsh1 expression extend into the VP (Fig. 8J,N). In contrast, in the Sey mutant, this dorsal limit of expression of Gsh1 is located much more ventrally at both rostral and medial levels at E13.5, and at rostral levels at E15.5 (Fig. 8C,G,K). In contrast, at medial levels at E15.5, a low level of Gsh1 expression expands into the pallium in Sey mutants (Fig. 8O). In Sey/Gsh2 double mutants at E13.5 and E15.5, the dorsal limit of Gsh1 expression resembles that of Gsh2 mutants (Fig. 8D,H,L,P). Thus, Pax6 and Gsh2 act combinatorially to repress Gsh1 expression in the ventral and lateral pallium.
Figure 8.
Gsh1 expression in Gsh2, Sey single, and Sey/Gsh2 double mutants at E13.5 and E15.5. At E13.5 Gsh1 is expressed in the MGE and LGE, with highest expression more ventrally (A, E). Arrows mark the dorsal limit of expression (A–P). In Gsh2 mutants, expression is similar to controls (B, F). In Sey mutants, expression is present in the MGE and ventral LGE only (C, G). In Sey/Gsh2 double mutants, expression is similar to that of Gsh2 mutants (D, H). In controls, at E15.5, expression is similar to E13.5 (I, M). In Gsh2 mutants, expression expands to the PSB (J, N). In Sey mutants, expression is similar to that at E13.5 (K, O). In Sey/Gsh2 double mutants, at rostral levels, the expression domain normal appears normal (L). However, at more medial levels, expression extends dorsally beyond the PSB into the pallium (P). n numbers are as follows: controls, n= 4 [E13.5], n= 3 [E15.5]; Gsh2−/−, n= 3 [E13.5], n=3 [E15.5]; Sey/Sey, n= 3 [E13.5], n= 3 [E15.5]; Sey/Sey;Gsh2−/−, n= 2 [E13.5], n= 3 [E15.5]. Scale bar: 200 μm.
Discussion
Gene expression studies have revealed that the embryonic telencephalon can be parcellated into refined molecular maps (Puelles et al. 2000; Medina et al. 2004; Flames et al. 2007). Combined with in utero transplantation and genetic fate mapping studies this work has unraveled the correlation between specific subpallial progenitor pools and the diversity of cortical interneurons in the adult brain (Nery et al. 2002; Butt et al. 2005; Flames et al. 2007; Fogarty et al. 2007; Wonders et al. 2008, Xu et al. 2008). This restricted potential of neural progenitors is also maintained in adulthood (Merkle et al. 2007). Moreover, to a large extent major aspects of the function of both intrinsic and extrinsic key players that pattern the telencephalon have been elucidated. However, our understanding of the genetic mechanisms that pattern specific telencephalic progenitor zones, such as PSB, remain unknown. In this study, we examined the development of the PSB, an important progenitor domain for the OB, amygdala and early cerebral cortical Cajal–Retzius populations. Our studies reveal that the PSB is a highly complex and dynamic telencephalic progenitor zone, comprised of multiple molecularly distinct progenitor pools. We also provide novel insight into the genetic regulation of their specification by Pax6 and Gsh2.
Combinatorial Codes of Gene Expression Define Unique Progenitor Domains at the PSB
The importance of intrinsic specification for neuronal output in the cerebral cortex was first suggested by the “protomap” theory (Rakic 1988). Furthermore, the parcellation of progenitor domains and the generation of restricted neuronal diversity were first appreciated in the spinal cord (reviewed in Jessell 2000). Collectively, these studies defined a paradigm in which regional expression of key genes (typically transcription factors) instruct cell fate decisions in a cell-autonomous manner, before or at the last cell division (reviewed in Dehay and Kennedy 2007). A major prediction of these studies is that progenitor pools express combinations of transcription factors, which endow these cells with unique fate potentials. Based on this idea, recent studies have comprehensively explored gene expression patterns of putative instructive factors in the subpallial proliferative zones. One study defined at least 18 molecular distinct progenitor domains in the embryonic subpallium (Flames et al. 2007). Moreover, at embryonic stages, postmitotic subpallial-derived cells express genes which encode proteins of a characteristically mature neuronal phenotype as early as E13.5 (Batista-Brito, Machold, et al. 2008). Collectively, these analyses have provided both an important reference and context to consider the spatio-temporal and genetic origins of neural diversity. However, to a large extent such studies have focused on the MGE, which generates the majority of cortical interneurons (Sussel et al. 1999).
In this study, we sought to extensively examine the molecular code of gene expression of the PSB. Our analysis using a battery of markers that delineate the dLGE (mTsh1, Sp8), VP (Sfrp2, Tgfα, and Dbx1), or both (Er81) subdomains reveals that the PSB also contains highly regulated patterns of gene expression. These combinatorial expression patterns highlight the importance of the PSB as a unique and highly heterogeneous telencephalic progenitor domain. Moreover considering that the PSB is a major source of cells that generates neuronal cell diversity in the limbic system and OB, we speculate that this molecular diversity is directly related to the vast array of neuronal subtypes that populate these mature telencephalic structures.
Function of Pax6 and Gsh2 in the Specification of the PSB Progenitor Domains
In addition to gene expression analysis in wild-type animals, we have examined the role of the homeodomain encoding genes Pax6 and Gsh2, both alone and in combination, in the specification of progenitor pools of the PSB. Overall, we find that Pax6 and Gsh2 function to regulate the expression of all PSB markers analyzed here. Previous analysis of the function of these genes from a number of groups has revealed that Pax6 and Gsh2 function as major regulators of dorsal/ventral patterning by acting in a genetic cross-repressive manner to specify proper cortical and striatal identity at either side of the PSB (Stoykova et al. 1996; Corbin et al. 2000; Stoykova et al. 2000; Toresson et al. 2000; Toresson and Campbell 2001; Yun et al. 2001). Consistent with this finding, the lack of both Pax6 and Gsh2 in mutant mice leads to a significant, but not complete, rescue of many of these dorsal/ventral patterning defects observed in single mutants (Toresson et al. 2000; Waclaw et al. 2004). However, because Pax6 (Sey) mutants exhibit ectopic pallial Gsh2 expression and Gsh2 mutants display ectopic subpallial Pax6 expression, a direct genetic requirement for each of these genes for PSB formation could not be differentiated from the consequences of ectopic expression of Gsh2 and Pax6.
By an in-depth analysis of Sey/Gsh2 double mutants, we reveal that both Pax6 and Gsh2 are required for the expression Er81, Sp8, mTsh1, Sfrp2, and Tgfα, as in the absence of both genes expression of these genes remains abnormal. In contrast, the regulation of Dbx1 expression, which marks VP progenitors, and EGFR, which marks cells of the LCS, is more complex, as the loss of Gsh2 on the Sey background rescues their expression. In addition, we demonstrate that dorsal telencephalic Sey mutant progenitors may be respecified to several dLGE cell lineages other than Sp8 as previously observed, and therefore importantly extend previous studies (Schuurmans et al. 2004; Kroll and O'Leary 2005; Gopal and Golden 2008), which have indicated differential patterning defects in the Sey cortex. Moreover, we show that this cortical misspecification also persists in the absence of both Pax6 and Gsh2 revealing a direct requirement for Pax6 in repression of ectopic dLGE identity in the lateral cortex.
In addition to the combinatorial function of Pax6 and Gsh2 in patterning of PSB progenitor pools, our study reveals novel individual roles for Gsh2 and Pax6. During embryogenesis, Gsh2 is expressed in a graded pattern in the subpallium with the highest expression at the PSB (Corbin et al. 2000; Toresson et al. 2000; Yun et al. 2001). Gsh2 mutant mice display cell proliferation defects in the SVZ of the LGE (Yun et al. 2001), as well as a loss of the dLGE marker Sp8 at E12.5, though interestingly this expression is partially recovered by E16.5 (Waclaw et al. 2006). Consistent with this finding, our study reveals that re-expression of Sp8 in the dLGE is initiated at E13.5, and is further improved by E15.5. This timing coincides with the increase in Gsh1 expression in the dLGE. As Gsh1 can partially rescue the striatal patterning defects observed in Gsh2 mutants (Toresson and Campbell 2001), it is therefore possible that Gsh1 performs a similar function in the restoration of Sp8 expression in the mutant dLGE that is reinitiated from E13.5. Our analysis of expression of mTsh1, which also marks the dLGE SVZ, importantly reveals that Sp8 and mTsh1 are differentially regulated by Gsh2. In contrast to the changes in expression of Sp8 in the absence of Gsh2, mTsh1 expression was grossly unaffected at E13.5. Interestingly, this regulation by Gsh2 is temporally dependent as Sp8 expression is re-initiated at later developmental time points, whereas mTsh1 expression is reduced. There are 2 non-mutually exclusive mechanisms by which this may be occurring: 1) the expression of mTsh1 and Sp8 in individual dLGE progenitor cells may be differentially regulated by Gsh2, or 2) mTsh1 and Sp8 mark separate progenitor subpopulations in the dLGE SVZ that are differentially regulated by Gsh2 function. In support of the latter explanation, analysis of patterns of protein expression in the PSB SVZ has shown a significant amount of molecular heterogeneity. For example, a subset, but not all, Sp8+ cells colocalize with Pax6 or Dlx5/6-GFP (Waclaw et al. 2006). Similarly, only some mTsh1+ cells express Dlx2 mRNA or DLX protein (as revealed by immunostaining with a pan-DLX antibody) (Caubit et al. 2005). Future genetic fate mapping analyses of both Sp8 and mTsh1 will be essential for unraveling the lineal relationships between these progenitor populations and their potential differential fates in the mature brain.
Previous analysis of Pax6 mutant mice has revealed numerous important functions for Pax6 in telencephalic development. These include cell-autonomous repression of ventral gene expression in the ventral and lateral pallium, the normal adhesiveness of cortical progenitor cells and their migration, axonal pathfinding, corticogenesis and arealization of the cerebral cortex (Schmahl et al. 1993; Stoykova et al. 1996, 1997, 2000; Götz et al. 1998; Estivill-Torrus et al. 2002; Jones et al. 2002; Jiménez et al. 2002; Talamillo et al. 2003; Tyas et al. 2003; Haubst et al. 2004; Holm et al. 2007; Manuel et al. 2007; Quinn et al. 2007). Consistent with previous studies, we find that the lack of functional Pax6 protein in the Sey mutant pallium results in the ectopic expression of subpallial markers (Stoykova et al. 1996, 2000; Chapouton et al. 1999; Corbin et al. 2000; Toresson et al. 2000; Yun et al. 2001; Kroll and O'Leary 2005), including several dLGE markers.
Based on the finding that both Sp8 and Er81 progenitors can give rise to calretinin-expressing OB cells (Waclaw et al. 2006; Allen et al. 2007), we hypothesize that ventralized dorsal telencephalic progenitors in the Sey mutant may produce a gamma-aminobutyric acidergic phenotype that derives from the ectopic expression of several PSB markers, such as Sp8, Er81, and perhaps mTsh1 as shown here. Importantly, this ventralization is generally independent of ectopic pallial Gsh2 expression, as the Sey/Gsh2 double mutants display a similar phenotype (Toresson et al. 2000).
In addition, our analysis of the VP markers Dbx1, Sfrp2, and Tgfα in single Gsh2 and Sey mutants and Sey/Gsh2 double mutants reveals an interesting and novel regulation of their expression by Pax6 and Gsh2. Previous studies of Sey and Gsh2 mutants, as well as our data shown here, reveal that Pax6 is required for the expression of each of these markers at the VP, whereas Gsh2 is required to restrict their expression from the dLGE domain. Our analysis of Sey/Gsh2 double mutants has uncovered an important differential regulation by Pax6 and Gsh2. As Sfrp2 and Tgfα expression remains absent in double mutants, this reveals that their expression is directly dependent on Pax6 and not repressed by ectopic Gsh2 pallial expression in Sey mutants. In contrast, Dbx1 expression is rescued in the double mutants. However, this expression is mislocalized to the LGE SVZ. Therefore, although Pax6 is required for Dbx1 expression at the VP, Gsh2 is also necessary to repress Dbx1 expression in the LGE. Thus, similar to the differential regulation of the dLGE markers mTsh1 and Sp8 by Gsh2, individual VP markers are differentially regulated by Pax6 (Sfrp2, Tgfα) or the combinatorial actions of both Pax6 and Gsh2 (Dbx1). This complex genetic regulation of PSB boundary formation by Pax6 and Gsh2 raises important questions regarding the spatial and functional heterogeneity of progenitor pools within the PSB.
Requirement of Pax6 and Gsh2 for Basal-Directed Migration
This analysis, along with previous studies, reveals that the PSB is a highly heterogeneous and complexly regulated progenitor region of the developing telencephalon. The PSB is the origin of the LCS and RMS, two major migratory streams in the developing telencephalon. The LCS populates the basal telencephalic limbic system, in particular the piriform and olfactory cortices and the amygdala (Hirata et al. 2002; Carney et al. 2006, Bai et al. 2008). The PSB also seeds the embryonic and postnatal SVZ stem cell niche and their subsequent progeny that migrate along the RMS to generate diverse subtypes of OB interneurons (Waclaw et al. 2006; Kohwi et al. 2007)
Our analysis of EGFR expression, a previously identified marker of LCS migratory cells (Caric et al. 2001), reveals that both the subpallial (Dlx2+) and pallial (Pax6+) subpopulations are both EGFR+. As Tgfα, a known ligand for EGFR, is expressed in the VP, it is possible that this signaling pathway is required for specification/migration of EGFR+ cells of the LCS. Indeed, the expansion of both Tgfα and EGFR in Gsh2 mutants and their loss in Sey mutants supports this notion. However, the re-expression of EGFR in Sey/Gsh2 double mutants, without rescue of Tgfα expression, reveals that this regulation is likely more complex, and may also/instead occur via Dbx1, as Dbx1 expression at the PSB is also restored in Sey/Gsh2 double mutants. In addition, our previous studies (Carney et al. 2006), as well as data shown here, reveal that Gsh2 is required for development of the Dlx2+ dLGE component of the LCS. Interestingly, as noted above, in this study we surprisingly reveal that at E13.5, the expression of mTsh1 in the dLGE and in putative migratory cells along the LCS appeared normal. However, 2 days later, we show reduced expression of mTsh1 in the dLGE SVZ and LCS. Thus, the complex Gsh2 regulation of specification of dLGE progenitor pools has consequences for the generation of the migratory populations that arise from this region. Some migratory populations (e.g., Dlx2+) are Gsh2-dependent, whereas other populations (e.g., early mTsh1) populations are Gsh2-independent. This observation is reminiscent of the complex genetic regulation of OB neurogenesis in which specific subpopulations are Dlx1/2-dependent and others Dlx1/2-independent (Long et al. 2007; Batista-Brito, Close, et al. 2008). As the PSB is a major progenitor pool for both the LCS and the RMS/OB (Wichterle et al. 1999; Stenman, Toresson, et al. 2003; Carney et al. 2006; Waclaw et al. 2006; Allen et al. 2007; De Marchis et al. 2007; Kohwi et al. 2007) the output of this embryonic heterogeneity may be directly linked to the functional complexity of neurons that populate both the mature basal telencephalic limbic system and postnatal SVZ/OB.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
Autism Speaks grant to J.C.; National Institute of Health (DA020140) to J.C.; and Cellular Imaging Core of Mental Retardation and Developmental Disabilities Research Center (P30HD40677).
Supplementary Material
Acknowledgments
We would like to thank Drs Kenny Campbell and Ronald Waclaw for advice on Gsh2 genotyping, the Gsh2 antibody, Sp8 probe and the Dlx5/6−cre-iresEGFP mice, and Dr Robert Hevner for the Tbr1 antibody. We are grateful to the following people for providing cDNAs: G. Fishell, Dbx1, Dlx2, Er81, Gsh1, Sfrp2; J. Rubenstein, Dlx5; C. Ragsdale, Tgfα; K. Campbell, Sp8; L. Fasano, mTsh1. We thank Dr Adan Aguirre for technical advice and Ms Berenice Alfonso for technical assistance at the start of this study. We also acknowledge members of the Corbin, Haydar and Zohn labs in the Center for Neuroscience Research for their helpful discussions and insight, and Drs Tarik Haydar, Vittorio Gallo, Adan Aguirre, and José Luis Olmos-Serrano for critical reading of the manuscript.
R.S.E.C. and J.C.G. devised the study and R.S.E.C. carried out the majority of experiments. L.A.C. and T.H. performed the Tgfα and Dbx1 in situ hybridization experiments, respectively, with tissue prepared and genotyped by R.S.E.C. K.M. provided further technical assistance. R.S.E.C. and J.C.G. analyzed the data and wrote the manuscript. All authors edited and approved the final manuscript. Conflict of Interest: None declared.
References
- Allen ZJ, 2nd, Waclaw RR, Colbert MC, Campbell K. Molecular identity of olfactory bulb interneurons: transcriptional codes of periglomerular neuron subtypes. J Mol Histol. 2007;38:517–525. doi: 10.1007/s10735-007-9115-4. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos S, Grove EA, Ragsdale CW. Identification of a Pax6-dependent epidermal growth factor family signaling source at the lateral edge of the embryonic cerebral cortex. J Neurosci. 2003;23:6399–6403. doi: 10.1523/JNEUROSCI.23-16-06399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Paramasivam M, Siddiqi F, Ackman JB, LoTurco JJ. The role of DCX and LIS1 in migration through the lateral cortical stream of developing forebrain. Dev Neurosci. 2008;30:144–156. doi: 10.1159/000109859. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci. 2008;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Machold R, Klein C, Fishell G. Gene expression in cortical interneurons is prescient of their mature function. 2008 doi: 10.1093/cercor/bhm258. Cereb Cortex. doi:10.1093/cercor/bhn258. [Epub ahead of print] PMID: 18250082 [PubMed—as supplied by publisher] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Waclaw RR, Campbell K, Potter SS, Scott WJ. Sp8 is crucial for limb outgrowth and neuropore closure. Proc Natl Acad Sci USA. 2003;100:12195–12200. doi: 10.1073/pnas.2134310100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Kim HJ, Puelles L, Porteus MH, Grippo JF, Rubenstein JL. The mouse Dlx-2 (Tes-1) gene is expressed in spatially restricted domains of the forebrain, face and limbs in midgestation mouse embryos. Mech Dev. 1993;40:129–140. doi: 10.1016/0925-4773(93)90071-5. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Puelles L, Porteus MH, Frohman MA, Martin GR, Rubenstein JL. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J Neurosci. 1993;13:3155–3172. doi: 10.1523/JNEUROSCI.13-07-03155.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Caric D, Raphael H, Viti J, Feathers A, Wancio D, Lillien L. EGFRs mediate chemotactic migration in the developing telencephalon. Development. 2001;128:4203–4216. doi: 10.1242/dev.128.21.4203. [DOI] [PubMed] [Google Scholar]
- Carney RS, Alfonso TB, Cohen D, Dai H, Nery S, Stoica B, Slotkin J, Bregman BS, Fishell G, Corbin JG. Cell migration along the lateral cortical stream to the developing basal telencephalic limbic system. J Neurosci. 2006;26:11562–11574. doi: 10.1523/JNEUROSCI.3092-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RS, Bystron I, López-Bendito G, Molnár Z. Comparative analysis of extra-ventricular mitoses at early stages of cortical development in rat and human. Brain Struct Funct. 2007;212:37–54. doi: 10.1007/s00429-007-0142-4. [DOI] [PubMed] [Google Scholar]
- Caubit X, Tiveron MC, Cremer H, Fasano L. Expression patterns of the three Teashirt-related genes define specific boundaries in the developing and postnatal mouse forebrain. J Comp Neurol. 2005;486:76–88. doi: 10.1002/cne.20500. [DOI] [PubMed] [Google Scholar]
- Chapouton P, Gartner A, Gotz M. The role of Pax6 in restricting cell migration between developing cortex and basal ganglia. Development. 1999;126:5569–5579. doi: 10.1242/dev.126.24.5569. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development. 2000;127:5007–5020. doi: 10.1242/dev.127.23.5007. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Nery S, Fishell G. Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat Neurosci. 2001;4(Suppl.):1177–1182. doi: 10.1038/nn749. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Rutlin M, Gaiano N, Fishell G. Combinatorial function of the homeodomain proteins Nkx2.1 and Gsh2 in ventral telencephalic patterning. Development. 2003;130:4895–4906. doi: 10.1242/dev.00717. [DOI] [PubMed] [Google Scholar]
- Dehay C, Kennedy H. Cell cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Bovetti S, Carletti B, Hsieh YC, Garzotto D, Peretto P, Fasolo A, Puche AC, Rossi F. Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: implication for intrinsic properties of the subventricular zone progenitor population. J Neurosci. 2007;27:657–664. doi: 10.1523/JNEUROSCI.2870-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstat DD, Liu JK, Mione M, Zhong W, Yu G, Anderson SA, Ghattas I, Puelles L, Rubenstein JL. DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J Comp Neurol. 1999;414:217–237. doi: 10.1002/(sici)1096-9861(19991115)414:2<217::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Estivill-Torrus G, Pearson H, van Heyningen V, Price DJ, Rashbass P. Pax6 is required to regulate the cell cycle and the rate of progression from symmetrical to asymmetrical division in mammalian cortical progenitors. Development. 2002;129:455–466. doi: 10.1242/dev.129.2.455. [DOI] [PubMed] [Google Scholar]
- Fernandez AS, Pieau C, Reperant J, Boncinelli E, Wassef M. Expression of the Emx-1 and Dlx-1 homeobox genes define three molecularly distinct domains in the telencephalon of mouse, chick, turtle and frog embryos: implications for the evolution of telencephalic subdivisions in amniotes. Development. 1998;125:2099–2111. doi: 10.1242/dev.125.11.2099. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marín O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal PP, Golden JA. Pax6-/- mice have a cell nonautonomous defect in nonradial interneuron migration. Cereb Cortex. 2008;18:752–762. doi: 10.1093/cercor/bhm114. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Haubst N, Berger J, Radjendirane V, Graw J, Favor J, Saunders GF, Stoykova A, Götz M. Molecular dissection of Pax6 function: the specific roles of the paired domain and homeodomain in brain development. Development. 2004;131:6131–6140. doi: 10.1242/dev.01524. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hirata T, Nomura T, Takagi Y, Sato Y, Tomioka N, Fujisawa H, Osumi N. Mosaic development of the olfactory cortex with Pax6-dependent and -independent components. Brain Res Dev Brain Res. 2002;136:17–26. doi: 10.1016/s0165-3806(02)00304-8. [DOI] [PubMed] [Google Scholar]
- Holm PC, Mader MT, Haubst N, Wizenmann A, Sigvardsson M, Gotz M. Loss- and gain-of-function analyses reveal targets of Pax6 in the developing mouse telencephalon. Mol Cell Neurosci. 2007;34:99–119. doi: 10.1016/j.mcn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Jessell T. Neuronal specification in the spinal cord: inductive signal and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jiménez D, López-Mascaraque L, de Carlos JA, Valverde F. Further studies on cortical tangential migration in wild type and Pax-6 mutant mice. J Neurocytol. 2002;31:719–728. doi: 10.1023/a:1025751914372. [DOI] [PubMed] [Google Scholar]
- Jones L, López-Bendito G, Gruss P, Stoykova A, Molnár Z. Pax6 is required for the normal development of the forebrain axonal connections. Development. 2002;129:5041–5052. doi: 10.1242/dev.129.21.5041. [DOI] [PubMed] [Google Scholar]
- Kim AS, Anderson SA, Rubenstein JL, Lowenstein DH, Pleasure SJ. Pax-6 regulates expression of SFRP-2 and Wnt-7b in the developing CNS. J Neurosci. 2001;21:RC132. doi: 10.1523/JNEUROSCI.21-05-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Petryniak MA, Long JE, Ekker M, Obata K, Yanagawa Y, Rubenstein JL, Alvarez-Buylla A. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27:6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum HI, Hussain RJ, Bronstein JM, Gall CM, Lee DC, Seroogy KB. Prenatal ontogeny of the epidermal growth factor receptor and its ligand, transforming growth factor alpha, in the rat brain. J Comp Neurol. 1997;380:243–261. doi: 10.1002/(sici)1096-9861(19970407)380:2<243::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kroll TT, O'Leary DD. Ventralized dorsal telencephalic progenitors in Pax6 mutant mice generate GABA interneurons of a lateral ganglionic eminence fate. Proc Natl Acad Sci USA. 2005;102:7374–7379. doi: 10.1073/pnas.0500819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, Ghattas I, Liu S, Chen S, Rubenstein JL. Dlx genes encode DNA-binding proteins that are expressed in an overlapping and sequential pattern during basal ganglia differentiation. Dev Dyn. 1997;210:498–512. doi: 10.1002/(SICI)1097-0177(199712)210:4<498::AID-AJA12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Long JE, Garel S, Alvarez-Dolado M, Yoshikawa K, Osumi N, Alvarez-Buylla A, Rubenstein JL. Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. J Neurosci. 2007;27:3230–3243. doi: 10.1523/JNEUROSCI.5265-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Sturgess K, Erdélyi F, Szabó G, Molnár Z, Paulsen O. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb Cortex. 2004;14:1122–1133. doi: 10.1093/cercor/bhh072. [DOI] [PubMed] [Google Scholar]
- Lu S, Bogarad LD, Murtha MT, Ruddle FH. Expression pattern of a murine homeobox gene, Dbx, displays extreme spatial restriction in embryonic forebrain and spinal cord. Proc Natl Acad Sci USA. 1992;89:8053–8057. doi: 10.1073/pnas.89.17.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Georgala PA, Carr CB, Chanas S, Kleinjan DA, Martynoga B, Mason JO, Molinek M, Pinson J, Pratt T, et al. Controlled overexpression of Pax6 in vivo negatively autoregulates the Pax6 locus, causing cell-autonomous defects of late cortical progenitor proliferation with little effect on cortical arealization. Development. 2007;134:545–555. doi: 10.1242/dev.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Medina L, Legaz I, Gonzalez G, De Castro F, Rubenstein JL, Puelles L. Expression of Dbx1, Neurogenin 2, Semaphorin 5A, Cadherin 8, and Emx1 distinguish ventral and lateral pallial histogenetic divisions in the developing mouse claustroamygdaloid complex. J Comp Neurol. 2004;474:504–523. doi: 10.1002/cne.20141. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Muzio L, DiBenedetto B, Stoykova A, Boncinelli E, Gruss P, Mallamaci A. Emx2 and Pax6 control regionalization of the pre-neuronogenic cortical primordium. Cereb Cortex. 2002;12:129–139. doi: 10.1093/cercor/12.2.129. [DOI] [PubMed] [Google Scholar]
- Nery S, Corbin JG, Fishell G. Dlx2 progenitor migration in wild type and Nkx2.1 mutant telencephalon. Cereb Cortex. 2003;13:895–903. doi: 10.1093/cercor/13.9.895. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Ciaranello RD, Rubenstein JL. Isolation and characterization of a novel cDNA clone encoding a homeodomain that is developmentally regulated in the ventral forebrain. Neuron. 1991;7:221–229. doi: 10.1016/0896-6273(91)90260-7. [DOI] [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Liu JK, Puelles L, Lo LC, Rubenstein JL. DLX-2, MASH-1, and MAP-2 expression and bromodeoxyuridine incorporation define molecularly distinct cell populations in the embryonic mouse forebrain. J Neurosci. 1994;14:6370–6383. doi: 10.1523/JNEUROSCI.14-11-06370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Rubenstein JL. Comparison of the mammalian and avian telencephalon from the perspective of gene expression data. Eur J Morphol. 1999;37:139–150. doi: 10.1076/ejom.37.2.139.4756. [DOI] [PubMed] [Google Scholar]
- Quinn JC, Molinek M, Martynoga BS, Zaki PA, Faedo A, Bulfone A, Hevner RF, West JD, Price DJ. Pax6 controls cerebral cortical cell number by regulating exit from the cell cycle and specifies cortical cell identity by a cell autonomous mechanism. Dev Biol. 2007;302:50–65. doi: 10.1016/j.ydbio.2006.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Remedios R, Huilgol D, Saha B, Hari P, Bhatnagar L, Kowalczyk T, Hevner RF, Suda Y, Aizawa S, Ohshima T, et al. A stream of cells migrating from the caudal telencephalon reveals a link between the amygdala and neocortex. Nat Neurosci. 2007;10:1141–1150. doi: 10.1038/nn1955. [DOI] [PubMed] [Google Scholar]
- Sahara S, Kawakami Y, Izpisua Belmonte JC, O'Leary DD. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2007;2:10. doi: 10.1186/1749-8104-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Schmahl W, Knoedlseder M, Favor J, Davidson D. Defects of neuronal migration and the pathogenesis of cortical malformations are associated with Small eye (Sey) in the mouse, a point mutation at the Pax-6-locus. Acta Neuropathol. 1993;86:126–135. doi: 10.1007/BF00334879. [DOI] [PubMed] [Google Scholar]
- Schuurmans C, Armant O, Nieto M, Stenman JM, Britz O, Klenin N, Brown C, Langevin LM, Seibt J, Tang H, et al. Sequential phases of cortical specification involve neurogenin-dependent and -independent pathways. EMBO J. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Pannese M, D'Esposito M, Stornaiuolo A, Gulisano M, Mallamaci A, Kastury K, Druck T, Huebner K, et al. Cloning and characterization of two members of the vertebrate Dlx gene family. Proc Natl Acad Sci USA. 1994;91:2250–2254. doi: 10.1073/pnas.91.6.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 2003;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Yu RT, Evans RM, Campbell K. Tlx and Pax6 co-operate genetically to establish the pallio-subpallial boundary in the embryonic mouse telencephalon. Development. 2003;130:1113–1122. doi: 10.1242/dev.00328. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Fritsch R, Walther C, Gruss P. Forebrain patterning defects in Small eye mutant mice. Development. 1996;122:3453–3465. doi: 10.1242/dev.122.11.3453. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Götz M, Gruss P, Price J. Pax6-dependent regulation of adhesive patterning, R-cadherin expression and boundary formation in developing forebrain. Development. 1997;124:3765–3777. doi: 10.1242/dev.124.19.3765. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Gruss P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J Neurosci. 1994;14:1395–1412. doi: 10.1523/JNEUROSCI.14-03-01395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykova A, Treichel D, Hallonet M, Gruss P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J Neurosci. 2000;20:8042–8050. doi: 10.1523/JNEUROSCI.20-21-08042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Szucsik JC, Witte DP, Li H, Pixley SK, Small KM, Potter SS. Altered forebrain and hindbrain development in mice mutant for the Gsh-2 homeobox gene. Dev Biol. 1997;191:230–242. doi: 10.1006/dbio.1997.8733. [DOI] [PubMed] [Google Scholar]
- Talamillo A, Quinn JC, Collinson JM, Caric D, Price DJ, West JD, Hill RE. Pax6 regulates regional development and neuronal migration in the cerebral cortex. Dev Biol. 2003;255:151–163. doi: 10.1016/s0012-1606(02)00046-5. [DOI] [PubMed] [Google Scholar]
- Toresson H, Campbell K. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development. 2001;128:4769–4780. doi: 10.1242/dev.128.23.4769. [DOI] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- Tyas DA, Pearson H, Rashbass P, Price DJ. Pax6 regulates cell adhesion during cortical development. Cereb Cortex. 2003;13:612–619. doi: 10.1093/cercor/13.6.612. [DOI] [PubMed] [Google Scholar]
- Vaughan TJ, Pascall JC, Brown KD. Nucleotide sequence and tissue distribution of mouse transforming growth factor-alpha. Biochim Biophys Acta. 1992;1132:322–324. doi: 10.1016/0167-4781(92)90170-5. [DOI] [PubMed] [Google Scholar]
- Vergaño-Vera E, Yusta-Boyo MJ, de Castro F, Bernad A, de Pablo F, Vicario-Abejon C. Generation of GABAergic and dopaminergic interneurons from endogenous embryonic olfactory bulb precursor cells. Development. 2006;133:4367–4379. doi: 10.1242/dev.02601. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ, 2nd, Bell SM, Erdelyi F, Szabo G, Potter SS, Campbell K. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Campbell K. The homeobox gene Gsh2 is required for retinoid production in the embryonic mouse telencephalon. Development. 2004;131:4013–4020. doi: 10.1242/dev.01272. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K, Garel S, Fischman S, Rubenstein JL. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J Comp Neurol. 2003;461:151–165. doi: 10.1002/cne.10685. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- Zembrzycki A, Griesel G, Stoykova A, Mansouri A. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev. 2007;2:8. doi: 10.1186/1749-8104-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.