Abstract
In α1-AT deficiency, a misfolded but functionally active mutant α1-ATZ (α1-ATZ) molecule is retained in the endoplasmic reticulum of liver cells rather than secreted into the blood and body fluids. Emphysema is thought to be caused by the lack of circulating α1-AT to inhibit neutrophil elastase in the lung. Liver injury is thought to be caused by the hepatotoxic effects of the retained α1-ATZ. In this study, we show that several “chemical chaperones,” which have been shown to reverse the cellular mislocalization or misfolding of other mutant plasma membrane, nuclear, and cytoplasmic proteins, mediate increased secretion of α1-ATZ. In particular, 4-phenylbutyric acid (PBA) mediated a marked increase in secretion of functionally active α1-ATZ in a model cell culture system. Moreover, oral administration of PBA was well tolerated by PiZ mice (transgenic for the human α1-ATZ gene) and consistently mediated an increase in blood levels of human α1-AT reaching 20–50% of the levels present in PiM mice and normal humans. Because clinical studies have suggested that only partial correction is needed for prevention of both liver and lung injury in α1-AT deficiency and PBA has been used safely in humans, it constitutes an excellent candidate for chemoprophylaxis of target organ injury in α1-AT deficiency.
In homozygous protease inhibitor phenotype ZZ (PIZZ) α1-AT deficiency, there is an ≈85–90% reduction in plasma α1-AT levels (1). Because it is susceptible to polymerization (2), the mutant α1-ATZ (α1-ATZ) molecule is retained in the endoplasmic reticulum (ER) rather than secreted. The defect in secretion of α1-ATZ is expressed in cells that constitute the major source of plasma α1-AT, liver cells, as well as in cells that may serve as local sites of synthesis, such as macrophages and epithelial cells (3). Both the α1-ATZ that can be extracted from liver tissue and the small amount that is secreted into the blood can inhibit neutrophil elastase activity, albeit at a somewhat reduced capacity compared with wild-type α1-AT (4–7). Individuals with this deficiency have a markedly increased risk of developing emphysema because of the decrease in number of α1-AT molecules in the lung that inhibit neutrophil elastase, cathepsin G, and proteinase 3. Interestingly, individuals with rare allotypic variants of α1-AT that are associated with 60–70% reduction in plasma α1-AT levels do not appear to have an increased risk of destructive lung disease (8). Moreover, recent studies of neutrophil elastolytic activity have suggested that relatively small increases in plasma α1-AT levels are needed to inhibit destructive proteolysis in deficient individuals (9).
Homozygous PIZZ α1-AT deficiency is also the most common genetic cause of liver disease in children (1). According to the “accumulation” theory (10), liver injury is caused by the hepatotoxic effect of α1-ATZ retained in the ER of liver cells. However, there is remarkable variability in the development of liver disease among affected PIZZ individuals. Some present in liver failure and require liver transplantation in the first several years of life whereas others do not manifest any clinical signs of liver disease for many years. A prospective nationwide screening study in Sweden has shown that 80–90% of PIZZ individuals identified prospectively have no clinical evidence of liver disease by 18 years of age, their age at the time of the last report on this population (11). Although some of these patients may have subclinical liver injury as evidenced by the fact that cirrhosis and hepatocellular carcinoma can be found incidentally at autopsy in PIZZ individuals who have died of other causes (12), the overwhelming clinical experience with this disease indicates that there is a high degree of genotype-phenotype variation in the effect of α1-AT deficiency on the development of liver disease and a number of PIZZ individuals are relatively “protected” from liver disease or have very slowly progressing liver disease. Our studies with genetically engineered fibroblast cell lines from PIZZ individuals carefully characterized for the presence or absence of liver disease indicate that “protection” from liver disease is correlated with efficient degradation of α1-ATZ in the ER (13). Thus, these studies have suggested that susceptibility to liver disease in α1-AT deficiency may be caused by a relatively small increase in the burden of α1-ATZ that accumulates in the ER. Taken together, clinical studies of emphysema and liver disease in α1-AT deficiency suggest that even partial correction of the secretory defect could protect α1-AT-deficient individuals from both liver and lung injury.
Recently, a class of compounds called chemical chaperones has been shown to reverse the cellular mislocalization or misfolding of several mutant plasma membrane, lysosomal, nuclear, and cytoplasmic proteins, including mutant cystic fibrosis transmembrane conductance regulator CFTRΔF508, prion proteins, mutant aquaporin molecules associated with nephrogenic diabetes insipidus, temperature-sensitive mutants of p53, pp60src, ubiquitin E1, and mutant β-galactosidase A associated with Fabry disease (14–18). In this study, we examined the effect of these compounds on the fate of α1-ATZ.
Materials and Methods
Cell Culture.
Human skin fibroblast cell lines from PIZZ individuals engineered for expression of α1-ATZ have been described (13, 19). The CJZ12B cell line is a human skin fibroblast cell line from a PIZZ individual without liver disease (“protected host”) that is engineered for stable expression of α1-ATZ and is relatively efficient in ER degradation of α1-ATZ (13). The murine hepatoma cell line Hepa 1–6 was engineered for stable expression of α1-ATZ for this study. It was generated by methods identical to the ones used for the human skin fibroblast cell lines with amphotropic recombinant retroviral particles carrying the human α1-ATZ gene (13). The clone used here is called Hepa1–6N2Z4. For experiments with chemical chaperones, the cells were preincubated at 37°C for 12–24 h in control medium or control medium supplemented with the designated chaperone at a concentration that represented the maximum tolerated by the cells without altering cell viability or total protein synthesis as assessed by trichloroacetic acid precipitation (13, 19). For each chaperone examined, the final concentrations used here were found to be similar to those observed for positive effects on other polypeptides in previous reports (14–18). Cells were then subjected to pulse-chase radiolabeling in the absence or presence of drug and the resulting cell lysates (IC) and cell culture fluid (EC) samples were analyzed by immunoprecipitation by using an antibody to α1-AT followed by SDS/PAGE/fluorography exactly as described (13, 19). Equivalent aliquots of cell lysates and cell culture fluid from each time point of each pulse-chase experiment were subjected to immunoprecipitation. The pulse period was 1 h.
In Vivo Delivery of 4-Phenylbutyric Acid (PBA) to PiZ Mice.
Seven mice homozygous for human α1-ATZ (Z11.03 homozygotes) (20) at 6–7 weeks of age were separated into two groups. Group 1 was fed by gavage PBA 560 mg/kg per day (14 mg/day in three divided doses) for 5 days. Group 2 was gavage fed vehicle (water) for 5 days. Treatment with PBA or vehicle was then stopped for a period of 2 days. By using a crossover design, a second trial period was then started. In this second trial period, group 1 was gavage fed vehicle and group 2 was gavage fed PBA for 5 days. Treatment with PBA or vehicle was then stopped again for a period of 2 days. Blood was drawn on the day before each trial period (day 0) and then on days 3, 5, 8, and 12 for each trial period and subjected to ELISA for human α1-AT. The protocol was approved by the Animal Studies Committee of Washington University School of Medicine.
ELISA for Human α1-AT.
Serum levels of α1-AT were quantified by ELISA with goat anti-human α1-AT IgG (Cappel) as coating antibody, rabbit anti-human α1-AT IgG (Boehringer Mannheim) as capture antibody, and peroxidase-conjugated goat anti-rabbit Ig (Dako) as detection antibody. The assay detected purified human α1-AT and α1-AT in human serum at concentrations as low as 0.1 ng/ml but did not detect any α1-AT in nontransgenic mouse serum.
Results and Discussion
Effect of Chemical Chaperones on Secretion of α1-ATZ in Cell Culture.
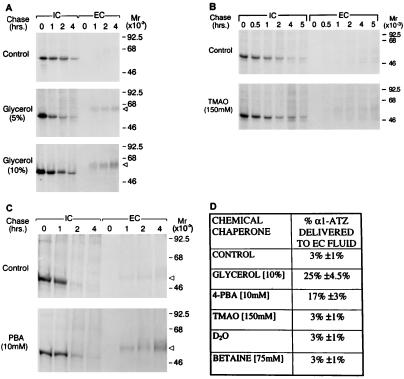
First we examined the effect of glycerol and trimethylamine oxide (TMAO) on secretion of α1-ATZ in a human fibroblast cell line engineered for stable expression of α1-ATZ (CJZ12B) and shown to recapitulate the defect known to occur in α1-AT deficiency. Glycerol and TMAO were selected because they appeared to be the most effective of the chemical chaperones in previous studies (14–18). Cells were incubated in either control medium or medium supplemented with 5 or 10% glycerol for 12 h at 37°C and were then subjected to a pulse-chase experiment (Fig. 1A). The results show that in controls, α1-ATZ is synthesized as a 52-kDa polypeptide, retained for 2 h, and then progressively disappears between 2 and 4 h of the chase period. Only a trace amount of the 55-kDa mature form of α1-ATZ can be detected in the cell culture fluid (EC). In cells treated with 5% glycerol, α1-ATZ is also synthesized as a 52-kDa polypeptide, but in this case, it disappears more rapidly, beginning between 1 and 2 h of the chase period and a substantial amount of α1-ATZ, in its mature 55-kDa form, is secreted into the EC fluid. In cells treated with10% glycerol, the same amount of 52-kDa α1-ATZ polypeptide is present in IC at t = 0 and disappears from IC at the same rate as in cells treated with 5% glycerol. However, there is an even greater increase in the amount of 55-kDa α1-ATZ polypeptide secreted into the EC fluid. These data indicate that glycerol mediates a significant increase in the secretion of α1-ATZ and that the positive effect of glycerol is concentration dependent. In contrast, TMAO had a minimal effect on secretion of α1-ATZ even at the highest concentration tolerated by the cells without a toxic effect (150 mM) (Fig. 1B). In fact, when three separate experiments with TMAO were subjected to densitometric analysis (Fig. 1D), there was no significant increase in secretion mediated by TMAO.
Figure 1.
Effect of chemical chaperones on secretion of α1-ATZ. CJZ12B cells (7, 8) were preincubated in control medium or medium supplemented with the designated drug as described in Materials and Methods. Cells were then subjected to pulse-chase radiolabelling, and the results were analyzed by immunoprecipitation followed by SDS/PAGE/fluorography. Molecular mass markers (in kDa) are shown on the right. The relative electrophoretic migration of the 55-kDa α1-ATZ polypeptide in the EC is indicated on the right by a triangular arrowhead. (A) CJZ12B cells incubated for 12 h in the absence or presence of 5 or 10% glycerol. (B) CJZ12B cells incubated for 12 h in the absence or presence of 150 mM TMAO. (C) CJZ12B cells incubated for 12 h in the absence or presence of 10 mM PBA. (D) Summary table of results from treatment of CJZ12B cells with several chemical chaperones. The data are expressed as percent α1-ATZ delivered to the EC after a 4-h chase, compared with the total α1-ATZ newly synthesized at t = 0 IC, as determined by densitometric analysis of gels. In each case, the pulse-chase experiment from the control and experimental conditions were exposed on the same film and a similar amount of α1-ATZ was present at t = 0 IC in the control and experimental conditions. Values represent the means ± 1.0 SD for the percent of α1-ATZ delivered to EC from three independent experiments in each case. The range of values for the control was 2.0–3.9%; glycerol, 23.0–27.2%; PBA, 15.0–18.4%; TMAO, 2.0–4.0%; D20, 2.0–4.0%; and betaine, 1.9–3.9%. Results were similar when phosphorimaging analysis was used to quantify results in two separate experiments for glycerol and PBA (data not shown).
Next, we examined the effect of PBA in this system. PBA is approved by the Food and Drug Administration for oral administration as an ammonia scavenger in the treatment of urea cycle disorders (21, 22). It has also recently been examined as a chemical chaperone for CFTRΔF508 (23, 24). Here, CJZ12B cells were incubated for 12 h at 37°C with 10 mM PBA and then subjected to pulse-chase analysis (Fig. 1C). The results show that there is a marked increase in the appearance of the 55-kDa mature form of α1-ATZ in the EC fluid in the presence of PBA. The results of multiple experiments with PBA and several other candidate chemical chaperones are shown in Fig. 1D. The results show that glycerol and PBA mediate approximately eight- and 5-fold increases in α1-ATZ secretion, respectively. TMAO, deuterated water, and betaine had no significant effect. The effect of PBA on secretion of α1-ATZ was not limited to the CJZ12B cell line. It also mediated an increase in secretion of α1-ATZ in the BWZ fibroblast cell line described in ref. 7 (data not shown).
We also examined the effect of PBA on secretion of α1-ATZ in a mouse hepatoma cell line engineered for stable expression of the human α1-ATZ gene, Hepa 1–6N2Z4 (Fig. 2). The results show that in the absence of PBA (control), there is a 52-kDa α1-ATZ polypeptide at t = 0 IC. This polypeptide is retained for 1 h and then progressively disappears between t = 2 and 3 h of the chase period with very little of the mature 55-kDa α1-ATZ polypeptide secreted into the EC medium. In the presence of PBA, a similar amount of the 52-kDa α1-ATZ polypeptide is present at t = 0 IC. There is no significant difference in the kinetics of its disappearance from IC during the chase period but here a substantial amount of the 55-kDa mature α1-ATZ polypeptide appears in the EC fluid. These data indicate that α1-ATZ has a similar fate in murine hepatoma cells as in human fibroblast cell lines and that PBA mediates an increase in secretion of α1-ATZ in both cell types.
Figure 2.
Effect of PBA on secretion of α1-ATZ in a murine hepatoma cell line. Hepa1–6N2Z4 cells were preincubated for 12 h at 37°C in control medium in the absence (control) or presence of 10 mM PBA and then subjected to pulse-chase radiolabeling and gel analysis as described in the legend of Fig. 1. Molecular mass markers (in kDa) are shown at the right. The relative electrophoretic migration of the 55-kDa α1-ATZ polypeptide in the EC is indicated on the right by a triangular arrowhead.
The effect of glycerol and PBA was specific for secretion of the mutant α1-ATZ. Neither compound altered the secretion of the endogenous complement protein factor H or total trichloroacetic acid-precipitable protein in CJZ12B cells (data not shown). Moreover, neither compound altered the secretion of endogenous wild-type α1-AT in the human hepatoma cell line HepG2 (data not shown). Finally, PBA did not significantly alter the rate of disappearance of α1-ATZ from the intracellular compartment (Figs. 1C and 2), suggesting that its effect predominantly involves an increase in translocation of α1-ATZ molecules from the ER into the rest of the secretory pathway rather than an effect on ER degradation of α1-ATZ.
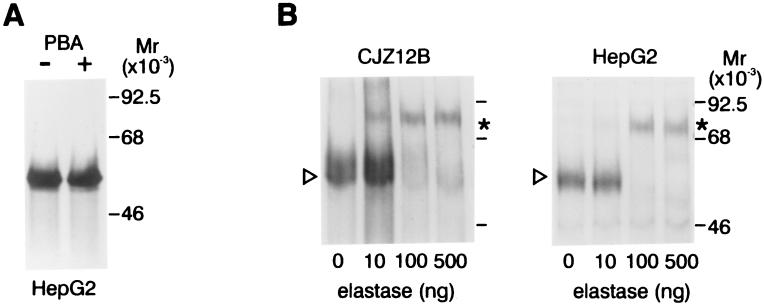
To determine whether PBA has other properties that are suitable for potential chemoprophylaxis in α1-AT deficiency, it was tested in two other ways. First, we examined the effect of PBA on the synthesis of α1-AT. For this, we used the well-differentiated human hepatoma cell line HepG2 in which endogenous expression and regulation of wild-type α1-AT appears to recapitulate, in most respects, its expression in liver cells in vivo (2) (Fig. 3A). The results show that PBA has no effect on the synthesis of α1-AT at concentrations and under conditions associated with its positive effect on secretion of α1-ATZ. This is an important result because it means that the drug can alter secretion without a concurrent increase in de novo synthesis of the mutant protein and, moreover, that its effects on α1-ATZ are not caused by transcriptional activation. Second, we examined the functional activity of α1-AT secreted in the presence of PBA (Fig. 3B). EC fluid from CJZ12B and HepG2 cells treated with PBA and then pulse radiolabeled to steady state was incubated with unlabelled neutrophil elastase and then subjected to SDS/PAGE and fluorography. These results show that most of the 55-kDa α1-AT secreted in each case is progressively converted to an ≈75-kDa high molecular weight complex with increasing concentrations of neutrophil elastase. There was no significant difference in the amount of elastase required to form a complex with mutant α1-ATZ secreted by CJZ12B as compared with wild-type α1-AT secreted by HepG2 cells. These results are similar to those observed when purified human plasma α1-AT or α1-AT secreted by Caco2 cells is reacted with neutrophil elastase (25). Mutant α1-ATZ in the EC fluid of glycerol-treated CJZ12B cells also formed complexes with neutrophil elastase as effectively as wild-type α1-AT from HepG2 cells (data not shown). Thus, the results indicate that the α1-ATZ secreted by PBA- or glycerol-treated cells is functionally active. These data do not, however, exclude the possibility that there is a change in the kinetics of association with neutrophil elastase. Neither PBA nor glycerol had any effect on the capacity of wild-type α1-AT secreted by HepG2 cells to form complexes with neutrophil elastase by using the same experimental protocol (Fig. 3B Left and data not shown).
Figure 3.
Other effects of PBA on α1-AT. (A) Effect of PBA on synthesis of endogenous wild-type α1-AT in the human hepatoma cell line HepG2. HepG2 cells were preincubated for 12 h at 37°C in control medium or medium supplemented with 10 mM PBA. The cells were then pulse labeled for 20 min and lysed, and the cell lysates analyzed by immunoprecipitation followed by SDS/PAGE/fluorography. (B) Functional activity of mutant α1-ATZ secreted by CJZ12B (Left) and wild-type α1-AT secreted by HepG2 (Right) cells after treatment with PBA. Cells were subjected to a pulse-chase experiment and EC was harvested after a chase period of 4 h. Aliquots of the EC were incubated for 30 min at 37°C in the absence or presence of purified human neutrophil elastase in increasing concentrations as shown at the bottom. The reaction was terminated by the addition of PMSF to 2 mM, and the reaction mixtures subject to immunoprecipitation and SDS/PAGE/fluorography. The relative migration of the 55-kDa native α1-ATZ in the EC was indicated by the arrowhead in the left margin. The ≈75-kDa α1-AT–elastase complex is indicated by an asterisk in the right margin.
Effect of PBA on Blood Levels of α1-ATZ in Vivo.
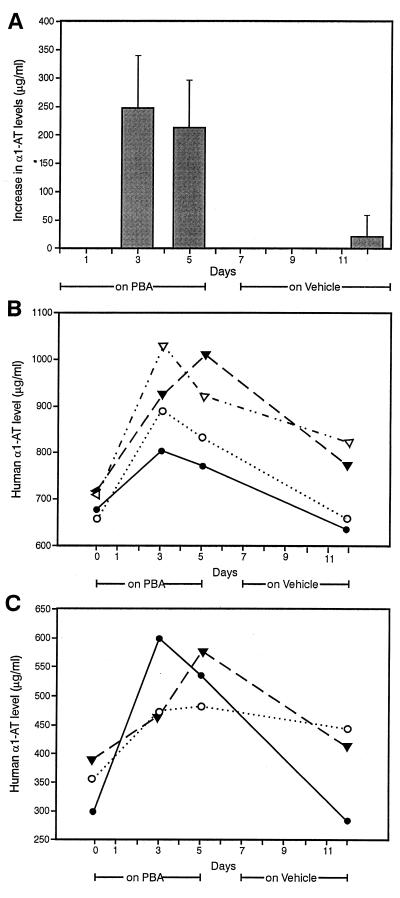
To determine whether chemical chaperones affect secretion of α1-ATZ in vivo, we administered PBA to mice transgenic for the human α1-ATZ gene [PiZ Z11.03 mice (20)] and measured serum levels of α1-AT by an ELISA specific for human α1-AT (Fig. 4). PBA at a dose of 560 mg/kg per day was administered by gavage for 5 days to 7 PiZ mice and was well tolerated without any apparent side effect. Interestingly, we found that baseline levels of human α1-AT were 600–700 μg/ml in four PiZ mice and 300–400 μg/ml in three other PiZ mice. During treatment with PBA (Fig. 4A), human α1-AT levels increased by ≈200–250 μg/ml at 3 days and remained elevated for the entire 5 days of the PBA-treatment period. The levels were at baseline when measured 7 days later (on day 12). The levels did not rise above baseline when the same mice were given vehicle (water) by gavage for 5 days either before or after the PBA-treatment period (data not shown). The increases in human α1-AT levels on days 3 and 5 were significantly different from that on day 12 (P < .001). Moreover, there was an increase in human α1-AT levels during PBA treatment in every single mouse (Fig. 4 B and C). In the PiZ mice with baseline α1-AT levels of 600–700 μg/ml, the effect of PBA resulted in blood levels of human α1-AT reaching ≈45–50% of the levels present in PiM (M3.03) (20) mice and normal humans (Fig. 4B). In the mice with lower baseline levels (200–300 μg/ml), blood levels reached ≈20–25% of the blood levels in PiM mice and normal humans (Fig. 4C).
Figure 4.
Effect of PBA on human α1-AT levels in PiZ mice. Seven PiZ mice were separated into two groups for administration of PBA or vehicle by gavage in a crossover design as described in Materials and Methods. (A) This graph shows the combined results for PBA in the two trial periods, including all seven PiZ mice at days 3 and 5 (while receiving PBA) and day 12 (off PBA for 7 days). The results are reported as means ± 1.0 SD for the increase in human α1-AT levels (μg/ml) compared with the baseline (day 0). The increases in blood levels of human α1-AT during PBA treatment on days 3 and 5 were significantly different from that on day 12 at P < .001 as determined by the Student's t test. There was no change in human α1-AT levels during administration of vehicle in any of the mice during either trial period (data not shown). (B) This graph shows the absolute levels of human α1-AT in four mice with baseline levels of 600–700 μg/ml. (C) This graph shows the absolute levels of human α1-AT in three PiZ mice with lower baseline levels (300–400 μg/ml).
Effect of Temperature on Secretion of α1-ATZ.
In previous studies showing the chaperone activity of glycerol and TMAO, the targets have been, for the most part, mutant proteins with temperature-sensitive protein folding defects (14–18), i.e., the mutant protein is able to adopt a translocation-competent folding state and reach its appropriate destination at the cell surface membrane or the nucleus when the temperature is lowered from 37°C to 27°C. We could not find any previous reports on how lower temperature affects α1-ATZ. Thus, we examined the fate/secretion of α1-ATZ in CJZ12B cells when the temperature is lowered from 37°C to 27°C (Fig. 5A). The results show that there is no increase in secretion of α1-ATZ, but there is a marked decrease in degradation at 27°C. Similar results were observed (data not shown) in a cell-free system that recapitulates proteasomal degradation of α1-ATZ (19). Thus, α1-ATZ is not a classical temperature-sensitive mutant but lowering the temperature inhibits its degradation. Finally, we examined the effect of increasing the temperature to 42°C on the fate/secretion of α1-ATZ (Fig. 5B). In this case, there is both inhibition of degradation and an increase in secretion of α1-ATZ. It was of interest to also see an ≈65-kDa polypeptide appear IC in a time-dependent fashion coincident with the retention of α1-ATZ between 1 and 4 h of the chase period at 42°C. The biochemical nature of this coprecipitating polypeptide is unknown at this time. It is not clear whether it reflects a member of the heat-shock protein family or another of the many changes that occur in cells at 42°C. Studies by Lomas et al. (26) have shown that there is increased polymerization of purified α1-ATZ in vitro when the temperature is increased from 37 to 42°C. The data presented here shows that there is enhanced secretion and diminished degradation of α1-ATZ in vivo when the temperature is increased to 42°C, indicating that the temperature has multiple and complex effects on the fate of α1-ATZ. There are two other important implications of the results in Fig. 5. First, chemical chaperones can alter the fate of mutant polypeptides in which the folding defect is not temperature sensitive. Second, inhibition of ER degradation is not necessarily associated with enhanced secretion of α1-ATZ. In fact, it appears that secretion and degradation may be separately affected by both physiologic and pharmacologic perturbations.
Figure 5.
Effect of temperature on the fate of α1-ATZ in CJZ12B cells. Cells were incubated for 12 h at normal growth temperature (37°C) or adjusted temperatures (27 or 42°C) and subjected to pulse-chase radiolabelling exactly as described in Materials and Methods. The relative migration of the 55-kDa α1-ATZ polypeptide in the EC is indicated by the arrowhead at the right margin. A 65-kDa polypeptide which coprecipitates with α1-ATZ in cell lysates at 42°C is indicated by an asterisk in the left margin.
Taken together, the results of this study demonstrate that the effects of chemical chaperones (particularly PBA) satisfy many of the criteria required for a potential chemoprophylactic strategy for liver and lung injury in α1-ATZ deficiency. PBA has been tolerated well after oral administration by children with urea cycle disorders without any significant toxic side effects (21, 22). It mediates an increase in secretion of functionally active α1-ATZ molecules in several different cell types, including cell culture model systems that recapitulate α1-ATZ deficiency with or without liver disease. It alters secretion without apparently increasing de novo synthesis or decreasing ER degradation. It does not appear to affect endogenous wild-type proteins. Finally, when administered by gavage feeding to PiZ mice, PBA is well tolerated and results in an increase in blood levels of α1-ATZ approaching 20–50% of the levels present in normal humans, which is very close to the levels predicted to protect the lungs from proteolytic damage (8, 9).
It is not yet known whether the α1-ATZ that reaches the lung of PiZ mice on PBA will retain its functional activity. A recent study by Elliott et al. (27) has identified polymers of α1-AT in the pulmonary lavage fluid of two out of five PIZZ patients with emphysema, presumably reducing the residual activity of the small amount of α1-ATZ present. However, one could argue that whatever effect PBA exerts on α1-ATZ within the intracellular portion of the secretory pathway, it will also have on α1-ATZ in the blood and body fluids.
It is also not clear whether long-term administration of PBA will reduce the load of α1-ATZ retained in the ER of liver cells. Nevertheless, with the new information provided by this report, it will now be possible to examine its effect on liver injury and development of hepatocellular carcinoma in transgenic mouse model systems. In fact, its long-term effect on the liver in PiZ mice will provide the first direct test of the “accumulation” theory for the pathogenesis of liver injury in α1-AT deficiency. It will also be important to determine the mechanism of its effect on secretion of α1-ATZ and whether chemical modification or alteration in pharmacological administration can result in an even more effective drug for chemoprophylaxis in α1-AT deficiency.
Acknowledgments
We thank Drs. Pam Zeitlin and William Welch for technical advice early in the studies, Dr. Jeff Teckman and other members of the Perlmutter laboratory for advice on the manuscript, and Mary Pichler for manuscript preparation. The studies reported in the manuscript were supported in part by National Institutes of Health Grants HL37784, DK52526, HDO7049, and DK56783.
Abbreviations
- α1-AT
α1-antitrypsin
- ER
endoplasmic reticulum
- α1-ATZ
mutant α1-AT type Z
- PIZZ
protease inhibitor phenotype ZZ homozygosity
- TMAO
trimethylamine oxide
- PBA
4-phenylbutyric acid
- IC
cell lysates
- EC
cell culture fluid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Perlmutter D H. In: Diseases of the Liver. Schiff E R, Sorrell M F, Maddrey W C, editors. Philadelphia: Lippincott; 1999. pp. 1131–1150. [Google Scholar]
- 2.Carrell R W, Lomas D A. Lancet. 1997;350:134–138. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- 3.Perlmutter D H, Kay R M, Cole F S, Rossing T H, Van Thiel D, Colten H R. Proc Natl Acad Sci USA. 1985;82:6918–6921. doi: 10.1073/pnas.82.20.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bathurst I C, Travis J, George P M, Carrell R W. FEBS Lett. 1984;177:179–183. doi: 10.1016/0014-5793(84)81279-x. [DOI] [PubMed] [Google Scholar]
- 5.Ogushi F, Fells G A, Hubbard R C, Straus S D, Crystal R G. J Clin Invest. 1987;89:1366–1374. doi: 10.1172/JCI113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahadeva R, Change W-S W, Dafforn T R, Oakley D J, Foreman R C, Calvin J, Wight D G D, Lomas D A. J Clin Invest. 1999;103:999–1006. doi: 10.1172/JCI4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llewellyn-Jones C G, Lomas D A, Carrell R W, Stockley R A. Biochim Biophys Acta. 1994;1227:155–160. doi: 10.1016/0925-4439(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 8.Crystal R G. J Clin Invest. 1990;85:1343–1352. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell E J, Campbell M A, Boukedes S S, Owen C A. J Clin Invest. 1999;104:337–344. doi: 10.1172/JCI6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrell R W. J Clin Invest. 1986;78:1427–1431. doi: 10.1172/JCI112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sveger T, Eriksson S. Hepatology. 1995;22:514–517. doi: 10.1002/hep.1840220221. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson S, Carlson J, Velez R. N Engl J Med. 1986;314:736–739. doi: 10.1056/NEJM198603203141202. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Whitman I, Molmenti E, Moore K, Hippenmeyer P, Perlmutter D H. Proc Natl Acad Sci USA. 1994;91:9014–9018. doi: 10.1073/pnas.91.19.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato S, Ward C L, Krouse M E, Wine J J, Kopito R R. J Biol Chem. 1996;271:635–638. doi: 10.1074/jbc.271.2.635. [DOI] [PubMed] [Google Scholar]
- 15.Tatzelt J, Pruisner S B, Welch W J. EMBO J. 1996;15:6363–6373. [PMC free article] [PubMed] [Google Scholar]
- 16.Tamarappoo B K, Verkman A S. J Clin Invest. 1998;101:2257–2267. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown C R, Hong-Brown L Q, Welch W J. J Clin Invest. 1997;99:1432–1444. doi: 10.1172/JCI119302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan J-Q, Ishii S, Asano N, Suzuki Y. Nat Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 19.Qu D, Teckman J H, Omura S, Perlmutter D H. J Biol Chem. 1996;271:22791–22795. doi: 10.1074/jbc.271.37.22791. [DOI] [PubMed] [Google Scholar]
- 20.Carlson J A, Rogers B B, Sifers R N, Finegold M J, Clift S M, DeMayo F J, Bullock D W, Woo S L. J Clin Invest. 1989;83:1183–1190. doi: 10.1172/JCI113999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maestri N E, Hauser E R, Bartholomew D, Brusilow S W. J Pediatr. 1991;119:923–928. doi: 10.1016/s0022-3476(05)83044-6. [DOI] [PubMed] [Google Scholar]
- 22.Brusilow S W. Pediatr Res. 1991;29:147–150. doi: 10.1203/00006450-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Rubenstein R C, Egan M E, Zeitlin P L. Clin Invest. 1997;100:2457–2465. doi: 10.1172/JCI119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubenstein R C, Zeitlin P L. Am J Respir Crit Care Med. 1998;157:484–490. doi: 10.1164/ajrccm.157.2.9706088. [DOI] [PubMed] [Google Scholar]
- 25.Perlmutter D H, Daniels J D, Auerbach H S, De Schryver-Kecskemeti K, Winter H S, Alpers D H. J Biol Chem. 1989;264:9485–9490. [PubMed] [Google Scholar]
- 26.Lomas D A, Evans D L, Finch J T, Carrell R W. Nature (London) 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 27.Elliott P R, Bilton D, Lomas D A. Am J Respir Cell Mol Biol. 1998;18:670–674. doi: 10.1165/ajrcmb.18.5.3065. [DOI] [PubMed] [Google Scholar]