Abstract
Aims
To classify the different types of anemia among moderately to severely disabled women living in the community and examine the relationship between types of anemia and mortality.
Methods
We studied anemia in 688 women, ≥65 years, in the Women's Health and Aging Study I, a population-based study of moderately to severely disabled older women living in the community in Baltimore, Maryland. Anemia was defined by World Health Organization criteria. Causes of anemia were classified as due to nutritional deficiencies (iron, folate, and B12 deficiencies), anemia of chronic inflammation, anemia with renal disease, and unexplained anemia.
Results
147 of 688 (21.4%) women were anemic (hemoglobin <12 g/dL). Of the 147 anemic women, 22 (15.0%) had anemia due to nutritional causes, 45 (30.6%) had anemia due to chronic inflammation, 29 (19.7%) had anemia and renal disease, and 51 (34.7%) had unexplained anemia. The proportions of those who died over five years among non-anemic women and women with anemia due to nutritional causes, chronic inflammation, renal disease, and unexplained anemia were 26.1%, 18.2%, 38.6%, 64.3%, and 33.3%, respectively (p<0.0001). Compared with non-anemic women, those with anemia and renal disease (HR 1.99, 95% CI 1.18-3.35, p=0.009) and anemia of chronic inflammation (HR 1.69, 95% CI 1.00-2.84, p=0.05) had higher risk of death.
Conclusions
Anemia is common among moderately to severely disabled older women living in the community, and about one-third of the anemia is unexplained. Anemia with renal disease and anemia of chronic inflammation are associated with a higher mortality.
Keywords: Aging, anemia, hemoglobin, inflammation, nutrition, women
Introduction
Anemia is common in older adults, and the prevalence of anemia increases with advancing age (1, 2). In the third National Health and Nutrition Examination Survey (1988-1994) (NHANES III), overall, 11.0% of men and 10.2% of women aged 65 and older were anemic, and the prevalence of anemia was greater than 20% among those aged 85 and older (3). Anemia has been associated with a wide spectrum of adverse outcomes among older adults (4), including reduced quality of life (5, 6), depression (7), decreased muscle strength (8), increased disability (9), higher risk of Alzheimer disease (10), and increased all-cause mortality (11-13). Anemia has also been linked with congestive heart failure (14) and impaired cognitive function (15). The reduction of oxygen-carrying capacity of the blood that occurs with anemia may account for fatigue, cardiovascular complications, and impaired physical performance (4).
Among adults ≥65 years in NHANES III, about one third of anemia was due to anemia of chronic inflammation or chronic renal disease, one third was due to nutrient deficiencies, and one third was unexplained anemia (3). The anemia of chronic inflammation, also known as the anemia of chronic disease, is commonly associated with conditions such as infections, cancer, autoimmune disease, organ transplant rejection, and chronic kidney disease (16). Deficiencies in iron, folate, and vitamin B12 may contribute to a substantial proportion of anemia in older adults. Iron deficiency anemia is the most common type of nutritional anemia among older adults and is often associated with occult blood loss (17). Folate and vitamin B12 are essential for the synthesis of DNA (18). After excluding iron and vitamin deficiencies, kidney disease, and chronic morbid conditions, alone or in combination, about one-third of the anemia in aging in NHANES III was unexplained (3). Another recent study conducted among residents in a skilled nursing facility showed that one-third of anemia in these older adults remained unexplained from a pathophysiological standpoint (19). The prevalence and types of anemia among older people in population-based studies is not well understood, and the relationship between the types of anemia and mortality has not been described in older adults. We characterized the prevalence and types of anemia and the relationship between types of anemia and mortality among older moderately to severely disabled women living in the community in the Women's Health and Aging Study I.
Methods
Subjects in this study were women, aged 65 and older, who participated in the Women's Health and Aging Study I (WHAS I), a population-based study designed to evaluate the causes and the course of physical disability in older women living in the community. WHAS I was recruited from an age-stratified random sample of women aged 65 years and older selected from Medicare enrollees residing in 12 contiguous zip code areas in Baltimore (20). Women were screened to identify self-reported physical disability that was categorized into four domains by report difficulty with tasks in the following areas: (1) mobility, (2) upper extremity function, (3) higher functioning household management, and (4) self-care. WHAS I enrolled the one-third most disabled women aged 65 and older, which were those with disability in two or more domains. Baseline examinations were conducted from 1992 to 1995 (20). Of the 1409 women who met study eligibility criteria, 1002 agreed to participate in the study in 1992. There were no major differences in sociodemographic or reported health characteristics between eligible participants and those who declined to participate (20). Standardized questionnaires were administered in the participant's home by trained interviewers. Two weeks later, a trained registered nurse conducted an examination of each study participant in her home, using a standardized protocol that included physical performance measures and a directed physical examination. Medical diagnoses such as osteoarthritis, diabetes, congestive heart failure, stroke, angina, cancer, and Parkinson's disease were determined using disease-specific algorithms and adjudication (20). The diagnosis of hypertension was based upon self-report.
Approximately 75% of women also consented to phlebotomy performed during a separate visit by a trained phlebotomist who followed a standardized protocol. A previous analysis has shown that those who did not participate in the blood drawing had lower education and a higher prevalence of frailty compared to those who participated in the blood drawing (21). Further details on the methods and sampling design of the WHAS studies are published elsewhere (20, 22). Non-fasting blood samples were obtained by venipuncture between 9 AM and 2 PM. Processing, aliquoting, and freezing were carried out at the Core Genetics Laboratory of The Johns Hopkins University School of Medicine following a standardized protocol. Blood samples were delivered to Quest Diagnostics Laboratories (Teterboro, New Jersey) on the day of blood drawing for complete blood count, folate, vitamin B12, and serum iron measurements. Serum vitamin B12 and folate were measured by immunoassay (RIA) (23). Serum erythropoietin was measured using enzyme-linked immunosorbent assay (ELISA) (EPO EIA, ALPCO Diagnostics, Windham, NH). Serum selenium was measured by graphite furnace atomic absorption spectrometry using a Perkin Elmer Analyst 600 with Zeeman background correction (24). Serum interleukin (IL)-6 was measured using ELISA (Quantikine, R & D Systems, Minneapolis, MN).
Anemia was defined as hemoglobin <12 g/dL. The types of anemia were defined using a framework similar to Guralnik and colleagues (3), except for the definition of iron deficiency, since the WHAS laboratory data did not include all the same indicators of iron status. Analyses for the present study are limited to 688 women who had complete data available for classification of anemias. Among women with hemoglobin <12 g/dL, iron deficiency anemia was defined as serum ferritin <12 mg/L, folate deficiency anemia was defined as serum folate <5.89 nmol/L, and anemia due to vitamin B12 deficiency was defined as serum B12 <200 pg/mL. Among anemic women, the anemia of chronic inflammation was defined as serum iron <60 ug/dL and serum ferritin >12 mg/L, and anemia due to renal disease was defined as creatinine clearance <30 mL/min. Unexplained anemia was defined as anemia that was not due to iron, folate, or vitamin B12 deficiencies or due to the anemia of chronic inflammation or renal disease. Women were categorized as non-frail or frail according to a standardized, validated definition of the frailty syndrome (25). Impaired cognition was defined as a Mini-Mental State Examination score of less than 24, a generally accepted cut-off for abnormal cognition (26). Vital status was determined on all women for whom baseline data on anemia were obtained through follow-up telephone interviews with living subjects, interviews with proxies, obituaries, and matching with the National Death Index from the baseline visit, 1992-1995 through the end of 2000. The Johns Hopkins University institutional review board approved the study protocol, and written informed consent was obtained from all participants.
Cox proportional hazards models were used to examine the survival probability over five years of follow-up for women by anemia status. Given differences in mean age of subjects across types of anemia, age-adjusted survival curves were used to calculate survival times (27). Other factors such as IL-6, selenium, and erythropoietin were not used for adjustment in survival curves because these factors may be causally involved in anemia and mortality. The statistical programs used were SAS (SAS Institute, Cary, NC).
Results
At baseline, 147 of 688 (21.4%) women were anemic. Of the 147 anemic women, 22 (15.0%) had anemia due to nutritional causes, 45 (30.6%) had anemia due to chronic inflammation, 29 (19.7%) had anemia due to renal disease, and 51 (34.7%) had unexplained anemia (Figure 1). Of the 29 women with anemia and renal disease, 14 met the criteria for anemia of chronic inflammation. Demographic and medical characteristics, serum IL-6, erythropoietin, and selenium concentrations, and the proportion of women who died over five years of follow-up are shown in Table 1. Women with the anemia of chronic inflammation/renal disease were older and a higher proportion of these women were black. The lowest level of education was in the unexplained anemia group. Mean serum selenium concentrations were lowest among women with the anemia of chronic inflammation/renal disease and highest among women who were not anemic. Geometric mean erythropoietin levels were highest among women with the anemia of chronic inflammation and lowest among those with renal disease. There were no significant differences in the prevalence of major diseases between non-anemic women and women with the four types of anemia except for diabetes, which was most common among women with anemia of chronic inflammation. The proportion of women with impaired cognition did not differ significantly by anemia status. A higher proportion of frail women had anemia with nutrient deficiencies and anemia with renal disease.
Fig. 1. Classification of anemia among women in the Women's Health and Aging Study I.
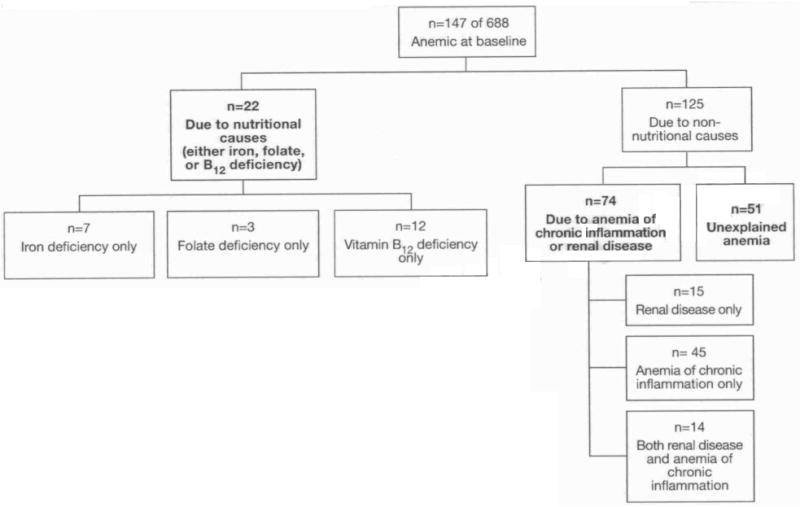
Table 1. Demographic characteristics, hemoglobin level, and prevalence of chronic conditions among women without anemia and with different types of anemia in the Women's Health and Aging Study I.
| Characteristic | Non-anemic (n=539) |
Anemic with Nutrient Deficiency (n=22) |
Anemic of Chronic Inflammation (n=45) |
Anemic with Renal Disease (n=29) |
Unexplained Anemia (n=51) |
p |
|---|---|---|---|---|---|---|
| Age, years | 77.0 (7.6) | 78.0 (8.6) | 71.1 (7.5) | 85.3 (6.6) | 77.8 (7.3) | <0.0001 |
| Black race (%) | 22.5 | 36.4 | 62.2 | 34.5 | 43.1 | <0.0001 |
| Education <12 years (%) | 59.6 | 86.4 | 73.3 | 75.9 | 90.2 | <0.0001 |
| Hemoglobin (g/L) | 13.4 (1.0) | 10.7 (0.8) | 10.8 (1.4) | 11.0 (0.9) | 11.3 (0.6) | <0.0001 |
| Erythropoietin (IU/mL) | ||||||
| Median (25th, 75th percentile) | 7.4 (3.8, 14.0) | 14.0 (10.1, 25.7) | 15.7 (7.4, 25.1) | 5.9 (3.3, 14.4) | 12.2 (3.9, 27.6) | <0.0001 |
| Selenium (μg/L) | 119 (19) | 114 (21) | 108 (14) | 110 (9) | 114 (15) | 0.0006 |
| Selenium in lowest quartile (%) | 21.8 | 40.0 | 47.6 | 39.3 | 24.5 | 0.0003 |
| IL-6, geometric mean and 95% CI | 3.78 (3.56, 4.01) | 4.88 (3.50, 6.81) | 5.92 (4.68, 7.49) | 4.08 (3.24, 5.14) | 4.09 (3.19, 5.25) | 0.003 |
| Condition (%) | ||||||
| Hypertension | 57.6 | 68.2 | 70.5 | 55.2 | 70.6 | 0.16 |
| Osteoarthritis | 53.2 | 54.6 | 55.6 | 37.9 | 58.8 | 0.74 |
| Diabetes mellitus | 15.9 | 4.6 | 31.1 | 10.3 | 23.5 | 0.1 |
| Congestive heart failure | 10.4 | 9.1 | 15.6 | 17.2 | 7.8 | 0.45 |
| Stroke | 5.6 | 0 | 6.7 | 10.3 | 2.0 | 0.43 |
| Angina | 22.8 | 13.6 | 28.9 | 34.5 | 23.5 | 0.32 |
| Cancer | 12.4 | 0 | 13.3 | 6.9 | 11.8 | 0.38 |
| Parkinson's disease | 2.0 | 4.6 | 0 | 0 | 3.9 | 0.2 |
| Impaired cognition1 | 16.3 | 18.2 | 22.2 | 24.1 | 19.6 | 0.6 |
| Frail2 | 23.8 | 45.5 | 33.3 | 44.8 | 31.4 | 0.01 |
| Died within five years (%) | 26.1 | 18.2 | 38.6 | 64.3 | 33.3 | 0.0006 |
Defined as score on Mini-Mental Status Exam <24 (Lezak 1995).
Defined according to Fried and colleagues (Fried 2002).
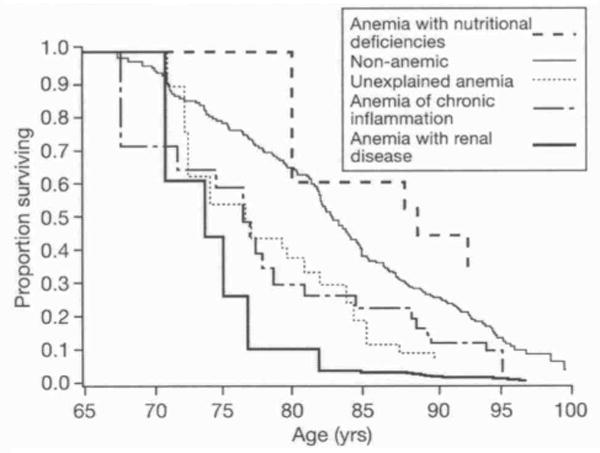
Of the 688 women who had anemia status assessed at baseline, 198 women (28.8%) died during the subsequent five years. The proportion of women who died was highest among those who had anemia due to renal disease (Table 1). The age-adjusted survival curves of women by anemia status are shown in Figure 2. In Cox proportional hazards regression with age as a time scale, outcome as death (26), and non-anemic as the reference category, risk of mortality was as follows: anemia with renal disease (HR 1.99, 95% CI 1.18-3.35, p=0.009), anemia of chronic inflammation (HR 1.69, 95% CI 1.00-2.84. p=0.05), unexplained anemia (HR 1.32, 95% CI 0.80-2.19, p=0.28), and anemia with nutritional deficiencies (HR 0.79, 95% CI 0.29-2.14, p=0.64).
Fig. 2. Five-year survival of women in the Women's Health and Aging Study I by anemia status.
Discussion
Anemia was present in over one-fifth of the women in this study population that represents moderately-severely disabled older women living in the community. The prevalence of anemia among women aged 65 years and older in WHAS I was higher than that reported for women of similar age in NHANES III (10.2%) (3) and in the population-based InCHIANTI study (11.5%) (8): this difference is likely due to the greater level of frailty, disability, and comorbidity among women in WHAS I. In this respect, the women in the study are similar to older women who are under medical care. Women with anemia were older, more likely to be black, and had a lower level of education than non-anemic women, which is consistent with previous observations (3, 28).
Among anemic women, over one-half had anemia of chronic inflammation and/or renal disease and over one-third had unexplained anemia. It is interesting to note that although women in WHAS I were moderate to severely disabled and had higher levels of morbidity compared with NHANES III and the InCHIANTI study, unexplained anemia accounted for about one-third of all anemia in these three studies (3). Unexplained anemia is currently a diagnosis of exclusion, and its pathogenesis remains obscure. Myelodysplastic syndrome and other uncommon causes of anemia, such as thalassemia minor, multiple myeloma, hereditary spherocystosis, autoimmune hemolytic anemia, and hypothyroidism could potentially contribute to unexplained anemia, but these causes have a relatively low prevalence (3). Two other potential causes of anemia that could contribute to unexplained anemia are low testosterone levels or low selenium (24). The relationship between serum testosterone levels and anemia among women in WHAS remains to be characterized. Low serum selenium seems unlikely to be a substantial cause of anemia among women with unexplained anemia, as, in the present study, mean selenium levels among women with unexplained anemia were nearly comparable with women who were non-anemic.
Women with unexplained anemia had lower erythropoietin levels than women with anemia due to nutrient deficiencies or chronic inflammation, but the erythropoietin levels were not as low as those found in women who had renal disease. It is not known whether unexplained anemia could be due to subclinical renal disease, and future research should be done to determine whether proteinuria and microalbuminuria are associated with unexplained anemia.
Previous studies have shown that anemia is associated with increased mortality among older adults (11, 13), but less is known about the relationship between the types of anemia and mortality. In age-adjusted analyses, when compared with non-anemic women, women with anemia and renal disease had the highest risk of death, followed by women with anemia of chronic inflammation. Unexplained anemia was associated with about a 30% increased risk of death but this did not reach statistical significance. The mortality rate among women with nutritional deficiencies and anemia was lower than that among women without anemia, and given the small numbers of women with nutritional deficiencies and anemia (22 women of whom four died), this may have been related to a small sample size in this group. About half of the group of women with anemia and renal disease also had chronic inflammation, and inflammation is a strong predictor of mortality among older adults with renal disease (29). Future work is needed to examine the relationship between types of anemia and mortality among less disabled, community-dwelling men and women.
Serum IL-6 was highest among women with the anemia of chronic inflammation and was lowest among non-anemic women. IL-6 is produced by macrophages and T lymphocytes and plays a central role in inflammation by inducing the production of acute phase proteins (30). IL-6 has been implicated as a key cytokine involved in the upregulation of hepcidin, a recently discovered peptide hormone that is produced by the liver and regulates iron metabolism by blocking iron absorption by the gut and release of iron from macrophages and the liver (31). In humans, hepcidin levels can be increased by experimental challenge with lipopolysaccharide (32) or IL-6 (33), but the relationship between hepcidin, anemia, and the low grade inflammatory state among older people living in the community remains uncharacterized and a major gap in knowledge.
In conclusion, the prevalence of anemia is high among older disabled women living in the community, and about one-third of the anemia is unexplained. Women who had anemia with renal disease and anemia of chronic inflammation were at significantly higher risk of death.
Acknowledgments
This work was supported by National Institute on Aging Grant R01 AG027012, AG029148, NIH-NCRR, OPD-GCRC grant RR00722, R01 AI41956, NIA Contract N01-AG12112, and the Intramural Research Program, National Institute on Aging, NIH.
References
- 1.Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116:3S–10S. doi: 10.1016/j.amjmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005;12:123–8. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 4.Lipschitz D. Medical and functional consequences of anemia in the elderly. J Am Geriatr Soc. 2003;51:S10–3. doi: 10.1046/j.1532-5415.51.3s.6.x. [DOI] [PubMed] [Google Scholar]
- 5.Thomas ML. Impact of anemia and fatigue on quality of life in cancer patients: a brief review. Med Oncol. 1998;15(suppl 1):S3–7. [PubMed] [Google Scholar]
- 6.Valderrabano F. Quality of life benefits of early anaemia treatment. Nephrol Dial Transplant. 2000;15(suppl 3):23–8. doi: 10.1093/oxfordjournals.ndt.a027972. [DOI] [PubMed] [Google Scholar]
- 7.Onder G, Penninx BW, Cesari M, et al. Anemia is associated with depression in older adults: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2005;60:1168–72. doi: 10.1093/gerona/60.9.1168. [DOI] [PubMed] [Google Scholar]
- 8.Cesari M, Penninx BW, Lauretani F, et al. Hemoglobin levels and skeletal muscle: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:238–41. doi: 10.1093/gerona/59.3.m249. [DOI] [PubMed] [Google Scholar]
- 9.Penninx BWJH, Pahor M, Cesari M, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–24. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- 10.Beard CM, Kokmen E, O'Brien PC, Anía BJ, Melton LJ., III Risk of Alzheimer's disease among elderly patients with anemia: population-based investigations in Olmsted County, Minnesota. Ann Epidemiol. 1997;7:219–24. doi: 10.1016/s1047-2797(97)00015-x. [DOI] [PubMed] [Google Scholar]
- 11.Izaks GJ, Westendorp RGJ, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–7. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 12.Kikuchi M, Inagaki T, Shinagawa N. Five-year survival of older people with anemia: variation with hemoglobin concentration. J Am Geriatr Soc. 2001;49:1226–8. doi: 10.1046/j.1532-5415.2001.49241.x. [DOI] [PubMed] [Google Scholar]
- 13.Chaves PHM, Xue QL, Guralnik JM, Ferrucci L, Volpato S, Fried LP. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J Am Geriatr Soc. 2004;52:1811–6. doi: 10.1111/j.1532-5415.2004.52502.x. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–44. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 15.Nissenson AR. Epoietin and cognitive function. Am J Kidney Dis. 1992;20(suppl 1):21–4. [PubMed] [Google Scholar]
- 16.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 17.Carmel R. Anemia and aging: an overview of clinical, diagnostic and biological issues. Blood Rev. 2001;15:9–18. doi: 10.1054/blre.2001.0146. [DOI] [PubMed] [Google Scholar]
- 18.Balducci L. Epidemiology of anemia in the elderly: information on diagnostic evaluation. J Am Geriatr Soc. 2003;51:S2–9. doi: 10.1046/j.1532-5415.51.3s.4.x. [DOI] [PubMed] [Google Scholar]
- 19.Artz AS, Fergusson D, Drinka PJ, et al. Mechanisms of unexplained anemia in the nursing home. J Am Geriatr Soc. 2004;52:423–7. doi: 10.1111/j.1532-5415.2004.52116.x. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. NIH Publication No. 95-4009. [Google Scholar]
- 21.Michelon E, Blaum C, Semba RD, Xue QL, Ricks MO, Fried LP. Vitamin and carotenoid status in older women: associations with the frailty syndrome. J Gerontol A Med Sci. 2006;61:600–7. doi: 10.1093/gerona/61.6.600. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the Women's Health and Aging Study. J Clin Epidemiol. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 23.Stabler SP, Allen RH, Fried LP, et al. Racial differences in prevalence of cobalamin and folate deficiencies in disabled elderly women. Am J Clin Nutr. 1999;70:911–9. doi: 10.1093/ajcn/70.5.911. [DOI] [PubMed] [Google Scholar]
- 24.Semba RD, Ferrucci L, Cappola AR, et al. Low serum selenium is associated with anemia among older women living in the community: the Women's Health and Aging Studies I and II. Biol Trace Elem Res. 2006;11:97–107. doi: 10.1385/BTER:112:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2002;56A:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 26.Lezak MD. Neuropsychological Assessment. Third. New York: Oxford University Press; 1995. [Google Scholar]
- 27.Lamarca R, Alonso J, Gomez G, Muñoz A. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53:M337–43. doi: 10.1093/gerona/53a.5.m337. [DOI] [PubMed] [Google Scholar]
- 28.Salive ME, Cornoni-Huntley J, Guralnik JM et al. Anemia and hemoglobin levels in older persons: relationship with age, gender, and health status. J Am Geriatr Soc. 1992;40:489–96. doi: 10.1111/j.1532-5415.1992.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 29.Honda H, Qureshi AR, Heimburger O, et al. Serum albumin, C-reactive protein, interleukin 6, and fetiun a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47:139–48. doi: 10.1053/j.ajkd.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late life diseases, and frailty. Annu Rev Med. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 31.Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12:107–11. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–6. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 33.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Investig. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]


