Abstract
In this study, a recombinant truncated West Nile virus envelope protein antigen (rWNV-E) was produced in serum-free cultures of the expresSF+ insect cell line via baculovirus infection. This production system was selected based on its use in the production of candidate human and animal vaccine antigens. A defined fermentation and purification process for the rWNV-E antigen was established to control for purity and immunogenicity of each protein batch. The material formulated with aluminum hydroxide was stable for greater than 8 months at 4°C. The recombinant vaccine candidate was evaluated for immunogenicity and protective efficacy in several animal models. In mouse and hamster WNV challenge models, the vaccine candidate induced viral protection that correlated with anti-rWNV-E immunogenicity and WNV neutralizing antibody titers. The rWNV-E vaccine candidate was used to boost horses previously immunized with the Fort Dodge inactivated WNV vaccine and also to induce WNV neutralizing titers in naïve foals that were at least 14-weeks of age. Furthermore, the vaccine candidate was found safe when high doses were injected into rats, with no detectable treatment-related clinical adverse effects. These observations demonstrate that baculovirus-produced rWNV-E can be formulated with aluminum hydroxide to produce a stable and safe vaccine which induces humoral immunity that can protect against WNV infection.
Keywords: West Nile virus, Envelope protein, Vaccine, Baculovirus, expresSF+ Insect cells
1. Introduction
West Nile virus (WNV) belongs to the flavivirus family and is prevalent in Africa, Southern Europe, Russia, the Middle East, India and Australia. It first appeared in North America in 1999 and disseminated through the continent in just a few years, becoming a major public health and veterinary concern [1–3]. WNV is transmitted primarily by Culex mosquitoes in a cycle involving birds as amplifying hosts and causes febrile illness that can lead to fatal meningitis or encephalitis in humans (particularly in the elderly), and in horses [4].
The WNV outbreak in North America coupled with the absence of effective treatment against WNV infection has triggered vaccine development efforts to prevent WNV infection of domestic animals and humans. As a result, a variety of WNV vaccine candidates have been developed in the last decade [4]. West Nile virions contain two surface proteins, membrane (M) and envelope (E); the E protein being the most immunogenic protein in all flaviviruses evoking the majority of neutralizing antibodies [4]. Three equine vaccines have been successfully commercialized in the USA. These equine vaccines include Innovator™ (FD, Fort Dodge, Princeton, NJ), formulated based on formalin-inactivated WNV [5], licensed by the USA Department of Agriculture in 2003; Recombitek® (Merial Ltd., Athens, GA), a Carbopol-adjuvanted recombinant replicative canarypoxvirus vaccine expressing prM (membrane precursor) and E transgenes [6] that was licensed in 2004; and pCBWN (Fort Dodge and Center for Disease Control and Prevention), a recombinant plasmid DNA expressing WNV membrane and envelope antigens, licensed as an equine vaccine in 2005 [7, 8].
There is no approved vaccine for WNV for human use; however, several vaccine candidates for WNV are being evaluated pre-clinically and clinically. The human vaccine candidates include recombinant DNA, attenuated live virus, and recombinant subunit vaccines. These candidates include: Chimerivax-WNV [9, 10], a live attenuated recombinant 17D-Yellow Fever (YF) vaccine strain where WN-prM/E genes were substituted for YF-prM/E genes; WN/DEN4Delta30 [11, 12], a live-attenuated WN-dengue-type 4 chimeric virus with WN-prM/E surface antigens in a dengue-4 virus backbone; MVSchw-sE(WNV), a recombinant live attenuated measles vaccine expressing WN-prM/E antigens [13]; and TRIP/sEWNV, a recombinant lentivirus expressing WN-prM/E [14]. Recombinant subunit vaccine candidates include soluble truncated recombinant E protein expressed in E. coli and Drosophila cells [15–18] or virus-like particles (VLP) expressed in Sf9 insect cells [19]. Recent data suggest that recombinant envelope domain III protein can also induce immune responses that protect against WNV infection [20–22].
This study focused on developing a recombinant subunit WNV vaccine candidate using the truncated viral E protein (rWNV-E) [15, 16, 23] as the target antigen. The truncated antigen includes the extracellular E protein domains I, II and III [23]. When the rWNV-E antigen was expressed in Drosophila insect cells [16], it was found more effective in eliciting protective antibodies than that produced in E.coli [15]. After this success in insect cells, we worked to develop an alternate method to manufacture the antigen under a process compatible with large-scale vaccine production and human clinical trials. To this end, we converted to a baculovirus-based production system (Protein Sciences Corporation) which has been qualified and used for cGMP manufacturing of candidate vaccine antigens for human clinical trials. Baculoviruses are non-infectious in humans by virtue of their narrow host range, which is restricted to a few taxonomically related insect species. Because the insects infected by baculoviruses are non-biting, humans generally do not have pre-existing immunity to the host expresSF+ insect cell proteins that could cause an allergic reaction to residual insect cell proteins in the vaccine preparation.
In this report, we describe the results of experiments with rWNV-E produced from baculovirus-infected expresSF+ (SF+) cells with production scales ranging from 1L to 10L cultures and typical purified product yields of 5 to 10 mg/L. Biochemical analyses demonstrated that the rWNV-E met defined product specifications and had appropriate immunoreactivity. Single vaccine doses (5μg) in a murine WNV lethal challenge model were used to optimize production and formulation with aluminum hydroxide. Immunogenicity and protective efficacy was assessed in mouse and hamster models of WNV infection. Immunogenic efficacy in previously vaccinated horses and naïve foals was compared to that of the commercially approved Innovator™ (Fort Dodge) vaccine, using WNV-neutralizing antibodies as surrogate markers. Finally, a GLP-compliant safety study was performed in rats. Overall, our results suggest that the SF+ cell-expressed rWNV-E vaccine candidate is stable, safe and induces humoral immune responses that can protect against WNV infection.
2. Material and methods
2.1. Expression and production of rWNV-E in SF+ cells
DNA encoding E protein amino-acid residues 1–406 of WNV strain 2741 (nucleotides 925 to 2142; GenBank accession no. AF206518) [16, 24] was inserted into the pPSC12 baculovirus transfer vector (Protein Sciences Corporation, Meriden, CT) using ligation-independent cloning. The recombinant plasmid directs the synthesis of a fusion protein consisting of the 18-amino acid AcNPV (Autographa californica nuclear polyhedrosis virus) chitinase secretory signal peptide, MPLYKLLNVLWLVAVSNA, followed by residues 1–406 of WNV E protein. DNA sequencing confirmed that no mutations were introduced into the sequence during the PCR amplification or the cloning process. The E protein transfer plasmid was co-transfected into Sf9 insect cells with BsuI linearized AcNPV genomic DNA. Homologous recombination between the transfer plasmid and the linearized genomic DNA rescued the virus. Recombinant virus identified by their plaque morphology was purified and used to infect cultures of the Sf9 insect cell line. Infected cells were screened for expression of recombinant protein by SDS-PAGE analysis and western blotting.
Passage 1 (P1) recombinant virus was propagated in Sf9 cells and all subsequent passages were with S. frugiperda expresSF+ cells (SF+ cells; Protein Sciences Corporation, Meriden, CT) in serum free medium (Protein Sciences Formulary Medium). Recombinant baculovirus propagated from a single plaque was used to generate a P3 “working virus bank” which was frozen and stored in liquid N2. Passage 4 or Passage 5 virus stocks derived from this bank were used for all subsequent infection of SF+ cells to produce rWNV-E batches. Briefly, rWNV-E was produced in 3L or 15L Applikon stirred tank bioreactors seeded with 1.0 × 106 SF+ cells/mL. The cells were infected with the rWNV-E baculovirus at a multiplicity of infection (MOI) of 1 after the SF+ cell density was between 2.0 and 2.5 × 106 cells/mL. The fermentations were harvested typically between 45 and 52 hours post infection when the cell viability had decreased to between 65 and 80%.
2.2. rWNV-E purification
rWNV E protein was extracted from SF+ cell pellets as follows: cell pellets were homogenized using a Polytron homogenizer in 20 mM Tris, 1% pluronic acid, pH. 8.0 and centrifuged for 30 minutes at 4500 rpm (~6000 × g). The supernatant was decanted and the remaining cell pellet was homogenized in 20 mM ethanolamine, 1% Triton X-100, 1mM EDTA, and centrifuged again. The pellet was washed a third time with phosphate buffered saline (PBS, pH 7.3) before being solubilized in 10 mM NaOH for one hour at 4°C followed by adjustment to pH 8.0 with 0.5 M sodium phosphate, pH 7.0. A DEAE anion exchange resin column step was utilized to remove contaminants that bound to the resin while solubilized rWNV-E protein flowed through the column. DEAE flow-through fractions were pooled, concentrated and buffer exchanged against 5 mM phosphate buffer pH 8.0, 150 mM NaCl. The protein concentration was determined by Bicinchoninic acid (BCA) Protein Assay (Pierce Biotechnology).
2.3.rWNV-E biochemical characterization
Polyacrylamide gradient gels were used to analyze rWNV-E-containing samples in Tris-Glycine buffer, in the absence of denaturing agent for native conditions, or in the presence of denaturing agent (SDS-PAGE), with or without reducing agent. Western blot analyses were performed according to standard procedures using PVDF membranes (Biorad), horse anti-rWNV-E antiserum [25] or its rabbit equivalent, diluted 1:10,000, and goat anti-horse or anti-rabbit IgG secondary antibody conjugated to alkaline phosphatase (Sigma) diluted 1:10,000. The colorimetric substrate BCIP/NBT (KPL) was used to detect the antigen-antibody complexes on the membrane. Size exclusion chromatography (Sephacryl 200, GE Healthcare Life Sciences) in phosphate buffered saline (PBS) was used to analyze the purified rWNV-E under native conditions.
A competitive ELISA method was developed to assess variations in immunoreactivity of purified rWNV-E protein. Ninety-six well ELISA plates were coated overnight at 4°C with rWNV-E (100 ng/well in carbonate buffer). Ten-fold serial dilutions (from 10 μg/ml to 0.001 μg/ml) of each rWNV-E antigen to be tested (competing antigen) were prepared in a solution containing 0.2 μg/ml horse polyclonal anti-E antibody (affinity-purified IgG) [25] in PBTM (PBS containing 0.1% Tween-20 and 0.5% fat-free dry milk) and incubated overnight at 4°C. The plates were then washed three times in PBT at room temperature (RT), blocked in PBTM for 30 minutes and incubated with each antigen-antibody mix for one hour at RT. Plates were washed three more times with PBT, incubated with anti-horse alkaline phosphatase (AP)-conjugate (Sigma) diluted 1:3000 in PBTM for one hour, and washed four times with PBT. The binding reaction was developed with pNPP AP substrate (Sigma) until an OD (405nm) of 1.5 was reached for control antigen at a concentration of 0.001 μg/ml.
The rWNV-E protein was assessed for glycosylation by digestion with peptide N-glycosidase F (PNGase F) and Endoglycosidase H (Endo H). Aliquots (3μg) of rWNV-E were treated with 2000 units of either enzyme in its respective buffer according to the manufacturer’s instructions (New England BioLabs), or without enzyme as a control. Digestions were carried out for 3 hours at 37°C. Samples were then analyzed by SDS-PAGE and Coomassie blue staining.
N-terminal amino-acid sequencing, electrospray mass spectrometry and Liquid Chromatography/Mass Spectrometry (LC MS/MS) peptide analyses of tryptic digests were performed by the W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University.
For mouse, hamster and horse injections, rWNV-E antigen was formulated with Alhydrogel™ adjuvant (Accurate) by mixing antigen and adjuvant at a ratio of 1μg of rWNV-E protein per 1μl of the 1.3% aluminum hydroxide suspension in 5 mM phosphate buffer and 150 mM NaCl, pH 8.0 for one hour at room temperature [16]. Binding of the protein to aluminum hydroxide suspension was assessed by SDS-PAGE (reducing conditions) followed by Coomassie blue staining, in which case the bound rWNV-E protein does not migrate through the gel.
Sterility testing was conducted by a methodology described in USP Sterility Tests <71> and 21 CFR 610.12. These methods entailed direct inoculation of the purified rWNV-E antigen preparations into Fluid Thioglycollate Medium (FTM) and Tryptic Soy Broth (TSB) medium and testing for bacterial or fungal growth over a two-week period.
Densitometry analysis of Coomassie blue-stained SDS-PAGE gels was used to estimate the purity of the rWNV-E protein. Stained gel images were analyzed using a BioRad GS 710 Image densitometer and corresponding Quantity One imaging and analysis software.
The LAL assay (Limulus Amebocyte Lysate, Endosafe kit, Charles River Labs) was used to determine endotoxin levels in many in-process materials and final purified protein concentrates. The assay quantifies the level of endotoxin by observation of the gel clotting caused by the interaction of endotoxins with the Limulus Amebocyte Lysate in serial dilutions of test samples.
The amount of residual non-specific baculovirus and host insect cell proteins was calculated using a western blot assay (Host Cell Protein Assay) employing a standard curve of known concentrations (40 ug to 1.6 ug) of a polyhedrin-negative baculoviral/insect cell lysate which was used as an immunogen to generate a rabbit polyclonal antisera. The immunoreactivity of HCP proteins in rWNV-E preparations was visually quantified in comparison to a standard curve.
2.4.Immunization of animals with rWNV-E vaccine candidate
All animal experiments were performed according to protocols approved by Institutional Animal Use Committees. C3H/HeN female mice (six week-old) (Charles River) were immunized sub-cutaneously with 100-μl single doses of rWNV-E adsorbed to Alhydrogel™. Control mice received injections of an equivalent amount of Alhydrogel™ adjuvant. The mice were challenged with 100 pfu of WNV isolate 2741 administered intraperitoneally two weeks later. Mice were bled via retroorbital sinus before challenge to test for serum antibody response. Morbidity and mortality were assessed twice daily for 21 days. All work with infected animals was carried out in biosafety level 3 (BL3) facilities.
Female Mesocricetus auratus hamsters (70 grams) (Charles River) received two 10 μg doses of Alhydrogel™-absorbed rWNV-E by sub-cutaneous injection, three weeks apart. Control hamsters received injections of an equivalent amount of Alhydrogel™ adjuvant in the same buffer conditions. At day 35, two weeks after the second immunization, hamsters were bled to determine antibody response. All animals were challenged at day 35 with 104 pfu of WNV isolate 2741 administered intraperitoneally. Morbidity and mortality were assessed twice daily for 21 days. The work was carried out in BL3 facilities.
Forty horses of different breeds, ages, and gender, with a documented history of WNV vaccination with Fort Dodge Animal Health Innovator™ killed virus (FD) vaccine in previous years, were selected for this study. Animals were randomized and groups were boosted with a single dose of either FD vaccine or rWNV-E Alhydrogel™-formulated vaccine. Twenty horses were injected with FD vaccine as recommended [5]. Twenty horses were injected intramuscularly with 50 μg of rWNV-E adsorbed to Alhydrogel™. Horses were examined for signs of adverse reactions for several hours following each injection. Horses were maintained on pasture and in barns. All the animals were bled at the beginning of the study and at regular intervals for a period of six months. Serum samples were analyzed for antibodies recognizing WNV-E protein by ELISA and for WNV neutralizing antibodies in a plaque reduction neutralizing assay. Five foals (4–15 weeks old) were immunized with rWNV-E vaccine and boosted after four weeks using the same conditions described above. Serum samples were collected one day prior to the first injection and at regular intervals for a period of 6 months and analyzed in ELISA and PRNT assays.
2.5.GLP-compliant toxicology study of rWNV-E vaccine candidate
The safety of the recombinant subunit WNV vaccine candidate was assessed in rat studies using multiple dose levels of rWNV-E antigen combined with Alhydrogel™. The study was conducted at the IIT Research Institute-Life Sciences Group, Chicago, IL. The experiment had four treatment groups, each composed of 10 male and 10 female Sprague-Dawley rats. The protocol included two injections, 15 days apart, of 0, 10, 50 or 100 μg of antigen mixed with 100 μl of Alhydrogel™ (1.3 % aluminum hydroxide) suspension in 0.5 ml. Dosages were administrated via intramuscular injection into the rear hind legs. The study extended a total of 45 days (30 days after last immunization). Serum was collected for antibody analysis prior to each inoculation, at study day 30 and at study-conclusion. Immunogenicity was assessed by determining anti-rWNV-E IgG titers by ELISA and virus neutralization titers by PRNT. Safety was assessed by monitoring the animals for the 45 days of the protocol. Methods used in the in-life study included: moribundicity/mortality observations, injured and diseased animals, body weight measurements, clinical pathology, blood chemistry, hematology, and coagulation. Vaccine candidate safety was also assessed at the macroscopic level and microscopic level (histopathology) in evaluations of tissues collected at the end of the study from the control (Alhydrogel™ alone) and high dose (100μg dose) groups (IIT Research Institute).
2.6.Detection of WNV-specific antibodies
The ELISA method used to detect anti-WNV-E antibodies present in mouse, hamster, rat and horse sera was similar to the one previously described [16], with the exception that the rWNV-E antigen coated on the plates was expressed in SF+ cells instead of Drosophila cells. Plates coated with both types of antigen produced comparable results. Color development of positive control wells was standardized to allow comparison of results from experiment to experiment. Serum dilutions used for ELISA data analyses yielded ODs in a linear range.
In vitro plaque reduction neutralization tests (PRNT) were used to evaluate the ability of serum samples to inhibit virus replication in cultured Vero cells. Sera from immunized hamsters were tested in an assay that was carried out in a BSL3 facility using WNV strain 2741 [16, 26]. Sera from immunized mice, horses and rats were tested in a BSL2 facility employing an assay that used an attenuated chimeric virus WNV/DEN4Delta30 (kindly provided by Dr. Steven Whitehead, NIAID, NIH, Bethesda, MD) [27–29]. Vero cells, maintained in Opti-MEM-Glutamax (Invitrogen) with 5% FBS and 100 μg/ml penicillin-streptomycin, were seeded in 24-well plates at 105 cells/well 24 hours before infection. Ten-fold serial Opti-MEM dilutions of heat-inactivated serum samples were mixed with virus (100 pfu/100 μl), maintained for about 10 minutes at RT, and added to the cells in culture for 1h at 37°C, in 5% CO2. Overlay medium (1% methylcellulose in OptiMEM-Glutamax with 2% FBS, 50 μg/ml gentamycin and 2.5 μg/ml amphotericin B) was then added to the cells and the plates were incubated at 37°C in 5% CO2 for 5 days. Viral plaque formation was detected by immunostaining using anti-flavivirus E protein monoclonal antibody 4G2, purified from HB112 hybridoma (ATCC) culture supernatant on HiTrap™ Protein G HP columns (GE Healthcare). Adherent cells were first fixed in 80% methanol, blocked in 5% skim milk in PBS (PBSM) for 10 minutes at RT and incubated with 10 μg/ml 4G2 IgG at 37°C in 5% CO2 for 90 minutes. Cells were washed twice in PBSM and incubated with a 1/2000 dilution of peroxidase-labeled anti-mouse antibody conjugate (Kirkegaard & Perry Laboratories, KPL) for 60 minutes at 37°C. Cells were washed twice in PBS and incubated in peroxidase substrate (TrueBlue, KPL) until plaque immunostaining was observed.
3. Results
3.1.Production and purification of rWNV-E expressed in expresSF+cells (SF+) from Spodoptera frugiperda
A recombinant baculovirus expressing WNV truncated envelope protein (amino-acid residues 1–406) (rWNV-E) was constructed, amplified and used to infect SF+ cells. Recovery of rWNV-E from cell culture supernatants was first attempted using a previously established purification procedure [16]. This procedure, however, yielded an average of only 0.25–0.5 mg/liter. As an alternative, an immuno-affinity approach to purify rWNV-E from the culture supernatant was also developed [23]. Although the process produced the recombinant protein that was used to determine the rWNV-E crystal structure, it did not yield more than an average of 0.5–1 mg/liter of cell culture supernatant. While both of these methods could be used to recover soluble E406 comparable in purity and immunogenicity to the well-characterized Drosophila-cell purified rWNV-E antigen [16], the relatively low yields associated with their preparation was not a suitable option for vaccine production. With the goal of preparing larger quantities of vaccine antigen, we took advantage of the fact that a significant amount of rWNV-E remained in the cell pellet fraction. We developed a procedure that yielded > 5 mg rWNV-E per liter of cell culture from cell pellets by disrupting the cells, and purifying rWNV-E with a series of extraction and column chromatography steps to remove contaminants. Reproducible purifications, with minimal protease contamination were obtained with this rapid purification method. Cell pellet rWNV-E was successfully purified from three 1L, three 2L and two 10L fermentation cultures using a GLP procedure.
3.2.Biochemical characterization of rWNV-E expressed in SF+cells
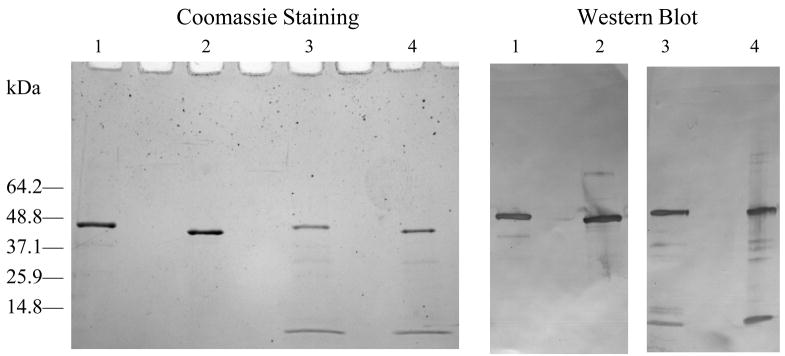
Each batch of purified rWNV-E was analyzed in terms of purity, folding and immunoreactivity. Analytical methods that compared purified SF+ cell-expressed rWNV-E with Drosophila cell-expressed rWNV-E, a known potent vaccine antigen in animal models of WNV infection, were employed to ensure that the process produces rWNV-E antigen with defined antigenicity. SDS-PAGE and western blot analyses (Figure 1) confirmed both the identity and integrity of the protein (~48 kDa monomer). Most preparations from cell pellets contained a ~10-kDa gel product that was identified as a rWNV-E fragment released from subdomain III, as determined by western blot and by tryptic digestion followed by LC MS/MS peptide analysis. rWNV-E protein has 12 cysteines that contribute to protein structure by forming six intra-molecular disulfide bonds. These disulfide bonds are normally formed as the protein passes through the intracellular secretion pathway [16]. Native polyacrylamide gel electrophoresis and size exclusion column chromatography confirmed the absence of high molecular weight, disulfide-linked aggregates (data not shown), suggesting an appropriate folding for the SF+ cell- purified rWNV-E.
Fig. 1.
SDS-PAGE analyses of purified rWNV-E. Coomassie blue staining, and western blot analyses comparing 1 ug of purified Drosophila-cell expressed rWNV-E, under reduced (lane 1) or non-reduced (lane 2) conditions with 1ug of SF+ cells-expressed rWNV-E, under reduced (lane 3) or non-reduced (lane 4) conditions.
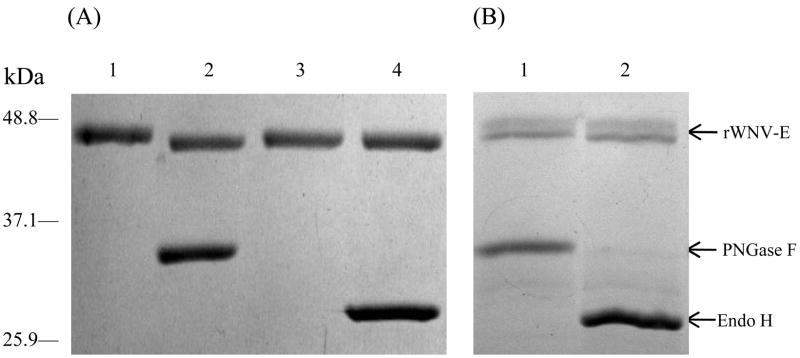
Previous studies of rWNV-E protein purified from the culture supernatant of insect cells (Drosophila S2 [16] and SF+ [23]) showed that it is a glycoprotein. A single N-linked glycosylation site, conserved in most flaviviruses, is located at Asn154. We used peptide N-glycosidase F (PNGase F, a 36-kDa amidase that removes N-linked carbohydrate structures) and Endoglycosidase H (Endo H, a 29-kDa enzyme which cleaves within the chitobiose core of high mannose and within some hybrid oligosaccharides in N-linked glycosylations) to assess the glycosylation pattern of rWNV-E batches (Figure 2). Results revealed that in addition to the usual N-linkage, the antigens purified from the cell pellets carried immature glycans characteristic of incompletely processed glycosylations, most likely at the same site. The presence of such incompletely processed structures was also confirmed by Electrospray Mass Spectrometry (EMS) analysis, adding about 3.5 kDa in molecular weight to the cell pellet purified rWNV-E (48.6 kDa), compared to the soluble Drosophila-cell expressed rWNV-E (44.9 kDa) [16].
Fig. 2.
rWNV-E glycosylation pattern assessed in glycosylation assays that utilized peptide N-glycosidase F (PNGase F) or Endoglycosidase H (Endo H). On a Coomassie blue-stained reducing SDS-PAGE: (A) Drosophila-cell produced rWNV-E digested with either PNGase F (lane 2) or Endo H (lane 4). Lane 1 and 3 correspond to non-digested controls. Only PNGase F induced a slight gel shift of ~ 1kDa (lane 2), confirming N-linked glycosylation. (B) Baculovirus-produced rWNV-E was separately digested and then mixed with non-digested controls with either PNGase F (lane 1) or Endo H (lane 2). Both PNGase F and Endo H induced a gel shift > 3kDa. rWNV-E (arrow) upper and lower bands respectively correspond to the non-digested and the digested protein forms.
Since the baculovirus-produced rWNV-E was extracted from the cell pellet, we investigated whether its signal peptide was properly removed. N-terminal amino acid sequencing identified two populations of rWNV-E in the purified samples produced by this method: one without the signal peptide and a second one containing the signal peptide. The ratio of the two populations ranged in different preparations from 1:1 to 3:1. The 1–2 kDa difference in molecular weight was not detected by SDS-PAGE. It is likely that the heterogenicity of glycosylation made the bands more diffuse and difficult to resolve.
Epitope presentation of each SF+ cells-purified rWNV-E protein batch was assessed by competitive ELISA. The purified rWNV-E vaccine antigen expressed in Drosophila cells [16], served as a positive control. Any other antigen presenting the same competitive profile was considered antigenically equivalent. Half maximal effective concentration (EC50) values were used as a indication of potency of an antigen produced through the purification process. The baculovirus-sourced rWNV-E purified according to our protocol and the Drosophila purified antigen presented comparable EC50 values (data not shown).
These data suggest that despite additional glycans, a subdomain III proteolytic fragment, and incomplete N-terminal processing, the overall immuno-reactivity of the cell lysate-sourced rWNV-E remains comparable to that of the Drosophila-expressed standard.
3.3.Product specifications and stability studies of bulk and adjuvanted rWNV-E vaccine antigen
Quality control analyses were performed to evaluate rWNV-E bulk production lots to determine if they met the specification criteria described in Table 1. The data indicated that the production process can consistently produce rWNV-E vaccine bulk antigen suitable for clinical testing.
Table 1.
Bulk rWNV-E product specifications
| QC test | Product specification |
|---|---|
| Appearance | Clear colorless solution |
| Identity | Confirmed by western blot |
| Purity (SDS-PAGE) | >98% |
| Protein Concentration | 100–600 μg/mL |
| pH | 8.0 |
| Formulation | 5 mM NaPO4, 150 mM NaCl |
| Endotoxin/Pyrogenicity | <500 EU/mL |
| Host Cell Protein | <1.3% |
| Potency EC50 | ≤0.1 g/ml |
| Sterility | Negative for bacterial and fungal contamination |
Bulk antigen underwent a three-month stability test at 2 to 8°C in a temperature-monitored refrigerator to evaluate the stability of rWNV-E in the final antigen buffer formulation. At each designated time-point, the bulk was aseptically sampled and evaluated for product specifications (Table 1). The results of the stability study for two batches indicated that rWNV-E protein remained intact during the three-month period.
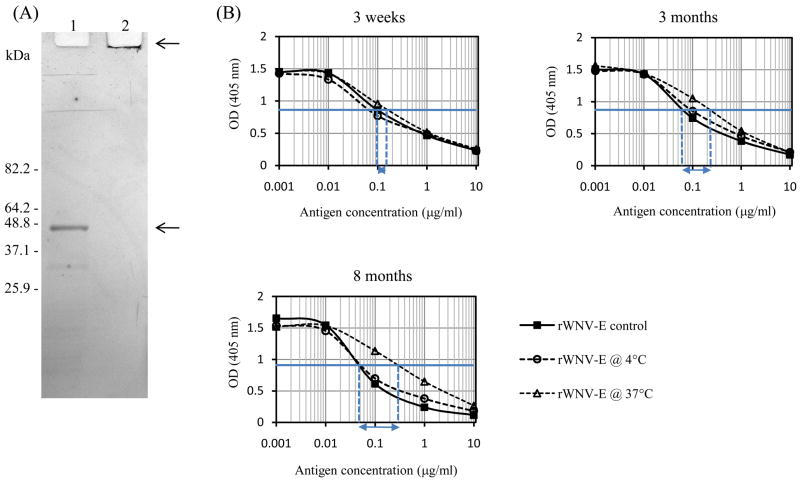
Alhydrogel™ (aluminum hydroxide) was chosen as adjuvant for our recombinant antigen. Aluminum adjuvants are widely used in licensed veterinary and human vaccines as safe stimulators of Th2 immunity [30]. Methods to adsorb rWNV-E protein to Alhydrogel™ were developed in previous studies with Drosophila cell-expressed rWNV-E [16]. Under such conditions, baculovirus-expressed rWNV-E antigen completely bound to Alhydrogel™. The antigen bound to Alhydrogel™ did not migrate through a polyacrylamide gel (Figure 3A). In addition, it effectively competed for antibody binding like non-conjugated rWNV-E (data not shown), suggesting that the adjuvanted antigen resembled the free antigen. The Alhydrogel™-adjuvanted vaccine antigen was used in a stability study conducted at both 4°C and 37°C. Stability was assessed by testing immuno-reactivity towards polyclonal anti-rWNV-E antibodies (Figure 3B). The formulated antigen exhibited long-term stability when stored at 4°C; no loss of immunoreactivity was observed (ΔEC50< 0.1) over the entire period of the study (8 months). When stored at 37°C, rWNV-E started to lose immunoreactivity within a few weeks, as previously described for the Drosophila-expressed antigen [31]. These data suggested that storage of the vialed vaccine candidate for extended period of time under refrigerated conditions would be acceptable.
Fig. 3.
rWNV-E adsorption to to Alhydrogel™ and stability study. (A) SDS-PAGE analyses of rWNV-E antigen before (lane 1, lower arrow) and after (lane 2, higher arrow) it was adsorbed to Alhydrogel™ for one hour at room temperature. The gel was stained with Coomassie blue. (B) Competitive ELISA analyses compared rWNV-E freshly adsorbed to Alhydrogel™ (rWNV-E control) with rWNV-E adsorbed to Alhydrogel™ and stored at 4°C or at 37° for three weeks, three months or eight months. EC50 values for control rWNV-E control and formulated rWNV-E stored at 37 °C are indicated by dashed vertical lines, and differences in value between the two were shown by horizontal arrows ( EC50).
3.4.Immunogenicity and protection against WNV lethal challenge in established animal models
Animal studies are an important factor in selecting and validating a vaccine production process. Ledizet et al. [16] previously reported that rWNV-E expressed in Drosophila S2 cells induced high antibody titers when combined with Alhydrogel™ and injected into mice or horses. Likewise, passively immunized mice injected with sera from actively immunized horses were resistant to challenge with a lethal WNV dose. Plaque reduction assays identified WNV neutralizing antibodies in sera from immunized horses. In the study reported here, the baculovirus-expressed rWNV-E vaccine candidate was tested for immunogenicity and protection in two different rodent models of WNV infection, mouse and hamster.
3.4.1. Efficacy in a murine model of infection
To validate the procedures developed to produce the formulated rWNV-E vaccine candidate, a number of formulated production batches were evaluated in a mouse challenge model for both immunogenicity and protection against a lethal dose of WNV. We specifically used single, low dose injections in the mouse studies to detect differences in immunogenicity between preparations and to optimize the production and formulation process.
To determine if Alhydrogel™ adjuvant stimulated immunity to baculovirus-produced recombinant antigen, mice were first immunized with 5 μg single doses of SF+ cell pellet-purified rWNV-E prepared with or without Alhydrogel™. Control mice received Alhydrogel™ without antigen. Two weeks after immunization, sera were collected for anti-rWNV-E IgG detection and mice were challenged. ELISA results presented in Table 2a indicate that rWNV-E combined with Alhydrogel™ was significantly more immunogenic than antigen alone (Unpaired t Test, p value < 0.01). The single-dose immunization with adjuvanted rWNV-E induced a wide range of anti-rWNV-E IgG levels in the mice. Control mice began to die at day 7 after challenge and were all dead by day 11. Immunization with non-adjuvanted antigen did not elicit anti-rWNV-E IgG antibody levels detectable by ELISA (Table 2a), however, 3 out of 10 of those mice survived viral challenge for 21 days. All of the mice immunized with 5 μg doses of adjuvanted antigen survived and produced antigen-specific antibodies, suggesting that rWNV-E formulation with Alhydrogel™ enhances protective immunity in mice.
Table 2.
Immunization of mice with a single dose of rWNV-E
| Experiment | Treatment group | Anti-WNV-E IgG |
|---|---|---|
| Mean OD ± S.D. (Range) | ||
| a | Alhydrogel™ alone control | 0.02 ± 0.005 (0.01–0.02) |
| Baculovirus-produced rWNV-E with Alhydrogel™ 5 μg | 0.47 ± 0.43 (0.03–1.49) | |
| Baculovirus-produced rWNV-E alone 5 μg | 0.02 ± 0.01 (0.01–0.04) | |
| b | Alhydrogel™ alone control | 0.01 ± 0.002 (0.004–0.01) |
| Baculovirus-produced rWNV-E with Alhydrogel™ 5 μg | 0.56 ± 0.52 (0.05–1.36) | |
| Drosophila-produced rWNV-E with Alhydrogel™ 5 μg | 0.46 ± 0.38 (0.06–1.25) | |
| c | Alhydrogel™ alone control | 0.09 ± 0.08 (0.08–0.10) |
| Baculovirus-produced rWNV-E with Alhydrogel™ 2.5 μg | 0.28 ± 0.20 (0.11–0.55) | |
| Baculovirus-produced rWNV-E with Alhydrogel™ 5 μg | 0.35 ± 0.32 (0.09–0.84) | |
| Baculovirus-produced rWNV-E with Alhydrogel™ 10 μg | 0.73 ± 0.46 (0.13–1.42) |
Serum samples were collected from groups of 10 mice, 2 weeks after immunization. Presence of IgG antibodies against rWNV-E was determined by ELISA at a 1:50 serum dilution.
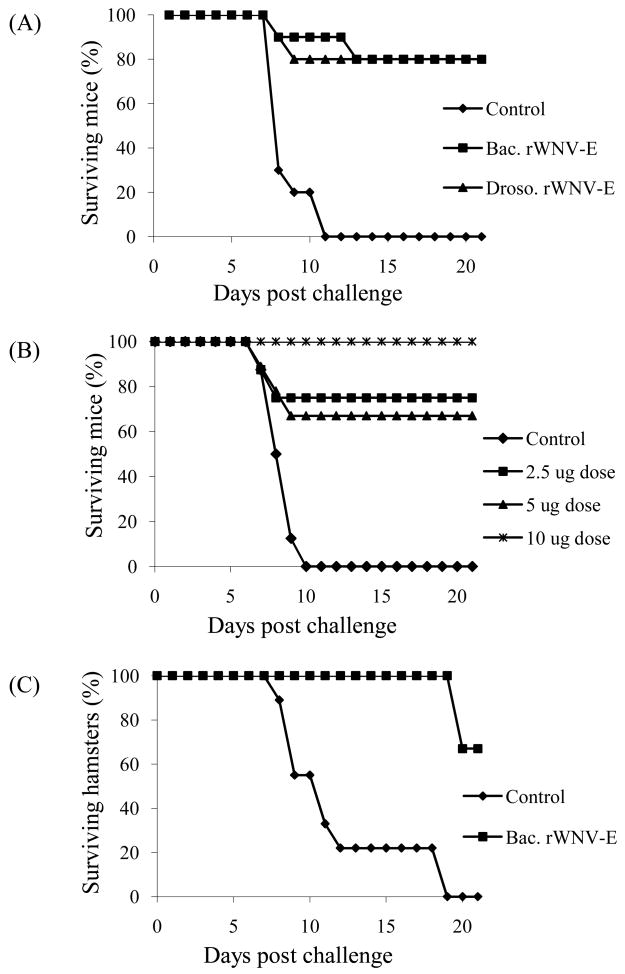
Our previous studies with the E. coli- or Drosophila-produced antigens used one or two 20 μg doses of antigens for murine immunizations. We used the WNV mouse challenge model to compare the well characterized Drosophila cell-produced rWNV-E antigen [16] with the baculovirus-produced antigen under the same formulation conditions (Table 2b, Figure 4A). Mice were immunized with single 5 μg doses and infected with WNV two weeks later. Prior to the viral challenge, serum samples were collected from each animal and IgG antibody levels were determined by ELISA (Table 2b). Although variability was observed in the anti-rWNV-E antibody levels induced in both treatment groups, both immunized group titers were significantly elevated compared to the control group (t Test, p value < 0.002). The mean anti-rWNV-E antibody levels were found to be statistically indistinguishable in the two groups of immunized mice (t Test, p value = 0.63). Likewise, survival curves after challenge with a lethal WNV dose showed no difference between the two groups of immunized mice (80% at 21 day endpoint) (Figure 4A). These data indicated that immunization with a single 5-ug dose of rWNV-E produced either in the Drosophila or in the baculovirus insect expression systems protected mice against WNV.
Fig. 4.
rWNV-E candidate vaccine induces protective immunity in mice and hamsters. (A) Groups of ten mice were injected with Alhydrogel™ alone (Control), single 5 μg-doses of SF+ cell-expressed rWNV-E antigen adsorbed to Alhydrogel™ (Bac. rWNV-E) or Drosophila cell-expressed rWNV-E adsorbed to Alhydrogel™ (Droso. rWNV-E). Mice were challenged with WNV after two weeks. (B) Groups of ten mice were injected with Alhydrogel™ control or single doses of 2.5, 5 or 10 μg of SF+ cell-expressed rWNV-E vaccine, and challenged with WNV two weeks later. Mouse survival was monitored for 21 days. (C) Groups of ten hamsters were injected three weeks apart with two 10 μg doses of rWNV-E vaccine candidate purified from SF+ cells (Bac. rWNV-E), or Alhydrogel™ control. The animals were challenged two weeks later with WNV.
We then investigated whether immunization with the baculovirus-expressed vaccine candidate induced a dose-dependent antibody response and protection against WNV. Groups of ten mice were immunized with 2.5, 5 or 10 μg single doses of Alhydrogel™-adjuvanted antigen. ELISA analyses demonstrated a dose-dependent increase in the mean anti-rWNV-E antibody level (Table 2c). Sera from each group of mice were pooled and tested for the presence of WNV neutralizing antibodies. PRNT50 titers in the pooled sera increased from <1:10 for the control group, to 1:40 for both the 2.5 and 5 μg doses, and 1:80 for the 10 μg-dose. The groups of mice injected with a single dose of vaccine candidate were 65–100% protected (Figure 4B). All mice in the control group died. These data indicate that single doses of rWNV-E vaccine candidate induces anti-rWNV-E IgGs, WNV neutralizing antibodies and viral protection that are dose dependent in a murine model.
3.4.2. Efficacy in a hamster model of infection
After establishing vaccine candidate efficacy in the mouse model, we used a hamster model of WNV infection [32] to confirm efficacy in a second animal species. The experiment evaluated immunogenicity and protection elicited by formulated baculovirus-expressed rWNV-E in naïve hamsters. Groups of 9 hamsters were injected with 10 μg doses of Alhydrogel™-adsorbed rWNV-E antigen or Alhydrogel™ alone at day 0, and received a second identical injection at day 21. Hamsters were then challenged at day 35 with 104 pfu of WNV isolate 2741 and followed for 21 days post infection. Serum samples were collected at day 35, before challenge, and tested for both anti-rWNV-E IgG and virus neutralizing antibody titers. Presence of IgG antibodies was determined by ELISA at a 1:400 dilution: mean ODs ± S.D (range) were 0.82 ± 0.52 (0.1–1.55) for the vaccinated group and 0.07 ± 0.005 (0.07–0.08) for the adjuvant control group (t Test, p < 0.001). The two dose immunization provided protection against WNV (70% survival; Figure 4C). All of the control animals died upon infection. When the two groups were compared for survival, a Chi square distribution of 0.00053 was obtained. Eight out of the nine immunized hamsters produced positive PRNT95 titers (1:20 to 1:80) in WNV strain 2741 neutralization assays. All control hamsters showed negative PRNT results. A Kandall tau Rank correlation comparing the immunized group IgG ELISA ODs and PRNT95 showed a Kandall tau value of 0.76 with a 2-sided p-value of 0.010; the same analysis applied to all animals (immunized and control groups) showed a Kandall tau value of 0.78 with a 2-sided p-value of 6.48 × 10−5. Our findings demonstrated an overall quantitative correlation between the humoral immune response to hamster vaccination as measured by the IgG ELISA and neutralizing antibody titers.
3.5. Immunogenicity study in horses
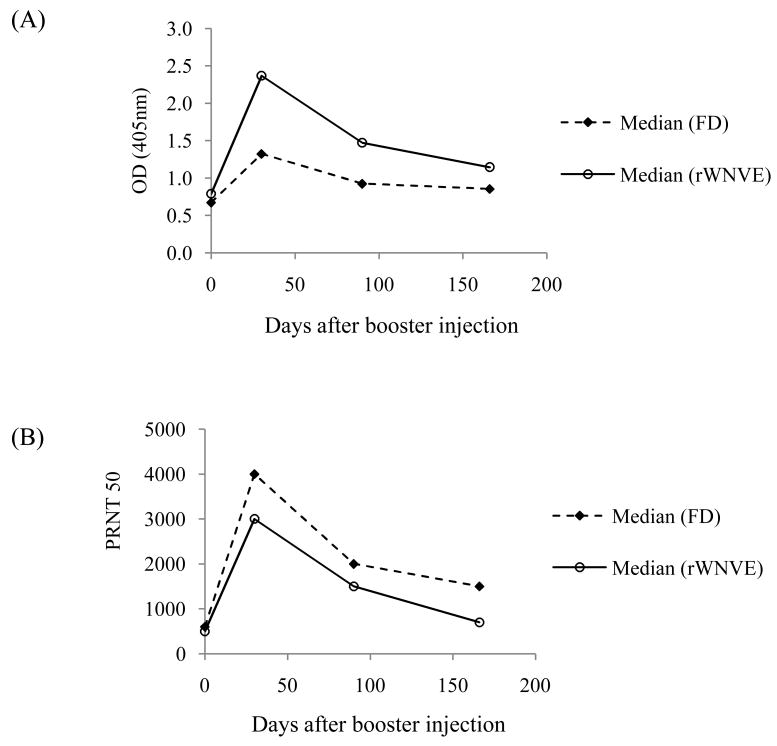
The utility of the rWNV-E vaccine for annual boosting of horses and its ability to elicit WNV neutralizing antibodies that persist throughout the spring and summer WNV season were evaluated in a six-month study. An initial study involved two groups of 20 horses each with a documented history of vaccination with Fort Dodge Animal Health Innovator™ (FD) killed WNV vaccine in the previous years. One group was boosted with Alhydrogel™-conjugated rWNV-E purified from SF+ cell pellets (50 μg/dose). The second group was boosted with FD commercial vaccine. Horses immunized with the FD vaccine had mild skin inflammation at the site of injection, as previously reported [5]. No sign of skin inflammation was observed when horses were injected with rWNV-E vaccine candidate. Horses were bled the day before administration of the single booster injection and at regular intervals for a period of 6 months. Serum anti-rWNV-E IgG titers and PRNT levels using the WNV/DEN4 Delta30 virus were determined for each horse at each time point.
Both vaccines boosted anti-rWNV-E IgG and WNV neutralizing antibody titers. All 20 horses from the FD group and 19 out of 20 horses from the rWNV-E group had increased WNV-E IgG titers after the booster. The one exception was a horse that had been refractory to FD immunizations in previous years. A second injection of rWNV-E vaccine was administrated to the horse and it elicited an antibody response comparable to that of all other animals in the group. All horses’ antibody titers, except that of the one described above, peaked at the day 30 time point after the booster injection. In Figure 5A, median ELISA OD values (serum dilution 1:200) were calculated for both groups of horses at each time point. The recombinant WNV-E vaccine boost induced a higher IgG reactivity to rWNV-E (p value < 0.005) than the FD commercial killed virus vaccine, and titers remained higher six months later. In Figure 5B, PRNT titers for each group were compared by using median PRNT50 values. These PRNT analyses showed no significant virus neutralizing titer differences between the two groups of horses for the six month period after boosting. Identical conclusions were obtained when geometrical mean rather than median ODs and PRNT50 were calculated.
Fig. 5.
Booster immunogenicity study in horses, comparing rWNV-E candidate vaccine with the Fort Dodge Animal Health Innovator™ killed virus (FD) vaccine. Horses were immunized in prior years. Twenty horses were boosted with FD vaccine. Twenty horses were immunized with a single dose of rWNV-E vaccine intramuscularly with 50 μg of rWNV-E adsorbed to Alhydrogel™. Serum samples were collected (n=20 for the FD group; and n=19 for the rWNV-E boosted horses that responded after a single injection) throughout the study, and analyzed for IgG antibodies recognizing rWNV E-protein by ELISA (serum dilution 1:200) (A) and for neutralizing antibodies in a plaque reduction assay (PRNT50) (B). ELISA OD median values for each time course were calculated for each group.
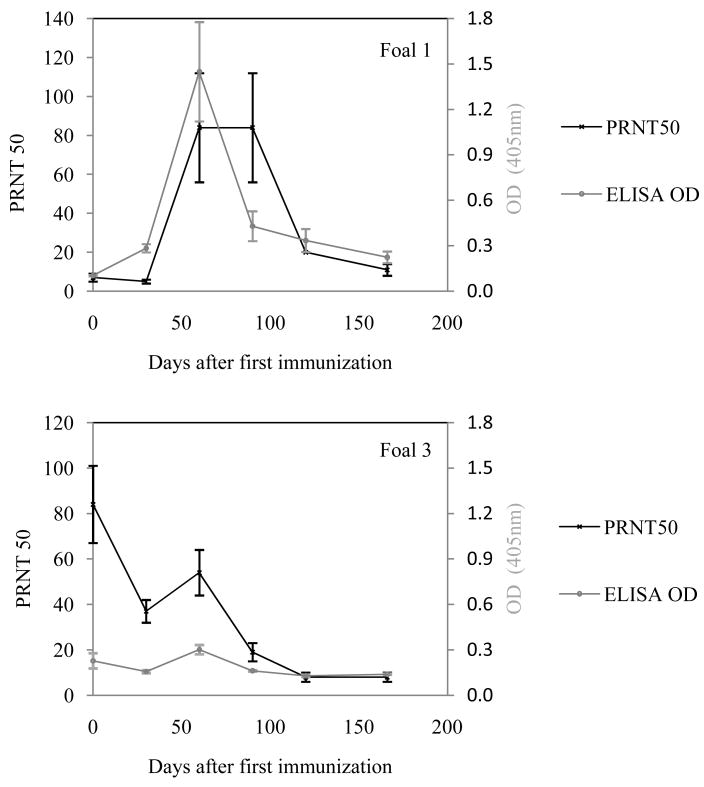
In a second study, five naïve foals were vaccinated and boosted with the rWNV-E vaccine candidate and evaluated for WNV antibodies at several time points. The foals received two injections of vaccine, four weeks apart. Foals 1 through 5 were respectively 15, 14, 11, 7 and 4 weeks old at the time of the first injection. Foals 1 and 2 responded positively to the vaccination with increased anti-rWNV-E IgG and PRNT50 titers peaking at day 60, or 30 days after the second injection (Foal 1, Figure 6A). The three other foals were all very young and did not respond very well to vaccination (foal 3, Figure 6B), most likely because of residual maternal antibodies interfering with the antigen; the corresponding mares in this study had been vaccinated with the FD vaccine in the previous years and all tested positive for anti-rWNV-E neutralizing antibodies the day the foals were vaccinated. We concluded that naïve foals should at least be 14 weeks old or have low residual maternal antibodies before they are vaccinated against WNV infection.
Fig. 6.
Immunogenicity study in naïve foals. Foals born in the spring of 2007 were immunized with rWNV-E vaccine and boosted after four weeks (Day 30) with 50 μg of rWNV-E candidate vaccine. Serum samples were collected at the time of the first injection and throughout the study and analyzed for anti-rWNV-E IgG antibodies and WNV/DEN4Delta 30 neutralizing antibodies in ELISA and PRNT assays, respectively. (A) Geometrical mean values for PRNT50 and ELISA OD (serum dilution 1:200) obtained for Foal 1. (B) PRNT50 and ELISA OD (serum dilution 1:200) values obtained for Foal 3.
Taken together, these results demonstrated that for horses previously immunized with FD vaccine, boosting with rWNV-E adjuvanted with Alhydrogel™ elicited neutralizing antibody titers in the range of those elicited by the commercial FD vaccine.
3.6. Rat toxicology study
A toxicology study to evaluate the safety of the baculovirus-expressed, cell pellet-derived recombinant subunit WNV vaccine candidate in rats was performed at the IIT Research Institute (Chicago, IL). The study included four treatment groups: an Alhydrogel™ control and Alhydrogel™ with rWNV-E in 10, 50, and 100 μg doses.
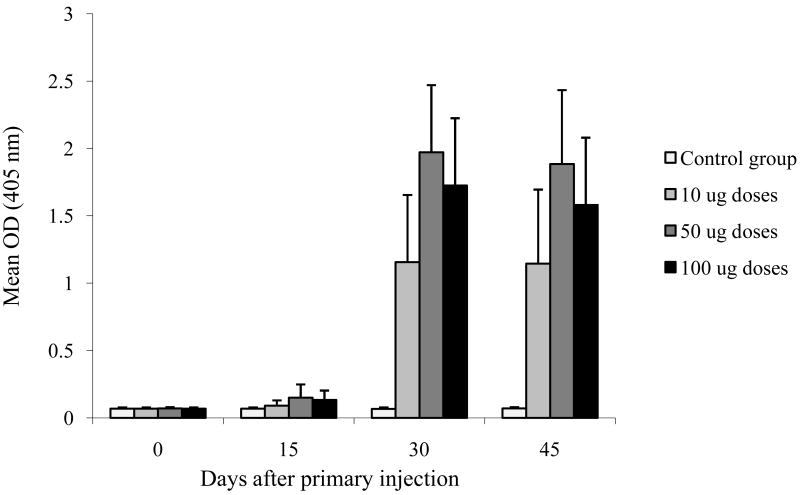
As part of the study, immunogenicity data were collected. Anti-rWNV-E IgG serum analyses were completed for all surviving rats (4 groups, n=19 or 20) at all time points (Day 0, 15, 30 and 45). Figure 7 depicts the results in mean OD (± S.D.) values obtained at 1:800 serum dilutions. In the three treatment groups containing antigen, antibody titers peaked at the day 30 time point, 15 days after boosting. Anti-rWNV-E antibody was not detected in the control group. No significant response difference was observed between males and females (data not shown). The day 30 antibody response increased as the dose increased from 10 to 50 μg (Unpaired t-Test p < 0.001). The 50 and 100-μg doses induced comparable antibody responses. Serum virus neutralizing antibody titers were determined for 7 animals per group using the WNV/DEN4Delta30 virus. Rats injected with Alhydrogel™ control (group 1) did not develop WNV neutralizing antibodies. Rats injected with rWNV-E (groups 2, 3 and 4) all developed WNV neutralizing antibodies. Although the PRNT50 values for immunized animals from groups 2, 3 and 4 ranged widely, titers were typically negative at the first injection, >1:10 at day 15, and peaked at the day 30 time point. PRNT50 geometrical mean titers at 0, 15, 30 and 45 days were: 10, 50, 1580, 1436 respectively for the 10 μg dose; 10, 69, 2747, 2554 respectively for the 50 μg dose; and 10, 101, 2068, 3657 respectively for the 100 μg dose. Data indicated a dose-response increase until 50-μg doses that then reached a plateau, confirming the ELISA results.
Fig. 7.
Immunogenicity study in rats. Four groups of 20 rats were immunized twice (Day 0 and Day 15) with 0, 10, 50 or 100 ug of rWNV-E vaccine candidate. Serum samples were collected throughout the study at day 0, 15, 30 and 45 and analyzed by ELISA for presence of anti-rWNV-E antibodies. Mean OD ± S.D. (serum dilution 1:800) for each group and each time point are presented.
Pathology studies showed no treatment-related effects of the immunogen. Results of the in-life study only reported the coincidental death of one male at the highest dose (100 μg) on study day 29. At necropsy this animal had calculi in the bladder, which was thought to have caused blockage and death unrelated to immunization. No other deaths were noted in the remaining study animals. Edema and occasional erythema were generally observed at the injection site after each administration in animals treated with the test article and the frequency of positive reactogenicity observations tended to be dose-related. Reactogenicity was transient, as the injection sites from all test-article treated animals returned to normal within six days after the administration, suggesting these findings may be of minimal toxicological significance. The final histopathology report, which included analyses of up to 40 tissues per animal, indicated no adverse effects due to administration of the rWNV-E. At the injection sites, aggregates of macrophages and/or lymphocytes were observed microscopically; these findings were considered a reaction to the adjuvant. All remaining microscopic findings were interpreted as incidental findings.
Overall, this vaccine toxicology study demonstrated that rWNV-E purified from SF+ cell pellets is immunogenic in rats, induces virus neutralizing antibodies and has minimal toxicity.
4. Discussion
The results reported herein describe a scalable baculovirus-insect cell expression and purification method to produce truncated recombinant WNV envelope protein (rWNV-E) antigen for use in a vaccine against WNV. Expression in expresSF+ cell cultures produced a purified rWNV-E antigen that induced virus neutralizing antibodies in multiple animal species when formulated with aluminum hydroxide, and elicited protective immunity in two rodent species.
The approach described in this study may present potential advantages over other WN virus vaccine development strategies. It uses a serum-free insect cell line that has been established as a stable, scalable, low cost and high yielding cell line for vaccine antigen production. The expresSF+ cells have been tested in accordance with FDA protocols and has been used for production of recombinant glycoproteins for therapeutics and vaccines that have been used in human clinical trials [33]. A rapid and low cost purification procedure extracted rWNV-E protein from the cell pellets with yields that reached up to 100 mg/10L with >98% purity and proven stability. Single doses of antigen as low as 2.5μg could induce WNV neutralizing antibodies and protect mice against WNV lethal infection (80% survival rate), using aluminum hydroxide, an adjuvant that is suitable for use in humans. Higher antigen doses and multiple injections triggered a stronger immune response in animals without any side effects.
Experimental recombinant envelope (E) protein subunit vaccines against WNV and other flaviviruses have been developed using several different methods [3, 16–18, 22, 34]. Such vaccines are known to elicit strong immune responses directed toward multiple protective E protein epitopes [16, 25]. The Drosophila cell expression system yields flavivirus E protein that is highly pure and that resembles the native protein [16–18, 35]. The baculovirus-expression system is another insect-cell system for the production of viral or parasitic antigens with vaccine potential for humans and animals [33, 36]. Recombinant truncated envelope proteins from dengue virus types 1, 2 and 3 have been produced in Sf9 cells and used successfully to stimulate dengue virus neutralizing antibodies in mice and protect them from an otherwise lethal virus challenge [37, 38]. In spite of the potential utility of the baculovirus system to produce recombinant subunit vaccines, no published study has demonstrated that it could be used for large scale production. In two rWNV-E crystallization studies [23, 39], recombinant soluble antigen was purified from baculovirus-insect cell supernatants. The crystal structure of SF+ cells-secreted recombinant envelope protein was resolved and adopted the same overall fold as that of the E proteins from dengue and tick-borne encephalitis viruses [23]. In this study we show that the cell-pellet extracted antigen resembles its secreted counterparts, since it is equally effective as a recombinant vaccine candidate that resembles the viral native protein. Despite structural differences that resulted from incomplete processing of the signal peptide and glycosylation, the cell pellet-derived rWNV-E entered the secretory pathway, folded properly, and induced WNV neutralizing antibodies that correlated with viral protection. The Drosophila cell expression system used an 18-amino acid Drosophila BiP secretory signal sequence to direct the recombinant protein through the secretory pathway. The baculovirus-SF+ approach used a heterologous AcNPV chitinase signal peptide. Neither system required the recombinant truncated envelope protein to be co-expressed with the prM protein, which prevents the native E protein from fusing with host cell membranes during transport in intracellular vesicles [40]. Our experience with baculovirus expression of WNV prM-E fusion protein was that it provided low yields of mature E protein. In this study, rWNV-E yields were improved by including detergents in the purification procedure that enabled rapid extraction of rWNV-E from the cell pellet fraction. This rWNV-E formed crystals under conditions established for x-ray crystallography of SF+-secreted rWNV-E [23], indicating proper folding of the cell pellet-derived rWNV-E (Yorgo Modis, personal communication).
SF+ cell pellet-purified rWNV-E vaccine candidate was compared to the commercially available Fort Dodge (FD) Innovator™ killed virus vaccine in an equine booster study. The two very different formulations can induce comparable anamnestic responses, with WNV neutralizing titers obtained within the same range. The results are consistent with our previous work that showed similar responses to vaccination of naïve horses [16]. Overall, our horse studies supported our rodent immunization results, suggesting that neutralizing antibodies recognizing the live virus were likely responsible for overall WNV protection.
In conclusion, in these studies we have established the baculovirus-insect SF+ cell expression system as a potential method to produce recombinant subunit flavivirus envelope antigens for animal and human vaccine development. The purified rWNV-E antigen, when formulated with Alhydrogel™, was stable, non-toxic, immunogenic in all four animal species tested and efficacious in mouse and hamster WNV challenge models.
Acknowledgments
The authors would like to thank Bonnie Hamid (Connecticut Agricultural Experiment Station, New Haven, CT) for her assistance with the hamster immunization and challenge studies and PRNT using WNV strain 2741. In addition we are grateful to Dr. Steve Whitehead (NIAID, NIH, Bethesda, MD) who provided attenuated chimeric virus WNV/DEN4Delta30 and PRNT protocols. We acknowledge Zehndong Wang and Valerie Mermall from Protein Sciences Corporation for their respective expert assistance in the experimental design and the purification process. We are thankful to Gia Barss and Stacey Bucko for their help in the horse immunizations and serum collections. This work was supported by grant U01AI061361, from the National Institutes of Allergy and Infectious Diseases to L2 Diagnostics, LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marfin AA, Gubler DJ. West Nile encephalitis: an emerging disease in the United States. Clin Infect Dis. 2001;33(10):1713–9. doi: 10.1086/322700. [DOI] [PubMed] [Google Scholar]
- 2.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2(9):519–29. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 3.Gould LH, Fikrig E. West Nile virus: a growing concern? J Clin Invest. 2004;113(8):1102–7. doi: 10.1172/JCI21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dauphin G, Zientara S. West Nile virus: recent trends in diagnosis and vaccine development. Vaccine. 2007;25(30):5563–76. doi: 10.1016/j.vaccine.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Ng T, Hathaway D, Jennings N, Champ D, Chiang YW, Chu HJ. Equine vaccine for West Nile virus. Dev Biol (Basel) 2003;114:221–7. [PubMed] [Google Scholar]
- 6.Grosenbaugh DA, Backus CS, Karaca K, Minke JM, Nordgren RM. The anamnestic serologic response to vaccination with a canarypox virus-vectored recombinant West Nile virus (WNV) vaccine in horses previously vaccinated with an inactivated WNV vaccine. Vet Ther. 2004;5(4):251–7. [PubMed] [Google Scholar]
- 7.Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75(9):4040–7. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196(12):1732–40. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arroyo J, Miller C, Catalan J, Myers GA, Ratterree MS, Trent DW, et al. ChimeriVax-West Nile virus live-attenuated vaccine: preclinical evaluation of safety, immunogenicity, and efficacy. J Virol. 2004;78(22):12497–507. doi: 10.1128/JVI.78.22.12497-12507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monath TP, Liu J, Kanesa-Thasan N, Myers GA, Nichols R, Deary A, et al. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci U S A. 2006;103(17):6694–9. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pletnev AG, Claire MS, Elkins R, Speicher J, Murphy BR, Chanock RM. Molecularly engineered live-attenuated chimeric West Nile/dengue virus vaccines protect rhesus monkeys from West Nile virus. Virology. 2003;314(1):190–5. doi: 10.1016/s0042-6822(03)00450-1. [DOI] [PubMed] [Google Scholar]
- 12.Pletnev AG, Swayne DE, Speicher J, Rumyantsev AA, Murphy BR. Chimeric West Nile/dengue virus vaccine candidate: preclinical evaluation in mice, geese and monkeys for safety and immunogenicity. Vaccine. 2006;24(40–41):6392–404. doi: 10.1016/j.vaccine.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Despres P, Combredet C, Frenkiel MP, Lorin C, Brahic M, Tangy F. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J Infect Dis. 2005;191(2):207–14. doi: 10.1086/426824. [DOI] [PubMed] [Google Scholar]
- 14.Iglesias MC, Frenkiel MP, Mollier K, Souque P, Despres P, Charneau P. A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity against West Nile virus. J Gene Med. 2006;8(3):265–74. doi: 10.1002/jgm.837. [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Anderson JF, Magnarelli LA, Wong SJ, Koski RA, Fikrig E. Immunization of mice against West Nile virus with recombinant envelope protein. J Immunol. 2001;167(9):5273–7. doi: 10.4049/jimmunol.167.9.5273. [DOI] [PubMed] [Google Scholar]
- 16.Ledizet M, Kar K, Foellmer HG, Wang T, Bushmich SL, Anderson JF, et al. A recombinant envelope protein vaccine against West Nile virus. Vaccine. 2005:3915–24. doi: 10.1016/j.vaccine.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Watts DM, Tesh RB, Siirin M, Rosa AT, Newman PC, Clements DE, et al. Efficacy and durability of a recombinant subunit West Nile vaccine candidate in protecting hamsters from West Nile encephalitis. Vaccine. 2007;25(15):2913–8. doi: 10.1016/j.vaccine.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieberman MM, Clements DE, Ogata S, Wang G, Corpuz G, Wong T, et al. Preparation and immunogenic properties of a recombinant West Nile subunit vaccine. Vaccine. 2007;25(3):414–23. doi: 10.1016/j.vaccine.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiao M, Ashok M, Bernard KA, Palacios G, Zhou ZH, Lipkin WI, et al. Induction of sterilizing immunity against West Nile Virus (WNV), by immunization with WNV-like particles produced in insect cells. J Infect Dis. 2004;190(12):2104–8. doi: 10.1086/425933. [DOI] [PubMed] [Google Scholar]
- 20.Chu JH, Chiang CC, Ng ML. Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection. J Immunol. 2007;178(5):2699–705. doi: 10.4049/jimmunol.178.5.2699. [DOI] [PubMed] [Google Scholar]
- 21.Martina BE, Koraka P, van den Doel P, van Amerongen G, Rimmelzwaan GF, Osterhaus AD. Immunization with West Nile virus envelope domain III protects mice against lethal infection with homologous and heterologous virus. Vaccine. 2008;26(2):153–7. doi: 10.1016/j.vaccine.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald WF, Huleatt JW, Foellmer HG, Hewitt D, Tang J, Desai P, et al. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J Infect Dis. 2007;195(11):1607–17. doi: 10.1086/517613. [DOI] [PubMed] [Google Scholar]
- 23.Kanai R, Kar K, Anthony K, Gould LH, Ledizet M, Fikrig E, et al. Crystal structure of West Nile virus envelope glycoprotein reveals viral surface epitopes. J Virol. 2006;80(22):11000–8. doi: 10.1128/JVI.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson JF, Andreadis TG, Vossbrinck CR, Tirrell S, Wakem EM, French RA, et al. Isolation of West Nile virus from mosquitoes, crows, and a Cooper’s hawk in Connecticut. Science. 1999;286(5448):2331–3. doi: 10.1126/science.286.5448.2331. [DOI] [PubMed] [Google Scholar]
- 25.Ledizet M, Kar K, Foellmer HG, Bonafe N, Anthony KG, Gould LH, et al. Antibodies targeting linear determinants of the envelope protein protect mice against West Nile virus. J Infect Dis. 2007;196(12):1741–8. doi: 10.1086/523654. [DOI] [PubMed] [Google Scholar]
- 26.Beaty BJ, Calisher CH, Shope RE, editors. Arboviruses. 6. American Public Health Association; Washington, DC: 1989. [Google Scholar]
- 27.Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg. 2001;65(5):405–13. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 28.Goncalvez AP, Men R, Wernly C, Purcell RH, Lai CJ. Chimpanzee Fab fragments and a derived humanized immunoglobulin G1 antibody that efficiently cross-neutralize dengue type 1 and type 2 viruses. J Virol. 2004;78(23):12910–8. doi: 10.1128/JVI.78.23.12910-12918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pletnev AG, Putnak R, Speicher J, Wagar EJ, Vaughn DW. West Nile virus/dengue type 4 virus chimeras that are reduced in neurovirulence and peripheral virulence without loss of immunogenicity or protective efficacy. Proc Natl Acad Sci U S A. 2002;99(5):3036–41. doi: 10.1073/pnas.022652799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82(5):497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 31.Wong SJ, Demarest VL, Boyle RH, Wang T, Ledizet M, Kar K, et al. Detection of human anti-flavivirus antibodies with a West Nile virus recombinant antigen microsphere immunoassay. J Clin Microbiol. 2004;42(1):65–72. doi: 10.1128/JCM.42.1.65-72.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao SY, Guzman H, Zhang H, Travassos da Rosa AP, Tesh RB. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis. 2001;7(4):714–21. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treanor JJ, Schiff GM, Hayden FG, Brady RC, Hay CM, Meyer AL, et al. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007;297(14):1577–82. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- 34.Putnak RJ, Coller BA, Voss G, Vaughn DW, Clements D, Peters I, et al. An evaluation of dengue type-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine. 2005;23(35):4442–52. doi: 10.1016/j.vaccine.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 35.Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427(6972):313–9. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 36.van Oers MM. Vaccines for viral and parasitic diseases produced with baculovirus vectors. Adv Virus Res. 2006;68:193–253. doi: 10.1016/S0065-3527(06)68006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Putnak R, Feighny R, Burrous J, Cochran M, Hackett C, Smith G, et al. Dengue-1 virus envelope glycoprotein gene expressed in recombinant baculovirus elicits virus-neutralizing antibody in mice and protects them from virus challenge. Am J Trop Med Hyg. 1991;45(2):159–67. doi: 10.4269/ajtmh.1991.45.159. [DOI] [PubMed] [Google Scholar]
- 38.Delenda C, Frenkiel MP, Deubel V. Protective efficacy in mice of a secreted form of recombinant dengue-2 virus envelope protein produced in baculovirus infected insect cells. Arch Virol. 1994;139(1–2):197–207. doi: 10.1007/BF01309465. [DOI] [PubMed] [Google Scholar]
- 39.Nybakken GE, Nelson CA, Chen BR, Diamond MS, Fremont DH. Crystal structure of the West Nile virus envelope glycoprotein. J Virol. 2006;80(23):11467–74. doi: 10.1128/JVI.01125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, Chen J, et al. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science. 2008;319(5871):1830–4. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]