Abstract
Objective
We aimed to identify clinico-radiological risk factors that may predict unfavorable neurological outcomes in traumatic brain injury (TBI), and to establish a guideline for patient selection in clinical trials that would improve neurological outcome during the early post TBI period.
Methods
Initial clinico-radiological data of 115 TBI patients were collected prospectively. Regular neurological assessment after standard treatment divided the above patients into 2 groups after 6 months : the Favorable neurological outcome group (GOS : good & moderate disability, DRS : 0-6, LCFS : 8-10) and the Unfavorable group (GOS : severe disability-death, DRS : 7-29 and death, LCFS : 1-7 and death).
Results
There was a higher incidence of age ≥35 years, low initial GCS score, at least unilateral pupil dilatation, and neurological deficit in the Unfavorable group. The presence of bilateral parenchymal lesions or lesions involving the midline structures in the initial brain CT was observed to be a radiological risk factor for unfavorable outcome. Multivariate analysis demonstrated that age and initial GCS score were independent risk factors. The majority of the Favorable group patients with at least one or more risk factors showed improvement of GCS scores within 2 months after TBI.
Conclusion
Patients with the above mentioned clinico-radiological risk factors who received standard treatment, but did not demonstrate neurological improvement within 2 months after TBI were deemed at risk for unfavorable outcome. These patients may be eligible candidates for clinical trials that would improve functional outcome after TBI.
Keywords: Traumatic brain injury, Unfavorable neurological outcome, Risk factors
INTRODUCTION
The general incidence of traumatic brain injury (TBI) in developed countries is approximately 200/100,000 per year2). In the United States, about 2,000,000 patients suffer from head injuries annually, 75,000-100,000 patients die from these injuries, and 70,000-90,000 patients suffer from debilitating neurological dysfunction states until death17). TBI has a peak incidence between 15-24 years of age6,17). Since it is prevalent in children and young adults, the result of TBI has a serious and devastating effect in social and economic terms.
The mainstay treatment for TBI is to reduce the extent of secondary brain damage after the primary insult, since the damage occurring at the time of trauma is generally not amenable to alteration. During the past several decades, many clinical or pharmacological trials have been performed to produce methods for reducing or minimizing secondary brain damage after TBI. However no clear benefits have been shown to date due to the complex pathophysiological mechanisms and heterogeneous patient population involved in TBI. Only the calcium channel blocker, nimodipine, has shown to reduce secondary brain damage in traumatic subarachnoid hemorrhage patients compared to controls, by preventing production of oxygen mediated free radicals4). However, it has been stressed that this therapy does not treat the underlying brain parenchymal damage, and its use is therefore still controversial15,16). Recently, transplantation of stem cells has been attempted in TBI animal studies to treat the brain injury directly, and results have shown improved neurological function of TBI animals10,12,18,21). However, since the proportion of transplanted stem cells which migrated to the injury site and differentiated into neural cells was minimal, it is generally considered that any improvement of neurological function is due to neuroprotection by prevention of secondary injury to neural cells rather than actual or real tissue repair. Therefore, in order to prevent the emergence of neurological sequelae after severe TBI, it is desirable that clinical trials such as stem cell transplantation should intercede in the early post TBI period. However, difficulties are envisaged in recruiting patients for new early post-TBI clinical trials and in evaluating therapeutic outcomes because of the complex pathophysiological mechanisms and various outcomes.
The authors of this prospective study attempted to identify clinical and radiological risk factors that may predict unfavorable neurological outcomes in the early period after TBI, and ultimately to establish a guideline for patient selection in clinical trials that would improve neurological outcome during the early post TBI period.
MATERIALS AND METHODS
Patients
From December 2004 to March 2006, 115 patients who were admitted to the neurosurgical department via the emergency room (ER) due to head trauma were included in this study. Standard care was based on the guidelines of the 1997 EBIC-Guidelines5,11,13). All enrolled study patients were subject to prospective clinical and radiological data from the time of admission to the ER, and were evaluated for neurological outcome 6 months after initial therapy.
Clinico-radiological factors
Clinical risk factor assessment consisted of data collection pertaining to age, sex, mental status, Glasgow coma scale (GCS) score, neurological deficits, pupil size, initial systolic blood pressure, routine laboratory data, traumatic vector, and combined extracranial injury.
To assess radiological risk factors, the locations of the traumatic parenchymal lesions as observed on the initial brain CT at the ER were classified as follows : absent, unilateral, bilateral, or lesions involving the midline structures (brainstem or corpus callosum).
Assessment of neurological outcome
GCS scores of each and all patients were assessed immediately upon admission and at regular intervals thereafter by a research nurse. Neurological outcome was evaluated with the Glasgow Outcome Scale (GOS) 6 months after TBI. Disability rating scale (DRS) and the Rancho Los Amigos Cognitive Scale (LCFS) were also assessed6,9). To investigate and analyze the clinical and radiological risk factors for unfavorable neurological outcome, the assessment score was used to define a Favorable group (GOS : good & moderate disability, DRS : 0-6, LCFS : 8-10) and an Unfavorable group (GOS : severe disability-death, DRS : 7-29 and death, LCFS : 1-7 and death). For those patients who died within 6 months after TBI, the last score was designated as the 6 month score.
Statistical analysis was conducted with the SPSS program (version 12.0). Chi-square test was utilized for categorical variables, independent sample t-test for continuous variables, logistic regression method for multivariate analysis. Statistical significance was defined as p value<0.05. Continuous variables were expressed as mean±standard error.
RESULTS
Clinical and radiological characteristics
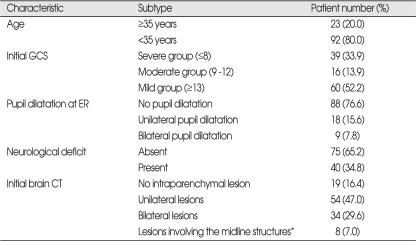
Among the total of 115 patients, 81 were male and 34 were female. The median age was 47.7 years (range, 16.8-85.2). Trauma vectors consisted of 37 motor vehicle accidents, 27 falling down injuries, 21 passenger accidents, and 30 miscellaneous injuries. The median GCS score at the time of arrival at the ER was 10.8 (range, 3-15), and 33.9% of patients were with a GCS score of less than 8, indicative of severe injury (Table 1). The number of patients with at least one pupil dilatation at the time of initial evaluation was 27 (23.4%). Forty (34.8%) patients who had neurological deficit were comprised of 9 with cranial nerve palsy, 6 with motor weakness, 1 with visual deficit, 4 with speech disturbance, 1 with cerebellar signs, and 19 patients with abnormal motor response and a GCS score of less than 8.
Table 1.
Clinical and radiological characteristics of 115 patients
*midline structures : corpus callosum or brain stem
There were 42 patients (36.6%) who demonstrated bilateral parenchymal lesions or lesions involving the midline structures on the initial brain CT at the ER (Table 1).
Thirteen of the 115 patients (11.3%) died from disease within 6 months after TBI. Eleven of the 13 patients died of increased intracranial pressure caused by the TBI itself, while 2 died from sepsis that progressed from pneumonia during treatment. The median survival time of the above 13 patients was 2 weeks (range, 1 day-20 weeks).
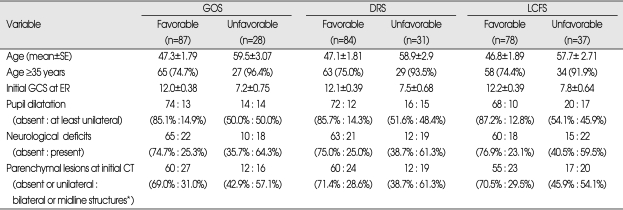
Neurological outcome assessment by GOS 6 months after the TBI showed that 87 patients were classified as the Favorable group and 28 as the Unfavorable group. The DRS method showed that the number of patients were 84 and 31 respectively, and 78 and 37 respectively by the LCFS method (Table 2).
Table 2.
Significant risk factors for the unfavorable neurological outcome (6 months after TBI) in all 3 assessment tools
*midline structures : corpus callosum or brain stem
Clinico-radiological risk factors for unfavorable outcome
Age, initial GCS score at ER, neurological deficit, at least unilateral pupil dilatation, and the presence of bilateral parenchymal lesions or lesions involving the midline structures on the initial CT showed significant difference between the Favorable and Unfavorable group when assessed by all 3 methods such as GOS, DRS and LCFS (Table 2).
The mean age of the Favorable group was 46.8-47.3 years according to the assessment methods, but this was significantly higher in the Unfavorable group (57.7-59.5 years). To interpret this result in detail, it was observed that the number of patients who were aged ≥35 years was significantly greater in the Unfavorable group (91.9-96.4%) compared to the Favorable group (74.4-75.0%). The mean initial GCS score in the Favorable group was 12.0-12.2, but this was significantly lower (7.2-7.8) in the Unfavorable group. At least unilateral pupil dilatation was observed in 45.9-50.0% of the Unfavorable group, which was significantly higher compared to the Favorable group (12.8-14.9%). Neurological deficits also occurred more frequently in the Unfavorable group (59.5-64.3%) compared to the Favorable group (23.1-25.3%). Besides age, initial GCS, pupil dilatation and neurological deficit, there was no significant difference between the 2 study groups with respect to sex, initial systolic blood pressure, routine laboratory data, traumatic vector, and combined extracranial injury.
The presence of bilateral parenchymal lesions or lesions involving the midline structures on the initial CT was significantly different between the Favorable and Unfavorable groups with regard to GOS, DRS, and LCFS assessment method (Table 2). While the incidence of bilateral parenchymal lesions or lesions involving the midline structures such as corpus callosum or brainstem on the initial CT was 28.6-31.0% in the Favorable group, it was significantly higher (54.1-61.3%) in the Unfavorable group (Table 2).
Result of multivariate analysis
The logistic regression method was utilized to analyze the following factors by multivariate analyses; age, initial GCS score at ER, neurological deficit, at least unilateral pupil dilatation, and initial CT findings (bilateral parenchymal lesions or lesions involving the midline structures). It was shown that age and initial GCS scores were independent risk factors for unfavorable outcome (p<0.001).
GCS changes between two groups
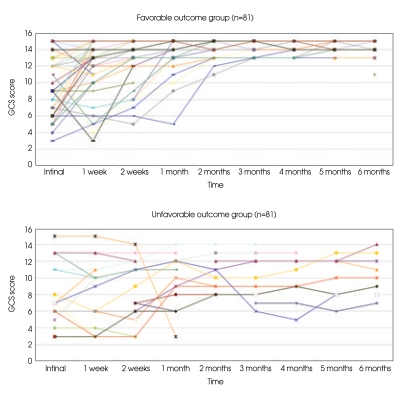
The changes in the GCS score according to progression of time were compared in the patients who had risk factors but showed different outcomes. There were 6 patients without any clinico-radiological risk factors; age ≥35 years, an initial GCS score of 8 or less, at least unilateral pupil dilatation, the presence of neurological deficit, initial CT findings of bilateral parenchymal lesions or lesions involving midline structure, while 109 patients had at least one of the above risk factors. According to the GOS at 6 months after TBI, 81/109 patients were Favorable group patients, while 28/109 were in the Unfavorable group. In contrast to the Unfavorable outcome group, majority of the Favorable group patients showed improvement of GCS scores within 2 months of the TBI (Fig. 1). DRS and LCFS also showed similar results (data not shown).
Fig. 1.
Diagram of Glasgow coma scale (GCS) scores after traumatic brain injury (TBI) in the patients who had at least one of the risk factors (n=109). Majority of the Favorable outcome group patients show improvement of GCS scores within 2 months of the TBI (upper), in contrast to those of the Unfavorable outcome group (lower).
DISCUSSION
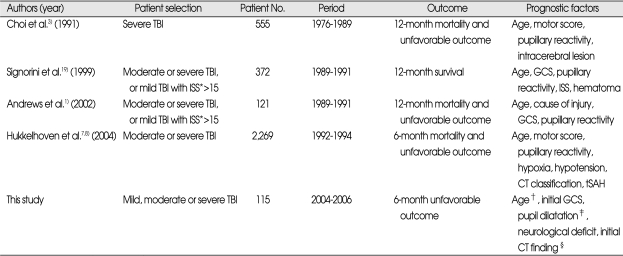
Many reports have been published to date that have attempted to predict prognostic factors for long term neurological outcome after severe or moderate TBI1,3,7,19). The reported prognostic factors are age, motor score, pupillary reactivity, intracerebral lesion, GCS, cause of injury, injury severity score, hypoxia, hypotension, the Traumatic Coma Data Bank (TCDB) CT classification, and traumatic subarachnoid hemorrhage1,3,7,14,19). Our series showed that age, at least unilateral pupillary dilatation, initial GCS, neurological deficit, and the presence of bilateral parenchymal lesions or lesions involving the midline structure of the initial CT were confirmed to be prognostic factors, and multivariate analysis demonstrated that age and initial GCS were independent prognostic factors (Table 3).
Table 3.
Reported prognostic factors after TBI in the literatures
*Injury Severity Score, †≥35 years, ‡at least unilateral pupil dilatation, §bilateral parenchymal lesion or lesions involving the midline structures the midline structures on the initial brain CT
The patients' age is known to be a primary risk factor for predicting neurological outcome after TBI. Older patients have decreased capacity for brain repair, and systemic complications are more frequent. Also, compared to younger patients, there is a higher incidence of systemic illnesses such as cardiovascular disease, diabetes, stroke and hence poorer outcome in older patients8,19). Our results also showed that mean age was greater in the Unfavorable group compared to the Favorable group (46.8-47.3 years vs. 57.7-59.5 years). In addition, patients who were older than 35 years comprised 91.9-96.4% in the Unfavorable group compared to the 74.4-75% of the Favorable group. To investigate other prognostic factors, Jiang et al.9) studied 846 severe TBI patients and analyzed the relationship between a GCS score of less than 8 and good recovery. They observed that in the good recovery group, the percentage of patients with GCS score from 8 to 3 were 39.88% (8 points), 37.61% (7 points), 32.16% (6 points), 21.69% (5 points), 16.18% (4 points), 6.98% (3 points), and they concluded that lower GCS scores correlated with lower chances of good recovery9). We also observed in this study that mean initial GCS scores were significantly lower in the Unfavorable group compared to the Favorable group (7.2-7.8 vs. 12.0-12.2), and it was also shown that age and initial GCS score were significantly different between the above two groups in multivariate analysis. In a study by Hukkelhoven et al.7) who studied TBI patients with respect to pupil reaction, unfavorable outcome in case of both pupil reaction was present in 459/1,518 (30%) patients, one pupil reaction in 178/317 (56%), and no pupil reaction in 225/314 (72%) patients. In a similar study, Jiang et al.9) showed good recovery percentage in 53.13% of patients with bilateral normal pupil response, 20.99% in those with unilateral abnormal pupil response, and 11.23% in those with bilateral abnormal pupil response. In contrast to the aforementioned studies, our evaluation of TBI patients focused on a more objective pupil dilatation rather than pupil reaction or response, and our analysis demonstrated that the percentage of patients with at least unilateral pupil dilatation at the time of ER visit was higher in the Unfavorable group compared to the Favorable group (45.9-50% vs. 12.8-14.9%). In addition to pupil dilatation, neurological deficit at ER which represent neural damage, was observed to be higher in the Unfavorable group (59.5-64.3%) compared to the Favorable group (23.1-25.3%).
As well as clinical features, brain CT findings are also factors for predicting prognosis for patients with TBI20). Hukkelhoven et al.7) reported that unfavorable outcome was observed among 238/932 (26%) of TCDB CT class I and II patients, 203/411 (49%) of class III patients, 48/86 (56%) of class IV, and 361/701 (51%) of TCDB CT V and VI patients. Wardlaw20) showed that brain CT findings of subdural hemorrhage, subarachnoid hemorrhage, parenchymal lesion including midline shifting or abnormal third ventricle were significantly related to low GCS scores and survival rates. In our study, we analyzed the locations of the traumatic parenchymal lesions on the initial brain CT at the ER and demonstrated similar results; the presence of bilateral parenchymal lesions or lesions involving the midline structures on the initial CT was 28.6-30.5% vs. 54.1-61.3% for the Favorable group and Unfavorable group, respectively. It is therefore considered that such radiological factors are also predictive of clinical outcome.
Recent studies have suggested that stem cell transplantation is beneficial for functional improvement in the animal model of TBI11,18). In these reports, the authors postulated that the therapeutic effect of transplanted stem cells might be caused by a neuroprotection rather than the substitution of damaged tissue. For maximum neuroprotection, the available animal study data to date suggest that clinical stem cell trials for functional recovery after human TBI should be instituted in the early post-trauma phase rather than in the late chronic phase. However, prognosis for TBI patients is affected by diverse and complex pathophysiological mechanisms, and even though poor neurological conditions may be present in the early phase of trauma, recovery usually occurs as time progresses. Therefore, in addition to studies that center on elucidating risk factors that predict poor neurological outcome, data on the timing of neurological improvement which did not exist in the past literature is needed. In this present study, the GCS score of Favorable outcome group and Unfavorable outcome group patients with at least one significant risk factor were assessed at intervals after TBI, and it was observed that Favorable outcome patients showed neurological improvement within 2 months of TBI. Therefore, the TBI patients with clinico-radiological risk factors who received standard treatment but did not show neurological improvement within 2 months are at risk for poor neurological outcome, and these patients should be provided with new clinical trials.
CONCLUSION
This study divided TBI patients into an Unfavorable group and a Favorable group, and assessed functional outcome 6 month after TBI with respect to GOS, LCFS, and DRS methods. The obtained clinico-radiological data was analyzed prospectively and the results showed that age ≥35 years, at least unilateral pupil dilatation, low initial GCS score, neurological deficit, and the presence of bilateral parenchymal lesions or lesions involving the midline structure of the initial CT were confirmed to be poor prognostic factors, and multivariate analysis demonstrated that age and initial GCS were independent prognostic factors. The majority of Favorable group patients with at least one risk factor showed improvement of GCS scores within 2 months of the TBI. Therefore, patients with the above mentioned clinico-radiological risk factors who received standard treatment, but who did not demonstrate neurological improvement within 2 months after TBI despite standard care were deemed at risk for Unfavorable neurological outcome. These patients may be eligible candidates for clinical trials that would improve functional outcome after TBI.
Acknowledgements
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (Grant No. 0412-DB00-010-0007).
References
- 1.Andrews PJ, Sleeman DH, Statham PF, McQuatt A, Corruble V, Jones PA, et al. Predicting recovery in patients suffering from traumatic brain injury by using admission variables and physiological data : a comparison between decision tree analysis and logistic regression. J Neurosurg. 2002;97:326–336. doi: 10.3171/jns.2002.97.2.0326. [DOI] [PubMed] [Google Scholar]
- 2.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury : a review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Choi SC, Muizelaar JP, Barnes TY, Marmarou A, Brooks DM, Young HF. Prediction tree for severely head-injured patients. J Neurosurg. 1991;75:251–255. doi: 10.3171/jns.1991.75.2.0251. [DOI] [PubMed] [Google Scholar]
- 4.Ercan M, Inci S, Kilinc K, Palaoglu S, Aypar Ü. Nimodipine attenuates lipid peroxidation during the acute phase of head trauma in rats. Neurosurg Rev. 2001;24:127–130. doi: 10.1007/pl00012396. [DOI] [PubMed] [Google Scholar]
- 5.Gunnarsson T, Fehlings MG. Acute neurosurgical management of traumatic brain injury and spinal cord injury. Curr Opin Neurol. 2003;16:717–723. doi: 10.1097/01.wco.0000102629.16692.ee. [DOI] [PubMed] [Google Scholar]
- 6.Hammond FM, Grattan KD, Sasser H, Corrigan JD, Bushnik T, Zafonte RD. Long-term recovery course after traumatic brain injury : a comparison of the functional independence measure and disability rating scale. J Head Trauma Rehabil. 2001;16:318–329. doi: 10.1097/00001199-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hukkelhoven CW, Steyerberg EW, Habbema JD, Farace E, Marmarou A, Murray GD, et al. Predicting outcome after traumatic brain injury : development and validation of a prognostic score based on admission characteristics. J Neurotrauma. 2005;22:1025–1039. doi: 10.1089/neu.2005.22.1025. [DOI] [PubMed] [Google Scholar]
- 8.Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marchall LF, et al. Patient age and outcome following severe traumatic brain injury : an analysis of 5600 patients. J Neurosurg. 2003;99:666–673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- 9.Jiang JY, Gao GY, Li WP, Yu MK, Zhu C. Early Indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma. 2002;19:869–874. doi: 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- 10.Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12:559–563. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- 11.Maas AI, Dearden M, Teasdale GM, Braakman R, Cohadon F, Iannotti F, et al. EBIC-guidelines for management of severe head injury in adults. Acta Neurochir (Wien) 1997;139:286–294. doi: 10.1007/BF01808823. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 13.Marik PE, Varon J, Trask T. Management of head trauma. Chest. 2002;122:699–711. doi: 10.1378/chest.122.2.699. [DOI] [PubMed] [Google Scholar]
- 14.Marshall LF, Marshall SB, Klauberg MR, Berkum VCM, Eisenberg HM, Jane JA, et al. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75:S14–S20. [Google Scholar]
- 15.Moppett IK. Traumatic brain injury : assessment, resuscitation and early management. Br J Anaesth. 2007;99:18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- 16.Murray GD, Taesdale GM, Schmitz H. Nimodipine in traumatic subarachnoid hemorrhage : a re-analysis of the HIT-I and HIT-II trials. Acta Neurochir (Wien) 1996;138:1163–1167. doi: 10.1007/BF01809745. [DOI] [PubMed] [Google Scholar]
- 17.Pickett W, Ardern C, Brison RJ. A population-based study of potential brain injuries requiring emergency care. CMAJ. 2001;165:288–292. [PMC free article] [PubMed] [Google Scholar]
- 18.Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, Raghupathi R, et al. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery. 2002;51:1043–1052. doi: 10.1097/00006123-200210000-00035. discussion 1052-1054. [DOI] [PubMed] [Google Scholar]
- 19.Signorini DF, Andrews PJ, Jones PA, Wardlaw JM, Miller JD. Predicting survival using simple clinical variables : a case study in traumatic brain injury. J Neurol Neurosurg Psychiatry. 1999;66:20–25. doi: 10.1136/jnnp.66.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wardlaw JM, Easton VJ, Statham P. Which CT features help predict outcome after head injury? J Neurol Neurosurg Psychiatry. 2002;72:188–192. doi: 10.1136/jnnp.72.2.188. discussion 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wennersten A, Meier X, Holmin S, Wahlberg L, Mathiesen T. Proliferation, migration, and differentiation of human neural stem/progenitor cells after transplantation into a rat model of traumatic brain injury. J Neurosurg. 2004;100:88–96. doi: 10.3171/jns.2004.100.1.0088. [DOI] [PubMed] [Google Scholar]