Abstract
Clear cell sarcoma (CCS), also called malignant melanoma of soft parts, is a rare malignant soft tissue tumor and is often associated with tendons or aponeuroses. Most of CCS involve extremities, especially lower extremities, but a tumor occurring in the trunk is rare. We report an extremely rare case of CCS originated in the upper thoracic back muscle. To our knowledge, this case is the second report of CCS of the back muscle.
Keywords: Clear cell sarcoma, Back muscle
INTRODUCTION
Clear cell sarcoma (CCS) is a rare and highly malignant neoplasia of difficult diagnosis, and shows melanocytic differentiation1,11). It is a tumor with a propensity for lymph node, lung, and bone metastasis1,19). CCS mainly occurs in young adults between the ages of 20 and 40 years16). The male to female ratio is almost equal15). Its prevalence ranges between 0.5% and 1% of all malignancies of the musculoskeletal system10). Most cases of CCS involve extremities, but a tumor occurring in the trunk is rare. We report an extremely rare case of CCS arising from the upper thoracic back muscle.
CASE REPORT
A 25-year-old man presented with a painless upper thoracic back mass for a period of 3 months; the mass was gradually increasing in size. Physical examination revealed a uniformly firm 2.5×3.5 cm mass in the right paramedian upper thoracic back region, at the T4 level. The skin appeared normal. The patient had no previous history of melanoma or other skin lesions.
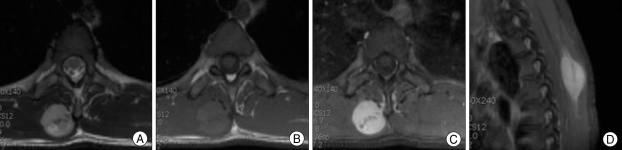
Thoracic spine computed tomography (CT) demonstrated a regular inhomogeneous enhancing 2.3×1.8×4.3 cm hypodense mass in the right paraspinal back muscle at the T4 level (Fig. 1). There were no adjacent bone structure involvement and no calcification in mass. On T2 weighted magnetic resonance (MR) images, the lesion showed intermediate high signal intensity, and it was slightly hyperintense to back muscles on T1 weighted images. Well-defined homogeneous enhancement was seen (Fig. 2). Total excision of the mass was performed. The lesion was a partly circumscribed dark gray mass and was firm.
Fig. 1.
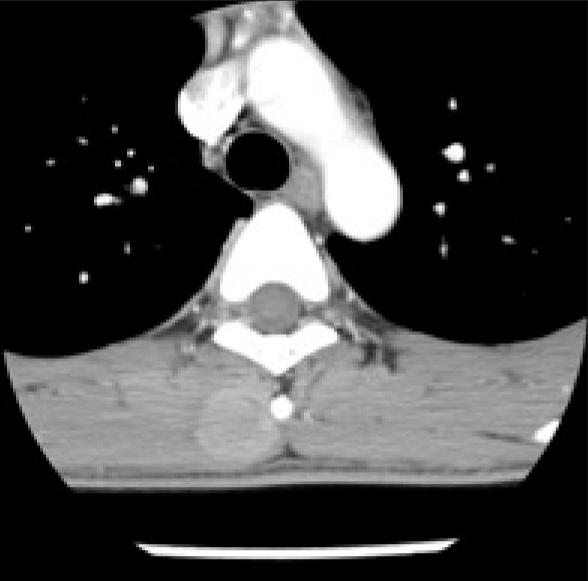
Computed tomographic scan of the thoracic spine show a rounded mass in the right paraspinal muscle at the T4 level. There was no calcification. Adjacent bone structure involvement was not seen.
Fig. 2.
T2-weighted (A) and T1-weighted (B) axial magnetic resonance images show a well-demarcated mass in the right paraspinal muscle. Homogeneous enhancement was seen (C and D).
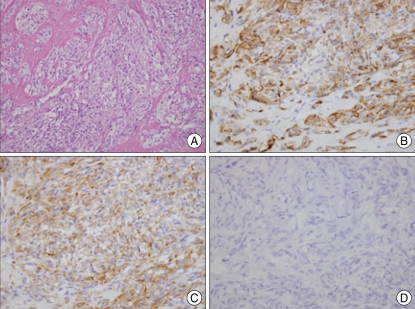
Histopathological examination revealed diffuse nests like infiltration of fusiform or spindle cells. Individual cells had prominent basophilic nucleoli and a distinct nuclear margin with clear cytoplasm. The neoplastic cells were positive for S-100, HMB-45, and vimentin, but negative for CD-117 (Fig. 3).
Fig. 3.
A photomicrograph shows round tumor cells with prominent basophilic nucleoli, distinct nuclear margins and clear cytoplasm (H & E, ×400) (A). Immunohistochemical stains of tumor cells show positive immunoreactivity for HMB-45 (B), vimentin (C), but no reactivity for CD-117 (D) (×400).
Postoperative investigations for metastasis were followed. Whole body PET CT showed no increased uptake lesions. On chest and abdominal CT, there were no abnormal metastatic lesions. A bone scan did not show increased uptake. The patient was referred to a dermatologist to check for a primary cutaneous malignant melanoma, but there were no other cutaneous lesions. All investigations to find a primary lesion elsewhere revealed no abnormality.
The tumor was therefore diagnosed as CCS of the back muscle.
Postoperative recovery was uneventful, and the patient is scheduled to undergo adjunctive radiotherapy.
DISCUSSION
CCS is a rare malignant soft tissue neoplasm that accounts for less than 1% of soft tissue malignancies8,10). CCS has a predilection for the extremities, in particular the lower extremities. Both the foot and the ankle are common sites of tumor appearance accounting for 33-65% of all reported cases13). The next most common sites are the knee, thigh, hand, forearm, elbow, and shoulder2). A tumor arising from the trunk is rare. Deenik et al.5) reported 30 patients with CCS between 1978 and 1992. None of these patients had a CCS in the trunk. Lucas et al.14) reported two cases involving the trunk among 35 CCS. Both cases showed chest wall involvement. Chung et al.3) reported on 141 patients with CCS between 1942 and 1980. They found only three patients whose tumors originated in the trunk; two tumors occurred on the abdominal wall, and one tumor occurred on the back. Therefore, among CCS involving trunk, only one case that originated from back muscle has been reported. To our knowledge, the case presented here is the second report of CCS of the back muscle.
Conventional radiographs of CCS are usually normal but may reveal a deep-seated radiopaque, soft tissue mass. Calcification in the tumor and local bone invasion are uncommon3). MR imaging is required to detect and characterize CCS. Due to the paramagnetic effect of the melanin pigment in CCS, most of the tumors show a hyperintense signal to muscle on T1 and hypointense signal on T2 weighted images4,10,12). The signal on T1 images is more specific for tumors displaying melanocytic differentiaion, whereas the signal on T2 images is rather variable4). The high signal intensity on T1 images can help to differentiate CCS from other strongly enhancing soft tissue tumors. Furthermore, tumors showing high signal on T1 images, such as lipomas, lipoblastomas and melanocytic schwannomas should be differentiated from CCS. In our case, MR findings of the tumor are compatible with CCS, as they showed a hyperintense signal to muscle on T1 images.
CCS is thought to derive from neural crest cells, and it shows melanocytic differentiation1,6,11). Therefore, these tumor cells often stain positive for the melanocyte immunohistochemical markers HMB-45 and S-1009). These markers are considerably helpful for distinguishing CCS from epithelial tumors and, especially, synovial sarcomas5). One of the main differential diagnoses of CCS is metastatic malignant melanoma17). CCS cannot be distinguished from malignant melanoma by immunohistochemical characteristics15). However, in contrast to malignant melanoma, CCS is characterized by the presence of a reciprocal cytogenetic translocation t(12;22) (q13;q12) that create a unique chimeric fusion of the Ewing sarcoma gene (EWS) and activating transcription factor 1 (ATF1) genes, which is found in more than 80% of reported cases6,9,18). Recently, Garcia et al.7) suggested that CD 117 immunoreactivity may be useful for separating melanoma from CCS; positive staining results exclude CCS, but are compatible with metastatic melanoma. Although we could not obtain cytogenetic results, the fact that there was no evidence of a primary skin lesion or metastatic lesions, taken together with our histopathological findings, support a diagnosis of CCS.
CONCLUSION
We present an extremely rare case of CCS originating from the back muscle. Since the prognosis of CCS is poor and unpredictable, and complete surgical tumor excision with radiation therapy for close resection margin is the only effective treatment. CCS is an important, although a rare condition, and should be considered in the diagnosis of patients with a soft tissue mass.
References
- 1.Albert MV, Verdeguer A, Fernandez JM, Castel V, Hernandez M, Oltra JS. Malignant melanoma of soft parts : a rare entity with a specific genetic marker. Pediatr Blood Cancer. 2006;46:659. doi: 10.1002/pbc.20659. [DOI] [PubMed] [Google Scholar]
- 2.Al-Nakshabandi NA, Munk PL. Radiology for the surgeon. Musculoskeletal case 38. Diagnosis : clear cell sarcoma of the foot. Can J Surg. 2007;50:58–59. [PMC free article] [PubMed] [Google Scholar]
- 3.Chung EB, Enzinger FM. Malignant melanoma of soft parts. A reassessment of clear cell sarcoma. Am J Surg Pathol. 1983;7:405–413. doi: 10.1097/00000478-198307000-00003. [DOI] [PubMed] [Google Scholar]
- 4.De Beuckeleer LH, De Schepper AM, Vandevenne JE, Bloem JL, Davies AM, Oudkerk M, et al. MR imaging of clear cell sarcoma (malignant melanoma of soft parts) : multicenter correlative MRI-pathology study of 21 cases and literature review. Skeletal Radiol. 2000;29:187–195. doi: 10.1007/s002560050592. [DOI] [PubMed] [Google Scholar]
- 5.Deenik W, Mooi WJ, Rutgers EJ, Peterse JL, Hart AA, Kroon BB. Clear cell sarcoma (malignant melanoma) of soft parts A clinicopathologic study of 30 cases. Cancer. 1999;86:969–975. [PubMed] [Google Scholar]
- 6.Dim DC, Cooley LD, Miranda RN. Clear cell sarcoma of tendons and aponeuroses : a review. Arch Pathol Lab Med. 2007;131:152–156. doi: 10.5858/2007-131-152-CCSOTA. [DOI] [PubMed] [Google Scholar]
- 7.Garcia JJ, Kramer MJ, Mackey ZB, O'Donnell RJ, Horvai AE. Utility of CD117 immunoreactivity in differentiating metastatic melanoma from clear cell sarcoma. Arch Pathol Lab Med. 2006;130:343–348. doi: 10.5858/2006-130-343-UOCIID. [DOI] [PubMed] [Google Scholar]
- 8.Gelczer RK, Wenger DE, Wold LE. Primary clear cell sarcoma of bone : a unique site of origin. Skeletal Radiol. 1999;28:240–243. doi: 10.1007/s002560050509. [DOI] [PubMed] [Google Scholar]
- 9.Henkle CT, Hawkins AL, McCarthy EF, Griffin CA. Clear cell sarcoma case report : complex karyotype including t(12;22) in primary and metastatic tumor. Cancer Genet Cytogenet. 2004;149:63–67. doi: 10.1016/S0165-4608(03)00295-4. [DOI] [PubMed] [Google Scholar]
- 10.Hermann G, Klein MJ, Springfield DS, Abdelwahab IF. Clear cell sarcoma (malignant melanoma of soft parts) : case report and review of the literature. Can Assoc Radiol J. 2002;53:237–240. [PubMed] [Google Scholar]
- 11.Hersekli MA, Ozkoc G, Bircan S, Akpinar S, Ozalay M, Tuncer I, et al. Primary clear cell sarcoma of rib. Skeletal Radiol. 2005;34:167–170. doi: 10.1007/s00256-004-0801-y. [DOI] [PubMed] [Google Scholar]
- 12.Hourani M, Khoury N, Mourany B, Shabb NS. MR appearance of clear cell sarcoma of tendons and aponeoroses (malignant melanoma of soft parts) : radiologic-pathologic correlation. Skeletal Radiol. 2005;34:543–546. doi: 10.1007/s00256-005-0893-z. [DOI] [PubMed] [Google Scholar]
- 13.Kazakos CJ, Galanis VG, Giatromanolaki A, Verettas DA, Sividis E. Clear cell sarcoma of the scapula. A case report and review of the literature. World J Surg Oncol. 2006;4:48. doi: 10.1186/1477-7819-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas DR, Nascimento AG, Sim FH. Clear cell sarcoma of soft tissues. Mayo Clinic experience with 35 cases. Am J Surg Pathol. 1992;16:1197–1204. doi: 10.1097/00000478-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Malchau SS, Hayden J, Hornicek F, Mankin HJ. Clear cell sarcoma of soft tissues. J Surg Oncol. 2007;95:519–522. doi: 10.1002/jso.20730. [DOI] [PubMed] [Google Scholar]
- 16.Murugan P, Basu D, Kumar S, Jagadish S. Clear cell sarcoma of the soft parts arising in the rectus abdominis in a child-aspiration cytology of a rare case. Cytojournal. 2007;4:15. doi: 10.1186/1742-6413-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suehara Y, Yazawa Y, Hitachi K, Terakado A. Clear cell sarcoma arising from the chest wall : a case report. J Orthop Sci. 2004;9:171–174. doi: 10.1007/s00776-003-0751-6. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira MR, Ribeiro FR, Cerveira N, Torres L, Amaro T, Henrique R, et al. Karyotypic divergence and convergence in two synchronous lung metastases of a clear cell sarcoma of tendons and aponeuroses with t(12;22)(q13;q12) and type 1 EWS/ATF1. Cancer Genet Cytogenet. 2003;145:121–125. doi: 10.1016/s0165-4608(03)00063-3. [DOI] [PubMed] [Google Scholar]
- 19.Xu GG, Chong YL, Cheong MO. Clear cell sarcoma of the rectus sheath. Singapore Med J. 2007;48:e203–e205. [PubMed] [Google Scholar]