Abstract
Objective
An acquired right-sided homonymous hemianopia can result in slowed left-to-right text reading, so-called hemianopic alexia (HA). Patients with HA lack essential visual information to help guide ensuing reading fixations. We tested two hypotheses: firstly, that practice with a visual rehabilitation method that induced small-field optokinetic nystagmus (OKN) would improve reading speeds in patients with HA when compared to a sham visual rehabilitation therapy; secondly, that this therapy would preferentially affect reading saccades into the blind field.
Methods
19 patients with HA were entered into a two-armed study with two therapy blocks in each arm: one group practiced reading moving text (MT) that scrolled from right-to-left, daily for two four week blocks (group1), while the other had sham therapy (spot-the-difference) for the first block and then crossed-over to MT for the second.
Results
Group 1 showed significant improvements in static text reading speed over both therapy blocks (18% improvement), while group 2 did not significantly improve over the first block (5% improvement) but did when they crossed-over to the MT block (23% improvement). MT therapy was associated with a direction-specific effect on saccadic amplitude for rightward but not leftward reading saccades.
Conclusion
OKN inducing therapy preferentially affects reading saccades in the direction of the induced (involuntary) saccadic component. This is the first study to demonstrate the effectiveness of a specific eye movement based therapy in patients with HA in the context of a therapy-controlled trial. A free web-based version of the therapy used in this study is available online to suitable patients with HA.
Introduction
Despite being able to adapt to various impairments caused by their hemianopia such as navigation though familiar environments, a sub-group of patients with a hemianopia that encroaches into or near foveal vision, fail to adequately compensate when reading text and indeed adopt an inefficient eye movement strategy when doing so,1 so-called hemianopic alexia (HA). Patients with HA produce many more saccades than normal subjects when reading along a line of text because the hemianopia deprives them of important visual information about the shape and location of ensuing words that normal readers use to guide a series of efficient reading saccades.2-4 This sensory deficit, resulting in a loss of ‘bottom-up’ information (information which depends directly on the nature of the external stimulus), causes a functional disturbance in the distributed neural network involved in producing reading saccades.5
Static text reading speeds in normal subjects can be exceeded when text is moved in quick jumps that simulate saccades while readers fixate centrally,6 or if the text is animated by making it scroll horizontally from right to left.7 This latter type of text, also called “Times Square” presentation, induces small-field optokinetic nystagmus (OKN) in the reader and, when used as part of a rehabilitation program, has been shown to improve subsequent reading performance on normal static text.4, 8 What has not been clear from previous studies is whether it is critical that the particular type of therapy-induced eye movement is of the OKN type, or whether any therapy that induces saccadic eye movements will suffice. We wished to investigate this by using two forms of oculomotor therapy in a group of patients with right-sided hemianopia causing HA: firstly, an OKN inducing reading therapy; secondly, a control or sham therapy that involved patients making voluntary saccades into their blind field with a non-reading task. We also wished to identify if any therapy-induced effects on reading saccades were direction specific, i.e.: whether patients’ initial landing positions within a word would move more to the right, ‘normalizing’ towards the preferred viewing location of normal readers,1, 9 and whether there would be a preferential effect on reading saccades into, as opposed to away from, the affected visual field.
Methods
Subjects, treatment and group allocation
Ethical approval for this study was granted by The Royal Free Hospital ethics committee and written consent obtained from all participants. 22 patients with a right-sided homonymous hemianopia that interfered with reading participated in this study, although three dropped-out before completing therapy. In the majority of cases the hemianopia was secondary to a posterior cerebral artery territory stroke (infarct or hemorrhage), but head injury and tumors were among the causative lesions (Table 1). All the patients had a fixed homonymous field defect that had been present for at least three months. Patients were allocated to one of two treatment groups to receive either two blocks of moving text (MT) group 1, or a first block of spot-the-difference (StD) therapy and then crossing over to moving text for the second block, group 2. Because of the small number of patients entering the study, we wished to ensure that the two treatment groups did not become unbalanced on a few key variables so patients were allocated using a modified minimization technique where cumulative measures of two factors (text reading speed (≤ 90 or >90 wpm) and degrees of sparing of right foveal/parafoveal (0 or 2 degrees) vision.) were used to minimize the difference between the two groups. The weighting used for allocation to the group with the lowest total was one10 We describe the minimization as modified because the first subject was not allocated randomly to either group, but deterministically placed in the group 1. Differences between the two groups on five variables were assessed using t-tests where normality assumptions were not violated as assessed by Shapiro-Wilk test or by a Mann-Whitney (MW) non-parametric test where they were. Results are shown in the last row of Table 1. There were no differences between the groups for the following variables: time since onset of hemianopia; degrees of sparing (with respect to the vertical meridian); single word reading speed; text reading speed. There was a significant difference between the groups for age at onset of hemianopia although these data were skewed by an outlier who had his hemianopia age five years old.
Table 1.
Demographic and reading speed data: the first 11 cases form group 1, the next eight group 2. Statistical comparisons were made between the two groups. P values for two-tailed t-tests or Mann-Whitney (italics) non-parametric test are shown in the final row
| Case | Sex | Age at symptom onset (years) | Time since symptom onset (years (months)) | Cause of hemianopia | Right visual field defect | Degrees of sparing of right visual field | Single word reading rate 3-letter words (sec) | Text reading rate (wpm) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 59 | 3 (4) | Tuberous Sclerosis | HS | 0 | 1.21 | 125 |
| 2 | M | 53 | 1 (3) | Infarct | HH | 2 | 1.09 | 59 |
| 3 | F | 67 | 2 (3) | Infarct | LQ | 0 | 1.06 | 86 |
| 4 | M | 5 | 37 | Head injury | HH | 2 | 0.75 | 151 |
| 5 | F | 55 | 3(11) | Tumor | HH | 0 | 0.76 | 113 |
| 6 | M | 33 | 1 (4) | Tumor | HH | 0 | 0.95 | 64 |
| 7 | M | 24 | 14 | Haemorrhage | HH | 2 | 0.90 | 58 |
| 8 | F | 34 | 2 (6) | Head injury | HH | 2 | 0.80 | 86 |
| 9 | M | 66 | 1 | Infarct | UQ | 0 | 1.22 | 99 |
| 10 | M | 51 | 2 (1) | Haemorrhage | HH | 2 | 0.98 | 54 |
| 11 | F | 20 | 14 | Porencephalic cyst | HH | 0 | 0.69 | 143 |
|
| ||||||||
| 12 | M | 61 | 1 (1) | Infarct | HH | 0 | 1.36 | 58 |
| 13 | M | 73 | 5 (2) | Infarct | HH | 2 | 0.82 | 95 |
| 14 | M | 57 | (6) | Infarct | HH | 0 | 1.00 | 83 |
| 15 | M | 67 | (9) | Infarct | LQ | 2 | 0.86 | 89 |
| 16 | M | 78 | (4) | Infarct | HH | 0 | 1.17 | 59 |
| 17 | M | 39 | 2(11) | Infarct | UQ | 0 | 1.03 | 63 |
| 18 | F | 71 | (3) | Infarct | HH | 0 | 0.78 | 98 |
| 19 | M | 59 | 2 (2) | Infarct | HH | 2 | 0.95 | 110 |
|
| ||||||||
| P | 0.014 | 0.117 | 0.736 | 0.576 | 0.337 | |||
The treatment blocks lasted for four weeks each. The patients were asked to keep diaries of how many minutes they spent on the therapies in each block. Data were collected at five time points (Figure 1): Neuropsychometric tests were only performed once at T1; text reading data (Neale analysis of reading, see below) was collected at T1-T5 with the baseline (B) measure being the average of the T1 and T2 scores; eye movements were recorded at either T1 or T2 (B), T3, T4 and T5. Single word reading data were measured at T1 and T4, as was visual field perimetry.
Figure 1.
Timeline for the two groups: G1 = group 1; G2 = group 2. The treatment blocks were either moving text (MT) or the sham therapy, spot-the-difference (StD). T1-5 are the five time points that data were collected. Text reading speeds were averaged over T1 and T2 to provide a baseline (B) measure.
General neuropsychological assessment
All patients were tested once (T1) on a battery of neuropsychological tests including the following: A test of general intellectual function, the Wechsler Abbreviated Scale of Intelligence (WASI);11 a test of visuo-spatial function, the Visual Object and Space Perception Battery (VOSP);12 a graded test of spelling;13 and Warrington’s recognition memory test for words and faces.14 Differences between the two groups on six neuropsychological variables were assessed as above and are presented in the Supplemental Table: E-Table 1. There were no differences between the groups on all of the variables.
Rehabilitation materials
The tapes were made by animating freely available text from a variety of Sherlock Holmes stories (http://www.citsoft.com/holmes3.html) across a computer screen from right-to-left (i.e.: horizontal motion was from the patients’ blind field into their seeing field), so-called Times Square presentation.7 After some minor preparations, such as deleting hyphens and adding a few spaces after each sentence, custom-written software was used to animate the text across the screen at a variable rate. The output from the graphics card was connected to the video-input on a VHS video recorder to produce the tapes. Tape speeds of: 85, 105, 143, 173, 205, 240, and 275 wpm were made with the text presented in Times New Roman font, 24 point, black on white, scrolling across the middle of the screen. Multiple copies were made and patients contacted one of us (G.A.S.) when they required a new (usually faster) tape. Patients were instructed to read the story on the tapes and try to follow it, although no tests of comprehension were made to check this.
‘Spot-the-difference’ tests were taken from a children’s puzzle booklet; the original cartoons were altered to remove text. The number of differences on each page varied between eight and 12. Patients were instructed to look for as many differences as possible between the two pictures, but were not told how many to expect, completing at least two cartoons over 20 min. Between 40 to 60 examples were selected randomly for each patient (out of a total of the 70 prepared tests), depending on their speed.
For both tasks (MT and StD), patients were asked to record in their diaries how long they spent on the tasks each day. The aim was to achieve a minimum of 400 minutes of rehab (20 sessions × 20 min) over approximately four weeks.
Text reading speeds
Text reading speeds were measured at all five timepoints (T1-T5), using passages 3, 4 and 5 of the Neale analysis of reading.15 This test has two parallel forms (A and B), which were alternated across the five time points (half the subjects started with ‘A’ and half with ‘B’). Subjects could self-correct errors, but were urged to move on if they got ‘stuck’ on a word for more than five seconds. The subjects were timed during oral reading of the passages and thus the time needed for articulation is included. Baseline scores (B) were calculated as the average of T1 and T2 scores. All data analyses were performed using SPSS version 12.0 (SPSS, Inc., Chicago IL), using a repeated measures ANOVA where the three time points (B, T3 and T4) were entered as within-subject factors, and the treatment group as a between-subject factor. Post-hoc paired t-tests were then used to test pairs of three time points for differences using a Bonferroni correction for multiple comparisons.
Single word reading speeds
Single word reading speeds were measured at two time points only, B and after both therapy blocks (T4), using 25 examples of three, five, seven and nine letter words taken from the MRC psycholinguistic battery,16 and were matched across word length groups on: frequency (range 1-350 per million on the Kucera and Francis scale) and imageability (range 525-600; total possible range 100-700). The words were displayed on a computer screen in the same font as the text used to record eye-movements (see below), reaction times were measured from stimulus presentation to initiation of vocalization using a voice key activated system; therefore, single word reading speeds cannot be simply extrapolated from text reading speeds. Reading slopes were calculated for each subject (time required to read words of increasing length in milliseconds per letter (mspl)), and this was analysed using a paired t-test (planned analysis). A subsequent paired analysis was carried out on each category of word length.
Recording and analysis of eye movements
Eye movements were recorded at T1 or T2 (B), T3, T4 and T5. The methods are as described previously,1 but in brief: eye movements were only made while the subjects read static text. Patients silently read three out of a corpus of 10 short (~50 words each) text passages extracted from newspaper journalism at each of the time points. The texts were displayed on a 22 in. monitor; each passage occupied at most nine lines of the display. Screen resolution was 1024 × 768 pixels, and text was rendered in 36-point Arial font as black on a white background. Viewing distance was between 60 and 80 cm; at this distance an average-width letter subtended 0.8° of visual angle. Comprehension was tested after reading each text by asking the participants to reiterate its content in order to provide evidence that they had read the texts. Eye movements were recorded with an SR EyeLink II video-based head-mounted eye tracking system (SR Research Ltd., Mississauga, Canada). Viewing was binocular, and the position of both eyes was sampled at 500 Hz. The patients’ eye position was calibrated using a 9-point grid. Drift correction was performed before each passage was displayed. Saccades were identified using the criterion of a 30°/s velocity and a 8000°/s acceleration. Fixation position and duration data from the left eye only were used. Data for the first word on the line and the word receiving the first fixation of the line (if not the first word) were eliminated from consideration. Measures that vary with word length (such as initial landing position) are reported in the text below, but are collapsed across the word length range (three to seven letters) in the table and figures.
One subject’s T4 data were corrupted and could not be used. Eleven of the 19 patients had no usable T5 eye movement recordings made. In order to increase the number of data points for each subject, T4 and T5 data were analysed together to provide combined ‘post-rehabilitation’ values. Data were entered into SPSS and analysed as outlined above in the text reading section.
Recording and analysis of visual field perimetry
Perimetry was performed twice, before and after completion of the therapy blocks (B and T4). Static fields were measured using the automated Humphrey field analyser II (Carl Zeiss Group, California, USA) analysis of the central 10 degrees of vision (central 10-2 threshold test). Dynamic fields were also measured using a Goldmann perimeter (Haag Streit, Köniz, Switzerland) when there were concerns over subject’s performance with the automated procedure (a false positive or false negative response rate greater than 15%). The procedures for determining the amount of field sparing were as reported previously.1 In the event of a discrepancy between the static and dynamic fields or pre and post therapy changes, the opinion of the orthoptist who performed the tests (Ms. Bronia Unwin) was sought. The precision of the static perimerty is two degrees and all the patients fell into one of two groups, either 0 or 2 degrees spared to the right of fixation.
Results
Three patients, all allocated to group 2, failed to complete the second block of therapy. The data from these subjects were excluded from all analyses, so n = 11 for group 1 and 8 for group 2. In tests between both groups, data from group 1 are always presented first with a colon separating the two group values.
Time in treatment blocks
There were no differences between the two groups in total time spent on rehabilitation tasks across both treatment blocks in minutes (range), 914 (865-955):901 (840-1000), t-test, t (10) 0.50, P = 0.628; or within groups for time spent on first versus second treatment blocks: 442:471, t-test, t (10) -1.16, P = 0.274; 443:459, t-test, t (7) -0.69, P = 0.513.
Text reading speeds
Between group effects
There was a main effect of time across both treatment groups: F(1,17) = 15.40, P < 0.001, but no interaction between treatment group and time, either comparing data from the three time points that spanned both blocks of therapy (B, T3 and T4): F(2,16) = 0.97, P < 0.388, or over the two that spanned just the first block (B and T3): F(1,17) = 1.27, P < 0.275. The two groups were analyzed separately below.
Within group effects: group 1
Results from group 1 revealed an overall effect of time: F(2,9) = 23.91, P < 0.001, with patients improving from a mean speed of 95 wpm at B to 103 at T3 and 112 at T4 (Figure 2A). Post hoc paired T-tests revealed improvements with all three comparisons using a Bonferroni correction for multiple comparisons (P 0.05/3 = 0.017): B-T3, t (10) = 3.77, P = 0.004; B-T4, t (10) = 8.01, P < 0.001; T3-T4, t (10) = 3.01, P = 0.013.
Figure 2.
Text reading times (y-axis, in words per minute) for both group 1 and group 2 at three time points (Baseline (B), T3 and T4). The group averages are shown in filled circles for group 1 (A) and filled squares for group 2 (B) with SEM error bars. Analysis was on the individual subjects’ reading times (open circles in both graphs). The average percentage increase is shown for both groups (C) across all four time points (B, T3, T4 and T5 (post-rehabilitation)).
Within group effects: group 2
Results from group 2 revealed an overall effect of time: F(2,9) = 19.39, P < 0.001, with patients improving from a mean speed of 82 wpm at B to 86 at T3 and 101 at T4 (Figure 2B). Post hoc paired T-tests revealed no improvements between B and T3 (after StD therapy) B-T3, t (7) = 1.93, P = 0.094; however, comparisons between baseline and T4, and T3 and T4 revealed improvements post MT treatment that survive a Bonferroni correction for multiple comparisons (P 0.05/3 = 0.017): B-T4, t (7) = 6.20, P < 0.001; T3-T4, t (7) = 3.74, P = 0.007.
Post-rehabilitation effects (T4-T5)
Reading times for both groups decreased after therapy stopped, but not significantly for group 2 (Figure 2C): reduction in mean at T4 from 101 wpm to 97 at T5, t (7) = 1.43, P = 0.196; and at marginal significance for group 1, reduction in mean at T4 from 118 wpm to 107 at T5, t (10) = 2.17, P = 0.055.
Single word reading speeds
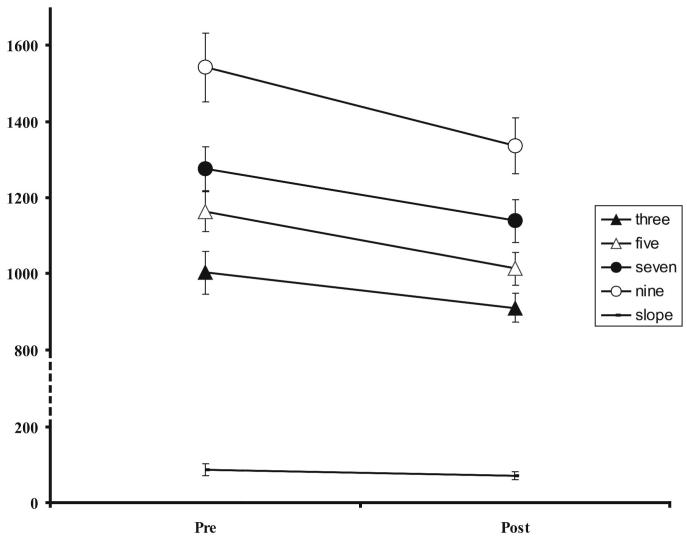
As there was no significant group by word-length interactions, data for both groups were pooled. There was no significant effect of therapy on the single word reading slopes (a planned analysis); however, patients read single words of all lengths (three, five, seven and nine letters long) significantly quicker post rehabilitation, although some of these effects are statistically marginal if a Bonferroni correction is made for multiple-comparisons (Figure 5 and Table 2 for means, t scores and associated P values).
Figure 5.
Average single word reading times and reading slopes pre and post rehabilitation with associated SEM (error bars). Y-axis is in milliseconds, note truncated scale. NB: data collapsed across both groups.
Table 2.
Mean single word reading speeds (across both treatment groups) for each class of letter length and reading slopes (ms per letter) pre and post rehabilitation. The analysis for the effect on slope was planned; the other four were not. A Bonferroni correction for these four multiple comparisons would place the cut-off for significance at 0.05/4 or P = 0.0125. All comparisons are significant at an uncorrected level
| Word length | Mean Pre (ms) | Mean Post (ms) | t(18) | P |
|---|---|---|---|---|
| 3 | 1002 | 909 | 2.57 | 0.019 |
| 5 | 1163 | 1012 | 5.02 | <0.001 |
| 7 | 1277 | 1139 | 2.57 | 0.019 |
| 9 | 1542 | 1336 | 2.63 | 0.017 |
|
| ||||
| Slope | 87 | 70 | 1.21 | 0.241 |
Eye movement data
Patients read short text passages silently while their eye movements were recorded. Three spatial and four temporal measures were analysed. Effects of time were tested across all therapy blocks (B, T3 and T4). Group by time interactions were tested both across all therapy blocks (B, T3 and T4), and over the first therapy block (B and T3) as this is the point where both groups should diverge the most, group 2 not having been exposed to MT at this time point.
Spatial characteristics
A. Saccadic amplitudes (progressive and regressive)
Comparison was made on the effect of therapy between progressive (rightward) and regressive (leftward) saccades. Both groups increased the size of their progressive saccades with a main effect of time: F(2,34) = 15.93, P < 0.001. Again there was also a group by time interaction at both T3: F(1,17) = 7.38, P = 0.015, and T4: F(2,34) = 4.46, P = 0.016, consistent with the therapeutic effect coinciding with the MT blocks rather than the StD block (Figure 3A). There was no main effect of time on regressive saccades: F(2,34) = 0.84, P = 0.44 (Figure 3B).
Figure 3.
Eye movement data: spatial characteristics. Average spatial saccadic measures across three time points (B, T3 and T4/T5) for both group 1 (filled circles) and group 2 (filled squares), and associated SEM (error bars) for the following reading measures: progressive (rightward) saccadic amplitude (A); regressive (leftward) saccadic amplitude (B); incoming saccadic amplitude (C). Y-axis shows amplitude in number of pixels. The average word-box widths for the 3-7 letter words in the materials were 93, 120, 142, 171, and 198 pixels respectively, and the average letter width was 25 pixels.
B. ‘Incoming’ saccadic amplitude
The ‘incoming’ saccade is a subgroup of all rightward saccades; it is the amplitude of the saccade that produces the first fixation within a word (whether launched from the preceding word or a previous word if the preceding word was not directly fixated). Once more there was both a main effect of time across both groups: F(2,34) = 7.87, P = 0.002, and a group by time interaction which was marginal at T3: F(1,17) = 3.98, P = 0.062, and significant at T4: F(2,34) = 3.88, P = 0.03 (Figure 3C).
C. Landing position
There was a non-significant trend for initial landing position to increase with time across both groups (the proportion-through-word measure included the preceding space): F(2,34) = 2.41, P = 0.105 (E-Table 2). On average, patients’ initial fixations were made 39% through a word at B, increasing to 42% at T4. This translated to a change from a fixation point just in front of the centre of the second letter to a point just behind it in a five-letter word.
Temporal characteristics
Patients in both groups improved over the course of the therapy as judged by the number of fixations per 100 words, with a main effect of time across both groups: F(2,34) = 29.78, P < 0.001, with a group by time interaction at both T3: F(1,17) = 33.45, P < 0.001, and T4: F(2,34) = 17.94, P < 0.001, consistent with the therapeutic effect coinciding with the MT blocks rather than the StD block (Figure 4A). This same effect was seen in the refixation proportion rates (the proportion of cases where a fixated word receives subsequent fixation(s) before the eye exits the word, for words of three to seven letters only), although the group by time interaction was of borderline significance at T4. Main effect of time: F (2,34) = 4.40, P =. 02; group by time interaction at T3: F (1,17) = 7.80, P = 0.013, and T4: F (2,34) = 2.76, P = 0.078 (Figure 4B).. There was no effect of therapy on mean fixation durations, although group 1 consistently had shorter fixation times than group 2: effect of group F (1,17) = 7.54, P = 0.014 (Figure 4C).
Figure 4.
Eye movement data: temporal characteristics. Average temporal saccadic measures across three time points (B, T3 and T4/T5) for both group 1 (filled circles) and group 2 (filled squares), and associated SEM (error bars) for the following reading measures: mean fixation rate, Y-axis = fixations per 100 words (A); refixation proportion, Y-axis = proportion through word (B); mean fixation duration, Y-axis = time in ms (C).
Analysis of perimetry
Only one of the 19 patients’ visual fields changed between B and T4, from 0 to 2 degrees of sparing. There was no change across the group: Wilcoxon Signed Ranks Test, Z -1.00, P = 0.317.
Discussion
A variety of interventional studies have attempted to treat acquired hemianopia by improving oculomotor behaviour on a series of visual search tasks or navigation through the environment,17 with only two studies focusing on HA.4, 8 However, this is the first study to attempt to control for the oculomotor effects of therapy by using a ‘sham’ therapy that induces eye movements.
Both patient groups showed significant improvements in their reading speeds and associated reading eye movements. Crucially, no significant improvements in these variables occurred in the first block of group 2 (the StD block), only after the MT therapy in both groups, suggesting that the therapeutic effect depended on this particular intervention. Both groups increased their text reading speeds by a similar amount over both blocks and because group 1 improved more in the second block compared to the first, as did group 2, there was no group by time interaction on this variable; however, when analyzed separately, the superior effect of MT versus StD therapy becomes clear. The StD therapy produced a non-significant 5% improvement versus baseline, the effect of the MT therapy versus the previous time point was 8% and 10% in group 1 ( = 18% overall), and 18% in the second block for group 2 (Figure 2C). Given that there were no significant differences in time-on-therapy both across and within groups, it is not clear why the magnitude of this effect was so pronounced in the second block of group 2. We used spot-the-difference as a sham task to attempt to control for subjects making saccades into their blind field, although the nature and size of saccades made during these tasks is likely to be different (involuntary vs. voluntary, smaller vs. larger). To systematically control for volitional (static vs. OKN), directional (L vs. R) and text vs. non-text elements would have required a fully-balanced design with eight rather than two groups; clearly, further studies are required to investigate which of these properties the therapeutic effect of moving text depends on.
The magnitude of improvement attributable to MT therapy in both groups (18% on average) is modest compared to that reported in two previous studies that used a very similar therapeutic task, 54% in the study by Kerkhoff et al. and 38% in the study by Zihl (both values calculated from improvements in mean reading times).4, 8 This may be due to a variety of demographic and methodological differences between the studies, but the most important one is probably time since infarct. In the Zihl study the subjects were in the post-acute phase after their stroke with the mean time since infarct being only 6 weeks; in the Kerkhoff study there was more of a range (3-220 weeks), with an average of 40 weeks; in contrast, the patients in our study ranged from 12-268 weeks (this is with the outlier who had their head injury aged five removed) with a mean of 96 weeks. Another important difference is the duration of therapy, which was 15 hours on average in our study but was open-ended in the Kerkhoff study, “[until] a performance plateau... was reached or when reading performance reached normal cut-off values.” although it is made clear that significant effects emerged after nine hours of total therapy on average. The Zihl study had a similar duration of therapy as our own, ~ 15 hours.
Regarding the eye movement data, the results of the temporal analyses mirror those seen in the text reading analysis, with effects of the group by time analysis most prominent at T3 where the groups would be expected to diverge maximally before group 2 received their MT therapy; patients read quicker, made fewer fixations per word and were less likely to make refixations within a word. This latter variable is probably the single most important cause of slowed reading in patients with HA.1 The improvement in the number of fixations did not come at a cost of increasing fixation times; however, neither did the fixation times decrease, as Zihl reported.4 The absence of a reduction in fixation time is evidence against the hypothesis that patients read faster post-therapy because their ‘central’ reading processes have been rehabilitated.
The therapy-related improvements in the spatial characteristics of patients’ eye movements are also interesting as they suggest a direction specific effect of the moving text on subsequent reading saccades into the blind field (i.e.: post-therapy voluntary saccades are positively influenced by the direction of the involuntary saccadic component of the OKN). Our initial hypothesis was that the horizontally scrolling text therapy would have an effect on the initial landing position within a word, driving it to the right so that more of the word would be appreciated by intact left foveal/parafoveal vision. Although there was a non-significant trend suggesting that this may be the case, the effect was small (E-Table 2). What seems to be happening is that the patients are making larger amplitude rightward saccades across text in general, this in turn leads to them not having to fixate as close to the end of a word before identifying it, so the launching site for their saccade onto the ensuing word tends to be closer to the beginning of a word then it was pre-therapy. The result of this is that the initial landing position does not change much, but the ‘incoming’ saccade increases in amplitude (Figure 3C). This direction-dependant result is different from that in the only other study to examine the effects of scrolling text on HAs reading eye movements.4 Zihl found that patients with a right-sided hemianopia and HA improved their saccadic amplitudes in both the right and left directions (magnitude ~17% for rightward saccades of 300ms duration, estimated from Figure 11D; no values given or estimable for leftward saccadic amplitudes). While we found a similar size of effect, 18% for group 1 and 12% increase for group 2, our results are clearly lateralized. This direction specific effect requires more investigation as we did not test the effects of inducing OKN in the opposite lateral direction.
Patients also read single words faster after rehabilitation and this could be one explanation as to why their text reading speeds improved. Patients with HA do have a word length effect not seen in normal subjects, although the resulting single word reading slopes are much shallower than those measured in patients with pure alexia.18 We found a non-significant decrease in the value of this slope, averaging 17 milliseconds per letter across both groups. We screened our patients for pure alexia (as patients with pure alexia often have an associated right-sided hemianiopia),18 and found no evidence for this condition in any of them, but we cannot completely eliminate the possibility that some had a mild ‘pure’ component to their reading disorder that we may have ‘inadvertently’ rehabilitated. There are two reasons why we think this is unlikely: firstly, despite speeding up on reading words of all lengths there was no significant effect on patients’ reading slopes, improving this measure has been considered a hallmark of inducing a therapeutic effect in patients with pure alexia.19 Second, there was no effect of therapy on decreasing fixation times, these are considerably longer for subjects with pure alexia.20
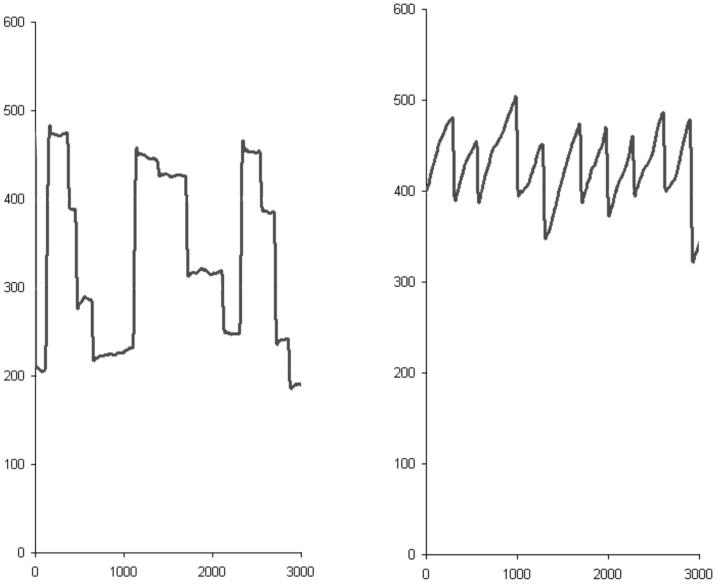
One final point that merits discussion, although it cannot be resolved by the data presented here, is how OKN-induced involuntary saccades may improve subsequent voluntary reading saccades. “The function of optokinetic nystagmus is thought to be the stabilisation of the eyes relative to space during slow head movements in the low frequency range”,21 although OKN is equally elicited by motion of the visual scene relative to the observer, such as when a subject views a nearby train pulling away from the platform. Our scrolling text stimuli produced what is more properly called small-field OKN, that is, the eye movement pattern associated with full-field OKN but without the vestibular component that causes a sensation of apparent self-motion.22 When a normal reader viewed the scrolling text, the mean amplitude of the saccadic component of the induced OKN was of a similar value to that when they viewed static text (Figure 6). Patients with HA make smaller amplitude rightward reading saccades than normal subjects but would be expected to have a similar mean amplitude for the saccadic component of their induced OKN as normal subjects, although this was not directly measured in our study. The OKN induced saccades of the HA patients are likely to have been of a larger amplitude than those produced by reading static text, and may have influenced the amplitude of post-therapy voluntary reading saccades in the same direction.
Figure 6.
Eye movements recorded from a normal subject reading both static text (left panel) and scrolling text (right panel). The static text consisted of three rows of four words that did not make a sentence in order to force the reader to fixate each word. The OKN inducing scrolling text also consisted of 12 words; the rate of motion was set to match the time it took to read the static text (3000 milliseconds, x-axis). The y-axis is in pixels. Declining values = eye movement to the right, ascending values = eye movement to the left. Note that the OKN induced saccades are of more uniform amplitude than the voluntary ones (near-vertical components of both scanpath traces).
The neural components that mediate saccadic eye movements are segregated throughout a large number of cortical and subcortical regions which contain at least 30 distinct saccade related cell classes.23 Evidence from ablation, stimulation, lesion and functional imaging studies suggest that the cortical and mesencephalic brain regions involved in the generation of different types of saccades are largely shared, although they differ in their specific response patterns. These areas descend onto pontine nuclei which are at the head of the final common motor output pathway to the lower motor neurons that produce horizontal eye movements.23, 24 The degree of anatomical and functional connectivity between cortical and sub-cortical regions that control different types of saccadic eye movement allow for one type of saccadic eye movement to potentially influence another, especially as the dynamic properties of the quick phase of OKN are similar to those of voluntary saccades.25
Acknowledgements
The authors would like to thank Ms Bronia Unwin for performing visual field perimetry on all the patients, Dr Nat Upton for his help with generating the rehabilitation materials and testing the index case. This work was funded by The Wellcome Trust (project grant no: GR063064AIA).
Footnotes
Disclosure: The authors report no conflicts of interest.
Website
Rehabilitation materials similar to the ones used in this study are available for use for patients with hemianopic alexia on a free access website. The site also contains diagnostic testing materials and reading tests to gauge the effects of therapy: http://www.ucl.ac.uk/mediares/mm/mm-projects/neurology/read_right/
Terms: Clinical trial [19], Rehabilitation [242], ‘Dyslexia’ Alexia preferred [315], ‘visual fields’ hemianopia preferred [196].
References
- 1.McDonald SA, Spitsyna G, Shillcock RC, Wise RJ, Leff AP. Patients with hemianopic alexia adopt an inefficient eye movement strategy when reading text. Brain. 2006;129:158–167. doi: 10.1093/brain/awh678. [DOI] [PubMed] [Google Scholar]
- 2.De Luca M, Spinelli D, Zoccolotti P. Eye movement patterns in reading as a function of visual field defects and contrast sensitivity loss. Cortex. 1996;32:491–502. doi: 10.1016/s0010-9452(96)80006-2. [DOI] [PubMed] [Google Scholar]
- 3.Rayner K, Inhoff AW, Morrison RE, Slowiaczek ML, Bertera JH. Masking of foveal and parafoveal vision during eye fixations in reading. J Exp Psychol Hum Percept Perform. 1981;7:167–179. doi: 10.1037//0096-1523.7.1.167. [DOI] [PubMed] [Google Scholar]
- 4.Zihl J. Eye movement patterns in hemianopic dyslexia. Brain. 1995;118:891–912. doi: 10.1093/brain/118.4.891. [DOI] [PubMed] [Google Scholar]
- 5.Leff AP, Scott SK, Crewes H, et al. Impaired reading in patients with right hemianopia. Ann Neurol. 2000;47:171–178. [PubMed] [Google Scholar]
- 6.Bouma H, de Voogd AH. On the control of eye saccades in reading. Vision Res. 1974;14:273–284. doi: 10.1016/0042-6989(74)90077-7. [DOI] [PubMed] [Google Scholar]
- 7.Kang TJ, Muter P. Reading dynamically displayed text. Behav Inf Technol. 1989;8:33–42. [Google Scholar]
- 8.Kerkhoff G, Munsinger U, Eberle-Strauss G, Stogerer E. Rehabilitation of hemianopic alexia in patients with postgeniculate visual field disorders. Neuropsychol Rehabil. 1992;2:21–41. [Google Scholar]
- 9.Rayner K. Eye guidance in reading: fixation locations within words. Perception. 1979;8:21–30. doi: 10.1068/p080021. [DOI] [PubMed] [Google Scholar]
- 10.Altman DG, Bland JM. Treatment allocation by minimisation. BMJ. 2005;330:843. doi: 10.1136/bmj.330.7495.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio: Harcourt Brace & Company; 1999. [Google Scholar]
- 12.Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St Edmunds: Thames Valley Test Company; 1991. [Google Scholar]
- 13.Baxter DM, Warrington EK. Measuring dysgraphia: a graded-difficulty spelling test. Behav Neurol. 1994;7:107–116. doi: 10.3233/BEN-1994-73-401. [DOI] [PubMed] [Google Scholar]
- 14.Warrington EK. Recognition Memory Test. Windsor: NFER Nelson; 1984. [Google Scholar]
- 15.Neale MD. Neale analysis of reading ability. Oxford, UK: NFER-Nelson; 1989. [Google Scholar]
- 16.Coltheart M. The MRC psycholinguistic database. Q J Exp Psychol A. 1981;33A:497–505. [Google Scholar]
- 17.Pambakian A, Currie J, Kennard C. Rehabilitation strategies for patients with homonymous visual field defects. J Neuroophthalmol. 2005;25:136–142. [PubMed] [Google Scholar]
- 18.Leff AP, Crewes H, Plant GT, Scott SK, Kennard C, Wise RJ. The functional anatomy of single-word reading in patients with hemianopic and pure alexia. Brain. 2001;124:510–521. doi: 10.1093/brain/124.3.510. [DOI] [PubMed] [Google Scholar]
- 19.Arguin M, Bub DN. Pure alexia: attempted rehabilitation and its implications for interpretation of the deficit. Brain Lang. 1994;47:233–268. doi: 10.1006/brln.1994.1051. [DOI] [PubMed] [Google Scholar]
- 20.Behrmann M, Shomstein SS, Black SE, Barton JJ. The eye movements of pure alexic patients during reading and nonreading tasks. Neuropsychologia. 2001;39:983–1002. doi: 10.1016/s0028-3932(01)00021-5. [DOI] [PubMed] [Google Scholar]
- 21.Davies R. Bedside neuro-otological examination and interpretation of commonly used investigations. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 4):iv32–44. doi: 10.1136/jnnp.2004.054478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandt T, Dichgans J, Koenig E. Differential effects of central verses peripheral vision on egocentric and exocentric motion perception. Exp Brain Res. 1973;16:476–491. doi: 10.1007/BF00234474. [DOI] [PubMed] [Google Scholar]
- 23.Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- 24.Buttner-Ennever JA, Horn AK. Anatomical substrates of oculomotor control. Curr Opin Neurobiol. 1997;7:872–879. doi: 10.1016/s0959-4388(97)80149-3. [DOI] [PubMed] [Google Scholar]
- 25.Garbutt S, Han Y, Kumar AN, Harwood M, Harris CM, Leigh RJ. Vertical optokinetic nystagmus and saccades in normal human subjects. Invest Ophthalmol Vis Sci. 2003;44:3833–3841. doi: 10.1167/iovs.03-0066. [DOI] [PubMed] [Google Scholar]