Abstract
Helicobacter pylori genomes contain about 30 hop genes that encode outer membrane proteins. H. pylori hopQ alleles exhibit a high level of genetic diversity, and two families of hopQ alleles have been described. Type I hopQ alleles are found more commonly in cag-positive H. pylori strains from patients with peptic ulcer disease than in cag-negative strains from patients without ulcer disease. In this study, we mutated hopQ in four H. pylori strains that each contained a type I hopQ allele, and then analyzed interactions of the wild-type and hopQ mutant strains with AGS cells. In comparison to the wild-type strains, two of the hopQ mutant strains exhibited increased adherence to AGS cells and two hopQ mutants did not exhibit any detectable differences in adherence. Higher levels of tyrosine-phosphorylated CagA were detected when AGS cells were co-cultured with a hyper-adherent hopQ mutant strain than when co-cultured with the corresponding wild-type strain. These data indicate that in some strains of H. pylori, the HopQ protein can attenuate bacterial adherence to gastric epithelial cells.
Keywords: Helicobacter pylori, HopQ, adherence, CagA
INTRODUCTION
Persistent colonization of the human stomach with H. pylori is a risk factor for peptic ulceration and adenocarcinoma. H. pylori genomes contain about 30 paralogous hop genes, which encode outer membrane proteins (Alm et al., 2000). Several Hop proteins mediate adherence of H. pylori to gastric epithelial cells. For example, BabA and SabA bind to fucosylated-Lewis B (Aspholm-Hurtig et al., 2004; Ilver et al., 1998) and sialyl-Lewis X antigens (Mahdavi et al., 2002), respectively, on the surface of gastric epithelial cells. The cell-surface receptors for other H. pylori Hop proteins with adhesive properties [including HopH (OipA) (Dossumbekova et al., 2006), HopC (AlpA), HopB (AlpB) (Odenbreit et al., 1999), and HopZ (Peck et al., 1999)] have not yet been identified. Several Hop proteins (e.g. HopV, HopW, HopX, and HopY) have been reported to function as porins (Alm et al., 2000; Peck et al., 2001).
One outer membrane protein, HopQ (omp27), is of interest because two highly divergent families of hopQ alleles have been identified (Cao & Cover, 2002). Type I hopQ alleles are found most commonly in H. pylori strains that contain the cag pathogenicity island (PAI), which encodes CagA and a type IV secretion system involved in the translocation of CagA into gastric epithelial cells (Backert & Selbach, 2008). CagA causes numerous alterations in gastric epithelial cells, including changes in cell morphology, increased cell scattering, disruption of tight junctions, and activation of β-catenin (Backert & Selbach, 2008; Hatakeyama, 2004). Several studies have reported an association between the presence of type I hopQ alleles and other H. pylori virulence markers, including type s1 vacA alleles (Cao & Cover, 2002; Lehours et al., 2004). Type II hopQ alleles are found more commonly in cag PAI-negative H. pylori strains than in cag PAI-positive strains (Cao & Cover, 2002). Geographical differences in the distribution of type I and type II hopQ alleles have been noted. For example, most H. pylori strains isolated in East Asia are cag PAI-positive and contain Type I hopQ alleles (Cao et al., 2005). Type II alleles are commonly found in H. pylori strains isolated in Western countries, but are uncommon among H. pylori strains from East Asia (Cao et al., 2005).
HopQ is known to be localized to the surface of H. pylori (Sabarth et al., 2005). However, nothing is known about functional properties of HopQ. Because HopQ is an outer membrane protein, we hypothesized that it might have a role in H. pylori adherence to gastric epithelial cells. In addition, because type I hopQ alleles are found predominantly in H. pylori strains containing the cag PAI, we hypothesized that HopQ might modulate one or more cag PAI-associated phenotypes. In this study, we selected four H. pylori strains containing type I hopQ alleles for analysis and generated isogenic hopQ mutant strains. We then analyzed properties of the hopQ mutant strains in comparison to the parental wild-type strains.
Materials and Methods
Bacterial strains
H. pylori strains 26695 SC#7 (Busler et al., 2006), J99 (Alm et al., 1999), J178 and 87-29 were used in this study. Each of these strains contains a type I hopQ allele (Cao & Cover, 2002). We also used a 26695 SC#7 Δcag PAI mutant strain, which lacks the entire cag PAI (Busler et al., 2006). For routine growth, H. pylori were maintained on trypticase soy agar plates containing 5% sheep blood. Sulfite-free Brucella broth containing 5% FBS (i.e. BB-FBS) was used for growth of H. pylori liquid cultures, and broth cultures were grown on a rotary shaker (150 rpm). The composition of the broth culture medium was modified from the original description by the use of Bacto proteose peptone instead of peptamin (Loh et al., 2007). Brucella-agar plates containing chloramphenicol 5 μg mL−1 were used for natural transformation experiments. All H. pylori cultures were grown at 37oC in room air supplemented with 5% CO2.
Plasmid construction and mutagenesis
The hopQ gene was amplified from H. pylori strain 26695 using primers BA7676 (5’ –AACGCTAAGGCTTTATGCTTATGCTC-3’) and AND2288 (5’-TTGTAATCAAAGAAACCATAA-3’) and cloned into a pGEM-T plasmid (Promega). A GPS-LS linker-scanning system (NE Biolabs) was then used to insert a unique PmeI site into the hopQ coding sequence via a transposon. This plasmid was digested with PmeI, and a chloramphenicol resistance (cat) cassette (Wang & Taylor, 1990) was ligated into the PmeI site. The resultant plasmid was used to transform H. pylori strains 26695 SC#7, J99, J178 and 87-29 and transformants were selected on Brucella agar plates supplemented with chloramphenicol (5 μg mL−1). Proper insertion of the cat cassette into the H. pylori chromosomal hopQ gene was confirmed by PCR analyses.
Analysis of H. pylori adherence to gastric epithelial cells
The human gastric adenocarcinoma cell line (AGS) was maintained in RPMI 1640 medium (GIBCO BRL) containing 10% FBS and grown at 37oC in 5% CO2. The cells were routinely subcultured every 2–3 days. An adherence assay modified from that described (Dossumbekova et al., 2006) was used to analyze H. pylori binding to AGS cells. Briefly, AGS cells were seeded into wells of a 24-well plate and grown overnight, prior to the addition of H. pylori. H. pylori were cultured in overnight in broth, and the number of H. pylori organisms added to cells was standardized based on measurements of optical density (OD600 of 1.0 = 109 CFU/ml). When serial dilutions of standardized H. pylori cultures were plated on solid media, there were no significant differences in the number of CFU/ml detected for wild-type and hopQ mutant strains. H. pylori were cocultured with AGS cells at various multiplicities of infection (MOI) for 1 h or 5 h at 37oC with gentle shaking (50 rpm) on an orbital shaker, and non-adherent bacteria were then removed by washing using PBS buffer. Bound cells were fixed with 4% paraformaldehyde for 15 min, followed by blocking in 10% normal goat serum for 1 h. After washing with PBS, the cells were incubated for 1 h with primary rabbit antiserum (1:500) generated against H. pylori soluble proteins (Cao et al., 1998). Following 3 washes in PBS, a cy3-conjugated anti-rabbit antibody (1:500, Sigma) was then added for 1 h. Cells were washed 3 times with PBS, and then were incubated for 15 min with trypsin (0.5 mg mL−1) to detach the cells, or treated with cyQUANT cell lysis buffer (Invitrogen), or 0.1% saponin in PBS to lyse the cells. The detached or lysed samples were removed, transferred to a 96-well assay plate (Corning), and fluorescence measured in a FLx800 microplate reader (BioTek).
As another approach to analyze H. pylori binding to AGS cells, H. pylori and AGS cells were co-cultured for 5 h at 37oC with gentle shaking as described above. The wells were washed 3 times with RPMI media to remove any unbound bacteria. The cells were then incubated in PBS containing 0.1% saponin (37oC, 15 min) (Kwok et al., 2002) and serial dilutions of the samples were cultured on blood agar plates. Colony forming units were enumerated 5 days after plating.
Immunoblot analysis of CagA expression
Broth cultures of H. pylori were grown overnight and inoculated into fresh brucella broth for 12 h before being harvested and lysed with NP-40 lysis buffer [150 mmol L−1 NaCl, 1% NP40, 50 mmol L−1 Tris-HCl (pH 8.0)] supplemented with protease inhibitors (Complete Mini; Roche Applied Science). The protein content of lysates was determined using the BCA reagent kit (Pierce). Following normalization of protein content, the proteins were separated using SDS-PAGE, and transferred to nitrocellulose membranes (Biorad). The membranes were blocked for 2 h in TBS-Tween (TTBS; Tris buffered saline containing 0.1% Tween 20) containing 3% milk, and subsequently probed with either polyclonal antiserum against CagA (1:6000 dilution, Austral Biologicals) or antiserum against H. pylori soluble proteins (1:10,000 dilution) (Cao et al., 1998). Following a 1 h incubation, the blots were washed with TTBS, and then incubated for 1 h with a HRP-conjugated goat anti-rabbit antibody. Labeled bands were visualized on X-ray film using chemiluminescent techniques, according to the manufacturer’s instructions (ECL Western Blotting Detection reagent, Amersham Bioscience). When reprobing of membranes was required, blots were incubated in Restore stripping buffer (Pierce) prior to the addition of a new primary antibody.
Analysis of CagA tyrosine phosphorylation
H. pylori were cultured in broth as described above and then co-cultured with AGS cells, using a 50:1 multiplicity of infection. Following a 5 h coculture, the cells were washed 3 times with ice-cold PBS, and then lysed with NP-40 lysis buffer containing protease inhibitors (Roche) and 2 mmol L−1 sodium orthovanadate. The protein concentration of each lysate was determined (BCA reagent kit, Pierce), and all samples were normalized for protein content. To detect tyrosine phosphorylated CagA, 10 μg of each sample was subjected to SDS-PAGE and western blot analyses using a 1: 6000 dilution of a mouse monoclonal anti-phosphotyrosine antibody (4G10) (Upstate Biotechnology). Following a 5 h incubation, the membranes were washed with TTBS and incubated for an additional 1 h with HRP-conjugated sheep anti-mouse IgG (1:10,000, Amersham Biosciences). Immunoreactive bands were then visualized using chemiluminescent techniques. Membranes were also probed with a polyclonal antibody against H. pylori CagA (1:6000, Santa Cruz), antiserum against H. pylori soluble proteins (anti-HP; 1:10,000) (Cao et al., 1998), and a goat anti-actin antibody (1:10,000, Santa Cruz). Secondary antibodies included a 1:6000 dilution of HRP-conjugated goat anti-rabbit IgG (for CagA and H. pylori soluble proteins), and a 1:10,000 dilution of HRP-conjugated anti-goat IgG (for actin; Santa Cruz). When reprobing of membranes was required, previously bound antibodies were removed using Restore stripping buffer (Pierce).
Analysis of IL-8 secretion
To monitor IL-8 secretion by AGS cells, supernatants from coculture experiments were clarified by centrifugation at 12,000 g. IL-8 levels in the supernatants were subsequently determined using an IL-8 cytometric bead assay (BD Biosciences), as per the manufacturer’s instructions. The samples were analyzed using a BD LSR II flow cytometer and a high throughput system. IL-8 standards provided with the IL-8 cytometric bead assay were used for determination of IL-8 concentrations. Data analysis was performed using FCAP Array Software v.1.0.1 (BD Bioscience, San Diego, CA).
RESULTS AND DISCUSSION
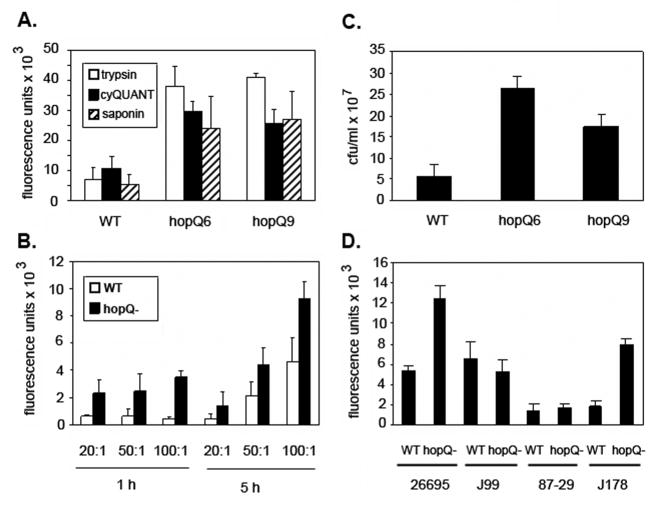
To study functional properties of HopQ, we first generated two independent hopQ mutants (hopQ6 and hopQ9) derived from H. pylori strain 26695 SC#7, as described in the Materials and Methods. To test the hypothesis that HopQ might have a role in the adherence of H. pylori to epithelial cells, we cultured wild-type H. pylori strain 26695 and its isogenic hopQ mutants with AGS cells, and the levels of H. pylori adherence were monitored as described in the Materials and Methods, using a polyclonal rabbit antiserum against H. pylori followed by a cy3-labeled anti-rabbit antibody. These studies revealed that the hopQ mutant strains bound to AGS cells to a significantly greater extent than did the wild-type strain at each of the tested MOI’s (Figure 1A, B). In addition, as shown in Figure 1C, the number of viable hopQ mutant bacteria recovered from these co-culture experiments was significantly higher than the number of wild-type bacteria recovered. We also analyzed the adherence properties of three other wild-type H. pylori strains (J99, J178, and 87-29) and the corresponding hopQ mutants. Similar to strain 26695, each of these strains contains a type I hopQ allele (Cao & Cover, 2002). As shown in Figure 1, a hopQ mutant derived from strain J178 exhibited significantly increased adherence to AGS cells compared to wild-type J178. In contrast, hopQ mutagenesis did not alter the binding of H. pylori strains J99 and 87-29 to AGS cells (Figure 1D). These data provide evidence that the HopQ outer membrane protein modulates H. pylori adherence in a strain-specific manner. Strain-specific variations have previously been detected with several other H. pylori phenotypes (Baldwin et al., 2007; Lee et al., 2006; Pflock et al., 2007; Tan et al., 2005).
Figure 1. Adherence of wild-type H. pylori and hopQ mutant strains to AGS gastric epithelial cells.
A) AGS cells were co-cultured with H. pylori wild-type strain 26695 (WT) or two isogenic hopQ mutant strains (hopQ6 and hopQ9) (MOI 50:1). After coculture for 1 h at 37oC, cells were fixed with paraformaldehyde and blocked with 10% goat serum. Cells were then incubated with a polyclonal antibody against H. pylori soluble proteins (1:500), followed by a cy3-labeled anti-rabbit antibody (1:500 dilution). The samples were treated with either 0.5 mg mL−1 trypsin, cyQUANT lysis buffer, or 0.1% saponin, and fluorescence was quantified using a FLx800 microplate reader (BioTek). B) H. pylori strain 26695 (WT) and an isogenic hopQ mutant strain were cocultured with AGS cells at MOI’s of 20:1, 50:1 and 100:1, and then the co-cultures were incubated with 0.5 mg mL−1 trypsin and analyzed as in Figure 1A. C) Following coculture of wild-type strain 26695 or hopQ mutant strains with AGS cells for 5h at 37o C, the cells were treated with PBS containing saponin (Kwok et al., 2002). Serial dilutions of samples were then plated on blood agar plates and colony forming units were enumerated 5 days after plating. D) H. pylori strains 26695, J99, 87-29, J178 and isogenic hopQ mutant strains were cocultured with AGS cells for 1 h. Fluorescent-based adherence assays were then performed as described above. For each of the panels, the results represent mean and standard deviation based on triplicate samples from a single experiment. All experiments were performed three times with similar results. Statistical significance was analyzed using Student’s t-test.
Because type I hopQ alleles are found predominantly in H. pylori strains containing the cag PAI, we hypothesized that HopQ might modulate one or more cag PAI-associated phenotypes. As a first step, we tested whether a wild-type H. pylori strain and a hopQ mutant strain differed in the expression of CagA. For these studies we analyzed H. pylori strain 26695 SC#7, which is known to translocate CagA into gastric epithelial cells (Busler et al., 2006), and an isogenic hopQ mutant. H. pylori strains were cultured in broth, and CagA expression was assessed by immunoblotting. As shown in Figure 2A, wild-type H. pylori strain 26695 and an isogenic hopQ mutant did not differ in CagA expression.
Figure 2. Analysis of CagA expression and CagA tyrosine phosphorylation.
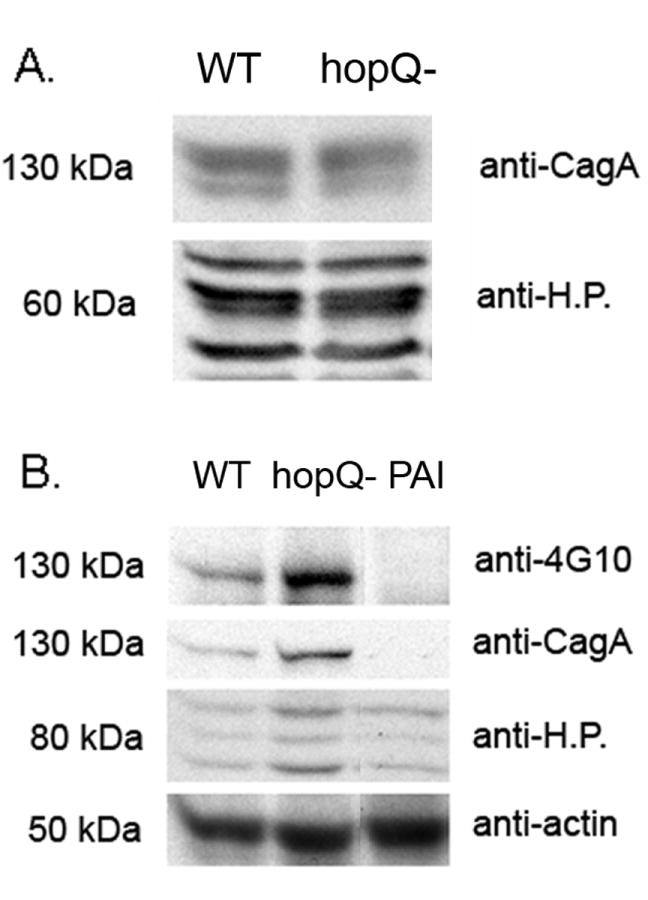
A) Immunoblot analysis of CagA expression in H. pylori strain 26695 (WT) and an isogenic hopQ mutant strain (hopQ-). H. pylori cells were cultured in brucella broth, and then lysed with NP-40 lysis buffer. Standardized quantities of H. pylori lysates (5 μg protein) were analyzed by immunoblotting. Membranes were immunoblotted sequentially with an anti-CagA antibody followed by an antiserum to soluble H. pylori proteins (anti-H.P.), as described in the Methods. B) Comparison of CagA translocation into AGS cells by H. pylori strain 26695 (WT), an isogenic hopQ mutant (hopQ-), or a Δcag PAI mutant strain (PAI). H. pylori cells were cultured in brucella broth and then were added to AGS cells (50:1 MOI) for 5 h. Samples were then washed with PBS, lysed with NP-40 lysis buffer, and standardized quantities of lysates (10 μg protein) were analyzed by immunoblotting. Membranes were sequentially probed with an anti-phosphotyrosine antibody (anti-4G10), anti-CagA, anti-actin, and antiserum to H. pylori proteins (anti-H.P.). The experiments were performed three times with similar results.
To determine whether expression of HopQ has any effect on CagA translocation into gastric epithelial cells, wild-type strain 26695 and an isogenic hopQ mutant strain were co-cultured with AGS cells, and the levels of tyrosine phosphorylated CagA were then analyzed as described in the Methods. As shown in Figure 2B, a higher level of tyrosine-phosphorylated CagA was detected in AGS cells co-cultured with the hopQ mutant than in AGS cells co-cultured with the wild-type strain. Similarly, a higher level of total CagA was detected in samples containing the hopQ mutant strain than in samples containing the wild-type strain. When the blots were reprobed with anti-actin antibody, the levels of actin were similar, indicating that there was minimal variation in the number of AGS cells present within each sample. When the blots were reprobed with an antiserum against soluble H. pylori proteins, immunoreactivity was stronger in samples containing the hopQ mutants than in samples containing the wild-type strain. This result is consistent with the data in Figure 1, showing that hopQ mutants derived from strain 26695 exhibit increased adherence to AGS cells compared to wild-type H. pylori. Increased bacterial adherence presumably facilitates increased translocation of CagA into host cells.
We also investigated the possibility that a hopQ mutation might alter the ability of H. pylori to stimulate IL-8 secretion by AGS cells. Wild-type H. pylori strain 26695 and an isogenic hopQ mutant strain each induced expression of IL-8 (Figure 3), whereas a cag PAI mutant strain did not (data not shown). There was no significant difference in the levels of IL-8 secretion stimulated by the wild-type strain and the hopQ mutant strain (Figure 3). The data shown in Figure 3 suggest that under the conditions of this assay, a sufficient number of adherent wild-type or mutant bacteria were present at each of the tested MOI’s to induce maximal levels of IL-8 induction, and therefore, despite a difference in the adherence of wild-type and hopQ mutant strains, no differences in the IL-8-inducing capacity of wild-type bacteria versus mutant bacteria were detected.
Figure 3. Analysis of IL-8 induction.
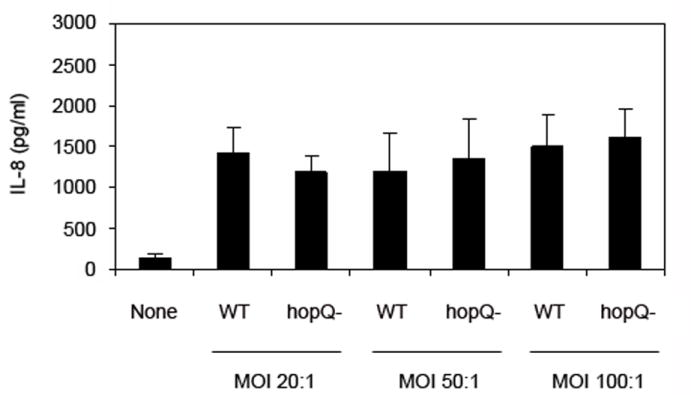
H. pylori wild-type strain 26695 (WT) and a hopQ mutant strain (hopQ-) were cultured in brucella broth and then added to AGS cells at MOI’s of 20:1, 50:1 and 100:1. Control AGS cells (labeled “none”) were maintained in medium alone. Following a 5 h incubation, supernatants were removed and the IL-8 content was quantified as described in the Materials and Methods. The results represent mean and standard deviation based on triplicate samples from a single experiment. No significant differences were detected when comparing the effects of wild-type versus hopQ mutant strains (Student’s t-test). This experiment was performed three times with similar results.
In conclusion, the results of this study indicate that the presence of HopQ modulates the adherence properties of H. pylori in a strain-specific manner. All of the strains tested in the current study contained type I hopQ alleles. Further studies will be required to determine whether type II HopQ proteins exhibit anti-adhesive properties, or whether this phenomenon is limited to type I HopQ proteins. The molecular basis for the observed increased adherence of 26695 and J178 hopQ mutant strains compared to the respective wild-type strains is not yet understood. One possibility is that the expression of HopQ on the bacterial cell surface may sterically block the adhesive properties of another bacterial adhesin. Another possibility is that HopQ is localized within a complex of molecules in the H. pylori outer membrane, and the presence or absence of HopQ may influence the adherence properties of other molecules in the complex. Interestingly, both H. pylori strains J99 and 87-29 are known to bind to express the BabA adhesin and bind Lewis B antigens, whereas strains 26695 and J178 are BabA negative and unable to bind to Lewis B (Hennig et al., 2004; Hennig et al., 2006). Based on this observation, it seems possible that the anti-adhesive properties of HopQ might be most readily detectable in H. pylori strains that lack adhesins such as BabA. As shown in Figure 2, higher levels of tyrosine-phosphorylated CagA were detected when AGS cells were co-cultured with a hyper-adherent hopQ mutant strain than when co-cultured with the corresponding wild-type strain. Therefore, by attenuating H. pylori adhesion to gastric epithelial cells, HopQ may downregulate H. pylori-induced gastric epithelial cell alterations.
Acknowledgments
This work was supported by NIH R01 DK53623, NIH R01 AI068009, and the Department of Veterans Affairs. We thank Ping Cao for technical assistance.
References
- Alm RA, Ling LS, Moir DT, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- Alm RA, Bina J, Andrews BM, Doig P, Hancock RE, Trust TJ. Comparative genomics of Helicobacter pylori: analysis of the outer membrane protein families. Infect Immun. 2000;68:4155–4168. doi: 10.1128/iai.68.7.4155-4168.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspholm-Hurtig M, Dailide G, Lahmann M, et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008 doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- Baldwin DN, Shepherd B, Kraemer P, Hall MK, Sycuro LK, Pinto-Santini DM, Salama NR. Identification of Helicobacter pylori genes that contribute to stomach colonization. Infect Immun. 2007;75:1005–1016. doi: 10.1128/IAI.01176-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busler VJ, Torres VJ, McClain MS, Tirado O, Friedman DB, Cover TL. Protein-protein interactions among Helicobacter pylori cag proteins. J Bacteriol. 2006;188:4787–4800. doi: 10.1128/JB.00066-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, McClain MS, Forsyth MH, Cover TL. Extracellular release of antigenic proteins by Helicobacter pylori. Infect Immun. 1998;66:2984–2986. doi: 10.1128/iai.66.6.2984-2986.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Cover TL. Two different families of hopQ alleles in Helicobacter pylori. J Clin Microbiol. 2002;40:4504–4511. doi: 10.1128/JCM.40.12.4504-4511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Lee KJ, Blaser MJ, Cover TL. Analysis of hopQ alleles in East Asian and Western strains of Helicobacter pylori. FEMS Microbiol Lett. 2005;251:37–43. doi: 10.1016/j.femsle.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Dossumbekova A, Prinz C, Mages J, et al. Helicobacter pylori HopH (OipA) and Bacterial Pathogenicity: Genetic and Functional Genomic Analysis of hopH Gene Polymorphisms. J Infect Dis. 2006;194:1346–1355. doi: 10.1086/508426. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- Hennig EE, Mernaugh R, Edl J, Cao P, Cover TL. Heterogeneity among Helicobacter pylori strains in expression of the outer membrane protein BabA. Infect Immun. 2004;72:3429–3435. doi: 10.1128/IAI.72.6.3429-3435.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig EE, Allen JM, Cover TL. Multiple chromosomal loci for the babA gene in Helicobacter pylori. Infect Immun. 2006;74:3046–3051. doi: 10.1128/IAI.74.5.3046-3051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Boren T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- Kwok T, Backert S, Schwarz H, Berger J, Meyer TF. Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipper-like mechanism. Infect Immun. 2002;70:2108–2120. doi: 10.1128/IAI.70.4.2108-2120.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WK, Ogura K, Loh JT, Cover TL, Berg DE. Quantitative effect of luxS gene inactivation on the fitness of Helicobacter pylori. Appl Environ Microbiol. 2006;72:6615–6622. doi: 10.1128/AEM.01291-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehours P, Menard A, Dupouy S, Bergey B, Richy F, Zerbib F, Ruskone-Fourmestraux A, Delchier JC, Megraud F. Evaluation of the association of nine Helicobacter pylori virulence factors with strains involved in low-grade gastric mucosa-associated lymphoid tissue lymphoma. Infect Immun. 2004;72:880–888. doi: 10.1128/IAI.72.2.880-888.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709–4715. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]
- Mahdavi J, Sonden B, Hurtig M, et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenbreit S, Till M, Hofreuter D, Faller G, Haas R. Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol Microbiol. 1999;31:1537–1548. doi: 10.1046/j.1365-2958.1999.01300.x. [DOI] [PubMed] [Google Scholar]
- Peck B, Ortkamp M, Diehl KD, Hundt E, Knapp B. Conservation, localization and expression of HopZ, a protein involved in adhesion of Helicobacter pylori. Nucleic Acids Res. 1999;27:3325–3333. doi: 10.1093/nar/27.16.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck B, Ortkamp M, Nau U, Niederweis M, Hundt E, Knapp B. Characterization of four members of a multigene family encoding outer membrane proteins of Helicobacter pylori and their potential for vaccination. Microbes Infect. 2001;3:171–179. doi: 10.1016/s1286-4579(01)01377-6. [DOI] [PubMed] [Google Scholar]
- Pflock M, Muller S, Beier D. The CrdRS (HP1365–HP1364) two-component system is not involved in ph-responsive gene regulation in the Helicobacter pylori Strains 26695 and G27. Curr Microbiol. 2007;54:320–324. doi: 10.1007/s00284-006-0520-9. [DOI] [PubMed] [Google Scholar]
- Sabarth N, Hurvitz R, Schmidt M, Zimny-Arndt U, Jungblut PR, Meyer TF, Bumann D. Identification of Helicobacter pylori surface proteins by selective proteinase K digestion and antibody phage display. J Microbiol Methods. 2005;62:345–349. doi: 10.1016/j.mimet.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Tan S, Fraley CD, Zhang M, Dailidiene D, Kornberg A, Berg DE. Diverse phenotypes resulting from polyphosphate kinase gene (ppk1) inactivation in different strains of Helicobacter pylori. J Bacteriol. 2005;187:7687–7695. doi: 10.1128/JB.187.22.7687-7695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Taylor DE. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene. 1990;94:23–28. doi: 10.1016/0378-1119(90)90463-2. [DOI] [PubMed] [Google Scholar]