Abstract
Cigarette smoke is a complex mixture consisting of more than 4500 chemicals, including several tobacco-specific nitrosamines (TSNA). TSNA typically form in tobacco during the post-harvest period, with some fraction being transferred into mainstream smoke when a cigarette is burned during use. The most studied of the TSNA is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). NNK has been shown to be carcinogenic in laboratory animals. Studies examining the carcinogenicity of NNK frequently are conducted by injecting rodents with a single dose of 2.5 to 10 μmol of pure NNK; the amount of NNK contained in all of the mainstream smoke from about 3700 to 14,800 typical U.S. cigarettes. Extrapolated to a 70-kg smoker, the carcinogenic dose of pure NNK administered to rodents would be equivalent to the amount of NNK in all of the mainstream smoke of 22 to 87 million typical U.S. cigarettes. Furthermore, extrapolating results from rodent studies based on a single injection of pure NNK to establish a causative role for NNK in the carcinogenicity of chronic tobacco smoke exposure in humans is not consistent with basic pharmacological and toxicological principles. For example, such an approach fails to consider the effect of other smoke constituents upon the toxicity of NNK. In vitro studies demonstrate that nicotine, cotinine, and aqueous cigarette “tar” extract (ACTE) all inhibit the mutagenic activity of NNK. In vivo studies reveal that the formation of pulmonary DNA adducts in mice injected with NNK is inhibited by the administration of cotinine and mainstream cigarette smoke. Cigarette smoke has been shown to modulate the metabolism of NNK, providing a mechanism for the inhibitory effects of cigarette smoke and cigarette smoke constituents on NNK-induced tumorigenesis. NNK-related pulmonary DNA adducts have not been detected in rodents exposed to cigarette smoke, nor has the toxicity of tobacco smoke or tobacco smoke condensate containing marked reductions in TSNA concentrations been shown to be reduced in any biological assay. In summary, there is no experimental evidence to suggest that reduction of TSNA will reduce the mutagenic, cytotoxic, or carcinogenic potential of tobacco smoke.
Keywords: mainstream cigarette smoke, tobacco-specific nitrosamines, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)
NNK AND OTHER TSNA IN TOBACCO AND TOBACCO SMOKE
Nitrosamines have been reported to be constituents of food, beverages, air, cosmetics, and industrial environments; accordingly, these chemicals have been an intensive topic of research and review for many years (IARC 17 1978; Banbury Report 1982; Loeppky and Michejda 1984; Magee 1996; Tricker 1997; Lin 1990; Preussmann and Eisenbrand 1984; Preston-Martin and Correa 1989; Magee 1989; Tricker et al. 1989; Startin 1996; Eisenbrand et al. 1996; Scanlan 1999;). It is commonly accepted that humans are exposed to nitrosamines on a daily basis; however, the precise levels of exposure and the significance of such exposure remains inconclusive (Tricker 1997).
Tobacco consumption represents an additional source of nitrosamine exposure. Tobacco-specific nitrosamines (TSNA) are a class of nitrosamines believed to occur only in tobacco, and have been reported as being present in a wide variety of tobacco-related products (Hoffmann et al. 1980; Adams et al. 1984; IARC 38 1985; Surgeon General’s Report 1989; Hoffmann et al. 1991; Tricker et al. 1991; Hoffmann et al. 1994; Hoffmann and Hoffmann 1997; Hoffmann et al. 1997; Hoffmann and Hoffmann 1998; Hecht 1999). Known TSNA include 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone [NNK], 4-(methylnitrosamino)-4-(3-pyridyl) butanal [NNA], N′-nitrosonornicotine [NNN], N′-nitrosoanabasine [NAB], N′-nitrosoanatabine [NAT], 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol [NNAL], 4-(methylnitrosamino)-4-(3-pyridyl)-l-butanol [iso-NNAL], and 4-(methylnitrosamino)-4-(3-pyridyl) butanoic acid [iso-NNAC] (Hecht and Tricker 1999). iso-NNAL and iso-NNAC rarely occur in mainstream cigarette smoke (Hecht and Tricker 1999). NNK, NNN, and NNAL are mutagenic in vitro and carcinogenic when administered to laboratory rodents (Boyland et al. 1964; Hoffmann et al. 1984). NAT and NAB demonstrate little or no mutagenic potential during in vitro testing nor carcinogenic activity in laboratory animals (Hoffmann et al. 1984; Padma et al. 1989).
Green and freshly harvested tobaccos are virtually free of TSNA (Green and Rodgman 1996; Caldwell and Conner 1990; Parsons et al. 1986; Spiegelhalder and Bartsch 1996; Brunnemann et al. 1982; Fischer et al. 1990). It is recognized that TSNA form during the post-harvest processing (e.g., curing) to which tobacco is subjected (Andersen et al. 1989; Djordjevic et al. 1989). Significant efforts have been expended toward studying the mechanism by which TSNA are formed (Peele 1995). TSNA are recognized as being formed when tobacco alkaloids (e.g., nicotine and nornicotine) are nitrosated (Tricker and Preussmann 1988). It has been postulated that, in the case of air curing of Burley tobacco, TSNA form as a result of microbial-mediated conversion of nitrate to nitrite, coupled with the subsequent reaction of nitrate-derived chemical species with alkaloids present in the tobacco (Peele et al. 1995; Chamberlain and Chortyk 1992; Hecht 1998; Hamilton et al. 1982; Burton et al. 1992; Bush et al. 1995; Wiernik et al. 1995). In addition, there have been studies examining the potential impact of factors such as temperature of curing barns, humidity, amount of nitrogenous fertilizer used in growing, and amount of shade vs. sunlight on TSNA formation (Tso 1990; Davis and Nielsen 1999). More recently, the presence of NOx gases produced from heating units used during flue-curing processes of Virginia tobacco has been shown to be a contributing factor to TSNA formation (Peele et al. 1999).
Typically, TSNA are not formed from tobacco pyrolysis; rather some fraction of the TSNA formed within tobacco during its curing process is transferred in mainstream tobacco smoke as preformed TSNA (Fisher et al. 1990). This is supported by the fact that the smoke generated by cigarettes made from low TSNA tobacco delivers low yields of TSNA in mainstream smoke (Peele et al. 1995; Doolittle et al. 2001). The amount of TSNA reported to be present in tobacco smoke varies among publications; probably due in part to differences in agricultural variations inherent in different crop years, tobacco curing techniques, the designs of the tested cigarette, the blends of tobacco used in cigarette manufacture, and smoking conditions. Furthermore, the various analytical methods employed to measure TSNA levels may contribute to this observed variability, leading some investigators to point out that earlier values reported in the scientific literature may be exaggerated due to artifact formation inherent in the earlier methodologies (Green and Rodgman 1996; Caldwell and Conner 1990).
Recent investigations have focused on the amount of TSNA, especially NNK, that a smoker is exposed to during smoking. Djordjevic et al. (2000) examined observed delivery of NNK and compared these data with delivery calculated using Federal Trade Commission (FTC) data. The actual observed delivery was nearly two times higher than delivery calculated using FTC data. Using observed delivery values (Djordjevic et al. 2000), a smoker of low-yield nicotine cigarettes (≤ 0.8 mg/cigarette) would be exposed to approximately 187 ng NNK/cigarette, while a smoker of medium-yield nicotine cigarettes (0.9 to 1.2 mg/cigarette) would be exposed to approximately 251 ng NNK/cigarette.
Some scientists have hypothesized that ingested tobacco alkaloids, such as nicotine, might contribute to TSNA formation within the human body (Hoffmann et al. 1994; Hecht and Hoffmann 1989). Others have published data that support the conclusion that endogenous TSNA formation does not occur (Fischer et al. 1990; Caldwell et al. 1991; Meger et al. 1995; Adlkofer 1995; Spiegelhalder and Fischer 1990; Hecht et al. 1999; Tricker et al. 1993). The hypothesis of endogenous TSNA formation conflicts with recent evidence that smoking cessation therapies that involve the administration of nicotine in the form of gum, patch, and/or inhalers do not lead to endogenous TSNA formation (Hecht et al. 1999).
GENOTOXICITY OF NNK
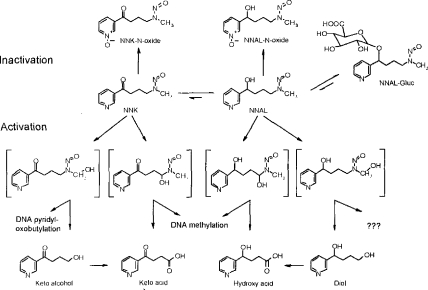
N-nitrosamines require metabolic activation by cytochromes P450 for the expression of genotoxicity. NNK metabolism by a P450-mediated α-hydroxylation pathway leads to several intermediates, some of which are genotoxic (Figure 1) (Hecht and Tricker 1999; Atalla and Maser 1999; Ren et al. 1999; Hecht et al. 1997). Moreover, NNAL, a genotoxic product of NNK carbonyl reduction may undergo α-hydroxylation resulting in the formation of additional genotoxic metabolites (Hecht and Tricker 1999; Atalla and Maser 1999; Ren et al. 1999; Hecht et al. 1997). Detoxication pathways include glucuronidation of NNAL and pyridine-N-oxidation of both NNK and NNAL (Hecht and Tricker 1999; Atalla and Maser 1999; Ren et al. 1999; Hecht et al. 1997).
Figure 1.
NNK metabolism pathways based on studies in laboratory animals. (From Hecht and Tricker, 1999.)
TSNA and cigarette smoke condensate are both mutagenic in the Ames assay in the presence of S9 metabolic activation (Palma et al. 1989; Lee et al. 1996). However, there is no evidence to suggest that the small amount of NNK in cigarette smoke contributes to the mutagenicity observed for cigarette smoke condensate. Approximately 200 μg of pure NNK is required to demonstrate mutagenicity in the Ames assay using strain TA1535, the most sensitive strain for base pair mutagens commonly associated with N-nitrosamines (Lee et al. 1996). The dose of NNK required to elicit a moderate mutagenic response (200 μg) is equivalent to the amount of NNK yielded by approximately 2985 Kentucky 1R4F reference cigarettes smoked under standard FTC smoking conditions (Borderding et al. 1997). Since the amount of cigarette smoke condensate present in approximately 0.01 1R4F cigarettes (100 μg of CSC) is sufficient to demonstrate a substantial mutagenic response in the Ames test, it follows that the mutagenic response is not being driven by the level of TSNA in CSC. Furthermore, mainstream smoke from cigarettes generated using low TSNA tobacco failed to demonstrate reduced mutagenic potential within the Ames assay (Doolittle et al. 2001). Therefore, several lines of experimental evidence indicate that there are insufficient quantities of TSNA in tobacco smoke to contribute to the mutagenicity of tobacco smoke observed in the Ames test.
TUMORIGENICITY OF NNK IN LABORATORY ANIMALS
Tobacco-specific nitrosamines such as NNK are rodent carcinogens (Hecht and Tricker 1999; Hecht et al. 1978; Hecht et al. 1988; Belinsky et al. 1990; Hoffmann et al. 1993; Hecht et al. 1989; Hecht et al. 1986; Hecht et al. 1983) when administered in pure form. Hecht et al. (1989) has shown that NNK induces lung adenomas in A/J mice in a dose-response manner within 4 months of a single intraperitoneal (i.p.) injection. Tobacco-specific nitrosamines have also been shown to be carcinogenic as a consequence of oral cavity implantation and skin painting (Hecht et al. 1986). At present, there are no published data demonstrating TSNA to be carcinogenic via inhalation or respiratory tract exposure.
The fact that systemically administered TSNA produce lung adenomas in rodents have led some investigators to hypothesize that TSNA may be an important risk factor for lung cancer development in smokers (Hecht et al. 1989; Hecht et al. 1986; Hecht et al. 1983). However, extrapolation of the carcinogenic dose used in rodents results in unachievable “pack-year equivalents” per smoker (discussed in next section). Furthermore, a simplistic extrapolation of results obtained with pure TSNA to tobacco smoke is not supported by published animal studies demonstrating that tobacco extracts and/or smoke may actually reduce the carcinogenicity of pure TSNA in rodents (Hecht et al. 1986; Finch et al. 1996). In one such study, a solution of NNN and NNK solubilized in water and administered to male F344 rats via oral swab induced statistically significant (p<0.05) increases in oral cavity tumors as compared to vehicle control (Hecht et al. 1986). However, when the animals received the same dose of NNN and NNK along with snuff extract, there was a statistically significant decrease in oral cavity tumors when compared with animals treated with pure NNN and NNK (Hecht et al. 1986).
A recent study has reported that whole-body inhalation exposure of A/J mice to 11% mainstream cigarette smoke and 89% sidestream cigarette smoke, used as an experimental surrogate for environmental tobacco smoke (ETS), resulted in a tumorigenic response provided that a 4 month post-exposure recovery period is incorporated into the experimental design (Witschi et al. 1997). A/J mice were similarly exposed to the same surrogate for whole ETS as well as HEPA-filtered ETS surrogate to remove the particulate phase of the smoke, so that the smoke consisted primarily of gas phase constituents (Witschi et al. 1997). Both exposures resulted in similar numbers of lung adenomas even though the concentration of NNK (mean ± SD) in the whole ETS surrogate and gas-phase ETS surrogate exposure atmospheres was 3.9 ± 3.5 and 0.29 ± 0.28 μg/m3, respectively. Based on these values, the authors concluded that NNK was not the causative agent in the observed adenomas (Witschi et al. 1997). Finally, smoke from cigarettes made with low TSNA tobacco gave essentially the same biological response in a 90-day inhalation study in rats as the smoke from cigarettes made without reduced TSNA tobacco (Kinsler et al. 2002). Moreover, a comparative 30-week dermal study using SENCAR mice and comparing the cigarette smoke condensate (CSC) from low TSNA tobacco with the CSC from cigarettes made without reduced TSNA tobacco showed no statistically significant differences in numbers of dermal tumors (Hayes et al. 2003).
DOSE COMPARISONS OF NNK USED IN ANIMAL STUDIES VERSUS SMOKER EXPOSURE
TSNA have been found to be carcinogenic in the lungs of rats, mice, and hamsters when injected systemically (Hecht et al. 1978; Hecht et al. 1988; Belinsky et al. 1990; Hoffmann et al. 1993; Hecht et al. 1989; Hecht et al. 1986; Hecht et al. 1983). However, as shown in Table 1 extrapolation of the carcinogenic dose used in rodent studies results in unachievable “pack-year equivalents” per smoker. Actually, to mimic die mouse exposure data, a smoker would need to smoke all of the cigarettes at once since rodents receive a single injection of NNK. Also, one needs to also assume 100% absorption of NNK from the smoke. These dose calculations are based on an average NNK yield of market full flavor cigarettes smoked under FTC conditions, and serve to compare the dosimetry reported in systemically injected mice versus the dose in cigarettes that a smoker would have to consume. When considering the relevance to human smokers of the doses employed during animal studies, it is important to remember that over 40 years of smoking, a three pack-a-day smoker would smoke 876,000 cigarettes, or 43,800 packs. The 876,000 cigarettes would be approximately 2% to 8% of the 11 to 43 million cigarettes required to yield the dose of NNK reported to be carcinogenic in mice scaled to human body weight.
Table 1.
Extrapolation of rodent bioassay results to human smokers.
| Animal Model | A/J Mouse | |
|---|---|---|
| Minimum Dose | Maximum Dose | |
| Total NNK Dose (mg/25 gram mouse) required to induce significant incidence of lung tumors | 0.52 mg | 2.07 mg |
| Total NNK Dose (mg/kg body weight) required to induce significant incidence of lung tumors | 20.8 mg | 82.8 mg |
| NNK/Cigarette (Chepiga, 2000) | 135 ng | |
| Total Cigarette Equivalence/kg Mouse Body Weight | ∼154,000 | ∼613,000 |
| Equivalent Dose of NNK in a 70 kg Smoker | 1456 mg | 5796 mg |
| Equivalent Number of Cigarettes | 10,785,185 | 42,933,333 |
| Comparison to Smoker (packs/day for 40 yrs) | 37 packs | 147 packs |
| Comparison to Smoker (Years of smoking 2 packs/day) | 739 years | 2941 years |
Some investigators have hypothesized a possible additive effect of individual TSNA in tobacco smoke. In the case of TSNA, there are 182 ng of NAT, 158 ng of NNN, and 135 ng of NNK per typical U.S. market full flavor cigarette (Chepiga et al. 2000); the total of all three TSNA would be about 475 ng per cigarette. Even assuming that NNN and NAT are as carcinogenic as NNK in rodents, which they are not (Hoffmann et al. 1984; Padma et al. 1989), one would still be considering unrealistic “pack-year equivalents” per smoker to yield the doses demonstrated to be carcinogenic in rodents (i.e., 10 to 40 packs per day for 40 years).
A recent study (Djordjevic et al. 2000) compared the amounts of NNK delivered to a smoker using the Federal Trade Commission (FTC) specified machine-smoking protocol (35-ml puff volume drawn for 2 s once per min) vs. data from actual smokers. Compared with the FTC protocol values, smokers of low-yield cigarettes (≤ 0.8 mg of nicotine per cigarette) and medium-yield cigarettes (0.9 to 1.2 mg of nicotine per cigarette) took statistically significantly larger puffs (48.6 and 44.1 ml, respectively) at statistically significantly shorter intervals (21.2 and 18.5 s, respectively), and drew larger total smoke volumes. Compared with the FTC yield for NNK per cigarette for Kentucky reference 1R4F, values of NNK per cigarette of smokers was approximately 2.5-fold higher for low-yield cigarettes and approximately 3.2-fold higher for medium-yield cigarettes. Using these values, a smoker would still need to smoke two packs per day for more than 300 years to be exposed to the low dose of NNK used in rodent studies and two packs per day for more than 1400 years for the equivalent of the higher dose used in rodent studies.
One of the more significant studies carried out to look at the dose-response relationship of the induction of pulmonary neoplasia in the Fischer 344 rat was by Belinsky et al. (1990). The lowest dose of NNK used to induce lung adenomas was 6 mg/kg, which is equivalent to over 44 thousand cigarettes/kg using 135 ng NKK/cigarette, as stated previously. The authors stated that this dose of NNK was similar to the dose of NNK that a smoker would be exposed to during a lifetime of smoking. This calculation apparently assumes that a smoker consumes 10 packs/day for 40 years.
INHIBITION OF THE BIOLOGICAL ACTIVITY OF NNK BY TOBACCO SMOKE AND SELECTED CONSTITUENTS
The tobacco-specific nitrosamine, NNK, requires metabolic activation to express its carcinogenic effects. However, there are competing detoxication pathways (Hecht 1994). The major metabolic pathway for NNK (in most tissues) involves conversion to NNAL via reduction of the NNK carbonyl group (Figure 1). This reaction occurs rapidly in rodents, primates, and human tissues (Smith et al. 1992; Castonguay et al. 1983). α-Hydroxylation of the methylene groups adjacent to the N-nitroso nitrogen of NNK and NNAL yields the corresponding keto acid and hydroxy acid, with liberation of the methylating agent, methanediazohydroxide. α-Hydroxylation of the methyl group in NNK ultimately yields the keto alcohol (also referred to as HPB), which can be oxidized to keto acid. The reactive intermediate, α-hydroxymethyl-NNK can decompose and react by pyridyloxobutylation of DNA and hemoglobin to form HPB-releasing adducts. α-Methyl hydroxylation of NNAL produces the major end product of 4-(3-pyridyl)butane-l,4,diol (diol), with no existing evidence to suggest that this metabolic pathway results in adduct formation (Richter et al. 2000). NNK and NNAL can be pyridine N-oxidized to form either the NNK or NNAL-N-oxide or can be conjugated to form NNAL-glucuronide, all of which are nongenotoxic metabolites that are readily excreted in urine.
Nicotine, cotinine, cigarette smoke, and aqueous cigarette “tar” extract (ACTE) have all been shown to inhibit the α-hydroxylation of NNK. Nicotine, as well as NNN and NAT demonstrate a dose-dependent inhibition of in vitro α-hydroxylation of NNK within rat oral tissue (Murphy and Heiblum 1990). Nicotine and cotinine both reduce NNK metabolic activation by α-hydroxylation in the isolated and perfused rat liver, but not in the isolated and perfused rat lung (Schulze et al. 1998). Nicotine significantly reduces in vivo metabolic activation of NNK and excretion of α-hydroxylation metabolites (Richter and Tricker 1994), as well as significantly reduces [5-3H] NNK binding of radioactivity (pyridyloxobutylation) to rat hemoglobin (Kutzer et al. 1994). Nicotine inhibits in vitro α-hydroxylation of NNK and protein binding (pyridyloxobutylation) in hamster lung explants (Schuller et al. 1991) and hepatic microsomal proteins (Castonguay and Rossignol 1992). Similar effects may occur in vivo since co-administration of nicotine results in a significant inhibition of NNK α-hydroxylation in the hamster (Richter et al. 2000). Compared to other rodent species, the hamster is relatively insensitive to NNK-induced lung tumorigenesis (Richter et al. 2000), most likely a consequence of limited NNK α-hydroxylation in the lung (Richter et al. 2000).
Lee et al. (1996) evaluated the mutagenicity of N-nitrosamines in the presence of nicotine and other structurally similar pyridine alkaloids. NNK, N-nitrosodimethylamine (NDMA), and NNN were tested in the Ames Salmonella typhimurium assay (in the presence of a metabolic activation system, S9) using strain TA1535, the most sensitive strain for base pair mutagens such as N-nitrosamines (Padma et al. 1989; Lee et al. 1996; Yahagi et al. 1977). Nicotine, cotinine, and aqueous cigarette “tar” extract (ACTE) all inhibited the mutagenicity of NDMA and NNK, while NNN mutagenicity was not affected. The induction of sister chromatid exchanges (SCE) in mammalian cells (CHO) by NNK in the presence of metabolic activation also was reduced significantly by nicotine and cotinine. Therefore, consistent with metabolism studies, nicotine and other tobacco constituents effectively inhibit the mutagenicity of NNK (Lee et al. 1996; Richter and Tricker 1994; Kutzer et al. 1994; Schuller et al. 1991).
While nicotine clearly inhibits the mutagenicity of NNK, other tobacco smoke constituents can also play a significant role. ACTE (aqueous cigarette tar extract), prepared from de-nicotinized cigarettes (containing significantly less nicotine [∼0.08 mg/cig] than the Kentucky 1R4F reference cigarette [∼0.9 mg/cig]) was tested for its effect on NNK mutagenicity (Lee et al. 1996). The inhibitory effects were almost identical suggesting that the inhibitory effect of ACTE on the mutagenicity of NNK is attributable to water-soluble constituents of cigarette smoke (Lee et al. 1996). The specific agent(s) in ACTE responsible for the inhibition of mutagenicity have not yet been identified.
NNAL is a potent pulmonary carcinogen in mice and rats (Hoffmann et al. 1993; Hecht et al. 1990) and is mutagenic in the Ames bacterial mutagenesis assay (Yahagi et al. 1977; Brown et al. 2001). Given the structural similarity between NNK and NNAL, and the metabolic activation of both by cytochromes P450, we hypothesized that there may be a similar inhibition of NNAL metabolism, and consequently, inhibition of the mutagenic activity of NNAL by tobacco smoke and its pyridine alkaloid constituents. In a recent study, we evaluated the ability of two pyridine alkaloids (nicotine and cotinine), as well as ACTE to inhibit the mutagenicity of NNAL as assessed by Salmonella typhimurium strain TA1535 in the presence of a metabolic activation system (S9) (Brown et al. 2001). Both pyridine alkaloids tested, as well as ACTE, inhibited the mutagenicity of NNAL in a concentration-dependent manner. These results demonstrate that tobacco smoke contains pyridine alkaloids, as well as other unidentified constituents that inhibit the mutagenicity of NNAL, a major metabolite of NNK (Brown et al. 2001). Due to the presence of these modulating agents in cigarette smoke, the biologically reactive dose of NNAL from cigarette smoking is likely to be much lower than predicted from studies comparing the biological activity of pure NNAL with plasma concentrations of NNAL.
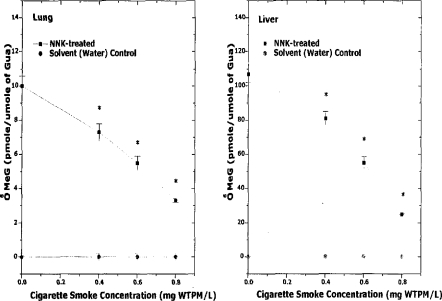
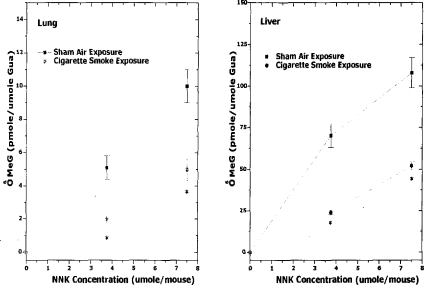
A single intraperitoneal injection of NNK induces the formation of O6-methylguanine in A/J mouse lung DNA (Brown et al. 1999; Peterson and Hecht 1991). O6-MeG is a promutagenic base that induces guanine (G) to adenine (A) transition (Ronai et al. 1993). Any inhibition of the P450 -mediated α-hydroxylation reaction would be expected to reduce the formation of DNA-reactive species from TSNA, hence reducing genotoxic, mutagenic, and tumorigenic activities. Exposure of A/J mice to mainstream cigarette smoke (0, 400, 600, or 800 mg TSP/m3) did not result in detectable levels of O6-MeG in either lung or liver (Figure 2) (Brown et al. 1999). Moreover, A/J mice co-exposed to mainstream smoke (0, 400, 600, or 800 mg TSP/m3) and a single i.p. administration of NNK (0, 3.75, or 7.5 μmol/mouse, sufficient to induce significant levels of O6-MeG adducts) resulted in a significant dose-dependent reduction in NNK-induced lung and liver O6-MeG (Figure 3) (Brown et al. 1999).
Figure 2.
Dose-dependent reduction of O6-MeG concentration by 1R4F cigarette smoke in A/J mice. Mice received a one-time, nose-only inhalation exposure of 1R4F cigarette smoke at 0, 0.4, 0.6, or 0.8 mg WTPM/L for 2 h to study the potential of cigarette smoke to inhibit NNK-induced O6-MeG formation. The dosing of NNK (7.5 μmol/mouse, ip) was performed at the midpoint of the 2-h exposure. Mice were euthanized 4 h after NNK treatment and lung and liver DNA was analyzed for O6-MeG by HPLC. (Mean ± SE; n = 18; * = p<0.05). (From Brown et al. 1999.)
Figure 3.
Effect of Kentucky reference 1R4F cigarette smoke (0.6 mg WTPM/L) on the lung and liver concentrations of O6-MeG in NNK-treated A/J mice. Mice received a one-time, nose-only inhalation regimen of either HEPA-filtered and humidified air (control) or 1R4F cigarette smoke at the previously determined MNLD (0.6 mg WTPM/L) for 2 h to monitor the effect of cigarette smoke on the concentration of O6-MeG in mice treated with NNK. A single ip dose of NNK (0, 3.75, or 7.5 μmol/mouse) was administered to mice at the midpoint of the 2-h exposure. Mice were euthanized 4 h after the NNK treatment, lung and liver DNA were analyzed for O6-MeG by HPLC. (Mean ± SE; n = 18; * = p < 0.05). (From Brown et al. 1999.)
In a recent study designed to study metabolic inhibition/competition, A/J mice were exposed to mainstream cigarette smoke from the 1R4F cigarette (600 mg TSP/ m3) for 2 h, followed by a single i.p. Injection of NNK (7.5 μmol/mouse). Results from these studies demonstrated that tobacco smoke exposure significantly reduced NNK metabolic activation to the hydroxy acid and keto acid by 15% (p=0.0029) and 42% (p<0.0001), respectively, compared with sham-exposed (control) animals (Brown et al. 2001). Thus, co-administration of cigarette smoke reduces the metabolic activation of NNK (via α-hydroxylation) to DNA-reactive methylating species, a critical step in the induction of lung tumorigenesis in the A/J mouse.
Finally, phenethyl isothiocyanate (PEITC) is an effective inhibitor of lung tumorigenesis induced in rats and mice by the tobacco-specific carcinogen NNK (Hecht et al. 2000). However, studies have failed to demonstrate a protective effect for PEITC on tobacco smoke carcinogenesis in rodent models (Witschi et al. 1998; Witschi et al. 1999) providing additional evidence that NNK is not the causative agent in animal models of tobacco-smoke carcinogenesis.
HUMAN BIOMARKERS OF NNK METABOLISM
Although some have assumed that NNK metabolism is similar in laboratory rodents and in man, recent data do not support this assumption. Studies examining urinary metabolites of nicotine, NNK, NNN, and NNAL in rats (when compared with humans) revealed significant differences (Trushin and Hecht 1999; Hecht et al. 1999). An initial hypothesis of these studies was that urinary (S)-hydroxy acid could be a potential urinary biomarker of NNK and NNN α-hydroxylation in smokers. However, researchers discovered that the metabolism was significantly different between rodents and humans. For example, in the rat it is possible to distinguish the hydroxy acid derived from nicotine from that derived from TSNA (Trushin and Hecht 1999); this was not possible in humans (Hecht et al. 1999). Furthermore, when metabolism of NNK within precision-cut rodent and human liver and lung slices was compared, metabolism to NNAL was significantly higher in human tissues than in rodent tissues (Castonguay et al. 1983).
Incubation of NNK (3 to 10 μM) with microsomes from human liver (Staretz et al. 1997) and lung (Smith et al. 1995) yields at least 95% NNAL, with little evidence of metabolism via α-hydroxylation, the predominant pathway in rodents (Hecht and Tricker 1999). Recent in vitro studies report that human buccal mucosa predominantly reduces NNK to NNAL (95 to 99%), in addition to metabolism via α-hydroxylation (0.6 to 3.8%) and pyridyl N-oxidation (0.3 to 2.2%) (Liu et al. 1993). In a study utilizing human lung slices (Castonguay et al. 1983), lung tissue demonstrated a low capacity to metabolize NNK. Metabolism proceeded mainly via a low Km (high affinity) reduction to NNAL (Km 0.5 μM; Vmax 388 fmol/min/mg protein and Km 39; Vmax 21380), with a lower potential to form methylating and/or pyridyloxobutylating species. Consistent with this, 7-methyl-2-deoxyguanosine DNA adduct levels in the lungs of smokers (and nonsmokers) cannot be explained by differences in tobacco exposure, with pyridyloxobutylation undetectable in smokers’ lungs (Blomeke et al. 1996). At a plausible level of NNK exposure, α-hydroxylation to the keto acid (Km 690; Vmax 13390) in the liver is unlikely due to the low Km high-affinity reduction to NNAL (Km 0.6; Vmax 254 and Km 44; Vmax 11340) (Castonguay et al. 1983).
Hemoglobin (Hb) adducts from TSNA have been suggested as biomarkers of exposure for both tobacco smoke and smokeless tobacco. The metabolic activation of both NNK and NNN results in a common Hb adduct, releasing 4-hydroxy-l-(3-pyridyl)-1-butanone (HPB) after alkaline hydrolysis. Initial biomonitoring studies reported adduct levels significantly higher in the blood of smokers than in nonsmokers (Carmella et al. 1990; Falter et al. 1994; Atawodi et al. 1998), while Hb adduct levels were significantly higher for users of smokeless tobacco when compared with smokers in two of the studies (Carmella et al. 1990; Falter et al. 1994). Urinary excretion of the NNK metabolites, NNAL and NNAL-Gluc are about 120 times higher in smokers than in nonsmokers, but HPB-releasing hemoglobin adducts derived from NNK and NNN in both smokers and nonsmokers are frequently not much higher than assay background amounts (Hecht and Tricker 1999). In a study in which smokers and smokeless tobacco users demonstrated approximately the same level of NNK and NNN uptake, the mean adduct level was approximately 7 times higher in smokeless tobacco users than in smokers (Carmella et al. 1990; Falter et al. 1994). This leads to the conclusion that adduct levels in smokers may be lower than expected due to induction of metabolizing enzymes that detoxify TSNA in smokers (Falter et al. 1994; Atawodi et al. 1998) and/or that TSNA activation is inhibited by other smoke constituents (Lee et al. 1996; Brown et al. 2001; Brown et al. 1999; Brown et al. 2001).
CONCLUSION
Is it prudent to reduce NNK and other TSNA in tobacco products? Sure it is, to the extent possible. Reduced TSNA in tobacco products will result in reduced exposure to TSNA. Will such reduction mean a reduced cancer risk? That cannot be determined until smokers have used reduced TSNA products for several years.
A review of the scientific literature suggests the following: (1) NNK, a tobacco-specific nitrosamine, is found in cured tobacco and in tobacco smoke; (2) in pure form, NNK is toxic and mutagenic to cultured cells in vitro; (3) in pure form, NNK is carcinogenic in experimental animals; (4) extrapolated to man and based on the minimum amount of NNK required to cause tumors in A/J mice (the most sensitive rodent model), the amount of NNK found in the smoke from millions of cigarettes would be required to provide a carcinogenic equivalent to smokers; (5) the mutagenicity and carcinogenicity of NNK can be inhibited by nicotine and cotinine, as well as additional unidentified constituents of cigarette smoke; and (6) CSC or smoke from reduced TSNA cigarettes is similar in toxicity, mutagenicity, and carcinogenicity to CSC or smoke from cigarettes with current levels of TSNA.
Based on our review of the published literature, we conclude that there is neither direct nor convincing evidence that NNK or TSNA in toto play a significant role in the increased risk of lung cancer associated with cigarette smoking. Furthermore, there is no compelling experimental evidence that reducing the levels of TSNA in tobacco smoke will have a significant impact on the lung cancer risks associated with cigarette smoking.
REFERENCES
- Adams JD, Lee SJ, Hoffman D. Carcinogenic agents in cigarette smoke and the influence of nitrate on their formation. Carcinogenesis. 1984;5(2):221–223. doi: 10.1093/carcin/5.2.221. [DOI] [PubMed] [Google Scholar]
- Adlkofer FX. Involvement of nicotine and its metabolites in the pathology of smoking-related diseases: Facts and hypotheses. In: Clarke PBS, Quik M, Adlkofer F, Thurau K, editors. Effects of Nicotine on Biological Systems II. Birkhauser Verlag; Basel, Switzerland: 1995. [Google Scholar]
- Andersen RA, Fleming PD, Burton HR, Hamilton-Kemp TR, Sutton TG. N′-Acyl and N′-nitroso pyridine alkaloids in alkaloid lines of Burley tobacco during growth and air curing. J Agric Food Chem. 1989;37(1):44–50. [Google Scholar]
- Atalla A, Maser E. Carbonyl reduction of the tobacco-specific nitrosamine 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone (NNK) in cytosol of mouse liver and lung. Toxicology. 1999;139:155–166. doi: 10.1016/s0300-483x(99)00114-6. [DOI] [PubMed] [Google Scholar]
- Atawodi SE, Lea S, Nyberg F, Mukeria A, Constantinescu V, Ahresn W, Brueske-Hohlfeld I, Fortes C, Boffetta P, Friesen MD. 4–Hydroxy-l– (3–pyridyl)-1–butanone-Hemoglobin adducts as biomarkers of exposure to tobacco smoke: Validation of a method to be used in multicenter studies. Cancer Epidemiol Biomarkers Prev. 1998;7:817–821. [PubMed] [Google Scholar]
- Magee Peter N., editor. Nitrosamines and Human Cancer. Cold Spring Harbor Laboratory; 1982. Banbury Report 12. [Google Scholar]
- Belinsky SA, Foley JF, White CM, Anderson MW, Maronpot RR. Dose-response relationship between O6–Methylguanine formation in Clara cells and induction of pulmonary neoplasia in the rat by 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone. Cancer Res. 1990;50:3772–3780. [PubMed] [Google Scholar]
- Blomeke B, Greenblatt MJ, Van Doan D. Distributions of 7–alkyl-2’-deoxyguanosine adduct levels in human lung. Carcinogenesis. 1996;17:741–748. doi: 10.1093/carcin/17.4.741. [DOI] [PubMed] [Google Scholar]
- Borgerding MF, Bodnar JA, Chung HL, Mangan PP, Morrison CC, Risner CH, Rogers JC, Simmons DF, Uhrig MS, Wendelboe FN, Wingate DE, Winkler LS. Chemical and biological studies of a new cigarette that primarily heats tobacco. Part 1. Chemical composition of mainstream smoke. Food Chem Tox. 1997;36:169–182. [PubMed] [Google Scholar]
- Boyland E, Roe FJC, Gorrod JW, Mitchley BCV. The carcinogenicity of nitrosoanabasine, a possible constituent of tobacco smoke. Br. J. Cancer. 1964;18:265–270. doi: 10.1038/bjc.1964.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BG, Chang C-JG, Ayres PH, Lee CK, Doolittle DJ. The effect of cotinine or cigarette smoke co-administration on the formation of O6–methylguanine adducts in the lung and liver of A/J mice treated with 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone (NNK) Tox Sci. 1999;47:33–39. doi: 10.1093/toxsci/47.1.33. [DOI] [PubMed] [Google Scholar]
- Brown B, Avalos J, Lee C, Doolittle D. The effect of tobacco smoke, nicotine, and cotinine on the mutagenicity of 4-(methylnitrosamino)-l–(3–pyridyl)-l–butanol (NNAL) Mul Res. 2001;494(1–2):21–29. doi: 10.1016/s1383-5718(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Brown B, Richter E, Tricker A, Doolittle D. The effect of a 2–h exposure to cigarette smoke on the metabolic activation of the tobacco-specific nitrosamine 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone (NNK) the A/J mice. Chemico-Biological Interactions. 2001;138:125–135. doi: 10.1016/s0009-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- Brunnemann KD, Hecht SS, Hoffmann D. N-nitrosamine: Environmental occurrence, in vivo formation and metabolism. J Toxicol-Clin Toxicol. 1982–3;19(6 & 7):661–688. doi: 10.3109/15563658208990397. [DOI] [PubMed] [Google Scholar]
- Burton HR, Naewanna KD, Bush LP. Distribution of tobacco constituents in tobacco leaf tissue. I. Tobacco-specific nitrosamines, nitrate, nitrite, and alkaloids. J Agric Food Chem. 1992;40:1050–1055. [Google Scholar]
- Bush LP, Cur M, Burton HR. Coresta Information Bulletin. 1995. Origin of nitrite-nitrogen for tobacco-specific N’-nitrosamine formation. Abstract 9814. [Google Scholar]
- Caldwell WS, Conner JM. Artifact formation during smoke trapping: An improved method for determination of N-nitrosamines in cigarette smoke. J Assoc Off Anal Chem. 1990;73(5):783–789. [PubMed] [Google Scholar]
- Caldwell WS, Greene JM, Plowchalk DR, deBethizy JD. The nitrosation of nicotine: A kinetic study. Chem Res Toxicol. 1991;4:513–516. doi: 10.1021/tx00023a003. [DOI] [PubMed] [Google Scholar]
- Carmella SG, Kagan SS, Kagan M, Foiles PG, Palladino G, Quart AM, Quart E, Hecht SS. Mass spectrometric analysis of tobacco-specific nitrosamine hemoglobin adducts in snuff dippers, smokers, and nonsmokers. Cancer Res. 1990;50:5438–5445. [PubMed] [Google Scholar]
- Castonguay A, Lin D, Stoner GD, Radok P, Furuya K. Comparative carcinogenicity in A/J mice and metabolism by cultured mouse peripheral lung of N′-nitrosonornicotine, 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone and their analogues. Cancer Res. 1983;43:1223–1229. [PubMed] [Google Scholar]
- Castonguay A, Stoner GD, Schut HAJ, Hecht SS. Metabolism of tobacco-specific N-nitrosamines by cultured human tissues. Proc Natl Acad Sci USA. 1983;80:6694–6697. doi: 10.1073/pnas.80.21.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castonguay A, Rossignol G. Modulation of the activation of 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone by hamster lung microsomes to protein alkylating species. Toxic in Vitro. 1992;6:397–404. doi: 10.1016/0887-2333(92)90046-t. [DOI] [PubMed] [Google Scholar]
- Chamberlain WJ, Chortyk OT. Effect of curing and fertilization on nitrosamine formation in bright and Burley tobacco. Beitr. Tobakforsch. 1992;15:87–92. [Google Scholar]
- Chepiga TA, Morton MJ, Murphy PA, Avalos JT, Bombick BR, Doolittle DJ, Borgerding MF, Swauger JE. A comparison of the mainstream smoke chemistry and mutagenicity of a representative sample of the US cigarette market with two Kentucky reference cigarettes (K1R4F and K1R5F) Food Chem Tox. 2000;38:949–962. doi: 10.1016/s0278-6915(00)00086-7. [DOI] [PubMed] [Google Scholar]
- Davis DL, Nielsen MT. Tobacco: Production, Chemistry and Technology. World Agriculture Series, CORESTA, Blackwell Science Ltd; Oxford, England: 1999. [Google Scholar]
- Djordjevic MV, Brunnemann KD, Hoffmann D. Identification and analysis of a nicotine-derived N-nitrosamino acid and other nitrosamino acids in tobacco. Carcinogenesis. 1989;10:1725–1731. doi: 10.1093/carcin/10.9.1725. [DOI] [PubMed] [Google Scholar]
- Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000;92:106–111. doi: 10.1093/jnci/92.2.106. [DOI] [PubMed] [Google Scholar]
- Doolittle DJ, Avalos JT, Bombick BR, Putnam KP, Nestor TB, Gentry JS. Biological studies on smoke condensates from cigarettes made with low nitrosamines flue-cured tobacco. Tox Sci. 2001;60:416. [Google Scholar]
- Eisenbrand G, Fuchs A, Koehl W. N-nitroso compounds in cosmetics, household commodities and cutting fluids. Eur J Canc Prev. 1996;5(1):41–46. [PubMed] [Google Scholar]
- Falter B, Kutzer C, Richter E. Biomonitoring of hemoglobin adducts: aromatic amines and tobacco-specific nitrosamines. Clin Investig. 1994;72:364–371. doi: 10.1007/BF00252829. [DOI] [PubMed] [Google Scholar]
- Finch GL, Nikula KJ, Belinsky SA, Barr EB, Stoner GD, Lechner JF. Failure of cigarette smoke to induce or promote lung cancer in the A/J mouse. Cancer Letters. 1996;99:161–167. doi: 10.1016/0304-3835(95)04059-5. [DOI] [PubMed] [Google Scholar]
- Fischer S, Spiegelhalder B, Eisenbarth J, Preussmann R. Investigations on the origin of tobacco-specific nitrosamines in mainstream smoke of cigarettes. Carcinogenesis. 1990;11(5):723–730. doi: 10.1093/carcin/11.5.723. [DOI] [PubMed] [Google Scholar]
- Green CR, Rodgman A. The Tobacco Chemists’ Research Conference: A half century forum for advances in analytical methodology of tobacco and its products. Recent Advances in Tobacco Science, Volume 22: Fifty years of tobacco science and technology; Symposium proceedings of the 50th meeting, Tobacco Chemists’ Research Conference; Richmond, VA. October 20–23, 1996.1996. [Google Scholar]
- Hamilton JL, Bush L, Lowe RH. Nitrate concentration changes during senescence and air curing of burley tobacco. Tob Sci. 1982;26:133–137. [Google Scholar]
- Hayes JR, Stravanja MS, Meckley DR, Van Kampen KR, Nelson PR, Ayres PH, Mosberg AT, Swauger JE. Comparative 30–week dermal tumor promotion study using SENCAR mice: comparison of cigarette smoke condensate from a reference cigarette containing direct-fire flue-cured tobacco and a test cigarette containing heat-exchange flue-cured tobacco. The Toxicologist. 2003;72(S-1):86. [Google Scholar]
- Hecht SS, Chen CB, Hirota N, Ornaf RM, Tso TC, Hoffmann D. Tobacco-specific nitrosamines: Formation from nicotine in vitro and during tobacco curing and carcinogenicity in Strain A mice. J Natl Cancer Inst. 1978;60(4):819–824. doi: 10.1093/jnci/60.4.819. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Adams JD, Numoto S, Hoffmann D. Induction of respiratory tract tumors in Syrian golden hamsters by a single dose of 4–(methylnitrosamino)-l–(3–pyridyl)-1–butanone (NNK) and the effect of smoke inhalation. Carcinogenesis. 1983;4:1287–1290. doi: 10.1093/carcin/4.10.1287. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, Hoffmann D. Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Research. 1986;46:4162–4166. [PubMed] [Google Scholar]
- Hecht SS, Abbaspour A, Hoffmann D. A study of tobacco carcinogenesis XLII. Bioassay in A/J mice of some structural analogues of tobacco-specific nitrosamines. Cancer Letters. 1988;42:141–145. doi: 10.1016/0304-3835(88)90251-0. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Morse MA, Amin S, Stoner GD, Jordan KG, Choi C, Chung FL. Rapid single-dose model for lung tumor-induction in A/J mice by 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone and the effect of diet. Carcinogenesis. 1989;10:1901–1904. doi: 10.1093/carcin/10.10.1901. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Hoffmann D. The relevance of tobacco-specific nitrosamines to human cancer. Cancer Surv. 1989;8:271–294. [PubMed] [Google Scholar]
- Hecht SS, Jordan KG, Choi C-I, Trushin N. Effects of deuterium substitution on the tumorigenicity of 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone and 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanol in A/J mice. Carcinogenesis. 1990;11:1017–1020. doi: 10.1093/carcin/11.6.1017. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Metabolic activation and detoxification of tobacco-specific nitrosamines—a model for cancer prevention strategies. Drug Metab Rev. 1994;26:373–390. doi: 10.3109/03602539409029803. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Spratt TE, Trushin N. Absolute configuration of 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanol (NNAL) formed metabolically from 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone (NNK) Carcinogenesis. 1997;18:1851–1855. doi: 10.1093/carcin/18.9.1851. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11(6):559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Tobacco smoke carcinogens and lung cancer. J Nat Canc Inst. 1999;91(14):1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Tricker AR. In: Analytical Determination of Nicotine and Related Compound and Their Metabolites. Gorrod JW, Jacob P III, editors. Elsevier; Amsterdam, The Netherlands: 1999. [Google Scholar]
- Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- Hecht SS, Hatsukami DK, Bonilla LE, Hochalter JB. Quantitation of 4–Oxo-4– (3–pyridyl) butanoic acid and enantiomers of 4–hydroxy-4– (3–pyridyl) butanoic acid in human urine: A substantial pathway of nicotine metabolism. Chem Res Toxicol. 1999;12:172–179. doi: 10.1021/tx980214i. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Kenney PM, Wang M, Trushin N, Upadhyaya P. Effects of phenethyl isothiocyanate and benzyl isothiocyanate, individually and in combination, on lung tumorigenesis induced in A/J mice by benzo [a] pyrene and 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone. Cancer Lett. 2000;150(1):49–56. doi: 10.1016/s0304-3835(99)00373-0. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Chen C-HB, Hecht S. The role of volatile and nonvolatile N-nitrosamines in tobacco carcinogenesis. In: Gori GB, Bock Fg, editors. Banbury Report 3: A Safe Cigarette? 1980. pp. 113–127. [Google Scholar]
- Hoffmann D, Rivenson A, Amin S, Hecht SS. Dose-response study of the carcinogenicity of tobacco-specific N-nitrosamine in F344 rats. J Cancer Res Clin Oncol. 1984;108:81–86. doi: 10.1007/BF00390978. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann L, Wynder EL. Lung cancer and the changing cigarette. In: O’Neill IK, Chen J, Bartsch H, editors. Relevance to human cancer of N-nitroso compounds, tobacco and mycoloxins. International Agency for Research on Cancer; Lyon: 1991. pp. 449–459. IARC Scientific Publications No. 105, [Google Scholar]
- Hoffmann D, Djordjevic MV, Rivenson A, Zang E, Desai D, Amin S. A study of tobacco carcinogenesis. LI. Relative potencies of tobacco-specific N-nitrosamines as inducers of lung tumours in A/J mice. Cancer Lett. 1993;71:25–30. doi: 10.1016/0304-3835(93)90092-n. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Brunnemann KD, Prokopczyk B, Djordjevic MV. Tobacco-specific N-nitrosamines and areca-derived N-nitrosamines: Chemistry, biochemistry, carcinogenicity, and relevance to humans. J Toxicol Environ Health. 1994;41:1–52. doi: 10.1080/15287399409531825. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health. 1997;50:307–364. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Djordjevic MV, Hoffmann I. The changing cigarette. Prev Med. 1997;26(4):427–434. doi: 10.1006/pmed.1997.0183. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann I. Tobacco Smoke Components (Letter to the Editor) Beit zur Tabak Inter. 1998;18(1):49–52. [Google Scholar]
- IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans . May, 1978. Some N-nitroso Compounds, Volume 17. [PubMed] [Google Scholar]
- IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans . 1985. Tobacco smoking, Volume 38. [PubMed] [Google Scholar]
- Kinsler S, Ayres PH, Mosberg AT. Rat subchronic inhalation study of smoke from cigarettes containing flue-cured tobacco cured either by direct-fired or heat exchanger curing processes. The Toxicologist. 2002;66:195. doi: 10.1080/08958370390217864. [DOI] [PubMed] [Google Scholar]
- Kutzer C, Richter E, Oehlmann C, Atawodi SE. Effects of nicotine and cotinine on NNK metabolism in rats. In: Clarke BPS, Quick M, Adlkofer F, Thurau K, editors. Effects of Nicotine on Biological Systems II. Birkhauser Verlag; Basel: 1994. pp. 385–390. [Google Scholar]
- Lee CK, Fulp C, Bombick BR, Doolittle DJ. Inhibition of mutagenicity of N-nitrosamines by tobacco smoke and its constituents. Mutat Res. 1996;367:83–92. doi: 10.1016/0165-1218(95)00078-x. [DOI] [PubMed] [Google Scholar]
- Lin JK. Nitrosamines as potential environmental carcinogens in man. Clin Biochem. 1990;23(1):67–7l. doi: 10.1016/0009-9120(90)90489-h. [DOI] [PubMed] [Google Scholar]
- Liu YK, Sundqvist, Belinsky SA, Castonguay A, Tjalve H, Grafstrom RC. Metabolism and macromolecular interaction of the tobacco-specific carcinogen 4–(methylnitrosamino)-l–(3–pyridyl)-l–butanone in cultured explains and epithelial cells of human buccal mucosa. Carcinogenesis. 1993;14:2383–2388. doi: 10.1093/carcin/14.11.2383. [DOI] [PubMed] [Google Scholar]
- Magee PN. The experimental basis for the role of nitroso compounds in human cancer. Canc Surv. 1989;8(2):207–239. [PubMed] [Google Scholar]
- Magee PN. Nitrosamines and human cancer: Introduction and overview. Eur J Canc Prev. 1996;5(1):7–10. doi: 10.1097/00008469-199609001-00002. [DOI] [PubMed] [Google Scholar]
- Meger M, Tricker AR, Scherer G, Adlkofer F, Klus H. 4–(N-methylnitrosamino)-1–(3-pyridyl)-1-butanol and its O-glucuronide in smoker and nonsmoker urine. 1st Annual Scientific Conference of the Society for Research on Nicotine and Tobacco; San Diego Bay, CA. March 24–25, 1995.1995. [Google Scholar]
- Murphy SE, Heiblum R. Effect of nicotine and tobacco-specific nitrosamines on the metabolism of NNN and NNK by rat oral tissue. Carcinogenesis. 1990;11:11663–1666. doi: 10.1093/carcin/11.9.1663. [DOI] [PubMed] [Google Scholar]
- Loeppky RN, Michejda CJ, editors. Nitrosamines and Related N-Nitroso Compounds-Chemistry and Biochemistry. American Chemistry Society Symposium Series 553; 1984. [Google Scholar]
- Padma PR, Amonkar AJ, Bhide SV. Mutagenic and cytogenetic studies of N’-nitrosonornicotine and 4–(methylnitrosamino)-1–(3-pyridyl)-1–butanone. Cancer Letters. 1989;46:173–180. doi: 10.1016/0304-3835(89)90127-4. [DOI] [PubMed] [Google Scholar]
- Parsons LL, Smith MS, Hamilton JL, MacKown CT. Nitrate reduction during curing and processing of burley tobacco. Tob Sci. 1986;30:100–103. [Google Scholar]
- Peele DM, Danehower DA, Coins GD. Coresta Information Bulletin. 1995. Chemical and biochemical changes during the flue-curing of tobacco. Abstract 9822. [Google Scholar]
- Peele DM, Riddick MG, Edwards ME, Gentry JS, Nestor TB. Formation of tobacco specific nitrosamines in flue-cured tobacco. Joint meeting of the CORESTA Agronomy & Phytopathology Study Groups; Suzhou, China. October 10–14, 1999.1999. CD-ROM version 11. [Google Scholar]
- Peterson LA, Hecht SS. O6–Methylguanine is a critical determinant of 4–(methylnitrosamino)-l–(3–pyridyl)-1–butanone tumorigenesis in A.J mouse lung. Cancer Pes. 1991;51:5557–5564. [PubMed] [Google Scholar]
- Preston-Martin S, Correa P. Epidemiological evidence for the role of nitroso compounds in human cancer. Canc Surv. 1989;8(2):459–473. [PubMed] [Google Scholar]
- Preussmann R, Eisenbrand G. N-Nitroso carcinogens in the environment. In: Searle CE, editor. Clinical Carcinogens. Vol. 2. American Chemical Society; Washington, D.C: 1984. pp. 829–868. ACS Monograph 182, [Google Scholar]
- Ren Q, Murphy SE, Danneneberg AJ, Park JY, Tephly TR, Lazarus P. Glucuronidation of the lung carcinogen 4–(methylnitrosamino)-1–(3–pyridyl)-1butanol (NNAL) by rat UDP-glucuronosyltransferase 2B1. Drug Metab Dis. 1999;27:1010–1016. [PubMed] [Google Scholar]
- Richter E, Tricker AR. Nicotine inhibits the metabolic activation of the tobacco-specific nitrosamine 4–(methylnitrosamino)-l–(3–pyridyl)-1–butanone in rats. Carcinogenesis. 1994;15:1061–1064. doi: 10.1093/carcin/15.5.1061. [DOI] [PubMed] [Google Scholar]
- Richter E, Friesenegger S, Engl J, Tricker AR. Use of precision-cut tissue slices in organ culture to study metabolism of 4–(methylnitrosamino)-l–(3–pyridyl)-1–butanone (NNK) and 4–(methylnitosamino)-l–(3–pyridyl)-1–bunaol (NNAL) by hamster lung, liver, and kidney. Toxicology. 2000;144:83–91. doi: 10.1016/s0300-483x(99)00193-6. [DOI] [PubMed] [Google Scholar]
- Ronai Z, Gradia S, Peterson LA, Hecht SS. G to A transitions and G-T transversion in codon 12 of the K-ras oncogene isolated from mouse lung tumors induced by 4–(methylnitrosamino)-l–(3–pyridyl)-1–butanone (NNK) and related DNA methylating and pyridyloxobutylating agent. Carcinogenesis. 1993;714:2419–2422. doi: 10.1093/carcin/14.11.2419. [DOI] [PubMed] [Google Scholar]
- Scanlan RA. N–nitroso compounds in carcinogenesis: an update of human exposure. American Chemical Society, Abstracts of Papers — Part 1, 217th ACS National Meeting; Anaheim, CA. March 21–25, 1999.1999. [Google Scholar]
- Schuller HM, Castonguay A, Orloff M, Rossignol G. Modulation of the uptake and metabolism of 4–(methylnitrosamino)-l–(3–pyridyl)-1–butanone by nicotine in hamster lung. Cancer Res. 1991;51:2009–2014. [PubMed] [Google Scholar]
- Schulze J, Schrader E, Foth H, Kahl GF, Richter E. Effect of nicotine or cotinine on metabolism of 4–(methylnitrosamino)-l–(3–pyridyl)-1–butanone (NNK) in isolated rat lung and liver. Naunyn-Schmiedeberg’s Arch Pharmacol. 1998;357:344–350. doi: 10.1007/pl00005177. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Guo Z, Gonzalez FJ. Metabolism of 4–(methylnitrosainino)-1–(3–pyridyl)-1–butanone (NNK) by human lung and liver microsomes and cytochrome P450 expressed in hepatoma cells. Cancel Res. 1992;52:1757–1763. [PubMed] [Google Scholar]
- Smith TJ, Stoner GD, Yang CS. Activation of 4–(methylnitrosamino)-1–(3–pyridyl)-1–butanone (NNK) in human lung microsomes by cytochrome P450, lipoxygenase, and hydroperoxides. Cancer Res. 1995;55:5566–5573. [PubMed] [Google Scholar]
- Spiegelhalder B, Fischer S. Formation of tobacco-specific nitrosamines. Abstract from the 15th International Cancer Congress, Hamburg, Germany, August 16–22, 1990. J Cancer Res Clin Oncol. 1990;116(2):1088. [Google Scholar]
- Spiegelhalder B, Bartsch H. Tobacco-specific nitrosamines. Eur J Cancer Prev. 1996;5(1):33–38. [PubMed] [Google Scholar]
- Staretz ME, Murphy SE, Patten CJ. Comparative metabolism of the tobacco-related carcinogens, benzo [a] pyrene, 4(methylnitrosamino)-1–(3–pyridyl)-1–butanone, 4–(methylnitrosamino)-1–(3–pyridyl)-1–butano, and N′-nitrosonornicotine in human hepatic microsomes. Drug Metab Dis. 1997;25:154–162. [PubMed] [Google Scholar]
- Startin JR. N-nitroso compounds in foods and drinks. Eur J Canc Prev. 1996;5(1):39. [Google Scholar]
- Tricker AR, Preussmann R. The occurrence of N-nitroso compounds [corrected] in zarda tobacco. Canc Lett. 1988;42:113–118. doi: 10.1016/0304-3835(88)90247-9. [DOI] [PubMed] [Google Scholar]
- Tricker AR, Spiegelhalder B, Preussmann R. Environmental exposure to preformed nitroso compounds. Canc Surv. 1989;8(2):251–272. [PubMed] [Google Scholar]
- Tricker AR, Ditrich C, Preussmann R. N-nitroso compounds in cigarette tobacco and their occurrence in mainstream tobacco smoke. Carcinogenesis. 1991;12(2):257–261. doi: 10.1093/carcin/12.2.257. [DOI] [PubMed] [Google Scholar]
- Tricker AR, Scherer G, Conze C, Adlkofer F, Pachinger A, Klus H. Evaluation of 4–(N-methylnitrosamino)-4–(3–pyridyl) butyric acid as a potential monitor of endogenous nitrosation of nicotine and metabolites. Carcinogenesis. 1993;14:1409–1414. doi: 10.1093/carcin/14.7.1409. [DOI] [PubMed] [Google Scholar]
- Tricker AR. N-Nitroso compounds and man: Sources of exposure, endogenous formation and occurrence in body fluids. Eur J Canc Prev. 1997;6(3):226–268. [PubMed] [Google Scholar]
- Trushin N, Hecht SS. Stereoselective metabolism of nicotine and tobacco-specific N-nitrosamines to 4–hydroxy-4–(3–pyridyl) butanoic acid in rats. Chem Res Toxicol. 1999;12:164–171. doi: 10.1021/tx980213q. [DOI] [PubMed] [Google Scholar]
- Tso TC. Production, Physiology, and Biochemistry of Tobacco Plant. Institute of International Development & Education in Agricultural and Life Sciences (IDEALS, Inc.); Beltsville, MD: 1990. [Google Scholar]
- U.S. Surgeon General . 1989. Reducing the health consequences of smoking: 25 years of progress. A report of the Surgeon General. U.S. Department of Health and Human Services, Office on Smoking and Health, Rockville, Maryland. [PubMed] [Google Scholar]
- Wiernik A, Christakopoulos A, Johansson L, Wahlberg I. Effect of air-curing on the chemical composition of tobacco. Rec Adv Tob Sci. 1995;21:39–80. [Google Scholar]
- Witschi H, Espiritu I, Maronpot RR, Pinkerton KE, Jones AD. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997;18(11):2035–2042. doi: 10.1093/carcin/18.11.2035. [DOI] [PubMed] [Google Scholar]
- Witschi H, Espiritu I, Yu M, Willits NH. The effects of phenethyl isothiocyanate, N-acetylcysteine, and green tea on tobacco smoke-induced lung tumors in strain A/J mice. Carcinogenesis. 1998;19:1789–1794. doi: 10.1093/carcin/19.10.1789. [DOI] [PubMed] [Google Scholar]
- Witschi H, Espiritu I, Uyeminami D. Chemoprevention of tobacco smoke-induced lung tumors in A/J strain mice with dietary myo-inositol and dexamethasone. Carcinogenesis. 1999;20:1375–1378. doi: 10.1093/carcin/20.7.1375. [DOI] [PubMed] [Google Scholar]
- Yahagi T, Nagao M, Seino Y, Matsushima T, Sugimura T, Okada M. Mutagenicities of N-nitrosamines on Salmonella. Mut Res. 1977;48:121–130. doi: 10.1016/0027-5107(77)90151-8. [DOI] [PubMed] [Google Scholar]