Abstract
The influence of low doses of guaiacol and ethanol, the natural effectors of lignin and phenolics transformations, on laccase and peroxidase activities produced by two strains of Basidiomycetes, Pleurotus sajor-caju and Trametes versicolor, was evaluated. Fungal mycelia were grown for 2 weeks on liquid media containing serial dilutions of guaiacol or ethanol ranging from 100−1 to 100−20 mol/L. Laccase and peroxidase activities in the medium were measured at the end of 2 weeks. The effect of low doses of guaiacol and ethanol on enzyme activities was manifested in an oscillating manner. Similar response patterns were observed when pure enzymes were exposed to the same serial dilutions of guaiacol and ethanol. T. versicolor cultures enriched with 40 mmol guaiacol (simulating natural environmental conditions) also displayed oscillating enzyme activity patterns in response to serial dilutions of guaiacol, but the maximum enzyme activity values were increased compared to those observed in cultures not receiving 40 mmol guaiacol. The differences between maxima and minima varied among the experimental groups and depended on the species of fungus, type of effector, and kind of enzyme. The results suggest the possibility of subtle regulation of enzymatic activity on the molecular level.
Keywords: laccase, peroxidase, Pleurotus sajor-caju, Trametes versicolor, ethanol, guaiacol, low doses
INTRODUCTION
The activities of enzymes catalyzing all metabolic pathways are in part regulated by low molecular effectors (Bonne 1997). Physiological events in cells are also regulated with the help of low molecular substances that influence the activity of respective receptors or enzymes in the metabolic pathway and regulate the speed of substrate flow by these pathways. The concentration of effectors is very important for initiation of events such as the activation of chemoreceptors or cascade of apoptotic processes in humans (Calabrese 2001a,b,c), as well as mechanisms of biochemical immunization in plants (Manniger et al. 1998; Katay and Tyihak 1998; Tyihak et al. 2002).
Rapoport and Luebering (1951) reported that diphosphoglycerate phosphatase is strongly activated by low concentrations of potential metabolic poisons, Hg(II) or Ag(I) ions. In 1964 Dixon and Webb reported that some effectors can act as poisons for some enzymes and activators for others. More recently, bimodal types of regulation of tyrosine kinase by low doses of the inhibitor genistein (Morimoto and Bonavida 1992) and of protein kinase C by very low concentration of some antioxidants (Maltseva 2002) have been described.
Effectors are usually divided into activators and inhibitors, but according to the hormetic principle the concept of activation and inhibition is strongly dependent on the concentration of effectors and their time of action (Calabrese and Baldwin 2000 and 2001a).
Fungi were chosen as the experimental model because they can be grown on various substrates and produce large amounts of extracellular enzymes, such as laccase and peroxidase, which cause the white rot of wood and are involved with the process of enzymatic delignification (Leonowicz et al. 1999; Luterek et al. 1998; Malarczyk and Widenska 2002). These enzymes are sensitive to the presence of various small molecular effectors that regulate the reactions involving the biodegradation of lignin and transformation of natural phenolics (Molitoris, 2001; Malarczyk et al. 2001). Many aromatic substances, mainly alcohols, aldehydes and acids such as ferulic, vanillic, and anisic are substrates for laccase. The induction of laccase is also possible with the same natural aromatic activators or similar synthetic substances such as 2,5-xylidin (Rogalski and Leonowicz 1992). Peroxidase, which requires H2O2 as a co-substrate, is often activated by the same substances. This article describes the effects of very low doses of guaiacol and ethanol on the activity of extracellular laccase and peroxidase in the liquid cultures of two fungi, Trametes versicolor and Pleurotus sajor-caju.
MATERIALS AND METHODS
Pure Enzymes
Cerrena unicolor laccase was purified in the Biochemistry Department, MCS University in Lublin according to the procedure described by Luterek et al. (1998). Horseradish peroxidase was purchased from Sigma Chemical Company.
Biological Models and Culture Conditions
Two species of fungi from the fungi collection of the Biochemistry Department, M.C.S. University, Lublin, Poland (i.e., strains Trametes versicolor, designated No. 20 and Pleurotus sajor-caju, designated No. 100 in the collection) were used as biological sources of laccase and peroxidase. Fungal mycelia were inoculated on the Lindeberg medium according to the method described by Luterek et al. (1998). After 10 days, the mycelia were homogenized with glass beads. Small conical flasks containing 10 ml medium were inoculated with the homogenate (0.5 ml homogenate/flask). Twenty-one flasks (a control and 20 serial dilutions of enzyme) were used in each experiment. Each experiment was replicated three times.
Preparation of Dilution and Stimulation Course
Serial dilutions (one part effector solution to 99 parts 75% ethanol) were prepared from solutions of guaiacol and ethanol (initial concentrations of 0.3 mol/L and 16 mol/L, respectively). The concentrations of the serial dilutions ranged from 100−1 to 100−20 mol/L [see Zenin’s refractometric studies (Zenin 1999a,b) for an explanation of molecular changes that may occur at ultra high dilutions]. Twenty μl of serial dilutions were added to the cultures every second day, beginning on day 3 of the experiment (a total of 120 μl/flask over the course of the 14-day experiment). Control cultures received after 14 days a total amount 120 μl/flask of 75% ethanol only. For experiments with pure enzymes, 200 μl of serial dilutions were added to each flask containing 200 μl of purified laccase or peroxidase in 700 μl of proper buffer and enzyme activities were measured at the end of 1 hour.
Enzyme Assays
Laccase activity was measured spectrophotometrically using syringaldazine (2.5 μM) as the substrate in 0.1 M citrate-phosphate buffer, pH 5.2 according to the method of Leonowicz and Grzywnowicz (1981). Peroxidase activity was measured using 4 μM of o-dianisidine in methanol and 10 mmoles of H2O2 as the substrate and co-substrate, respectively, in 0.1 M acetate-Na buffer, pH 5.5 according to the method of Claiborne and Fridovich (1979). Enzyme activities were expressed in nkatals where one nkatal is defined as the enzymatic degradation of one nmole of substrate during 1 sec and one international unit of enzymatic activity correspond to 16.67 nkatals.
Statistical Analysis
The results were analyzed by two-way ANOVA using the software package STATGRAPHICS version 2.6. Line regressing analyses were performed for the data from all 14 experiments presented in Table 1 and Figures 1 to 4. Trend lines are showed in all figures with proper equations. The values of maximal and minimal points (lying above or below the trend lines) were summarized and put in Figure 5 to illustrate how various dilutions of guaiacol and ethanol can work to activate and inhibit laccase and peroxidase activities (see also Javjock and Lewis, 2002).
Table 1.
Summary of data from the 14 experiments.
| No | Fungus or pure enzyme | Kind of enzymatic activity | Specific activity (nkatals/mg prot.) | Effector | –* (Maximum – Minimum) (nkatals) | Quantity of transits with J/U shapes |
|---|---|---|---|---|---|---|
| 1 | P.s-c. | Peroxidase. | 460 | ethanol | 200 | 4/2 |
| 2 | P.s-c. | Peroxidase. | guaiacol | 60 | 2/1 | |
| 3 | P.s-c. | Laccase | 4 155 | ethanol | 626 | 4/2 |
| 4 | P.s-c. | Laccase | guaiacol | 1724 | 3/1 | |
| 5 | Tr. vers. | Peroxidase. | 548 | ethanol | 664 | 3/1 |
| 6 | Tr. vers. | Peroxidase. | guaiacol | 680 | 2/2 | |
| 7 | Tr. vers. | Laccase | 5 020 | ethanol | 471 | 3/2 |
| 8 | Tr. vers. | Laccase | guaiacol | 1030 | 5/2 | |
| 9 | Tr. vers.** | Peroxidase. | 1000 | guaiacol | 2570 | 5/3 |
| 10 | Tr. vers.** | Laccase | 45 000 | guaiacol | 5870 | 3/2 |
| 11 | Peroxidase (pure) | Peroxidase.. | 2 720 | guaiacol | 88 | 4/2 |
| 12 | Peroxidase (pure) | Peroxidase | ethanol | 65 | 2/0 | |
| 13 | Laccase (pure) | Laccase | 600 000 | guaiacol | 20 | 6/4 |
| 14 | Laccase (pure) | Laccase | Ethanol | 40 | 4/2 |
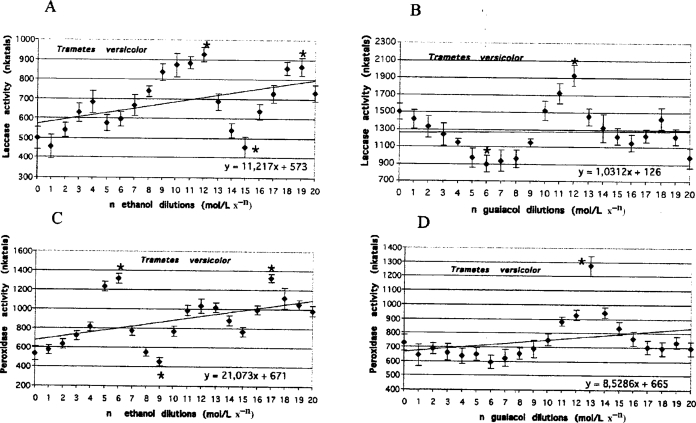
Figure 1.
Laccase activities measured on day 15 in the media of Trametes versicolor cultures grown in serial dilutions of ethanol and guaiacol, respectively (A and B). Peroxidase activities measured on day 15 in the media of Trametes versicolor cultures grown in serial dilutions of ethanol and guaiacol, respectively (C and D). Lines represent the results of trend analysis. The error bars mean the values (+/− SD) counted from the data of three replicate experiments. Mean values followed by asterisk (*) differ significantly (p=0.05) from control value according to results from STATSGRAPHICS program.
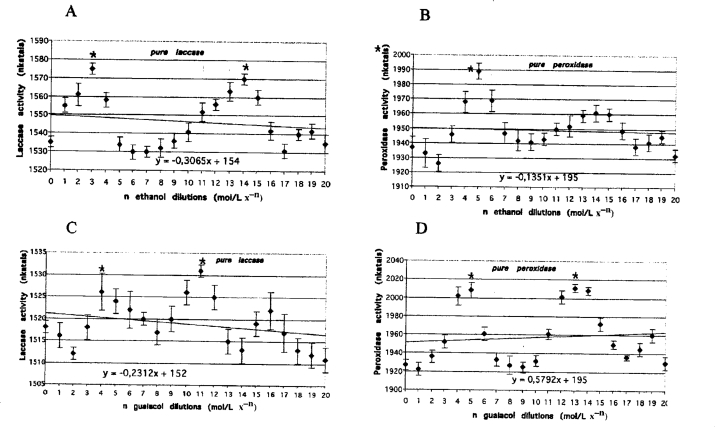
Figure 4.
Purified laccase (A and B) and pure peroxidase (C and D) activities measured after one hour of incubation with guaiacol or ethanol dilutions. Lines represent the results of trend analysis. The error bars mean the values (+/− SD) counted from the data of three replicate experiments. Mean values followed by asterisk (*) differ significantly (p=0.05) from control value according to results from STATSGRAPHICS program.
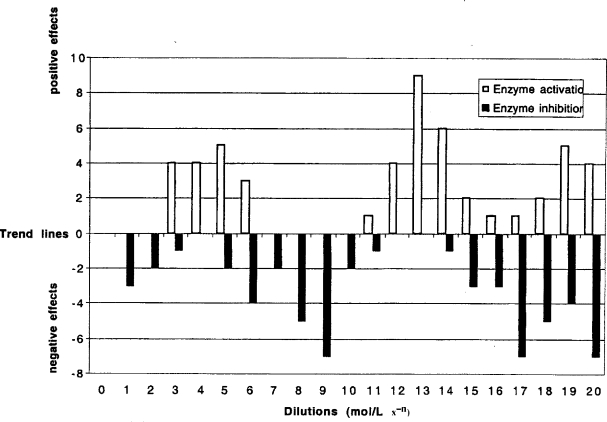
Figure 5.
Comparative analysis for the data from all 14 experiments presented in Table 1 and Figures 1 to 4. Trend lines are showed in all these figures and the values of maximal and minimal points (lying above or below the trend lines) were summarized to illustrate the activating or inhibiting possibilities of various dilutions of guaiacol and ethanol against laccase and peroxidase activities.
RESULTS
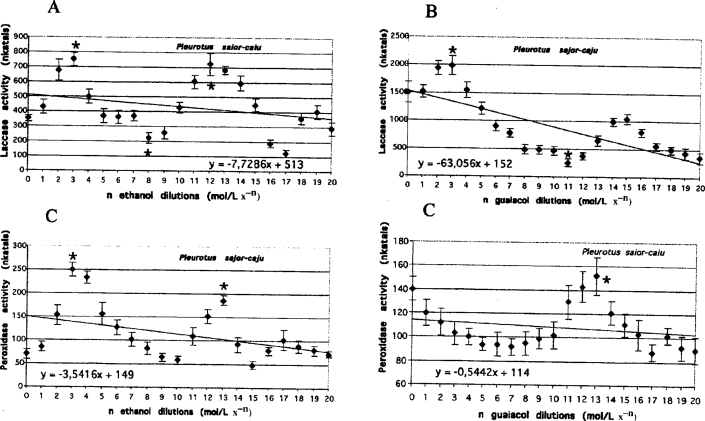
Oscillatory changes were observed in laccase and peroxidase activities in the media of both fungal species exposed to serial dilutions of guaiacol and ethanol. The oscillations were characterized by a similar number of maximal and minimal levels of activity (Figures 1 and 2, Table 1 no. 1 to 8).
Figure 2.
Laccase activities measured on day 15 in the media of Pleurotus sajor-caju cultures grown in serial dilutions of ethanol and guaiacol, respectively (A and B). Peroxidase activities measured on day 15 in the media of Pleurotus sajor-caju cultures grown in serial dilutions of ethanol and guaiacol, respectively (C and D). Lines represent the results of trend analysis. The error bars mean the values (+/− SD) counted from the data of three replicate experiments. Mean values followed by asterisk (*) differ significantly (p=0.05) from control value according to results from STATSGRAPHICS program.
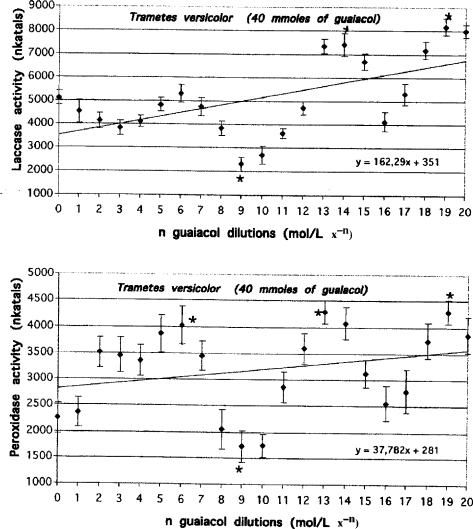
Similar oscillatory changes were observed in 40 mmol guaiacol-enriched cultures of T. versicolor exposed to serial dilutions of guaiacol. However, the distance between the maximal and minimal activity values increased more dramatically. For peroxidase it reached approximately 2570 nkatals (Figure 3, Table 1 no. 9) and for laccase it reached up to 5870 nkatals (Figure 3, Table 1 no. 10). The corresponding values for non-activated cultures of T. versicolor were approximately 680 nkatals for peroxidase and 1030 nkatals for laccase.
Figure 3.
Laccase and peroxidase activities measured on day 15 in the media of Trametes versicolor cultures enriched with 40 mmol of guaiacol and exposed to serial dilutions of guaiacol. Lines represent the results of trend analysis. The error bars mean the values (+/− SD), which were calculated from the data of three replicate experiments. Mean values followed by asterisk (*) differ significantly (p=0.05) from control value according to results from STATSGRAPHICS program.
Oscillatory changes were also observed in solutions of purified laccase and peroxidase, although the distance between the maximal and minimal activity values were much lower compared to those obtained from the fungal culture media (i.e., for peroxidase the distance between maximum and minimum did not exceed 90 nkatals (Figure 4 C and D, Table 1 no. 11 and 12) and for laccase the distance did not exceed 40 nkatals (Figure 4 A and B, Table 1 no. 13 and 14). The frequency of oscillations in enzymatic activity, however, was similar to that observed for the enzymes in fungal cultures.
Figure 5 represents an assessment of the combined data for the 14 different experiments and indicates the most likely probability of observing a maximal enzymatic activity.
DISCUSSION
Guaiacol and ethanol are found in the natural environment and essential for the metabolism of the fungi Trametes and Pleurotus. Increasing dilutions of both effectors showed the exponential dependence between the enzymatic protein reaction and the effector’s concentration. The oscillatory character of activity changes was observed for both enzymes in showing systematically repeated frequencies (Figure 5), indicating the tendency of some effector’s dilutions to stimulate the enzyme activity while other dilutions inhibit.
It should be emphasized that in all experimental conditions the oscillatory patterns were similar. However, the distance between maximum and minimum of activity did vary among the experimental groups and depended on the species of fungus, type of effector and kind of enzyme. Generally, the laccase activity level in the Trametes cultures was always higher than that in the Pleurotus cultures, which had an effect on the distance between the maximum and minimum activity values. Guaiacol showed a greater stimulation of laccase activity in both Trametes and Pleurotus cultures, whereas ethanol showed a greater stimultaion of peroxidase activity in the Pleurotus cultures.
In the case of Trametes cultures enriched with 40 mmol of guaiacol, statistically significant (p=0.05; see Figure 3) increases in the activities of both enzymes were observed in response to serial dilutions of guaiacol. Maximum laccase activity increased to approximately 8000 nkatals compared with 504 nkatals in the control cultures. Maximum peroxidase activity increased to 4250 nkatals compared to 54 nkatals in the control cultures. It is conceivable that this activation can take place in nature in in vivo conditions during fungal growth. Low concentrations of guaiacol may be generated during fungal metabolism of various phenol substances.
Changes in activity of both enzymes (Table 1) assume the shape of the letters J or U described as characteristic of hormesis (Calabrese 2001). The occurrence of oscillatory patterns of enzyme activation/inhibition in response to effectors should be examined for other species of fungi and other enzymes. The findings reported here suggest the possibility of subtle regulation of enzymatic activity on the molecular level and clarification of these processes should be the focus of future research.
Acknowledgments
This work was supported by European Community (Contract ICA2-CT-2000-10050), the Polish Committee for Scientific Investigations 139/E-339/SPUB-M-5PR-UE/DZ 280/200 and BS/BiNoZ/4.
REFERENCES
- Bonne C. From stimulus to message in biological systems, an illustrated survey (review) In: Bastide M, editor. Signals and Images. Kluver Academic Publishers; 1997. pp. 90–93. [Google Scholar]
- Calabrese E. Dopamine: biphasic dose responses. Crit Rev Toxicol. 2001a;31(4–5):63–583. doi: 10.1080/20014091111839. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Opiates: biphasic dose responses. Crit Rev Toxicol. 2001b;31(4–5):585–604. doi: 10.1080/20014091111848. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Apoptosis: biphasic dose responses. Crit Rev Toxicol. 2001c;31(4–5):607–615. doi: 10.1080/20014091111866. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Baldwin L. Chemical hormesis: its historical foundations as a biological hypothesis. Hum Exp Toxicol. 2000;19:2–32. doi: 10.1191/096032700678815585. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Baldwin L. Hormesis: a subordination generalizable and unifying hypothesis, Crit Rev Toxicol. 2001a;31(4–5):353–424. doi: 10.1080/20014091111730. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Baldwin L. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci. 2001b;62:330–338. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Baldwin L. U-shaped dose-responses in biology, toxicology, and public health. Annu Rev Public Health. 2001c;22:15–33. doi: 10.1146/annurev.publhealth.22.1.15. [DOI] [PubMed] [Google Scholar]
- Clairborne AC, Fridovich I. Chemical and enzymatic intermediates in the peroxidation of o-dianisidine by horseradish peroxidase. Biochemistry. 1979;18:2324–2330. doi: 10.1021/bi00578a029. [DOI] [PubMed] [Google Scholar]
- Dixon M, Webb EC. Enzyme cofactors (IX) In: Dixon M, Webb EC, editors. Enzymes. Longmans; 1964. pp. 420–429. [Google Scholar]
- Javjock MA, Lewis PG. Implications of hormesis for industrial hygiene. BELLE Newsletter. 2002;10(3):2–7. doi: 10.1191/0960327102ht264oa. [DOI] [PubMed] [Google Scholar]
- Katay G, Tyihak E. Effect of 1-methylascorbigen on the resistance potential of plants to pathogen. Acta Biologica Hungarica. 1998;49(2–4):429–437. [PubMed] [Google Scholar]
- Leonowicz A, Grzywnowicz K. Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as a substrate. Enzyme Microbiol Technol. 1981;3:55–62. [Google Scholar]
- Leonowicz A, Matuszewska A, Luterek J, Zigenhagen D, Wojtas-Wasilewska M, Cho N-S, Hofrichter M, Rogalski J. Bidegradation of lignin by white rot fungi. Fungal Genet Biol. 1999;27:175–185. doi: 10.1006/fgbi.1999.1150. [DOI] [PubMed] [Google Scholar]
- Luterek J, Gianfreda L, Wojtas-Wasilewska M, Cho N-S, Rogalski J, Jaszek M, Malarczyk E, Staszczak M, Fink-Boots M, Leonowicz A. Activity of free and immobilized extracellular Cerrena unicolor laccase in water miscible organic solvents. Holzforschung. 1998;52:589–595. [Google Scholar]
- Malarczyk E, Jarosz-Wilkolazka A, Jaskowska A, Kochmanska-Rdest J. Cultures of Basidiomycetes as the object of homeopathic experiments. Int J Medicinal Mushrooms. 2001;3(2–3):177–178. [Google Scholar]
- Malarczyk E, Widenska A. Relationship between tyrosinase, laccase, peroxidase and superoxide dismutase activities during the development of Pleurotus cystidiosus. Intern J Medical Mushrooms. 2002;4:49–57. [Google Scholar]
- Maltseva L. Bimodal type of regulation of protein kinase C by antioxidants down to ultra-low doses. Materials of Intern. Conference: Non-linear dose-response relationships in biology, toxicology and medicine; Amherst, MA, USA. June 11–13.2002. [Google Scholar]
- Manninger K, Csösz M, Tyihak E. Induction of resistance of wheat plants to pathogens by pretreatment with N-methylated substances. Acta Biologica Hungarica. 1998;49(2–4):275–281. [PubMed] [Google Scholar]
- Molitoris H-P. Mushrooms and man in medicine, myth and religion. Int J Medicinal Mushrooms. 2001;3:97–98. [Google Scholar]
- Morimoto H, Bonavida B. Diphteria-toxin and Pseudomonas A toxin-mediated apoptosis: ADP-ribosylation of EF-2 is required for DNA fragmentation and cell lysis and synergy with TNF-alfa. J Immunol. 1992;49:2089–2094. [PubMed] [Google Scholar]
- Rapoport S, Luebering J. Glycerate-2,3-diphosphatase. J Biol Chem. 1951;189:683–690. [PubMed] [Google Scholar]
- Rogalski J, Leonowicz A. Phlebia radiata laccase forms induced by veratric acid and xylidine in relation to lignin peroxidase and manganese-dependent peroxidase. Acta Biotechnol. 1992;12:213–221. [Google Scholar]
- Tyihák E, Steiner U, Schönbeck F. Time- and dose-dependent double immune response of plants to pathogens. Modern Fungicide and Antifungal Compounds III, 13th Intern Reinhardsbrunn Symp; May 14th–18th 2001; Friedrichroda, Germany. 2002. pp. 187–196. (AgroConcept GmbH Bonn) [Google Scholar]
- Zenin SV. The complex formation of acetonitril and methyl alcohol with water. Russian Journal of Physical Chemistry (Zhurnal Fizitcheskoj Chimii) 1999a;73(5):835–838. [Google Scholar]
- Zenin SV. A new method of the scientific description of traditional medicine. Russian Journal: Narodnaja Medicina of Russia (theory and practice) 1999b;3:6–7. [Google Scholar]