Abstract
Chronic low-level exposure to environmental toxins, including cadmium (Cd), is a growing problem in the industrialized world. One promising strategy for protection from these toxins is the use of low-dose exposure of environmental chemicals to induce cell tolerance and recovery, a phenomenon known as “protective hormesis”. Hormetic [low-dose stimulatory] effects occur in a variety of systems and with a number of chemicals. Cd is a potent carcinogen in rodents and has also been linked to human lung and prostate cancers. In the present study, we have evaluated the protective effects of low and ultra-low dose, long-term Cd exposure in the normal human prostate cells, RWPE-1. Cells were exposed to low and ultra-low doses (0, 0 (S−36), 10−6, 10−7, 10−18, 10−21, 10−32, or 10−36 M) of Cd for 20 weeks followed by treatment with 10−5 M Cd for another 8 weeks. Continuous exposure of RWPE-1 cells to 10−5 M Cd results in malignant transformation. However, cells pretreated with low and ultra-low doses of Cd had delayed transformation compared with controls. In addition, the number of transformed cell mounds was lower in pretreated cells indicating that low and ultra-low dose exposure had protective effects against high-dose Cd induced carcinogenesis. The expression of metallothionein (MT), the primary Cd detoxification protein, was induced by low-dose exposure to Cd and maintained during the 20 weeks. In addition, MT-1G mRNA was up-regulated 2- to 3-fold by low-dose and ultralow-dose Cd exposures and may be the mechanism of protective hormesis in this model. MT-1G mRNA might also serve as a biological indicator of very low-dose environmental Cd exposure.
Keywords: Cd, metallothionein (MT), prostate, low dose, ultra-low-dose, hormesis
INTRODUCTION
In the modern era, humans and other living organisms are frequently exposed to environmental pollutants such as heavy metals that have significant adverse biological effects. However, exposure to low doses of toxic agents are reported to have beneficial protective effects in a number of models (Davis and Svendsgaard, 1990; Stebbing 2000; Calabrese and Baldwin 2001), a phenomenon known as “protective hormesis”. Hormesis is the stimulatory effect of low-dose toxin exposures and has been demonstrated in a wide variety of organisms and for a large number of chemicals and radiation (Calabrese and Baldwin 2003). Cadmium (Cd) is an environmental pollutant with a number of harmful effects and is linked to human cancers of lung (Stayner et al. 1992), nasal sinuses (Jarup et al. 1998), and prostate (Elghany et al. 1990; Armstrong and Kazantzis 1985). It is classified as human carcinogen by The International Agency for Research on Cancer (1993) and The National Toxicology Program (2000). However, biologically advantageous effects of low-level Cd exposure have been reported in different models (Weis and Weis 1986; Herkovits and Perez-Coll 1995). Metallothioneins (MTs) are cysteine-rich, low-molecular-weight metal-binding proteins that play a major role in the protection of cells from heavy metals such as Cd. In a study with mouse cells (Damelin et al., 2000) hormetic activity from low levels of cadmium chloride was correlated with increased levels of Hsp 70 and metallothionein (MT) indicating a stress response. Achanzar et al. (Achanzar et al. 2001) have shown that exposure of normal human prostate epithelial cells to cadmium induces malignant transformation. They have also demonstrated acquisition of apoptotic resistance in these cadmium-transformed cells (Achanzar et al. 2000). The development of resistance to the cancer chemotherapeutic agent adriamycin in cadmium-resistant DU-145 human prostate cancer cells was demonstrated by Webber et al (Webber et al. 1988). Hormetic effects of Cd preexposure were also demonstrated during fin regeneration in fish (Weis and Weis 1986). In the present study we have attempted to determine the protective hormetic activity of Cd, using human prostate cells. Normal prostate cells were exposed for prolonged periods to different low and ultra-low doses of Cd and were analyzed for their expression of MT both at protein and mRNA levels. In addition, long-term preexposure to low and ultra-low dose Cd was examined for its ability to protect prostate cells from malignant transformation from exposure to higher Cd doses.
MATERIALS AND METHODS
Cells
Human normal prostate cell line RWPE-1 (Bello et al. 1997; Webber et al. 1997) was kindly provided by Dr. Mukta M. Webber, Department of Zoology, Michigan State University, East Lansing, MI. These cells were grown in Keratinocyte-serum-free medium supplemented with 5 ng/ml epidermal growth factor (EGF) and 50 (μg/ml bovine pituitary extract (BPE) in the presence of antibiotic-antimycotic solution. Cells were maintained in a humidified incubator containing 5% CO2 at 37°C.
Cd Preparation and Cell Treatment
Cd solutions were prepared from a 0.01 M CdCl2 (Sigma-Aldrich Corp., St. Louis, MO) stock solution by 10-fold serial dilutions with Cd-free water. Between each dilution they were subjected to 120 manual succussions. RWPE-1 cells were exposed to Cd at 0, 0 (S−36), 10−6, 10−7, 10−18, 10−21, 10−32 and 10−36 M concentrations for 20 weeks. 0 (S−36) denotes a treatment with water that was processed in the same way as Cd using serial dilutions and succussions but with no Cd added. After 20 weeks, these cells were challenged with a higher concentration of Cd (10−5 M) for 8 weeks. This exposure is known to induce malignant transformation of RWPE-1 cells (Achanzar et al. 2001). Cells were subsequently grown in Cd-free medium for another 4 weeks. Cells were subcultured weekly and subdivided for measurement of cell growth, cell viability, RNA extraction, and MT assays.
Chemical Analysis of Low and Ultra-Low Dose Cd Preparations and Media
All solutions and media were analyzed weekly for any extraneous Cd and other metal contaminants as follows. Cd concentration in samples was determined using a graphite furnace atomic absorption spectrometer (GFAAS), (Perkin Elmer Analyst 800). Operating conditions included the use of an EDL lamp, wavelength 228.8 nm and slit width 0.7 nm. The background detection limit (BDL) is 0.005 μg/l. The BDL was determined as two times the standard deviation (2SD) from an analysis of 20 consecutive blank solutions with no Cd added. The method’s detection limit is between 0.025 and 0.050 μg/l. A four-point calibration curve (standard range from 0.025 to 3.0 μg/l) was used to determine the Cd concentration in each of the tested solutions. Sample blanks, certified standard reference materials (NIST, Gaithersburg, MD), and spiked samples (i.e., samples containing a known amount of an analyte) were used for quality assurance. Furthermore, inductively coupled plasma-optical emission spectrometry (ICP-OES) (Perkin Elmer Optima 3000) was used to test the dilutions for purity and contaminants. A similar quality assurance procedure based on the use of sample blanks, standards (0.1 to 30 mg/l), certified standard reference materials and spiked samples were also used to conduct the ICP-OES measurements. The ICP-OES method was used to determine and monitor the concentration of 29 elements (Ag, Al, As, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, Hg, K, Li, Mg, Mn, Mo, Na, Ni, Pb, Sb, Se, Si, Sn, Sr, Ti, Tl, V, and Zn).
Cell Growth and Viability Assays
RWPE-1 cells were seeded at a concentration of approximately 1 × 106 cells/T-75 flask. CdCl2 was added in appropriate concentrations, and the cells were passed once weekly. During subculture, cells were counted in a suspension after trypsinization for each treatment. Cd treated RWPE-1 cells were plated in 96 well tissue culture plates in a range of 3.0 × 104 cells per well in a final volume of 100 μl. After 24 to 48 h, viability was determined using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromid) assay using Cell Proliferation Kit I (Roche Applied Science, Indianapolis, IN) as per the manufacturer’s instructions. Briefly, 10 μl of MTT labeling reagent was added and incubated for approximately 4 h in a humidified incubator at 37°C, 5% CO2. Solubilization solution (100 μl) was then added and incubated again overnight. The spectrophotometrical absorbance was measured with a microtiterplate reader at a wavelength of 570 nm (Dynatech Laboratories, Chantilly, VA).
MT Protein Measurement
MT protein was quantified, by using the 109Cd-hemoglobin assay (Eaton and Toal 1982). Following Cd treatment, cells were harvested with trypsin and lysed in cold 10 mM Tris HC1 (pH 7.4) by passing through a 24-gauge needle. Total protein concentration of each sample was determined by using BCA protein assay kit (PIERCE, Rockford, IL). Aliquots of the sample were mixed with CdCl2 solution containing 109Cd and hemoglobin. The mixture was heated for 2 min at 100°C and centrifuged at 10,000 × g. This step is repeated one more time and radioactivity in supernatant was measured. MT levels were normalized to cellular protein.
mRNA Analysis by RT-PCR
Cells were harvested and total RNA was prepared using Trizol method (Life Technologies, Gaithersburg, MD). PCR primer oligonucleotides for the isoforms of MT were as described by Mididoddi et al. (1996) and Barnes et al. (2000). Hsp 70 primers were purchased from StressGen Biotechnologies Corp, Victoria, BC, Canada. Glyceraldehyde-6-phosphate dehydrogenase (G6PDH) primers were obtained from Chemicon International Inc. (Temecula, CA) and were used to determine the equal levels of target RNAs. Complementary DNA was synthesized using 1 to 2 μg of total RNA and Superscript II RNase H-reverse transcriptase (Life Technologies, Gaithersburg, MD) following the manufacturer’s instructions. PCR reaction mixture contained 10 pmol of each primer pair and 2.5 U of Taq DNA polymerase (Life Technologies, Gaithersburg, MD). Linear range of amplification of each gene was determined by carrying out 18 to 35 cycles in increments of 3 cycles each. Based on the results, amplification reactions were carried out through 20 to 35 cycles (95°C to 30 s; 55°C to 45 s; 72°C to 45 s), using 10% of cDNA. PCR products (10 μl each) were analyzed by electrophoresis on 2% agarose gels. Densitometric analysis of the PCR products was performed with Instant Imager (Packard, Meriden, CT).
mRNA Analysis by Ribonuclease Protection Assay (RPA)
mRNA expression of stress genes in RWPE-1 cells treated with Cd were analyzed using Riboquant multiprobe ribonuclease protection assay kit (BD Biosciences, Palo Alto, CA) and North2South Biotin in vitro transcription kit (PIERCE, Rockford, IL). The protocols used for the RPA were according to the manufacturer’s instructions. Briefly, biotinylated RNA probe was prepared by transcribing the hStress-1 template set (bcl-x, p53, GADD45, c-fos, p21, bax, bcl-2, mcl-1, L32, and GAPDH) using T7 RNA polymerase. 20 μg of total RNA from each sample was hybridized with the labeled probe at 56°C for 12 to 14 h. Free probe and unhybridized RNA were digested with RNases for 45 min at 30°C. The RNase protected probes were purified and resolved on denaturing urea polyacrylamide gels (Invitrogen, Gaithersburg, MD). Protected fragments were transferred to Biodyne nylon membrane (PIERCE, Rockford, IL) by electrical transfer at around 100 V for 1 h. Protected probes were detected by SuperSignal RPA III chemiluminescent Detection Kit (PIERCE, Rockford, IL) and the membrane was exposed to X-ray film for 1 to 2 min. L32 and GAPDH mRNAs are housekeeping genes and served as controls in the assay to ensure equal loading of RNAs.
Statistical Analysis
All data are expressed as mean ± SEM of 3 to 5 sets of analysis. Data were analyzed by using one-way ANOVA and the Tukey-HSD multiple comparisons test for post hoc analysis (SPSS Ver. 10.1, SPSS Inc., Chicago, IL). Significance is defined as a p-value of less than 0.05.
RESULTS
Chemical analysis for Cd dose in preparations and screening for 29 other potential contaminants verified that Cd exposure levels were those predicted by dilution level with 10−6 M measuring approximately 0.5 mg/1 and exposure levels below this measuring below the detection limit. No evidence of absorption of change in Cd level exposures dilutions was evident over the 20 weeks. Silicon from the glassware remained constant in all samples at approximately 0.5 mg/1 throughout the experiment (data not shown).
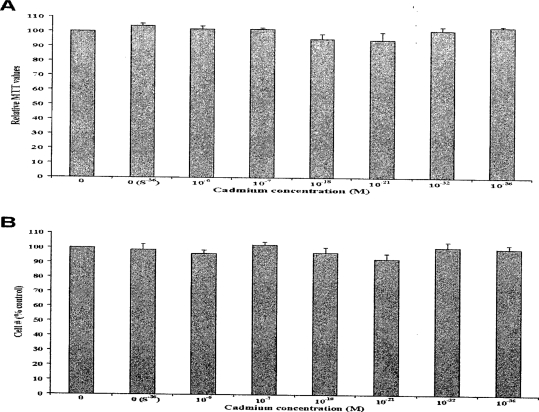
In order to study the effect of Cd on RWPE-1, cells were exposed to 0, 0 (S−36), 10−6, 10−7, 10−18, 10−21, 10−32, and 10−36 M Cd for 20 weeks. Cell proliferation was determined by measuring the number of attached cells during each passage. At these doses of Cd, no significant changes occurred in the cell morphology (data not shown) and growth compared to control as shown in Figure 1A from a representative data set of one passage. Cytotoxicity as measured by MTT assay also showed no significant differences between Cd treated and untreated control RWPE-1 cells (Figure 1B). Taken together, the results of these two assays suggest that at these doses Cd did not cause any significant inhibitory or proliferative changes on cell growth.
Figure 1.
Effect of Cd treatment on RWPE-1 cell growth and viability. (A) Representative data from cells treated with various concentrations of Cd [0, 0 (S−36), 10−6, 10−7, 10−18, 10−21, 10−32, or 10−36 M]. Attached cells were counted during each passage and expressed as percentage of control and no significant differences were noted. (B) Cell viability as assayed by MTT from three sets also indicate no cytotoxicity.
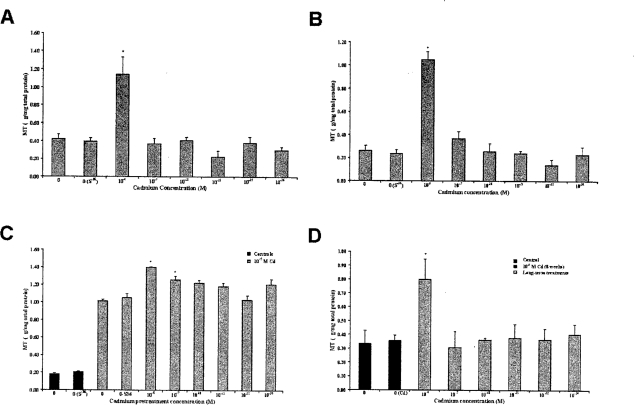
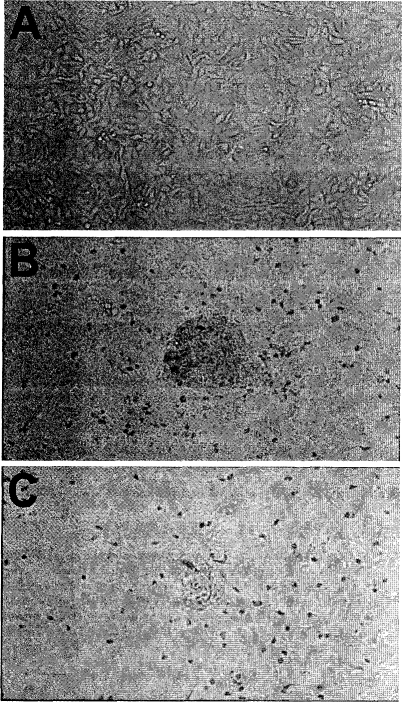
To determine the modulations in the expression of MT as a consequence of Cd treatment, MT protein measurement was performed by 109Cd-hemoglobin assay (Figure 2). Treatment of RWPE-1 cells with Cd (10−6, 10−7, 10−18, 10−21, 10−32, or 10−36 M)doses resulted in significant increase in MT protein expression only for 10−6 M concentration. A representative data set for MT protein levels from the third week of treatment is shown in Figure 2A. Furthermore, this increased MT level was maintained during the prolonged Cd exposure through 20 weeks (Figure 2B). Challenging these 20 weeks low-dose Cd pretreated cells with 10−5 M Cd for another 8 weeks increased in MT levels further for all treatments (Figure 2C). Although the increase was higher in all Cd pretreated cells, it was statistically significant only for the 10−6 and 10−7 M Cd pretreated cells when compared to passage matched positive controls. Growing these pretreated cells (10−6 to 10−36 M Cd for 20 weeks followed by treatment with 10−5 M Cd for 8 weeks) in normal media (0 M Cd) for another 4 weeks resulted in transformation. However, the cells pretreated with low and ultralow doses of Cd were slow in developing the transformed cell mounds compared to controls (Figure 3). Also, the number of transformed cell mounds was 35% less in pretreated cells compared to controls, indicating a protective effect from low-dose pretreatments. Analysis of these cells showed significant difference in MT levels between 10−6 M Cd treatment and controls (Figure 2D). Treatment with succussed water [Cd 0 (S−36)] did not show any significant differences, and the cell behavior and expression pattern were similar to normal cells (Figures 2A, B, and C).
Figure 2.
Effect of Cd on MT protein expression in RWPE-1 cells as analyzed by 109Cd-hemoglobin assay. Cells were continuously treated with various concentrations of Cd for 20 weeks. MT expression: (A) at 3rd week and (B) at 20th week resulting in a significant increase in MT for 10−6 M Cd concentration. C. Cd pretreated cells, exposed to 10−5 M Cd for 8 weeks showed increased MT levels and were significant for 10−6 or 10−7 M Cd pretreatments. (D) Cells from C were grown for additional 4 weeks in normal Cd-free media. 10−6 M Cd-pretreated cells maintained significantly increased MT level. Values are mean ± SEM of three analyses. *P<0.05 Cd treated compared with controls.
Figure 3.
Growth of RWPE-1 cells after Cd treatments. (A) Monolayer of cells in untreated control. (B) Cells after treatment with 10−5 M cd for 8 weeks followed by growth in normal media show clumping, whereas (C) similar treatment to longterm Cd pretreated cells resulted in clumps of smaller size and fewer in number.
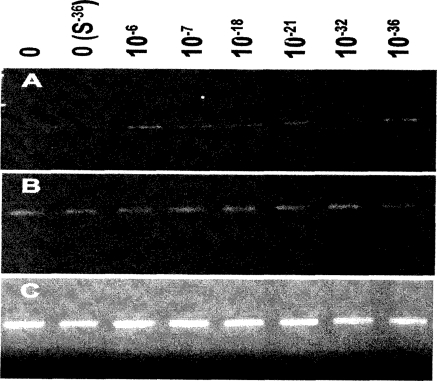
We have investigated the mRNA expression by semiquantitative RT-PCR for all 10 functional MT isoforms in RWPE-1 cells subjected to Cd treatment as detailed above. No significant differences were observed for any of the isoforms between controls and Cd exposed cells except for MT-1G. Increased expression (2- to 3-fold) of isoform MT-1G with Cd exposure is maintained for all low and ultra-low levels (Figure 4) and throughout the treatment duration of 20 weeks. No significant differences in mRNA levels were observed between controls of low dose Cd exposed cells for stress genes bcl-x, p53, GADD45, c-fos, p21, bax, bcl-2, mcl-1 as analyzed by RPA (data not presented). No significant changes occurred in the mRNA expression of Hsp 70 as analyzed by RT-PCR for any of the treatments (Figure 4).
Figure 4.
Effect of low-does Cd on mRNA expression of MT-1G, Hsp 70, and GAPDH in RWPE-1 cells as analyzed by RT-PCR. Representative picture showing PCR products from cells treated with low and ultralow doses of Cd for 20 weeks. (A) Increased levels of MT-1G mRNA (2- to 3-fold) and densitometric analysis from three experiments show no significant difference between Cd-treated specimens (data not shown). (B) No significant changes were observed in Hsp 70 levels. (C) Equal amounts of RNA were checked by analyzing GAPDH expression.
DISCUSSION
Hormetic effects of harmful substances, have been reported since the 19th century by Schulz (1887, 1888), yet their mechanisms remain largely uninvestigated. Bukowski and Lewis (2000) have reviewed hormetic patterns in a wide range of examples including humans and concluded that it may be a universal phenomenon. The idea that low-dose stimulatory effects may be useful for subsequent cellular protection is a new concept; however, its scope and mechanisms are largely unknown. We have explored this phenomenon by analyzing changes in mRNA and protein levels in normal prostate cells exposed to low and ultralow dose Cd. The results obtained in this study indicate that prolonged exposure to low dose Cd induces tolerance toward higher doses of Cd and induces metallothionein expression. Higher levels of MT were maintained even after 4 weeks of growth in Cd-free media when followed by treatment with a higher concentration of Cd. In addition, MT mRNA is upregulated by both low and ultralow Cd exposure, although almost exclusively in the MT-IG isoform. These observations are consistent with the findings of Croute et al. (2000), where human lung cells (A549) exposed to 0.1, 1, or 10μM Cd for 4 or 31 days overexpressed MT. In addition, prolonged exposure of mice to low dose Cd through diet resulted in inhibition of spontaneous carcinogenesis, which was attributed to increased MT production (Nishiyama et al. 2003). Others have also found that exposure of mice to low dose Cd for 11 to 12 weeks does not cause pronounced cytogenetic effects (Osipov 2002). Pretreatment with low dose Cd has been shown by several groups to induce tolerance against lethal dose of Cd in rats (Klaassen and Liu 1997; Delbancut et al. 1997). Similarly, Bufo arenarum embryos developed significant resistance against Cd toxicity with low-dose Cd pretreatment (Herkovits and Perez-Coll 1995). B6C3F1 mice treated with nitrosodiethylamine followed by Cd treatment suppressed spontaneous liver tumors and reduced the incidence of lung tumors (Waalkes et al. 1991, 1996), and again a role for metallothionein was demonstrated. There are also reports demonstrating beneficial effects of low-dose treatments other than Cd (Linde et al. 1994).
It is well known that MT plays a major role in the cellular kinetics and metabolism of Cd. Multiple functions of MTs, including their role in protection against Cd toxicity, are reviewed by Klaassen et al. (1999). Mammalian MTs belong to Class I and are composed of four subfamilies MT-1, MT-2, MT-3, and MT-4. A total of 10 functional and 6 nonfunctional MT isoforms have been identified in humans (Quaife et al. 1994; Palmiter et al. 1992; West et al. 1990; and Stennard et al. 1994). The role of these subfamilies and isoforms in metal homeostasis and detoxification are not completely clear. Only 1 of the 10 isoforms, MT-1G, showed a change in mRNA expression in the long-term, low-, and ultralow-dose exposed cells. No expression of MT-1G was detected either in normal human prostate tissues or in human prostate cancer cell lines DU-145, PC-3, and LNCaP (Garett et al. 2000). The induction of MT-1G with all low doses and its continued expression during the 20-week Cd treatment suggests a functional significance for this isoform in cell transformation even in the absence of increased MT production. Other isoforms did not have similar mRNA up-regulation patterns, suggesting that they may not be mediators of low-dose Cd-induced protection. However, they may be contributing for overall protein content.
Continuous treatment with 10 μM Cd induces transformation of RWPE-1 cells and acquires apoptotic resistance, forming cell mounds (Achanzar et al. 2001, 2002). The change in cellular sensitivity after exposure to low-dose Cd and the modulation of MT expression is one possible explanation for the reduced transformation observed in our experiments. Hormetic effect of a 0.5 mg/liter Cd preexposure during fin regeneration in fish was significant over 0.1 mg/1 treatment (Weis and Weis 1986). The lack of hormetic response at lower dose treatment may be due to inadequate preexposure time. Besides MTs, there may be other cellular processes that contribute for protection, and these need to be evaluated. We found both reduced and delayed transformation in cells preexposed to low- and ultralow dose Cd when MT-1G mRNA levels were up-regulated regardless of whether MT proteins were also effected. This suggests a central role for MT-1G mRNA in cellular protection from high dose Cd. In addition, MT-1G may be a sensitive biomarker for the detection of low and ultralow-dose environmental exposure to Cd.
In conclusion, our experiments show that long-term exposure of normal prostate cells to Cd at low and ultralow-doses impart biological activity resulting in protection against toxic doses. These observations also provide insights into possible mechanisms of action and are relevant to furthering our understanding of hormetic phenomenon. Further studies are in progress using microarray technology in order to examine possible low-dose Cd-induced gene expression signatures.
Acknowledgments
This work was supported by NIH (NCCAM) grant R21-AT00270-01 and by the Samueli Institute for Information Biology. The authors are grateful to Dr. Mukta M. Webber, Michigan State University, for kindly providing us with the RWPE-1 cells used in this study and to Drs. William Achanzar and Michael Waalkes of the National Institute of Environmental Health Sciences, NIH, for their help in background and development of the concept. The opinions or assertions contained herein are the private views of the authors and should not be construed as official or necessarily reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense.
REFERENCES
- Achanzar WE, Webber MM, Waalkes MP. Altered apoptotic gene expression and acquired apoptotic resistance in cadmium-transformed human prostate epithelial cells. Prostate. 2002;52:236–244. doi: 10.1002/pros.10106. [DOI] [PubMed] [Google Scholar]
- Achanzar WE, Diwan BA, Liu J, Quader ST, Webber MM, Waalkes MP. Cadmium-induced malignant transformation of human prostate epithelial cells. Cancer Res. 2001;61:455–458. [PubMed] [Google Scholar]
- Achanzar WE, Achanzar KB, Lewis JG, Webber MM, Waalkes MP. Cadmium induces c-myc, p53, and c-jun expression in normal human prostate epithelial cells as a prelude to apoptosis. Toxicol Appl Pharmacol. 2000;164:291–300. doi: 10.1006/taap.1999.8907. [DOI] [PubMed] [Google Scholar]
- Armstrong BG, Kazantzis G. Prostatic cancer and chronic respiratory and renal disease in British cadmium workers. Br J Ind Med. 1985;42:540–545. doi: 10.1136/oem.42.8.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NL, Ackland ML, Cornish EJ. Metallothionein isoform expression by breast cancer cells. IntJ Biochem Cell Biol. 2000;32:895–903. doi: 10.1016/s1357-2725(00)00024-8. [DOI] [PubMed] [Google Scholar]
- Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis. 1997;18:1215–1223. doi: 10.1093/carcin/18.6.1215. [DOI] [PubMed] [Google Scholar]
- Bukowski JA, Lewis RJ. Hormesis and health: A little way of what you fancy may be good for you. South Med J. 2000;93:371–374. [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis:a generalizable and unifying hypothesis. Crit Rev Toxicol. 2001;31:353–424. doi: 10.1080/20014091111730. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol Sci. 2003;71:246–250. doi: 10.1093/toxsci/71.2.246. [DOI] [PubMed] [Google Scholar]
- Croute F, Beau B, Arrabit C, Gaubin Y, Delmas F, Murat J-C, Soleihavoup J-P. Pattern of stress protein expression in human lung cell-line A549 after short- or long-term exposure to cadmium. Environ Health Perspect. 2000;108:55–60. doi: 10.1289/ehp.0010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damelin LH, Vokes S, Whitcutt JM, Damelin SB, Alexander JJ. Hormesis:a stress response in cells exposed to low levels of heavy metals. Hum Exp Toxicol. 2000;19:420–430. doi: 10.1191/096032700678816133. [DOI] [PubMed] [Google Scholar]
- Davis JM, Svendsgaard DJ. U-shaped dose-response curves:their occurrence and implications for risk assessment. J Toxicol Environ Health. 1990;30:71–83. doi: 10.1080/15287399009531412. [DOI] [PubMed] [Google Scholar]
- Delbancut A, Barouillet MP, Cambar J. Signals and Images. Dordrecht/London: Kluwer Academic Publishers; 1997. Evidence and mechanistic approach of the protective effects of heavy metal high dilutions in rodents and renal cell cultures; pp. 71–82. [Google Scholar]
- Eaton DL, Toal BF. Evaluation of the Cd/Hemoglobin affinity assay for the rapid determination of metallothionein in biological tissues. Toxicol Appl Pharmacol. 1982;66:134–142. doi: 10.1016/0041-008x(82)90068-0. [DOI] [PubMed] [Google Scholar]
- Elghany NA, Schumacher MC, Slattery ML, West DW, Lee JS. Occupation, cadmium exposure and prostate cancer. Epidemiology. 1990;1:107–115. doi: 10.1097/00001648-199003000-00005. [DOI] [PubMed] [Google Scholar]
- Garrett SH, Sens MA, Shukla D, Flores L, Somji S, Todd JH, Sens DA. Metallothionein isoforms 1 and 2 gene expression in the human prostate: down-regulation of MT-IX in advanced prostate cancer. Prostate. 2000;43:125–135. doi: 10.1002/(sici)1097-0045(20000501)43:2<125::aid-pros7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Herkovits J, Perez-Coll CS. Increased resistance against cadmium toxicity by means of pretreatment with cadmium/zinc concentrations in Bufo arenarum embryos. Biol Trace Elem Res. 1995;49:171–175. doi: 10.1007/BF02788966. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer . International Agency for Research on Cancer Monographs on the Evaluation of the Carcinogenic Risks to Humans. Vol. 58. Lyon: IARC Scientific Publications; 1993. Beryllium, cadmium, mercury and exposure in the glass manufacturing industry; pp. 119–137. [ISBN:0250–9555]. [PMC free article] [PubMed] [Google Scholar]
- Jarup L, Bellander T, Hogstedt C, Spang G. Mortality and cancer incidence in Swedish battery workers exposed to cadmium and nickel. Occup Environ Med. 1998;55:755–759. doi: 10.1136/oem.55.11.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Liu J. Role of metallothionein in cadmium-induced hepatotoxicity and nephrotoxicity. Drug Metab Rev. 1997;29:79–102. doi: 10.3109/03602539709037574. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Liu J, Choudhuri S. Metallothionein:An intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol. 1999;39:267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- Linde K, Jonas WB, Melchart D, Worku F, Wagner H, Eitel F. Critical review and meta-analysis of serial agitated dilutions in experimental toxicology. Human Exper Toxicol. 1994;13:481–492. doi: 10.1177/096032719401300706. [DOI] [PubMed] [Google Scholar]
- Mididoddi S, McGuirt JP, Sens MA, Todd JH, Sens DA. Isoform-specific expression of metallothionein mRNA in the developing and adult human kidney. Toxicol Lett. 1996;85:17–27. doi: 10.1016/0378-4274(96)03632-6. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program . Ninth report on carcinogens. Research Triangle Park; NC: 2000. [Google Scholar]
- Nishiyama S, Itoh N, Onosaka S, Okudaira M, Yamamoto H, Tanaka K. Dietary cadmium inhibits spontaneous hepatocarcinogenesis in C3H/HeN mice and hepatitis in A/J mice, but not in C57BL/6 mice. Toxicol Appl Pharmacol. 2003;186:1–6. doi: 10.1016/s0041-008x(02)00029-7. [DOI] [PubMed] [Google Scholar]
- Osipov AN, Pomerantseva MD, Ramaiya LK, Sypin VD, Shevchenko VA. estimation of DNA-protein cross-links, abnormal sperm heads and micronuclei in mice continuously exposed to heavy metals and gamma-radiation at low doses. Metal Ions Biol Med. 2002;7:336–341. [Google Scholar]
- Palmiter RD, Findley SD, Whitmore TE, Durnam DM. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci USA. 1992;89:6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife CJ, Findley SD, Erickson JC, Froelick GJ, Kelly EJ, Zambrowicz BP, Palmiter RD. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994;33:7250–7259. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- Schulz H. Zur lehre von der arzneiwirdung. Virchows Archiv fur Pathologische Anatomie und Physiologie fur Klinische Medizin. 1887;108:423–445. [Google Scholar]
- Schulz H. Uber Hefegifte. Pflugers Archiv fur die gesamte Physiologie des Menschen und der Tiere. 1888;42:517–541. [Google Scholar]
- Stayner L, Smith R, Thun M, Schnorr T, Lemen RA. A dose response analysis and quantitative assessment of lung cancer risk and occupational cadmium exposure. Ann Epidemiol. 1992;2:177–194. doi: 10.1016/1047-2797(92)90052-r. [DOI] [PubMed] [Google Scholar]
- Stebbing AR. Hormesis:interpreting the beta-curve using control theory. J Appl Toxicol. 2000;20:93–101. doi: 10.1002/(sici)1099-1263(200003/04)20:2<93::aid-jat640>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Stennard FA, Holloway AF, Hamilton J, West AK. Characterization of six additional human metallothionein genes. Biochim Biophys Acta. 1994;1218:357–365. doi: 10.1016/0167-4781(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Diwan BA, Rehm S, Ward JM, Moussa M, Cherian MG, Goyer RA. Down-regulation of metallothionein expression in human and murine hepatocellular tumors:association with the tumor-necrotizing and antineoplastic effects of cadmium in mice. J Pharmacol Exp Ther. 1996;277:1026–1033. [PubMed] [Google Scholar]
- Waalkes MP, Diwan BA, Weghorst CM, Bare RM, Ward JM, Rice JM. Anticarcinogenic effect of cadmium in B6C3F1 mouse liver and lung. Toxicol Appl Pharmacol. 1991;110:327–335. doi: 10.1016/s0041-008x(05)80015-8. [DOI] [PubMed] [Google Scholar]
- Webber MM, Rehman SM, James GT. Metallothionein induction and deinduction in human prostates carcinoma cells: relationship with resistance and sensitivity to adriamycin. Cancer Res. 1988;48(16):4503–4508. [PubMed] [Google Scholar]
- Webber MM, Bello D, Kleinman HK, Hoffman MP. Acinar differentiation by nonmalignaut immortalized human prostatic epithelial cells and its loss by malignant cells. Carcinogenesis. 1997;18:1225–1231. doi: 10.1093/carcin/18.6.1225. [DOI] [PubMed] [Google Scholar]
- Weis P, Weis JS. Cadmium acclimation and hormesis in Fundulus heteroclitus during fin regeneration. Environ Res. 1986;39:356–363. doi: 10.1016/s0013-9351(86)80061-5. [DOI] [PubMed] [Google Scholar]
- West AK, Stallings R, Hildebrand CE, Chiu R, Karin M, Richards RI. Human metallothionein genes:structure of the functional locus at 16q 13. Genomics. 1990;8:513–518. doi: 10.1016/0888-7543(90)90038-v. [DOI] [PubMed] [Google Scholar]