Abstract
Sex steroids have a role in modulating responses that extends beyond reproduction. The current study investigated the influence of the sex steroid 17β-oestradiol on hypothalamic-pituitary-adrenal (HPA) and behavioural responses to acute or repeated restraint stress. Ovariectomized rats treated with 17β-oestradiol or peanut oil via a subcutaneous silastic capsule were subjected to daily handling (non stressed), acute (single, 1 h) or daily (10 days, 1 h/day) restraint stress. Blood collected at the end of stress revealed that 17β-oestradiol treatment augmented the corticosterone response to acute restraint. After daily exposure to restraint, the corticosterone response was noticeably diminished in untreated females but 17β-oestradiol-treated rats still showed an exaggerated response compared to castrated, untreated females. Brain tissue collected 3 h after the end of restraint was probed using isotopic in situ hybridization for corticotropin-releasing factor (CRF) and vasopressin gene expression in the paraventricular nucleus (PVN) of the hypothalamus. 17β-oestradiol treatment at the higher dose (120 μg/ml) decreased basal CRF mRNA. Stress caused an increase in CRF mRNA expression in 17β-oestradiol-treated rats but not in the vehicle group. Repeated restraint stress caused an increase in PVN parvocellular vasopressin gene expression, which was more pronounced in 17β-oestradiol-replaced rats. Animals were exposed to the elevated plus maze for 5 min as a test for anxiety. Non-stressed control rats with or without 17β-oestradiol replacement spent the same percentage amount of time exploring the open arms of the maze. Previous exposure to acute restraint stress caused a marked reduction in the time spent exploring the open arms, indicating an increase in anxiety levels in these rats; this effect was observed in both vehicle and 17β-oestradiol-treated rats. After repeated restraint stress, 17β-oestradiol-replaced rats spent as much time exploring the open arms of the maze as controls, indicating adaptation. By contrast, nonreplaced rats were still showing a significant reduction in open arm exploration after repeated restraint. The present study presents novel data showing that the HPA axis remains reactive to repeated stress in 17β-oestradiol-treated ovariectomized rats, but stress-induced anxiety behaviour is reduced.
Keywords: stress, oestradiol, adaptation, anxiety, CRF, AVP
The ability of an animal to mount an appropriate response to a stimulus that poses a threat to homeostasis is essential for survival. The hypothalamic-pituitary-adrenal (HPA) axis is a major component of the stress response and sex differences in HPA function have been reported. For example, females exhibit a higher basal rate of adrenocorticotropin (ACTH) and corticosterone secretion than males (1). It has been shown that female rats respond to various acute stressors, such as ether, restraint or electric shock, with a greater release of corticosterone than males (2, 3).
Several studies have reported the existence of gender differences on behavioural tests that measure anxiety. For example, female rodents exhibit less anxiety on the elevated plus maze by spending more time on the open arms than males (4). Similarly, after acute stress, males show a more pronounced reduction in open arm exploration than females (5). The influence of gender may vary with different stressors. For example, crowding has been shown to be stressful for males whereas isolation is more stressful for females (6). Similarly, group-housing has a positive effect on the ability of females to adapt to repeated foot-shock (7, 8). Male rats show a more pronounced up-regulation of corticotropin-releasing factor (CRF) mRNA in the paraventricular nucleus (PVN) after chronic stress than females (9). Ovarian hormones are implicated in these sex differences. Basal and stress-activated corticosterone levels have been shown to vary across the oestrous cycle, being at their maximal during the proestrous phase when circulating 17β-oestradiol levels are high (10). Anxiety-like behaviour of female rodents has been reported to alter across the oestrous cycle (11), implicating activational effects of gonadal steroids on emotional behaviour. Various stressors, such as foot shock (12), CRF administration (13), restraint (14) and novelty (15), have been shown to result in greater stress-induced increase in ACTH and corticosterone levels ovariectomized 17β-oestradiol-treated compared to nonreplaced rats.
The HPA axis adapts its activation in the face of a repeated stressor. Repeated exposure to restraint causes a reduction in the magnitude of the ACTH and corticosterone response in male rats (16, 17). Coincident changes in CRF and vasopressin mRNA expression are observed in the hypothalamic PVN. The number and transcript density of parvocellular neurones expressing vasopressin is increased after repeated stress (18, 19). Similarly, colocalization of vasopressin with CRF vesicles in the external zone of the median eminence is increased (20). A few studies have looked at sex differences in the adaptation to repeated stress, and demonstrated an effect of gender (21, 22). In addition, it has been shown that 17β-oestradiol modulates neurotransmitter levels after chronic restraint stress (23). Although there is a wealth of literature addressing the nature and manner of the adaptation of the HPA response in males, the role of gonadal steroids in females has been neglected.
Previous exposure to stress has been demonstrated to have an anxiogenic effect on subsequent plus maze behavioural testing. For example, exposure to just a single session of acute restraint stress, followed by exposure to the plus maze, results in a reduction in the amount of time spent in the open arms compared to nonstressed controls (24). This response habituates in male rats after repeated stress (25). The present study examined how 17β-oestradiol treatment affected the adaptation of the HPA (both plasma corticosterone and gene expression in the PVN) and anxiety response to restraint stress under acute and repeated conditions. It shows that 17β-oestradiol appears to facilitate adaptation to stress.
Materials and methods
Animals
Virgin female Lister Hooded rats (Harlan, Bicester, UK) weighing between 190 and 220 g at the start of the experiment were used. They were housed in groups of 4-5 per cage (cage size 36.5 × 53 × 20 cm) in a controlled environment (temperature 21 °C and humidity 55%), with ad libitum access to food (standard rat chow) and water. Animals were kept on a 12 : 12 h reverse light/dark cycle (lights on 22.00 h), and were left undisturbed for at least 4 days on arrival. They were then handled daily for at least 1 week before experimental manipulations were carried out. All experiments were carried out in accordance with the Animals Scientific Procedures Act (1986).
Experimental groups
Animals were randomly assigned to one of six groups (n = 7-8 per group): (i) ovariectomy + 120 μg/ml 17β-oestradiol (Sigma-Aldrich Co Ltd, Poole, Dorset, UK): (a) unstressed, (b) acutely stressed and (c) repeatedly stressed; (ii) ovariectomy + peanut oil implant: (a) unstressed, (b) acutely stressed and (c) repeatedly stressed. In experiment 2, an extra three groups were used: (iii) ovariectomy + 40 μg/ml 17β-oestradiol: (a) unstressed, (b) acutely stressed and (c) repeatedly stressed. Animals were housed in same stress groups.
Surgery
All rats were ovariectomized under isofluorane/N2O anaesthesia via a dorsal incision. At the time of surgery, rats were implanted with a 25 mm silastic capsule (0.062′ inner diameter; 0.03′ outer diameter) subcutaneously. The capsule was filled with 40 μl of fluid then the ends were sealed with Type A silastic glue (BDH Laboratory Supplies, VWR, Lutterworth, Leics, UK) so that 20 mm was left for active release of the steroid. Implants were preincubated over night in 0.01 m phosphate-buffered saline (PBS) to activate release. Animals were allowed to recover for at least 7 days before stress procedures. During the recovery period, rats were given a prophylactic dose of aureomycin (Fort Dodge Animal Health, Hedge End, Southampton, UK) in their drinking water.
Stress procedure
Experiment 1: effect of 17b-oestradiol on adaptation of stress-induced plasma corticosterone levels
Animals in the stress groups were subjected to either a single 60-min restraint session for the acute or repeated daily 60-min restraint sessions for 10 days (repeated stress). Animals were transferred from the holding room to a separate testing room where they were placed in a plexiglass tube (internal diameter 5.5 cm) and placed in the testing cage. The stress sessions were carried out during the dark phase between 11.30 h and 14.30 h. Rats assigned to the repeated stress groups were subjected to daily restraint on days 8-17 post surgery. On day 17, acute stress rats were subjected to a single session of restraint stress after which they were immediately killed. Control rats were handled daily and killed 17 days after surgery. All rats were transferred to another procedure room immediately after the last session of restraint and killed by a lethal dose of sodium pentobarbitone.
Experiment 2: effect of 17b-oestradiol on changes in PVN CRF and vasopressin mRNA expression
The experiment was conducted as described for experiment 1 except that the rats were returned to their home cage in the holding room at the end of the last session of restraint. Animals were then killed 3 h after the termination of restraint stress and the brains processed as described below.
Experiment 3: behavioural testing on the elevated plus maze
The rats were tested on an elevated plus maze. Behavioural testing was conducted during the dark phase, 4 h after the end of the last stress session. Briefly, the plus maze consisted of two open arms: 50 × 10 cm, and two closed arms which were 50 × 10 × 40 cm. The central platform was 10 × 10 cm and the entire apparatus was mounted on a metal base 50 cm above the floor. Animals were placed in the central square facing an open arm. The rats were allowed to explore freely for 5 min. During this period, the amount of time spent on the open arms was recorded, as was the number of arm entries. The behavioural testing carried out under red light.
Blood and tissue collection
Blood was removed at the time of sacrifice via cardiac puncture using heparinized syringes and transferred into centrifuge tubes. Tubes were centrifuged at 3000 r.p.m. for 8 min after which the plasma was collected and stored at -20 °C before assaying for corticosterone. Corticosterone was measured by radioimmunoassay using a standard protocol. The tracer used was corticosterone [1,2,6,7-3H(N)] (Perkin Elmer Life Sciences, Cambridge, UK). Intra- and inter assay coefficient of variation was 3.9% and 5.1%, respectively. 17β-oestradiol was measured using a commercial 125I radioimmunoassay kit (Diagnostic Systems Laboratories, Oxford UK). The assay was carried out on samples obtained from a separate group of rats 17 days after surgery. Intra-assay coefficient of variation was 5.9% and sensitivity of the kit was 0.6 pg/ml.
Brains were collected and frozen on dry ice and stored at -70 °C until sectioning in a cryostat. A 1-cm piece of uterus was collected from each rat postmortem and weighed to assess efficacy of both ovariectomy and 17β-oestradiol replacement.
In situ hybridization
Brains were sectioned in a cryostat at -20 °C at 12 μm and thaw-mounted onto poly L-lysine coated microscope slides (BDH Laboratory Supplies). Sections were left to air dry for at least 1 h and then fixed in 4% paraformaldehyde in PBS for 15 min at 4 °C followed by two 5-min washes in PBS. The sections were then dehydrated through a series of 70%, 80%, 90% and 95% ethanol for 3 min each. The slides were then stored at 4 °C in sealed plastic tubs containing 95% ethanol. Before hybridization, slides were removed from the ethanol and air-dried on slide racks for least 1 h.
Probes for CRF and vasopressin mRNA were 42 and 30 base oligonucleotides complimentary to the exonic mRNA coding for CRF and vasopressin (obtained by ‘blast’ searches). CRF oligonucleotide was an antisense 42mer sequence, GGC CCG CGG CGC TCC AGA GAC GGA TCC CCT GCT CAG CAG GGC. Vasopressin was a 30mer sequence, GGT GAG GCG GAA AAA ACC CTC TCG ACA CTC. Probes were tail-end labelled with 35S as follows: 4 μl of purified oligonucleotide (5 ng/μl) was added to 4 μl of 5 × Buffer (Roche Ltd, Lewes, UK). 1.25 μl of 1 m cobalt chloride (Roche) was added followed by 8 μl of DEPC-treated water. 3 μl of 35S-deoxyadenosine triphosphate (10 mCi/ml) (Amersham Biosciences, Little Chalfont, Bucks, UK) was added followed by 2 μl of terminal deoxynucleotide tranferase enzyme (Roche). The reaction mixture was incubated at 37 °C for 30 min. After the incubation period, the reaction was quenched by adding 12 μl of DEPC-treated water. Purification of labelled probe from unincorporated nucleotides was carried out by passing the reaction mixture through a G-50 sephadex microspin column (Amersham Biosciences) and centrifuged at 3400 r.p.m. 2 μl of 1 m dithiothreitol was added to the labelled probe. The labelled probe was then diluted to give a specific activity of 5000 c.p.m. μl-1 in a hybridization buffer (containing 50% deionized formamide, 20% 20x SSC, 10 × Denhardts solution, 0.2% Salmon sperm DNA, 10% dextran sulphate, 0.5 m sodium phosphate and 0.1 m sodium pyrophosphate). 100 μl of hybridization mixture was added to each slide that was then coverslipped and sealed along the edges with DPX mountant (to prevent the sections from drying out). The slides were then incubated overnight in a fan oven at 43 °C. After incubation, the DPX was peeled off and the coverslips removed in 1 × SSC solution keeping the slides immersed at all times. The slides were then washed twice for 30 min each wash in 1 × SSC at 55 °C in a shaking bath, rinsed briefly in water and dehydrated in 300 mm ammonium acetate dissolved in 70% ethanol for 3 min. The sections were then left to air dry for at least 2 h before exposure to X-ray film.
Autoradiography
Hybridized sections were exposed to Kodak BioMax MR autoradiographic film, in a light-proof X-ray film cassette (Amersham Biosciences) along with a set of 14C labelled standards (range 30-862 mCi/μg) (Amersham Biosciences). The duration of the exposure varied, according to the strength of the oligonucleotide signal, 1 day for vasopressin and 7 days for CRF. Films were developed under red light in an automated film processor (Fuji Medical Film Processor, FPM-100A) (Fuji Photo Film UK, London, UK). Due to the much higher level of vasopressin expression emanating from the magnocellular cells, it became necessary for cellular resolution, thus emulsion autoradiography was used. Sections processed for vasopressin mRNA were coated with Ilford UK LM-1 emulsion under red light conditions and stored in a light proof box with desiccant at 4 °C for 3-5 days. Slides were developed with 9% Kodak D-19 solution (Kodak Ltd, Hemel Hempstead, Herts, UK) for 5 min at room temperature, rinsed in 1% acetic acid solution for 3 min (stop solution), fixed in 25% Ilford fixer for 5 min and then washed under running water for 30 min. The sections were then stained in 0.5% cresyl violet acetate solution, rinsed in water, dehydrated through a series of ascending alcohol concentration (70%, 80%, 95%, 100% ethanol) and defatted in Histoclear (BDH Laboratory Supplies) before coverslipping with DPX mountant. Adjacent series of sections were stained for reference purposes.
Analysis and quantification
For quantification of CRF mRNA in the PVN, the optical density of the autoradiographic images was measured using a Macintosh-based image analysis system (NIH Image). The optical densities of three to four consecutive sections per rat were analysed bilaterally and the average value for each rat was used to calculate group means. The number of parvocellular cells expressing vasopressin was counted bilaterally for each rat. Three sections were analysed for each rat and an average cell count per section was used to calculate group means.
Statistical analysis
Neuroendocrine data were analysed by two-way univariate analysis of variance (anova) with stress and 17β-oestradiol treatment as factors. Behavioural data were analysed using a multivariate anova with percentage time spent in the open arms, open arm entries and total number of arm entries as variables, and stress and 17β-oestradiol treatment as factors. Subsequent analysis on each variable was carried out using two-way univariate anova with stress and 17β-oestradiol treatment as factors. Post-hoc analysis was carried out (if the anova was significant) using Bonferroni multiple comparisons test. Significance was accepted at the α = 0.05 level. Data were log transformed as necessary.
Results
Experiment 1
Corticosterone (Fig. 1)
Fig. 1.
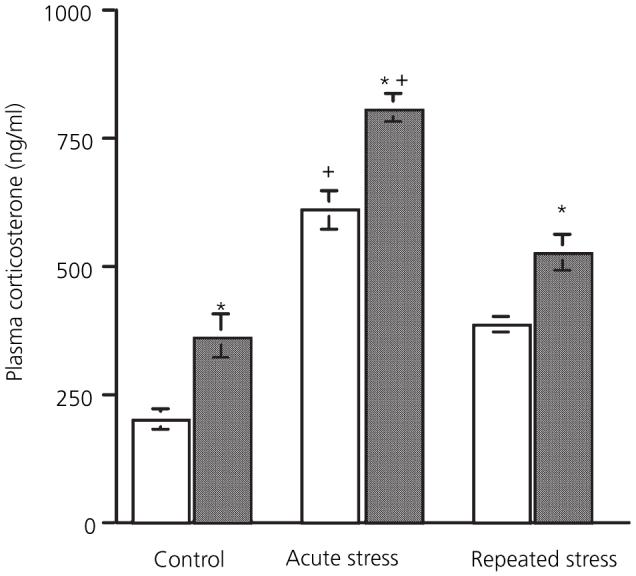
Effect of 17β-oestradiol treatment on adaptation of the corticosterone response to stress. □, Vehicle;  , 120 μg/ml 17β-oestradiol *Significantly different from corresponding vehicle group; +significantly different from control group (P < 0.05, Bonferroni).
, 120 μg/ml 17β-oestradiol *Significantly different from corresponding vehicle group; +significantly different from control group (P < 0.05, Bonferroni).
Blood samples were taken immediately after the end of the stress to ensure that plasma corticosterone levels represent the falling phase of the stress response. There was a significant main effect of both stress [F(2,36) = 86.62, P < 0.001] and 17β-oestradiol treatment [F(1,36) = 40.74, P < 0.001] on corticosterone levels. However there was no interaction between the two factors [F(2,36) = 0.52, not significant], indicating that 17β-oestradiol increased corticosterone levels independently of stress. Post-hoc analysis showed that levels in the 17β-oestradiol group were more elevated compared to the vehicle group after acute (day 1) stress (P < 0.01). After repeated stress (day 10), the corticosterone response had diminished, with both vehicle and 17β-oestradiol-treated groups exhibiting lower levels than corresponding acute stress groups (P < 0.001). After repeated stress, mean corticosterone levels in the 17β-oestradiol group were still higher than vehicle (P < 0.05). Thus, in all groups (non stress, acute and repeated stress), 17β-oestradiol-replaced rats exhibited higher corticosterone levels compared to corresponding vehicle groups.
Experiment 2
Adrenal and thymus weights
17β-oestradiol replacement overall significantly increased adrenal weight [F(2,47) = 4.1, P = 0.023] and stress had no significant effect [F(2,47) = 0.65, not significant] on adrenal weight (Table 1). There was no significant interaction between the two factors.
Table 1.
Mean Adrenal Weight (mg/100 g Body Weight)
| Treatment | Control | Acute stress | Repeated stress |
|---|---|---|---|
| Vehicle | 27.8 ± 2.1 | 27.0 ± 0.91 | 27.1 ± 1.8 |
| 40 μg/ml /17β-oestradiol | 31.7 ± 1.5 | 30.2 ± 1.8 | 31.0 ± 2.3 |
| 120 μg/ml /17β-oestradiol | 32.7 ± 0.9 | 34.4 ± 3 | 29.7 ± 2 |
There was a significant main effect of 17β-oestradiol treatment on thymus weight [F(2,47) = 15.88, P < 0.001] but no significant effect of stress [F(2,47) = 0.5, not significant] (Table 2). There was no significant interaction between the two factors. 17β-oestradiol replacement significantly reduced thymus weights only in the 120 μg/ml repeatedly stressed group compared to corresponding vehicle group (P < 0.05).
Table 2.
Mean Thymus Weight (mg/100 g Body Weight)
| Treatment | Control | Acute stress | Repeated stress |
|---|---|---|---|
| Vehicle | 209.6 ± 11.5 | 229.3 ± 12.4 | 211.7 ± 9.6 |
| 40 μg/ml /17β-oestradiol | 200.4 ± 8.3 | 216.1 ± 8 | 197.2 ± 7.9 |
| 120 μg/ml /17β-oestradiol | 184.3 ± 10.5 | 154.6 ± 3.9 | 165.6 ± 12.3* |
Significantly different from corresponding vehicle group (P < 0.05 Bonferroni).
CRF mRNA
The expression of CRF mRNA in the PVN is shown in Figs 2a and 3. There were significant main effects of stress [F(2,44) = 7.8, P < 0.001] and 17β-oestradiol treatment [F(2,44) = 5.44, P < 0.005] on CRF mRNA levels in the PVN. There was also a significant interaction between the two factors [F(4,44) = 10.5, P < 0.001]. Post-hoc analysis revealed that basal expression of CRF was significantly lower in rats receiving implants containing 120 μg/ml 17β-oestradiol compared to vehicle and 40 μg/ml 17β-oestradiol control rats (P < 0.001 in both instances). Neither acute nor repeated stress had any effect on CRF expression in vehicle-treated rats, compared to vehicle controls (not significant in both instances). Similarly rats with 40 μg/ml 17β-oestradiol implants showed no change in CRF expression after acute or repeated restraint stress compared to corresponding controls (not significant). There was no difference in CRF expression between corresponding vehicle and 40 μg/ml 17β-oestradiol-replaced rats at any condition (not significant in all instances). Animals with 120 μg/ml 17β-oestradiol implants did show an increase in expression after acute stress compared to corresponding control rats (P < 0.001). This increase was still evident after 10 days of restraint (P < 0.001).
Fig. 2.
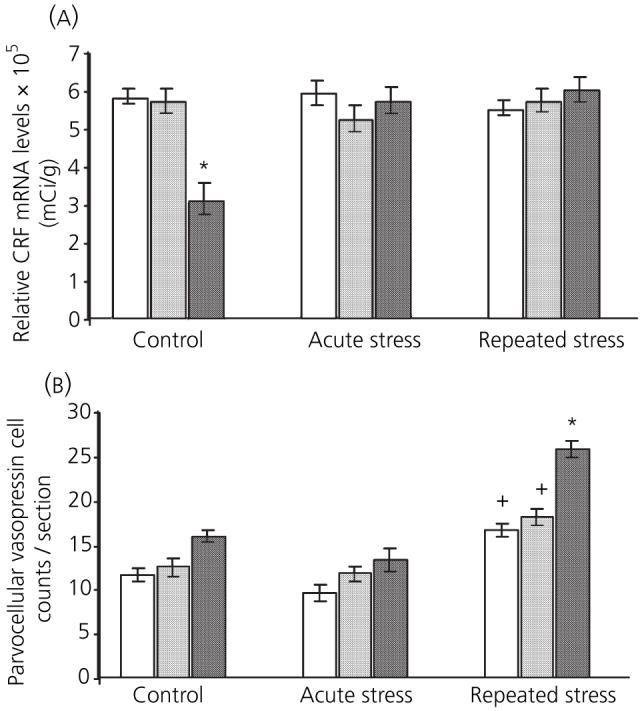
Effect of 17β-oestradiol treatment on paraventricular nucleus mRNA expression 3 h after acute or repeated restraint stress. □, vehicle;  , 40 μg/ml 17β-oestradiol; ■, 120 μg/ml 17β-oestradiol. (a) basal corticotropin-releasing factor (CRF) mRNA levels were significantly lower in rats receiving 120 μg/ml 17β-oestradiol implants. (b) After repeated restraint, there was an increase in parvocellular cells expressing vasopressin mRNA. Animals with 120 μg/ml 17β-oestradiol implants had significantly higher counts of vasopressin expressing parvocellular cells compared to vehicle. (*P < 0.05 and +P < 0.001, Bonferroni).
, 40 μg/ml 17β-oestradiol; ■, 120 μg/ml 17β-oestradiol. (a) basal corticotropin-releasing factor (CRF) mRNA levels were significantly lower in rats receiving 120 μg/ml 17β-oestradiol implants. (b) After repeated restraint, there was an increase in parvocellular cells expressing vasopressin mRNA. Animals with 120 μg/ml 17β-oestradiol implants had significantly higher counts of vasopressin expressing parvocellular cells compared to vehicle. (*P < 0.05 and +P < 0.001, Bonferroni).
Fig. 3.
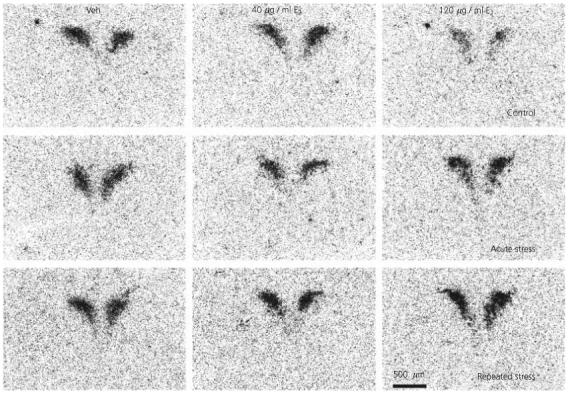
Effect of 17β-oestradiol treatment on corticotropin-releasing factor (CRF) expression in the paraventricular nucleus after restraint stress. Lightfield photomicrographs of CRF mRNA in situ hybridization signal in representative sections. Veh, Vehicle; E2, 17β-oestradiol.
Vasopressin mRNA
The expression of CRF mRNA in the PVN is shown in Figs 2b and 4. There were significant main effects of stress [F(2,35) = 71.6, P < 0.001] and 17β-oestradiol treatment [F(2,35) = 30.51, P < 0.001] on the number of vasopressin expressing parvocellular cells in the PVN; the interaction between the two factors was also significant [F(4,35) = 6.1, P < 0.01]. Post-hoc analysis showed that, after acute stress, there was no change in the number of vasopressin expressing parvocellular cells in any of the groups. After repeated restraint stress, there was a significant increase in the number of parvocellular cells expressing vasopressin in all three treatment groups compared to corresponding controls (P < 0.05). This increase was significantly higher in the repeatedly stressed rats receiving 120 μg/ml 17β-oestradiol implants compared to vehicle-treated rats (P < 0.001). The increase in vasopressin after repeated restraint stress was significantly higher in rats replaced with 120 μg/ml 17β-oestradiol compared to the 40 μg/ml group (P < 0.001).
Fig. 4.
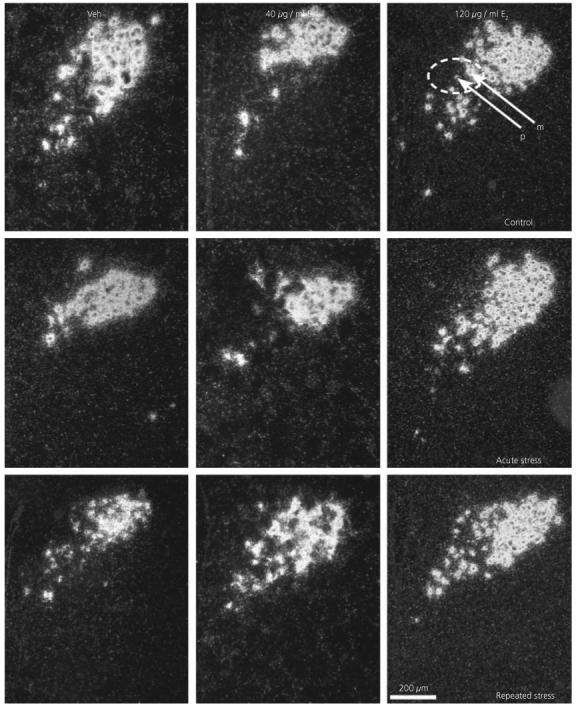
Effect of 17β-oestradiol treatment on vasopressin expression in the PVN after restraint stress. Darkfield photomicrographs of vasopressin mRNA in situ hybridization signal in representative sections. Veh, Vehicle; E2, 17β-oestradiol; m, magnocellular cell; p, parvocellular cell.
Experiment 3: plus maze behaviour (Fig. 5)
Fig. 5.
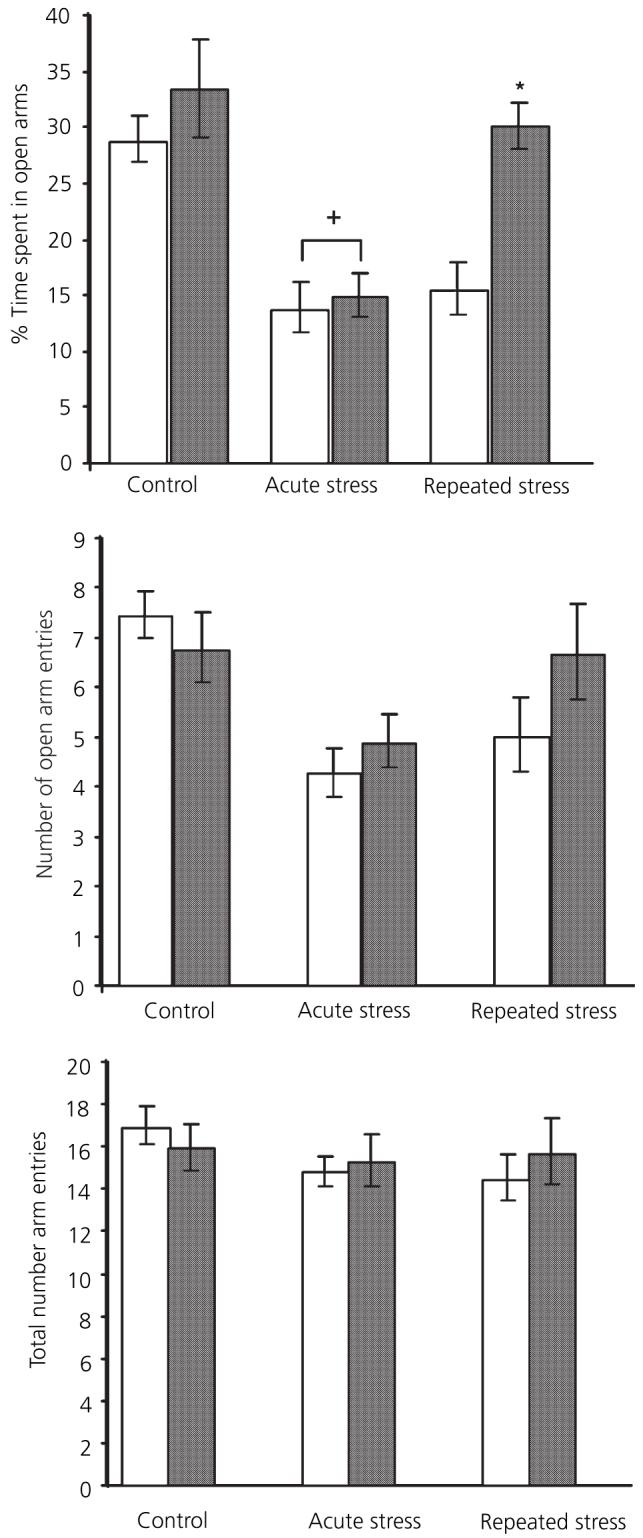
Effect of restraint stress and 17β-oestradiol on plus maze behaviour. □, Vehicle;  , 120 μg/ml 17β-oestradiol. +Significantly different from vehicle control; *significantly different from vehicle repeated restraint group (P < 0.05, Bonferroni).
, 120 μg/ml 17β-oestradiol. +Significantly different from vehicle control; *significantly different from vehicle repeated restraint group (P < 0.05, Bonferroni).
Percentage time spent on open arms
There were significant main effects of stress [F(2,44) = 15.7, P < 0.001] and 17β-oestradiol treatment [F(1,44) = 7.46, P < 0.01] on the percentage time spent on the open arms. The interaction between the two factors was just outside significance [F(2,44) = 3.1, p = 0.055, significant). Post-hoc analysis showed that not acute restraint stress significantly reduced the percentage time spent out in the open arms in both vehicle and 17β-oestradiol rats compared to corresponding controls (P = 0.005). There was no significant difference between vehicle and 17β-oestradiol-treated groups after acute stress (not significant). After repeated stress, vehicle-treated rats still spent less time out on the open arms than corresponding controls (P = 0.008). By contrast, repeatedly stressed 17β-oestradiol-replaced rats had adapted, spending as much time out in the open arms as the corresponding nonstressed controls (not significant). Vehicle-treated rats spent significantly less time on the open arms compared to the 17β-oestradiol-treated rats (P = 0.014). There was no significant difference between vehicle and 17β-oestradiol control (nonstressed) rats (not significant).
Number of arm entries
There was no effect of stress on the total number of arm entries made [F(2,44) = 1, P = 0.38, not significant] and no effect of 17β-oestradiol treatment [F(1,44) = 0.1, not significant]. However, there was a significant main effect of stress on the number of open arm entries [F(2,44) = 7.2, P = 0.002] but no significant effect of 17β-oestradiol treatment [F(1,44) = 0.9, not significant]. There were no effects of either stress or 17β-oestradiol on closed arm entries (F < 1 in both cases; not significant).
17β-oestradiol increased uterine weight
Uterine weight was used as a bioassay to determine the efficacy of the ovariectomy and 17β-oestradiol replacement procedure. The results showed a significant effect: vehicle (4.2 ± 0.3) < 40 μg/ml 17β-oestradiol (9.2 ± 0.5) < 120 μg/ml 17β-oestradiol (12.3 ± 0.9) mg/100 g body weight.
Determination of plasma 17β-oestradiol levels
In a separate experiment, plasma 17β-oestradiol levels were measured 17 days after surgery in ovariectomized rats receiving silastic implants containing 17β-oestradiol (40 or 120 μg/ml). Levels were: 40 μg/ml 17β-oestradiol implant, 16 ± 1.9 pg/ml; 120 μg/ml 17β-oestradiol implant, 45 ± 3.7 pg/ml.
Discussion
There was a robust increase in plasma levels of corticosterone after acute restraint stress, and 17β-oestradiol-treated rats exhibited a more pronounced response. After repeated stress, the plasma corticosterone response in all treatment groups had diminished, indicating habituation, although levels were still accentuated in 17β-oestradiol-treated rats. Habituation of the corticosterone response to repeated restraint stress in the rat has been demonstrated on numerous studies on males (18, 26, 27). An interesting feature of this study is that although 17β-oestradiol-replaced females have enhanced stress-induced corticoster-one levels, the rate of adaptation of the corticosterone response was similar across the groups. This implies that the magnitude of initial corticosterone secretion and adaptation of the corticosterone response are differentially controlled by 17β-oestradiol. This could be due to several reasons. First, it has been shown that 17β-oestradiol increases glucocorticoid synthesis in the adrenal gland (28, 29). 17β-oestradiol-replaced rats in the current study tended to have larger adrenal glands (and smaller thymus glands), which may reflect increased release of corticosterone. Alternatively, although ACTH levels were not measured in this study, previous studies have reported augmented acute stress-induced ACTH secretion in 17β-oestradiol-replaced female rats (2, 10). ACTH secretion is subject to negative feedback control by glucocorticoids (30). 17β-oestradiol has been strongly implicated in reduced efficacy of glucocorticoid negative feedback inhibition (12, 31). This would result in a greater ACTH response and subsequent corticosterone secretion.
In a separate experiment (data not shown), we compared uterine weights across the oestrous cycle with those of ovariectomized replaced female rats using several doses (40, 120, 240 μg/ml 17β-oestradiol). We found that 120 μg/ml resulted in uterine weights comparable to rats in proestrous (when 17β-oestradiol levels are at their maximal). 17β-oestradiol replacement at the higher dose (120 μg/ml) used in the definitive experiments resulted in significantly lower basal expression of CRF compared to vehicle-treated females. This finding corroborates a previous study (32). Higher basal corticosterone levels may result in increased negative feedback, which may partly contribute to decreased mRNA levels. In support of this, decreases in basal CRF mRNA levels were found to be dependent upon the integrity of the adrenal glands (32). It could be argued that the elevated basal corticosterone levels observed in rats with the higher dose of 17β-oestradiol may be nullified by 17β-oestradiol up-regulation of plasma CBG levels (33). It appears unlikely to be the case in this study because 17β-oestradiol-replaced rats had smaller thymus glands than vehicle-treated rats, suggesting exposure to higher levels of corticosterone (34). It is possible that chronic 17β-oestradiol treatment may decrease CRF mRNA levels directly. A functional ERE has been identified in the human CRF gene (35). One study reported that 17β-oestradiol increased CRF mRNA levels in vitro (35). A later report from the same laboratory, using a different cell line, found that 17β-oestradiol decreased CRF transcription (36). In intact female rats, basal CRF mRNA levels have been to found to be maximal during proestrous when 17β-oestradiol levels are high (37). This suggests that, in cycling rats at least, 17β-oestradiol has an excitatory effect on the HPA axis, whereas in the present study chronic 17β-oestradiol (120 μg/ml) replacement decreased basal CRF mRNA levels. This implies that there is a differential effect of dynamic versus static 17β-oestradiol levels on HPA activity.
After acute and repeated stress, CRF mRNA levels in rats receiving 120 μg/ml 17β-oestradiol implants had increased. Because we were unable to measure prestress levels at all the times and treatment points, whether or not this represents an acute effect of stress remains uncertain. The important point is that neither vehicle nor lower dose 17β-oestradiol-treated rats exhibited any change in CRF mRNA levels compared to control (unstressed) values. Several studies in male rats have demonstrated an increase in CRF mRNA levels after acute restraint stress (18, 19). Levels in our study were only examined at one time point (i.e. 3 h after stress) and it is possible, although unlikely, that stress-induced changes in CRF mRNA levels could have been observed at other time points. We have data showing that CRF is not increased 1 h after stress in vehicle-treated ovariectomized rats (unpublished data). Previous studies have demonstrated stress-induced increases in CRH hnRNA within 30 min, with a peak in mRNA levels 2-3 h later (18, 38). This suggests that the time point chosen in this study was within the range to detect stress-induced changes in CRF mRNA levels, although this awaits verification with a thorough time course. We carried out the experiment during the dark phase, and this may account for the discrepancy between the current findings and previous studies. This explanation appears unlikely because a previous study using the same stress regimen found an increase in CRF mRNA expression after acute stress (19). The time point of 3 h poststress was chosen to accommodate the different stress-induced responses of both CRF and vasopressin mRNA. Although CRF gene expression occurs within 30 min of exposure to a stressor, that of vasopressin is slower, with detectable changes in vasopressin hnRNA occurring 1-2 h after exposure (39). It is also possible that the already high basal CRF levels in nonreplaced rats may have resulted in a ceiling effect, but levels in adrenalectomized females can rise to even higher levels, which makes this explanation less likely. The relationship between 17β-oestradiol status and the stress induced increases in CRF mRNA is likely to be a complex one with altered glucocorticoid feedback playing a key role. In another study, we found that adrenalectomy caused an increase in CRF mRNA expression in ovariectomized vehicle-treated rats (unpublished data). It is possible that the unstressed vehicle-treated rats were chronically stressed, masking any effects of the restraint stress procedure itself. However, we believe this to be an unlikely explanation because we have found that these rats have larger thymus glands than 17β-oestradiol-treated controls. Chronically stressed rats show thymic involution as a result of chronic exposure to glucocorticoids (34), which is an effect that we did not observe in nonstressed vehicle-treated rats.
Differences between 17β-oestradiol and vehicle-treated rats in the mRNA expression of vasopressin, the other major ACTH secretagogue, were also evident after repeated stress, although subject to the same reservations of whether these represent altered baseline or acute responses to stress. Due to the high variability in silver grain density as a result of the emulsion protocol, cell counts of vasopressin-positive parvocellular neurones were used to monitor changes in expression. The cells we measured were carefully chosen from the dorsomedial part of the PVN, which are largely, although not entirely, distinct from the magnocellular region. These cells have both autonomic and median eminence connections (40). Several studies have shown that manipulations which alter mRNA expression levels also result in changes in the number of cells expressing vasopressin (41). Under basal conditions, the number of medial PVN parvocellular cells expressing vasopressin was low across all the treatment groups. In the present study, acute stress did not appear to have any effect on vasopressin expression in any of the groups. This agrees with previous investigations that demonstrated either no or a very small increase in parvocellular vasopressin expression after acute stress in males (19, 42). After repeated stress, there was a clear increase in parvocellular vasopressin mRNA expression across the groups, as shown by previous studies in males (18, 19, 43).
The novel finding was that this increase was markedly potentiated in rats replaced with the higher dose of 17β-oestradiol. The site of oestrogen modulation of the HPA axis is not fully elucidated and may occur at several sites, including the adrenal gland, anterior pituitary, hypothalamus and extrahypothalamic regions. Receptors for oestrogen receptors (ER), ERα and/or ERβ, are expressed in all of these regions (44, 45). Although ERβ-immunoreactivity has been identified in the medial parvocellular part of the PVN, few vasopressin neurones colocalize with the receptor (46). This suggests that the mechanism of 17β-oestradiol action is unlikely to be directly on the parvocellular neurones. However, the results of a recent study suggest that ERβ in the PVN may regulate corticosterone responses to stress (47). It is also possible that 17β-oestradiol may modulate afferent inputs into the parvocellular PVN. For example, the PVN receives a substantial excitatory input from brainstem catecholaminergic neurones, and oestrogen has been shown to modulate noradrenaline turnover (48), as well as alpha-1β adrenoreceptor expression in the hypothalamus (49). It appears unlikely that reduced corticoid feedback was responsible (50, 51) because the high-dose 17β-oestradiol rats had higher levels of corticosterone compared to vehicle-treated ones.
Another novel finding in this study was the emergence of a difference between 17β-oestradiol and vehicle-treated rats after repeated stress in the elevated plus maze, a recognized test of anxiety-like behaviour (52). Previous exposure to a single session of restraint stress noticeably and significantly reduced open arm time compared to controls in an equivalent manner in vehicle and 17β-oestradiol groups. This anxiogenic effect of acute restraint has been shown in previous studies on male rats (53). However, after 10 daily sessions of restraint, the anxiogenic action of previous stress was no longer observed in 17β-oestradiol-treated rats (i.e. they had completely habituated to the stressor). By contrast, the vehicle-treated group still exhibited pronounced signs of anxiety-like behaviour (decreased percentage time in the open arms). Neither stress nor 17β-oestradiol treatment altered the total number of arms entered or the number of closed arms (i.e. locomotion). Control (unstressed) rats spent a similar amount of percentage time in the open arms (approximately 20%) irrespective of 17β-oestradiol treatment.
The apparent discrepancy between the habituation of the corticosterone and behavioural response to repeated stress might imply that the underlying processes are distinct. On the other hand, increased glucocorticoids may enhance memory for adverse effects (54, 55). The latter suggests that 17β-oestradiol may aid habituation of behaviour by increasing glucocorticoid response to stress. Whether this is so remains to be determined. CRF is thought to play an important role in the coordination of behavioural responses to stress and in anxiety (56). There were no discernible differences in CRF mRNA expression in the PVN between groups after repeated stress in our experiment. This is not surprising because it is thought that the CRF containing neurones involved in this process are distinct from the hypophysiotropic PVN population and, as such, are also differentially regulated by stress; for a review, see (57). Several studies have demonstrated the expression of both or either of the oestrogen receptors in central areas implicated in the expression of anxiety or fear-related behaviours, including the amygdala and the lateral bed nucleus of the stria terminalis (45). 17β-oestradiol administration alters the activity of amygdala neurones (58). The role of the amygdala in anxiety is well-established. The effects of 17β-oestradiol may also be indirect via alteration of neurotransmitter and peptidergic systems. The 5-HT and noradrenergic systems have been implicated in behavioural anxiety-related responses, and 17β-oestradiol modulates the transmitter turnover and the expression of receptors in both these systems. For example, 17β-oestradiol has been shown to down-regulate the 5-HT1A (59), and up-regulate the 5HT2A receptors (60). Furthermore, 17β-oestradiol and vehicle rats exhibit different profiles of central serotonin and noradrenaline metabolites after chronic stress in a region-specific manner (23). Additionally, 17β-oestradiol increases neuropeptide Y expression, a peptide with inhibitory effects on anxiety-related behaviours (61).
In conclusion 17β-oestradiol decreased basal levels of CRF in ovariectomized female rats, but not vasopressin. 17β-oestradiol increased the corticosterone response to stress and, although subsequent adaptation occurs during repeated stress, levels in 17β-oestradiol-treated females remained higher than untreated ovariectomized rats. The process of adaptation or habituation of CRF mRNA is not altered by 17β-oestradiol, in contrast to vasopressin mRNA, which is enhanced in chronically stressed rats by 17β-oestradiol. Levels of anxiety, as measured in the plus maze, adapt in 17β-oestradiol-treated female rats, but not in those without replacement hormone. Tajen together, this suggests that 17β-oestradiol plays a significant role in the way that female rats adapt to repeated stress.
Acknowledgements
This study was supported by the Medical Research Council. We thank Sarah Cleary and Helen Shiers for assistance with the assays and Adrian Newman for photography.
References
- 1.Lesniewska B, Nowak M, Malendowicz LK. Sex differences in adrenocortical structure and function. XXVIII. ACTH and corticosterone in intact, gonadectomised and gonadal hormone replaced rats. Horm Metab Res. 1990;22:378–381. [PubMed] [Google Scholar]
- 2.Lesniewska B, Miskowiak B, Nowak M, Malendowicz LK. Sex differences in adrenocortical structure and function. XXVII. The effect of ether stress on ACTH and corticosterone in intact, gonadectomized, and testosterone- or estradiol-replaced rats. Res Exp Med (Berl) 1990;190:95–103. doi: 10.1007/pl00020011. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R. Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci. 1998;16:289–295. doi: 10.1016/s0736-5748(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 4.Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 5.Steenbergen HL, Heinsbroek RP, Van Hest A, Van de Poll NE. Sex-dependent effects of inescapable shock administration on shuttlebox-escape performance and elevated plus-maze behavior. Physiol Behav. 1990;48:571–576. doi: 10.1016/0031-9384(90)90302-k. [DOI] [PubMed] [Google Scholar]
- 6.Brown KJ, Grunberg NE. Effects of housing on male and female rats: crowding stresses male but calm females. Physiol Behav. 1995;58:1085–1089. doi: 10.1016/0031-9384(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 7.Westenbroek C, Den Boer J, Ter Horst G. Gender-specific effects of social housing on chronic stress-induced limbic FOS expression. Neuroscience. 2003;121:189–199. doi: 10.1016/s0306-4522(03)00367-1. [DOI] [PubMed] [Google Scholar]
- 8.Westenbroek C, Ter Horst G, Roos M, Kuipers S, Trentani A, Den Boer J. Gender-specific effects of social housing in rats after chronic mild stress exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:21–30. doi: 10.1016/s0278-5846(02)00310-x. [DOI] [PubMed] [Google Scholar]
- 9.Duncko R, Kiss A, Skultetyova I, Rusnak M, Jezova D. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology. 2001;26:77–89. doi: 10.1016/s0306-4530(00)00040-8. [DOI] [PubMed] [Google Scholar]
- 10.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 11.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plusmaze test. Physiol Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 12.Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- 13.Elkind-Hirsch KE, Valdes CT, Rogers DG. Does estradiol treatment normalize the hypothalamic-pituitary-adrenal axis in streptozotocin-induced ovariectomized diabetic female rats? Horm Metab Res. 1991;23:481–485. doi: 10.1055/s-2007-1003734. [DOI] [PubMed] [Google Scholar]
- 14.McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–247. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- 15.Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Herbert J. Alterations in sensitivity to intracerebral vasopressin and the effects of a V1a receptor antagonist on cellular, autonomic and endocrine responses to repeated stress. Neuroscience. 1995;64:687–697. doi: 10.1016/0306-4522(94)00413-y. [DOI] [PubMed] [Google Scholar]
- 17.Kant GJ, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL. Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav. 1985;22:631–634. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- 18.Ma XM, Lightman SL. The arginine vasopressin and corticotrophin-releasing hormone gene transcription responses to varied frequencies of repeated stress in rats. J Physiol. 1998;510:605–614. doi: 10.1111/j.1469-7793.1998.605bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinnock SB, Herbert J. Corticosterone differentially modulates expression of corticotropin releasing factor and arginine vasopressin mRNA in the hypothalamic paraventricular nucleus following either acute or repeated restraint stress. Eur J Neurosci. 2001;13:576–584. doi: 10.1046/j.0953-816x.2000.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Goeij DC, Kvetnansky R, Whitnall MH, Jezova D, Berkenbosch F, Tilders FJ. Repeated stress-induced activation of corticotropin-releasing factor neurons enhances vasopressin stores and colocalization with corticotropin-releasing factor in the median eminence of rats. Neuroendocrinology. 1991;53:150–159. doi: 10.1159/000125712. [DOI] [PubMed] [Google Scholar]
- 21.Haleem DJ, Kennett G, Curzon G. Adaptation of female rats to stress: shift to male pattern by inhibition of corticosterone synthesis. Brain Res. 1988;458:339–347. doi: 10.1016/0006-8993(88)90476-3. [DOI] [PubMed] [Google Scholar]
- 22.Karandrea D, Kittas C, Kitraki E. Contribution of sex and cellular context in the regulation of brain corticosteroid receptors following restraint stress. Neuroendocrinology. 2000;71:343–353. doi: 10.1159/000054555. [DOI] [PubMed] [Google Scholar]
- 23.Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 24.Padovan CM, Guimaraes FS. Restraint-induced hypoactivity in an elevated plus-maze. Braz J Med Biol Res. 2000;33:79–83. doi: 10.1590/s0100-879x2000000100011. [DOI] [PubMed] [Google Scholar]
- 25.Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10:3003–3007. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- 26.Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress. chronic stress and habituation. Physiol Behav. 1988;43:47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Herbert J. The effect of long-term castration on the neuronal and physiological responses to acute or repeated restraint stress: interactions with opioids and prostaglandins. J Neuroendocrinol. 1995;7:137–144. doi: 10.1111/j.1365-2826.1995.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 28.Kitay JI. Effect of oestradiol in adrenal corticoidogenesis: an additional step in steroid biosynthesis. Nature. 1966;209:808–809. doi: 10.1038/209808a0. [DOI] [PubMed] [Google Scholar]
- 29.Nowak KW, Neri G, Nussdorfer GG, Malendowicz LK. Effects of sex hormones on the steroidogenic activity of dispersed adrenocortical cells of the rat adrenal cortex. Life Sci. 1995;57:833–837. doi: 10.1016/0024-3205(95)02015-b. [DOI] [PubMed] [Google Scholar]
- 30.Dallman MF. Control of adrenocortical growth in vivo. Endocr Res. 1984;10:213–242. doi: 10.1080/07435808409036499. [DOI] [PubMed] [Google Scholar]
- 31.Peiffer A, Lapointe B, Barden N. Hormonal regulation of type II glucocorticoid receptor messenger ribonucleic acid in rat brain. Endocrinology. 1991;129:2166–2174. doi: 10.1210/endo-129-4-2166. [DOI] [PubMed] [Google Scholar]
- 32.Paulmyer-Lacroix O, Hery M, Pugeat M, Grino M. The modulatory role of estrogens on corticotropin-releasing factor gene expression in the hypothalamic paraventricular nucleus of ovariectomized rats: role of the adrenal gland. J Neuroendocrinol. 1996;8:515–519. doi: 10.1046/j.1365-2826.1996.04835.x. [DOI] [PubMed] [Google Scholar]
- 33.Feldman D, Mondon CE, Horner JA, Weiser JN. Glucocorticoid and estrogen regulation of corticosteroid-binding globulin production by rat liver. Am J Physiol. 1979;237:E493–E499. doi: 10.1152/ajpendo.1979.237.6.E493. [DOI] [PubMed] [Google Scholar]
- 34.Akana SF, Cascio CS, Shinsako J, Dallman MF. Corticosterone. narrow range required for normal body and thymus weight and ACTH. Am J Physiol. 1985;249:R527–R532. doi: 10.1152/ajpregu.1985.249.5.R527. [DOI] [PubMed] [Google Scholar]
- 35.Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makrigiannakis A, Zoumakis E, Margioris AN, Stournaras C, Chrousos GP, Gravanis A. Regulation of the promoter of the human corticotropin-releasing hormone gene in transfected human endometrial cells. Neuroendocrinology. 1996;64:85–92. doi: 10.1159/000127103. [DOI] [PubMed] [Google Scholar]
- 37.Bohler HC, Jr, Zoeller RT, King JC, Rubin BS, Weber R, Merriam GR. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res Mol Brain Res. 1990;8:259–262. doi: 10.1016/0169-328x(90)90025-9. [DOI] [PubMed] [Google Scholar]
- 38.Kovacs KJ, Sawchenko PE. Regulation of stress-induced transcriptional changes in the hypothalamic neurosecretory neurons. J Mol Neurosci. 1996;7:125–133. doi: 10.1007/BF02736792. [DOI] [PubMed] [Google Scholar]
- 39.Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus. cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 41.Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 42.Ma XM, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997;138:4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- 43.Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 44.Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol. 1998;19:253–286. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- 45.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 46.Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. Proc Natl Acad Sci USA. 1998;95:3281–3286. doi: 10.1073/pnas.95.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–845. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- 48.Wise PM, Rance N, Barraclough CA. Effects of estradiol and progesterone on catecholamine turnover rates in discrete hypothalamic regions in ovariectomized rats. Endocrinology. 1981;108:2186–2193. doi: 10.1210/endo-108-6-2186. [DOI] [PubMed] [Google Scholar]
- 49.Petitti N, Karkanias GB, Etgen AM. Estradiol selectively regulates alpha 1B-noradrenergic receptors in the hypothalamus and preoptic area. J Neurosci. 1992;12:3869–3876. doi: 10.1523/JNEUROSCI.12-10-03869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma XM, Levy A, Lightman SL. Rapid changes of heteronuclear RNA for arginine vasopressin but not for corticotropin releasing hormone in response to acute corticosterone administration. J Neuroendocrinol. 1997;9:723–728. doi: 10.1046/j.1365-2826.1997.00646.x. [DOI] [PubMed] [Google Scholar]
- 51.Kovacs KJ, Foldes A, Sawchenko PE. Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J Neurosci. 2000;20:3843–3852. doi: 10.1523/JNEUROSCI.20-10-03843.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hogg SA. review of the validity and variability of the elevated plusmaze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 53.Padovan CM, Del Bel EA, Guimaraes FS. Behavioral effects in the elevated plus maze of an NMDA antagonist injected into the dorsal hippocampus: influence of restraint stress. Pharmacol Biochem Behav. 2000;67:325–330. doi: 10.1016/s0091-3057(00)00361-0. [DOI] [PubMed] [Google Scholar]
- 54.Roozendaal B. Stress and memory. opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 55.Cordero MI, Kruyt ND, Merino JJ, Sandi C. Glucocorticoid involvement in memory formation in a rat model for traumatic memory. Stress. 2002;5:73–79. doi: 10.1080/1025389029000124404. [DOI] [PubMed] [Google Scholar]
- 56.Buwalda B, de Boer SF, Van Kalkeren AA, Koolhaas JM. Physiological and behavioral effects of chronic intracerebroventricular infusion of corticotropin-releasing factor in the rat. Psychoneuroendocrinology. 1997;22:297–309. doi: 10.1016/s0306-4530(97)00032-2. [DOI] [PubMed] [Google Scholar]
- 57.Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids. implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 58.Womble MD, Andrew JA, Crook JJ. 17 beta-Estradiol reduces excitatory postsynaptic potential (EPSP) amplitude in rat basolateral amygdala neurons. Neurosci Lett. 2002;331:83–86. doi: 10.1016/s0304-3940(02)00871-6. [DOI] [PubMed] [Google Scholar]
- 59.Osterlund MK, Hurd YL. Acute 17 beta-estradiol treatment down-regulates serotonin 5HT1A receptor mRNA expression in the limbic system of female rats. Brain Res Mol Brain Res. 1998;55:169–172. doi: 10.1016/s0169-328x(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 60.Sumner BE, Fink G. The density of 5-hydoxytryptamine2A receptors in forebrain is increased at pro-oestrus in intact female rats. Neurosci Lett. 1997;234:7–10. doi: 10.1016/s0304-3940(97)00651-4. [DOI] [PubMed] [Google Scholar]
- 61.Sahu A, Crowley WR, Kalra SP. Hypothalamic neuropeptide-Y gene expression increases before the onset of the ovarian steroid-induced luteinizing hormone surge. Endocrinology. 1994;134:1018–1022. doi: 10.1210/endo.134.3.8119137. [DOI] [PubMed] [Google Scholar]


