Summary
Among humans, face recognition involves highly specialized cognitive and neural processes that enable the recognition of specific individuals [1–5]. While comparative studies suggest similar cognitive processes underlie face recognition in chimpanzees and humans [6–8, SOM#1], it remains unknown whether chimpanzees also show face-selective activity in ventral temporal cortex. This study is the first to examine regional cerebral glucose metabolism using 18F-Flurodeoxyglucose Positron Emission Tomography in chimpanzees after they performed computerized tasks matching conspecifics’ faces and nonface objects (SOM#2). A whole brain analysis comparing these two tasks directly in five chimpanzees revealed significant face-selective activity in brain regions known to comprise the distributed cortical face processing network in humans, including superior temporal sulcus and orbitofrontal cortex [9–11]. In order to identify regions that were exclusively active during one task, but not the other, a resting-state condition was subtracted from each task and the activity exclusive to each task was identified. This revealed numerous distinct patches of face-selective activity in the fusiform gyrus that were interspersed within a large expanse of object-selective cortex. This pattern suggests similar object form topography in the ventral temporal cortex of chimpanzees and humans, in which faces may represent a special class of visual stimulus.
Results
Whole Brain Analyses: Face versus Object
The first analysis revealed numerous brain regions that showed greater metabolic activity during the face matching task when compared directly to the object matching task using the contrast: face minus object (see Table 1). Figure 1 illustrates face-selective activity in the posterior superior temporal sulcus (STS) and orbitofrontal cortex overlaid on a 3D rendering of a chimpanzee MRI. These regions comprise part of the distributed cortical network for face processing in humans [9–11]. Notably absent from this analysis was activity in the fusiform gyrus, the primary region where face-selective activity is found in humans using the comparable analysis [2–3].
Table 1.
A listing of brain regions identified in chimpanzees as being significantly more active during the face-matching compared to object-matching task (p< 0.05, 1-tailed). This was derived using a whole-brain analysis including only clusters of two or more contiguous voxels where the volume of activation was > 20 mm3.
| Brain region | Volume (mm3) | p-value | Side of activation |
|---|---|---|---|
| Dorsal primary motor/medial parietal cortex | 460.60 | 0.001 | L |
| Intraparietal sulcus | 243.31 | 0.002 | R |
| Parieto-occipital sulcus/posterior cingulate | 218.23 | 0.003 | Bilat |
| Anterior cingulate | 188.93 | 0.005 | R |
| Medial prefrontal cortex | 179.55 | 0.006 | L |
| Intraparietal sulcus | 151.89 | 0.01 | L |
| Middle STS/insula | 121.42 | 0.019 | L |
| Lateral primary motor cortex | 112.98 | 0.023 | L |
| Ventromedial orbitofrontal cortex/medial orbital cortex | 101.26 | 0.03 | R |
| Anterior cingulate | 94.23 | 0.035 | L |
| Posterior superior temporal sulcus (STS) | 83.92 | 0.045 | R |
| Posterior STS | 80.17 | 0.05 | L |
| Medial parietal cortex | 80.17 | 0.05 | L |
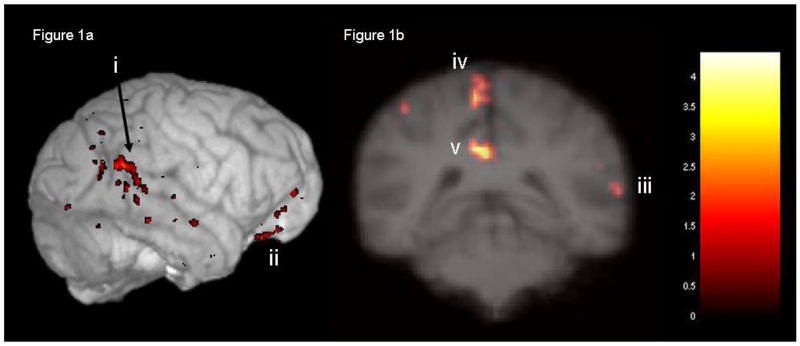
Figure 1.
illustrates the results of a whole brain analysis comparing metabolic brain activity during the face versus object-matching task (p< 0.05, uncorrected). Figure 1a shows face-selective activations in the right posterior STS (i) and orbitofrontal cortex (ii) overlaid on a 3D reconstruction of the average chimpanzee MRI (chimplate). Figure 1b illustrates face-selective activations in right posterior STS (iii), left primary motor/medial parietal cortex (iv), and posterior cingulate (v) overlaid on a coronal slice of the chimplate.
Whole Brain and ROI Analyses: Face and Object versus Rest
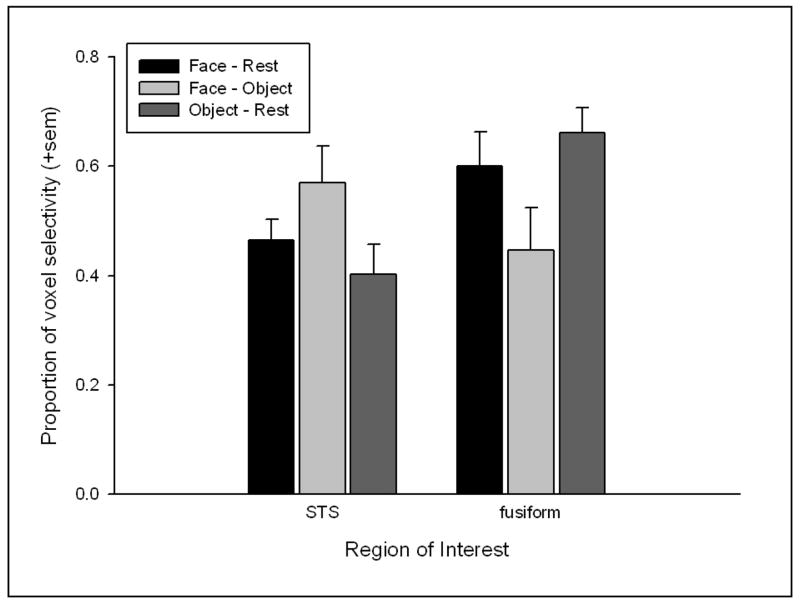
A second analysis used a specific region of interest (ROI) approach to compare the proportion of task-specific voxels in each contrast (FR = face minus rest, OR = object minus rest, and FO = face minus object) in both the fusiform gyrus and posterior STS of each individual subject (see SOM #3). These means (+sem) are plotted in Figure 2. This illustrates that the fusiform gyrus had more face- and object-selective voxels than the posterior STS when compared to rest, but that there were more face-selective voxels in the posterior STS than fusiform gyrus when compared to the object task.
Figure 2.
The mean proportion of face- and object-selective voxels in the fusiform gyrus and posterior STS identified using the following contrasts, face minus rest (FR), object minus rest (OR) and face minus object (FO).
These results are not surprising as the ventral temporal cortex in humans is known to be highly selective for a variety of object categories, not just faces [10, 12–16]. Moreover, the definitive face-selective region in humans, the fusiform face area, or FFA, [2,4] is small (about a cm3) and its location is highly variable across individuals (see SOM #4), so it is not surprising that the group analysis presented here did not identify a putative FFA in the chimpanzee brain, which is about a third of the volume of the human brain. To overcome these issues, human studies use functional ROI approaches to first localize the FFA from surrounding object-selective cortex and then later compare neural responses to faces and control stimuli in these functionally defined regions [17]. Because the FDG-PET procedures used in the present study only allow for one experimental condition per scan, data are still being acquired that would enable a similar procedure to be implemented in the chimpanzee. The present data set, however, could conceivably be used as the functional localizer data for future studies.
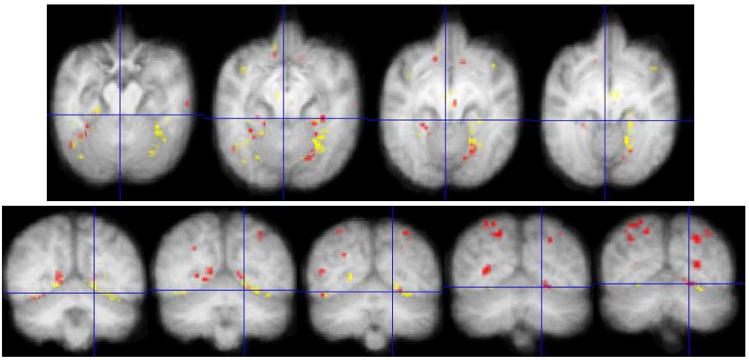
A final analysis aimed to identify regions that were exclusively face-selective or object-selective across the whole brain in chimpanzees by comparing these conditions to the resting-state, when subjects are awake and resting quietly in their home cage, which is known to activate social cognition networks in chimpanzees and humans [18–19]. Figure 3 illustrates the location of face- and object-selective activity overlaid on several axial and coronal slices through the chimpanzee brain. According to the analysis, red regions show voxels that were significantly (p< 0.05) and uniquely active for the FR contrast, but showed no activation in the OR contrast, and the yellow regions indicate voxels that were significantly and uniquely active for the OR contrast, showing no activation in the FR contrast. Figure 3 shows the location of face-selective activity to be tightly interspersed within a large object-responsive territory in the ventral temporal cortex, particularly the right fusiform gyrus. In addition to fusiform gyrus, face-selective activity was found in nucleus accumbens, superior temporal gyrus, posterior STS, supramarginal gyrus, posterior parietal cortex/angular gyrus, and the parieto-occipital sulcus. Additional object-selective activity was found in precentral gyrus, insula, and thalamic nuclei. Both contrasts activated nonoverlapping regions in ventromedial orbitofrontal cortex, inferior frontal gyrus, parahippocampal cortex, and the calcarine fissure.
Figure 3.
The location of unique face- and object-selective activity in the chimpanzee brain. The results of repeated measures ANOVA in SPM5 showing the location of face and object-selective (p< 0.05) activity compared to rest were first binarized, and then added to form the union of these results (FR+OR). Each binarized condition was then subtracted from the binarized union to reveal regions that were uniquely face-selective [bin(FR+OR) − binOR], or uniquely object-selective [bin(FR+OR) − binFR]. Red patches show voxels that were significantly more active in the FR contrast, but showed no activation in the OR contrast. Yellow regions show voxels that were significantly more active during the OR contrast, but showed no activation in the FR contrast. These activations are overlaid on an average chimpanzee MRI brain in the axial (top row) and coronal (bottom row) planes. Images are in neurological convention (left is left).
These regions may represent part of a distributed neural system for face processing in chimpanzees, as proposed in humans, where the initial visual analysis of faces activates regions in the occipitotemporal cortex, and then additional processing may take place in the fusiform gyrus, STS and extended regions [9]. Although the data from this study do not permit definitive demonstration of a chimpanzee FFA homologue, face-selective regions were found to be distributed throughout the brain, particularly in the right posterior fusiform gyrus, which were tightly interspersed within regions of object-selective cortex. This is consistent with the existence of a specific sub-region within the ventral visual cortex of chimpanzees for processing unique classes of objects: faces.
General Discussion
In order to understand whether a particular behavior, such as expertise in face recognition, represents a unique specialization in humans, comparative data are essential. Previous behavioral studies in chimpanzees have demonstrated many of the same cognitive specializations for face processing as humans, such as rapid individuation of faces [6], species-specific face-inversion effects [7], and utilization of 2nd-order relational information [8]. Behavioral evidence for similar face expertise in monkeys has not been strongly established [20, but see 21]. Moreover, while monkeys have a series of interconnectived face-selective brain regions, these patches lie along the lateral aspect of the temporal lobe, within the STS and on the adjacent inferior temporal convexity [22–24, 26], not in the ventromedial aspect of the posterior temporal lobe where face-selective activity is primarily observed in humans, and is reported here in chimpanzees. Thus, there is currently no strong evidence to suggest that macaque face-selectivity is in a region that could be considered homologous to the fusiform gyrus. The functional topography of these areas, however, appears quite similar in all species in that the face-selective regions are embedded within object-selective cortex [22].
Thus, the data presented here support similar neural substrates for face processing in chimpanzees and humans distributed across the whole brain and, specifically, by revealing face-related activity in the fusiform gyrus, orbitofrontal cortex, and posterior STS. Collectively, these similarities and differences suggest that the last common ancestor of macaques, chimpanzees, and humans may have shared a set of neurocognitive mechanisms used to process faces and other relevant visual stimuli, but that additional neural mechanisms evolved in the common ancestry of chimpanzees and humans, pushing these regions into ventromedial temporal cortex, and enabling greater expertise in analyzing and individuating faces. Further studies will be needed before the evolutionary details of these specializations become clearer, such as whether these skills are present in other great apes.
Experimental Procedures
Subjects and Procedure
Five adult chimpanzees (3 males, 14 – 18 years of age) participated in these studies. All subjects had prior experience matching faces using computerized tasks (SOM #1) [6–8]. The stimuli used in this study were novel photographs of individuals who were personally unfamiliar to the subjects, and clip art objects that did not show any faces or face-like images (SOM #2). Prior to each task, the subject was given a 15 mCi dose of [18F]-FDG (2–3 ml volume mixed in approximately 50 ml of Kool-Aid™ sweetened with Splenda™). Subjects then began working on either the face-matching task or the object-matching task, performing without interruption for the entire [18F]-FDG uptake period, approximately 45–60 minutes, when the absorption of [18F]-FDG into the brain begins to asymptote. Subjects were then anesthetized and transported to the Emory University Hospital’s Center for Positron Emission Tomography where they received a 3D whole-brain scan using a Siemens High Resolution Research Tomograph under propofol anesthesia (10 mg/kg/hr) following established procedures [27–28, SOM #5].
Image Processing and Data Analysis
Each subject’s PET scan (face, object and rest conditions) was coregistered to their own MRI. These were converted to a binary mask and applied to the co-registered PET scans to effectively strip away non-brain information. Each stripped and coregistered PET scan was then normalized to its average whole-brain activity so that regional cerebral glucose metabolism (rCGM) could be compared across conditions and between subjects. These scans were then spatially normalized to an average chimpanzee MRI template (chimplate, SOM #5) and these were analyzed using repeated measures ANOVAs in SPM5 where task condition was the within-subject factor. Subsequent t-tests were used to identify which brain regions were significantly more active during one task compared to another. The first analysis compared the face-matching and object-matching tasks directly (p< 0.05) using the contrast: face minus object (FO). This analysis used a more liberal threshold than is typically assessed in neuroimaging studies that was motivated by the small sample size and to provide descriptive analysis of the extended neural regions activated across the whole brain.
Second, for each contrast: face minus rest (FR), object minus rest (OR) and face minus object (OR), the proportion of task-specific voxels was calculated using an ROI-based analysis. The ROIs included were the fusiform gyrus and the posterior STS (see SOM #3). The task specific activity in each ROI was calculated by dividing the number of positive voxels by the size of the individual ROI. Thus, for the contrast FR, positive voxels were those more active for faces compared to rest.
Finally, the face- and object-selective topography of the chimpanzee brain was analyzed by identifying voxels that were unique to each condition when compared to a resting-state baseline. Regions that were significantly (p< 0.05) more face- (FR) and object-selective (OR) when compared to rest were identified using SPM5. These t-map images were then binarized and combined to produce an image showing the union of activity in the two contrasts (binFR+binOR). Each of the original binarized t-map images, either binFR or binOR, were then subtracted from the binarized union revealing the regions that were uniquely active in each individual contrast. In this manner, regions of activity that were shared by each contrast were effectively removed, resulting in activity that was inclusive only of regions unique to that contrast. Unique “face-selective” regions were plotted from the t-map resulting from [bin(FR+OR) – binOR] and unique “object-selective” regions were plotted from the t-map resulting from [bin(FR+OR) – binFR]. These results should be interpreted cautiously because the binarizing procedure may overinflate true differences between the tasks1.
Supplementary Material
Acknowledgments
This investigation was supported by RR-00165 from the NIH/NCRR to the Yerkes National Primate Research Center (YNPRC), R01-MH068791 to LA Parr, NIGMS T32 GM008605 training grant to E. Hecht, and The Center for Behavioral Neuroscience IBS#9876754, a Science and Technology Center Program of the National Science Foundation. The Yerkes National Primate Research Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. Helpful input was provided by Nancy Kanwisher and three anonymous reviewers. Thanks to Jim Rilling for providing the resting-state brain scans, Sheila Sterk for animal assistance, Delicia Votaw and Margie Jones for scanning assistance, and the veterinary and animal care staff at the YNPRC.
Footnotes
A voxel that is slightly greater in condition A compared to condition B, e.g., 0.5 vs. 0.4, when binarized becomes assigned to condition A. Thus, some voxels that were identified as selective for condition A but not also selective for condition B may indeed be only slightly more selective for A. The same inflation would apply for voxels identified as selective for condition B but not also selective for condition A.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maurer D, Le Grand R, Mondloch CJ. The many faces of configural processing. TICS. 2002;6:255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- 2.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. J Cog Neurosci. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- 4.Yovel G, Kanwisher N. Face perception: domain specific, not process specific. Neuron. 2004;44:747–748. doi: 10.1016/j.neuron.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Gauthier I, Tarr MJ, Moylan J, Skudlarski W, Core JC, Anderson AW. The fusiform “face area” is part of a network that processes faces and the individual level. J Cog Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- 6.Parr LA, Winslow JT, Hopkins WD, de Waal FBM. Recognizing facial cues: Individual recognition in chimpanzees (Pan troglodytes) J Comp Psychol. 2000;114:47–60. doi: 10.1037/0735-7036.114.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parr LA, Dove TA, Hopkins WD. Why faces may be special: Evidence for the inversion effect in chimpanzees (Pan troglodytes) J Cog Neurosci. 1998;10:615–622. doi: 10.1162/089892998563013. [DOI] [PubMed] [Google Scholar]
- 8.Parr LA, Heintz M, Akamagwuna U. Three studies of configural face processing by chimpanzees. Brain and Cog. 2006;62:30–42. doi: 10.1016/j.bandc.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. TICS. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 10.Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representation of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 11.Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res Bull. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Kreiman G, Koch C, Fried I. Category specific visual responses of single neurons in the human medial temporal lobe. Nature neurosci. 2000;3:946–953. doi: 10.1038/78868. [DOI] [PubMed] [Google Scholar]
- 13.Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. PNAS. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerlach C, Law I, Gade A, Paulson OB. Categorization and category effects in normal object recognition: a PET study. Neuropsychologia. 2000;38:1693–1703. doi: 10.1016/s0028-3932(00)00082-8. [DOI] [PubMed] [Google Scholar]
- 15.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 16.Grill-Spector K, Kushnir T, Hendler T, Edelman S, Itzchak Y, Malach R. A sequence of object-processing stages revealed by fMRI in the human occipital lobe. Hum Brain Mapp. 1998;6:316–328. doi: 10.1002/(SICI)1097-0193(1998)6:4<316::AID-HBM9>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxe R, Brett M, Kanwisher N. Divide and conquer: in defense of functional localizers. NeuroImage. 2006;30:1088–1096. doi: 10.1016/j.neuroimage.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 18.Rilling JK, Barks SK, Parr LA, Preuss TM, Faber TL, Pagnoni G, Bremner JD, Votaw JR. A comparison of resting state brain activity in humans and chimpanzees. PNAS. 2007;104:17146–17151. doi: 10.1073/pnas.0705132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilback L, Eickhoff SB, Rotorska-Jagiela A, Fink GR, Vogeley K. Minds at rest: social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consci Cog. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Parr LA, Heintz M, Pradhan G. Rhesus monkeys lack face expertise. J Comp Psychol. 2008 doi: 10.1037/0735-7036.122.4.390. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahl CD, Logothetis NK, Hoffman KL. Individuation and holistic processing of faces in rhesus monkeys. Proc Biol Sci. 2007;274:2069–2076. doi: 10.1098/rspb.2007.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsao DY, Freiwald WA, Knutsen TA, Madndeville JB, Tottell RBH. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinsk MA, DeSimone K, Moore T, Gross CG, Kastner S. Representations of faces and body parts in macaque temporal cortex: A functional MRI study. PNAS. 2005;102:6996–7001. doi: 10.1073/pnas.0502605102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrett DI, Mistlin AJ, Chitty AJ, Smith PAJ, Potter DD, Borennimann R, Harries MH. Specialized face processing and hemispheric asymmetry in man and monkey: evidence from single unit and reaction time studies. Behav Brain Res. 1988;29:245–258. doi: 10.1016/0166-4328(88)90029-0. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial expression and gaze-selected responses in the monkey amygdala. Curr Biol. 2007;17:1–7. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 27.Phelps EA, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in human with 18-F-2-fluro-2-deoxy-d-glucose: validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- 28.Phelps ME, Mazziotta JC. Positron emission tomography: human brain function and biochemistry. Science. 1985;228:799–809. doi: 10.1126/science.2860723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.