Abstract
Background
There are documented associations between elevated maternal corticotropin-releasing hormone (CRH) levels and adverse pregnancy outcomes. However, reports of these findings often lack sufficient detail and rationale regarding the bioassay methodology. This shortcoming can be problematic for researchers who do not possess in-depth laboratory sciences knowledge but who want to include bioassays in their investigations or to evaluate published reports. The quality and reliability of CRH measurement results can be significantly affected by variables encountered during sample collection, processing, storage, and bioassay. Thus, it is important to establish research laboratory protocols that are based on well-informed rationales and to carefully consider and control for relevant variables.
Approach
A synthesis of laboratory sciences literature regarding variables affecting CRH measurement in pregnancy is presented. Additionally, consultation with experienced researchers provided an in-depth understanding of CRH measurement. From these sources, a laboratory protocol for clinical research was developed.
Results
Multiple variables that are specific to the reliability of CRH measurement in pregnancy have been identified. These include sample collection methods, sample processing, sample integrity, sample storage, and the actual assay selected.
Conclusion
The reliability of CRH measurements can be significantly improved by identifying and controlling for variables encountered during sample collection, processing, storage, and bioassay. Adequate methodological details are difficult to glean solely from the published literature, thus consultation with well-informed researchers is necessary. A protocol for CRH bioassay in clinical research is proposed.
Keywords: corticotropin-releasing hormone, pregnancy, bioassay methodology
Anumber of researchers have investigated the role of corticotropin-releasing hormone (CRH) in normal and abnormal pregnancies (Lockwood et al., 1996; McLean & Smith, 2001; Siler-Khodr et al., 2003; Smith, Mesiano, & McGrath, 2002; Wadhwa et al., 2004). Associations have been reported between higher levels of CRH and pregnancy adversity, such as preterm birth and hypertension (Hobel, Arora, & Korst, 1999; Ruiz, Fullerton, Brown, & Dudley, 2002; Wadhwa et al., 2004), thus stimulating increased research interest. Although CRH is rarely detectable in the circulation of normal, nonpregnant, and early gestational women, it is present in increasing quantities throughout normal pregnancy, particularly during the second half of gestation (Lockwood et al., 1996). As opposed to normal hypothalamic CRH production outside of pregnancy (in which the CRH is taken up rapidly in the hypophyseal portal blood system, and therefore not seen in the peripheral circulation; Chrousos, 2000), CRH production during pregnancy takes place in the placenta, and the hormone circulates peripherally (McLean & Smith, 2001). Furthermore, placental CRH production is thought to play a prominent role in the normal hormonal, physical, and maturational preparation of the maternal-fetal-placental unit for human labor and birth (Challis, Matthews, Gibb, & Lye, 2000; Norwitz, Robinson, & Challis, 1999).
Although there are numerous reports of CRH measurement, they provide insufficient detail regarding the bioassay methodology and rationale and little discussion on maximizing the quality of results. This shortcoming is problematic for researchers who do not possess in-depth laboratory sciences knowledge but who desire to include CRH bioassays in their investigations. Furthermore, this lack of detail and rationale makes it difficult to fully evaluate and compare the results from various studies. As is the case for all bioassays, the quality of CRH measurements can be affected by many variables, such as specimen collection techniques, processing, and storage as well as the actual assay used (i.e., enzyme-linked immunosorbent assay [ELISA] vs. radioimmunoassay [RIA]; Guder, Narayanan, Wisser, & Zawta, 2003). Unexpected results can also be obtained when care has not been taken to adhere to bioassay steps and instructions as provided by the reference labs (Peninsula, 2006). Moreover, CRH measurement is not common in the research laboratory and is extremely rare in the diagnostic lab. Researchers can benefit from expanding their knowledge of bioassay research to the analyte of interest (such as CRH) prior to including these often costly assays in their research studies. Such an approach can enhance the design, conduct, and results of bioassay research as well as the ability to accurately evaluate associations that may exist.
The purpose of this article is to address bioassay research methodology, highlighting the issues involved in developing a clinical research protocol specifically for CRH measurement in pregnancy. Accordingly, we conducted a synthesis of laboratory sciences literature specifically focusing on the variables affecting the quality of CRH measurement. Due to the lack of adequate detail and rationale reported in the literature, we also consulted with researchers with expertise in CRH measurement. Using the results of this synthesis and consultation, we developed a proposed laboratory protocol for clinical research.
Variables Affecting the Quality of CRH Measurement
We have identified two categories of variables that affect the quality of the results of CRH measurement: preanalytical variables and the specific bioassay chosen.
Preanalytical Variables
Preanalytical variables (those in play prior to the actual conduct of the assay) can markedly affect the results of CRH measurement (Guder et al., 2003). These variables, which are displayed in Figure 1, affect the reliability of the process of recovering the CRH molecule for measurement purposes.
Figure 1.

Preanalytical variables affecting CRH bioassay results. NOTE: CRH = corticotropin-releasing hormone.
Researchers must answer several questions to determine how best to ensure that the integrity and quality of a sample collected from a study participant will be maintained prior to measurement. Each specific bioassay, including CRH measurement, has its own unique set of preanalytical variables that must be considered. For example, does plasma or serum provide the best recovery of the CRH molecule? Is the time of day of sample collection relevant? Do temperature, time, or sample additives contribute to CRH degradation?
Plasma vs. serum
In the case of CRH, most references support the use of plasma as opposed to whole blood or serum (Evans, Livesey, Ellis, & Yandle, 2001; Guder et al., 2003), which necessitates the use of a collection tube (i.e., a “lavender top” tube) that allows for the separation of plasma from whole blood. Plasma is the “virtually cell-free supernatant following centrifugation of whole blood, the coagulability of which is inhibited by the addition of anticoagulants” (Guder et al., 2003, p. 32), and thus use of plasma avoids potential interferences from cellular, platelet, and metabolic products (as contained in serum). Moreover, Evans et al. (2001) reported a significant difference in CRH assay results between plasma and serum samples (46% higher in serum; p > .05), suggesting that the cellular products of the serum sample rendered falsely elevated CRH values. Conversely, one study reported preliminary testing that indicated similar stability of CRH in plasma and serum samples, thus lending support to the researchers’ use of maternal serum samples that had been stored for 6−10 years for CRH measurement (Holzman, Jetton, Siler-Khodr, Fisher, & Rip, 2001). Notably, those study data were not published, nor was a discussion/rationale provided regarding conflicting reports.
In most bioassay measures, whole blood and hemolysis contribute to blood cell interference in peptide measurement, thus altering the results (Guder et al., 2003). Furthermore, blood constituents (e.g., proteases) and blood cell breakdown can degrade protein molecules, also contributing to inaccurate results (Guder et al., 2003). Finally, it is not known whether CRH and/or its metabolites in urine or other body fluids (i.e., saliva, vaginal secretions) accurately reflects the circulating or bioactive CRH levels. Thus, these matrices may not be adequate when measuring CRH. Based on this review, we selected plasma as the material of choice for the proposed laboratory protocol developed in this article.
Timing of sample collection
Unlike with many hormones (e.g., cortisol), CRH production and secretion do not follow a circadian rhythm. Rather, CRH is continuously produced and released during pregnancy by the placenta in a pulsatile fashion (Petraglia et al., 1994). This quality means that there is nothing to mandate that sample collection occur at a specific time during the 24-hr day, thus allowing the researcher to schedule sample collection with convenience for the participant as well as the research team in mind. Therefore, our proposed laboratory protocol does not provide a specific time for sample collection.
Effects of time and temperature
Time and temperature have well-documented influences on many analytes (Ellis, Livesey, & Evans, 2003; Guder et al., 2003), including CRH (Evans et al., 2001), prompting many researchers to adopt a “gold standard” time and temperature approach, which includes chilled centrifugation and preparation of plasma within 2 hr of collection followed by either conduct of the assay or placement of the sample into frozen storage (Lockwood & Kuczynski, 2001; McGrath et al., 2002; Sibai et al., 2005). Given that many studies, in particular large epidemiological studies, cannot feasibly follow this standard, other approaches have been sought. Evans et al. (2001) concluded that CRH may remain stable for up to 120 hr in plasma samples that are kept at 4°C (typical laboratory refrigeration temperature). However, at a temperature of 30°C (temperatures encountered in hotter climates or during transport in a car in summer temperatures), CRH is stable in plasma only up to 18 hr after collection. Furthermore, Strong et al. (2006) reported a correlation coefficient exceeding .96 when comparing the gold standard approach and a delayed sample processing of up to 22 hr after collection, suggesting that CRH could be reliably measured with a delayed approach in samples that are kept refrigerated/chilled at 4°C prior to the delayed processing (centrifugal separation of plasma from the whole blood). This report conflicts with the often strong recommendation that the processing of samples occur as soon as possible to avoid hemolysis and interference with CRH measurement. In our proposed laboratory protocol the impact of time and temperature has been balanced with the realities of clinical research. Thus, the final protocol allows for delayed processing of the sample (within 12 hr of collection) and recommends keeping the samples continuously chilled at 4°C.
Sample additives/preservatives
Additives to collection tubes serve the purpose of preservation of the analyte. For example, the addition of the anticoagulant ethylenediaminetetra-acetic acid (EDTA) to the collection tube effectively renders, after centrifugation, a plasma sample in which the cellular and metabolic blood constituents do not remain to interfere with the bioassay (Guder et al., 2003). Although anticoagulants can markedly affect some analytes, EDTA appears to have no altering affect on CRH (Evans et al., 2001). Given the long-standing nature and effectiveness of obtaining plasma samples by using a collection tube that contains EDTA, the proposed laboratory protocol includes this standard practice.
Some researchers report adding a protease inhibitor (i.e., aprotinin 500 KIU/1 ml blood) to their samples (Hobel & Culhane, 2003; Mancuso, Schetter, Rini, Roesch, & Hobel, 2004; Sandman et al., 2006). A protease inhibitor prevents enzymatic breakdown of sample components, particularly peptides (such as the CRH molecule). Although no published studies could be found that directly compare the efficacy of processing samples for CRH measurement with and without a protease inhibitor, it has been informally reported that as much as 20−25% of CRH is lost due to degradation of the molecule before assay unless samples are processed immediately (C. Arora, personal communication, October 4, 2006; R. J. Ruiz, personal communication, November 11, 2006). Given the realities of clinical research, which often preclude immediate processing of samples, the addition of a protease inhibitor might be quite important, but the rationale for doing so is not supported in the current literature. Furthermore, adding aprotinin use to a laboratory protocol can be cumbersome and expensive, a likely reason that many researchers do not include the use of a protease inhibitor. Based on the understanding, however, of the adverse effects of protease degradation upon protein molecules (i.e., CRH), our proposed laboratory protocol includes the use of aprotinin as an additive to the evacuated tubes prior to sample collection.
Sample processing
Processing involves separation of the serum or plasma from other components of the blood (i.e., platelets, red blood cells [RBCs], and white blood cells [WBCs]), which is accomplished via centrifugation. A standard red top tube, either barrier or nonbarrier, produces serum after the sample is allowed to clot for 30−60 min (to inactivate/remove the clotting factors) then centrifuged at 3,000 rpm for 10 min (Guder et al., 2003). A standard lavender top tube (containing an anticoagulant such as EDTA) is centrifuged at 3,000−4,500 rpm for 15 min to obtain a platelet-free plasma (Guder et al., 2003). Centrifugation is commonly done at room temperature, but some researchers use refrigerated centrifugation (Hobel, Arora, et al., 1999; Sorem et al., 1996; Torricelli et al., 2006).
After centrifugation, the serum or plasma lies above the “pellet” of RBCs/WBCs. Aliquots of plasma or serum can then be gently siphoned from the top of the sample and placed into storage or immediately used in bioassays. If the samples are not immediately used for bioassay measurement, appropriate storage becomes an issue. Our proposed laboratory protocol uses the above standard approach to centrifugation under refrigeration in light of the adverse effects of temperature.
Long-term sample storage
Researchers commonly desire to store samples for later use, either to take advantage of the cost-effectiveness of running assays in batches or to run assays after biomarkers of research interest have been identified. The main questions regarding sample storage are related to appropriate duration and temperature. The effect of repeated thaw/refreeze episodes is also of interest.
If plasma samples are not immediately assayed (within 3−5 days), it is recommended that they be placed in frozen storage of at least −20°C (Guder et al., 2003). A number of researchers report storage of samples at −20°C to −30°C (Holzman et al., 2001; Petraglia et al., 1994; Ruiz et al., 2002), and many report storage of samples at −70°C to −80°C, presumably to maintain stability of the samples for longer storage durations (Hobel, Dunkel-Schetter, Roesch, Castro, & Arora, 1999; Mancuso et al., 2004; Sandman et al., 2006; Strong et al., 2006; Wadhwa et al., 2004). Although storage for longer than 2 years is generally not recommended, no published studies could be found that compared the results of CRH assays of samples stored for various lengths of time (e.g., 2−6 months vs. 2 years vs. 5 or more years) and at various temperatures (e.g., −20°C vs. −70°C) with the gold standard of immediate bioassay. In fact, some studies have used samples held for as long as 6−10 years (Holzman et al., 2001). Moreover, nearly all study reports fail to mention the length of sample storage prior to CRH bioassay. This failure raises concern about the quality of these samples, particularly at higher (i.e., −20°C) storage temperatures.
Repeated thawing and refreezing of samples is not recommended, as it may affect the sample integrity (Guder et al., 2003). However, Holzman et al. (2001) subjected samples to thawing and refreezing cycles and reported no significant loss of CRH. As previously mentioned, these data were not published and must be considered with caution. In summary, the determining factor is primarily the length of storage in relation to temperature levels maintained. In general, lower temperatures provide for better stability of an analyte for longer periods of time. Thus, the proposed laboratory protocol recommends storage of all plasma samples at −80°C immediately after the samples have been prepared to best preserve the integrity of the samples for a duration of 2 years or less.
Transportation of samples
In clinical research, samples are frequently transported from the collection site to be processed, stored, and/or assayed in another location (e.g., a central research lab). Transportation requires maintenance of the integrity of the sample, primarily via controlled temperature, and avoidance of excessive disturbance of the sample. Therefore, consideration must be given to appropriate transport procedures based on the stage of sample processing. Accordingly, during transport samples should not be subjected to freezing temperatures or excessive heat (e.g., a vehicle without air-conditioning in the summer) (Guder et al., 2003). Furthermore, unless a barrier collection tube has been used, centrifuged samples should not be transported as this often causes remixing of the samples, and repeat centrifugation is not recommended (Guder et al., 2003). However, aliquots removed from centrifuged samples can easily be transported, chilled or frozen, as long as stable temperatures are maintained. Furthermore, frozen samples can be transported over longer distances without undue loss of quality if they are kept frozen throughout transport (e.g., through the use of dry ice; Guder et al., 2003). To balance the realities of clinical research against maintenance of the sample integrity, the proposed laboratory protocol directs that collected samples be transported (less than 30 min transport time) unprocessed to the central lab in a cooler with freezer packs to maintain a chilled condition. Processing should then occur immediately on arrival at the central research lab.
Plasma extraction
An extraction process essentially removes the CRH from the plasma, thus avoiding measurement interference from other plasma proteins, such as the CRH-binding protein (CRH-BP; Guder et al., 2003). One laboratory surveyed strongly recommended an extraction process prior to any CRH measurement, in spite of the fact that an “extraction-free” assay product is available (Peninsula, 2006). Linton et al. (1995) report that 45−81% of the CRH molecule is rendered unavailable for measurement due to the interference of CRH-BP, therefore creating serious undermeasurement of total CRH levels in the unextracted samples. Complicating the issue further, these researchers documented an increasing interference of CRH-BP in the presence of increasing CRH levels. Finally, it is known that CRH binding to CRH-BP is highly variable between individuals (Linton et al., 1995); thus it is not possible to make adjustments for a calculated standard level of binding across individuals. However, two studies document the use of a tandem ELISA process in which extraction was not used. These detailed reports describe a process by which researchers account for the total plasma CRH by conducting an ELISA for the CRH/CRH-BP complex first followed by an ELISA for the unbound CRH (Behan et al., 1996; Erickson et al., 2001).
Most researchers do choose to use an extraction process, but they vary in the methods they use (methanol vs. C18 Sep-Column). In the few studies that actually report the percentage of CRH recovery, the methods appear to render similar percentages of CRH recovery, with methanol achieving 50% (Ruiz et al., 2002), 87.5% (Holzman et al., 2001), and as much as 103% (McGrath et al., 2002), and C18 Sep-Column achieving 60−80% (Sorem et al., 1996) and (inversely proportional to the volume of plasma) 52−85% (one cartridge) to 79−88% (two tandem cartridges) (Emanuel et al., 1994). The more obvious differences between the two methods lie in the cost, ease of use, and commercial availability of the products involved. Table 1 summarizes these differences. Sep-Pak 18 is a commercially produced kit with a higher associated cost than methanol extraction. Methanol must be kept ice cold, and both methods require high-speed concentrating (drying) centrifugation. Both methods render a product that is essentially lyophilized (freeze dried) CRH. This lyophilized CRH can be used immediately (preferred) or stored frozen. It must be resuspended in assay buffer at the time of the actual bioassay measurement. The proposed protocol uses the methanol extraction method due to the significantly smaller costs and relative ease of the method. As recommended, the extraction process occurs immediately prior to the actual conduct of the assay.
Table 1.
Comparison of CRH Extraction Methods
| Extraction Method | Cost | Time Requirement | Processing | Extraction Efficiency |
|---|---|---|---|---|
| C18 Sep-Columna | One “kit” extracts 50 plasma samples for US$130 (US$2.60 per sample) | Approximately 24 hr | Requires evaporation via centrifugal concentrator | 52−88% recovery of the CRH moleculeb |
| Methanol | Approximately US$3.50 for 50 plasma samples (US$0.07 per sample) | Approximately 24 hr | Requires evaporation via centrifugal concentrator | 50% to > 90% recovery of the CRH molecule |
Commercially available “kit” (e.g., Peninsula Labs).
Recovery percentage inversely proportional to the volume of plasma extracted.
Selection of the Assay
After considering and controlling for the preanalytical variables discussed thus far, the research team must choose the most appropriate assay for CRH measurement. This decision rests largely on factors such as cost, ease and, most importantly, reliability/sensitivity of the tests available. Three tests have documented use in the research literature: RIA, ELISA, and immunoradiometric assay (IRMA). IRMA has been less often used and reported in the literature and therefore will not be further discussed in this report.
Behan et al. (1996, p. 2584) point out that ELISAs are “nonisotopic, are often more sensitive, are easier to operate, have a rapid turnover time and broader range of detection... are generally more reliable” than conventional RIAs. However, most studies report the use of RIA in spite of the drawbacks of radioisotope use and the comparatively decreased ease of running the assay. The rationale for assay selection is rarely mentioned or discussed in the literature. However, a contributing factor for the selection of RIA may be that a majority of the initial studies measuring CRH were conducted using the gold standard RIA technology of the day (1980s and early 1990s). Since that time, ELISA and IRMA technology have been well developed for many analytes but not for CRH. Two studies reporting the use of ELISA for CRH measurement did not discuss the selection rationale, the limitations of the assay, or the advantages and disadvantages of its use (Erickson et al., 2001; Sibai et al., 2005). As previously discussed, Behan et al. (1996) reported the development of a tandem ELISA procedure for measurement of CRH with apparently excellent results, but this method has not been further tested in clinical research.
Table 2 provides a comparison of the general characteristics of ELISA and RIA for the measurement of CRH. In spite of the negative aspect of radioisotope use with the RIA methodology, it has been selected for the proposed protocol due to its extensive use reported in the literature. Furthermore, commercial laboratories and published reports document greater sensitivity with RIA.
Table 2.
Characteristics of Radioimmunoassay (RIA) and Enzyme-Linked Immunosorbent Assay (ELISA) for CRH Measurement
| Assay | Cost per Samplea | Assay Durationb | Use of Radioisotopes | Intra-assay/Interassay CV (%)c | Specificity (% Cross-reactivity) | Sensitivity (ng/ml)d |
|---|---|---|---|---|---|---|
| ELISA | US$10−18 | 4.5−6 hr | No | 3−8/5−10 | 100 (human); 0 (ACTH) | 0.08−0.3 |
| RIA | US$12−14; US$242e | 3 days | Yes | 2−6/8.5−10 | 100 (human); 0 (ACTH) | 0.00095−0.02 |
Note: ACTH = adrenocorticotropin-releasing hormone; CV = coefficient of variability; CRH = corticotropin-releasing hormone.
Average direct cost per sample if a commercially prepared “kit” is used that simultaneously runs 41 samples in duplicate during a “batch” testing process, along with standards and controls.
Does not include extraction time, which is usually an additional 24 hr.
Can vary widely between labs and individuals performing the assays. These are reported by the manufacturers of the bioassay products.
All original reports of sensitivities have been converted to ng/ml for ease of comparison.
Cost per individual sample, as opposed to samples as part of a batch, as quoted by a local/regional commercial reference laboratory.
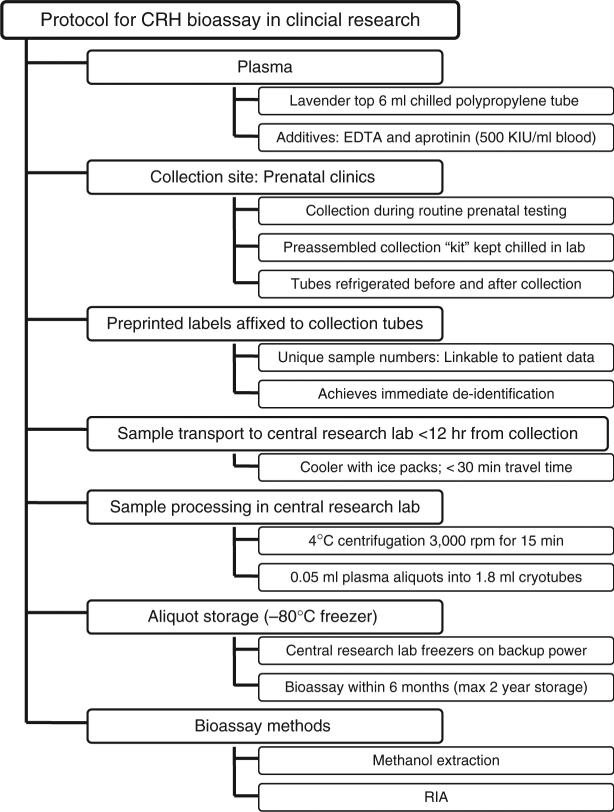
Protocol Development
In developing the laboratory protocol provided here (outlined in Figure 2), we considered how to maximize the recovery of CRH and diminish the degradation of the CRH molecule while also accommodating the needs of a clinical setting. We developed this protocol, based on this review of the published literature as well as on direct consultation with knowledgeable researchers, for use in clinical research studies of CRH and pregnancy. Variations of this protocol are being used with apparent success in a number of research studies (Hobel, Arora et al., 1999; Mancuso et al., 2004; Sandman et al., 2006; Wadhwa et al., 2004).
Figure 2.
Laboratory protocol for CRH bioassay in clinical research. NOTE: EDTA = ethylenediaminetetra-acetic acid; CRH = corticotropin-releasing hormone.
Discussion
In developing a lab protocol, consultation with well-informed researchers who are familiar with CRH measurement is essential, given that sufficient methodological details are difficult to glean solely from the literature. For researchers who will be including CRH measurement in their protocols, the choices are to learn about and conduct your own assays (if adequate lab facilities and knowledgeable support are available) or, alternatively, to send the samples to a reputable lab with the expertise in CRH measurement.
Well-informed approaches to CRH measurement will be rewarded with higher quality results and, importantly, have a defensible justification for the laboratory protocols. Although no consensus has been reached regarding a gold standard approach to CRH measurement, particularly in clinical research, information is available that can inform protocol development. However, there is a need for investigations that make direct comparisons between extraction methods (i.e., methanol vs. C18 Sep-Columns) and bioassay techniques (i.e., RIA vs. ELISA vs. IRMA) and examine the efficacy of the use of protease inhibitors during sample collection and processing steps. Furthermore, given the danger of working with radioisotopes, it would be relevant to advance the development of a reliable and sensitive ELISA bioassay for the measurement of CRH. It is also important to acknowledge that appropriate safety precautions are outlined by individual institutions and laboratory services and that these guidelines must be followed precisely to avoid potential harm to those conducting any bioassay techniques.
Identifying a “gold standard” approach for the measurement of CRH in clinical research would be the ideal. Such a standard would enable researchers to more accurately measure CRH and to interpret and compare the results from different studies as well as conduct confirmatory replication studies.
Acknowledgments
This work has been supported by a National Research Service Award from the National Institute of Nursing Research, National Institutes of Health (1 F31 NR010046−01), a March of Dimes Foundation 2006 Graduate Research Award, and an American College of Nurse-Midwives Foundation 2006 Graduate Research Award. We would like to thank Dr. Chander Arora (Cedars Sinai Medical Center) and Dr. George Chrousos (National Institutes of Health, Bethesda) for their kind and informative support.
References
- Behan DP, Khongsaly O, Liu XJ, Ling N, Goland R, Nasman B, et al. Measurement of corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in human plasma by two-site enzyme-linked immunoabsorbant assay. The Journal of Clinical Endocrinology and Metabolism. 1996;81:2579–2586. doi: 10.1210/jcem.81.7.8675581. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocrine Reviews. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The HPA axis and the stress response. Endocrine Research. 2000;26:513–514. doi: 10.3109/07435800009048562. [DOI] [PubMed] [Google Scholar]
- Ellis J, Livesey JH, Evans MJ. Hormone stability in human whole blood. Clinical Biochemistry. 2003;36:109–112. doi: 10.1016/s0009-9120(02)00440-x. [DOI] [PubMed] [Google Scholar]
- Emanuel RL, Robinson BG, Seely EW, Graves SW, Kohane I, Saltzman D, et al. Corticotrophin releasing hormone levels in human plasma and amniotic fluid during gestation. Clinical Endocrinology (Oxford) 1994;40:257–262. doi: 10.1111/j.1365-2265.1994.tb02477.x. [DOI] [PubMed] [Google Scholar]
- Erickson K, Thorsen P, Chrousos G, Grigoriadis DE, Khongsaly O, McGregor J, et al. Preterm birth: Associated neuroendocrine, medical, and behavioral risk factors. The Journal of Clinical Endocrinology and Metabolism. 2001;86:2544–2552. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Livesey JH, Ellis MJ, Yandle TG. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clinical Biochemistry. 2001;34:107–112. doi: 10.1016/s0009-9120(01)00196-5. [DOI] [PubMed] [Google Scholar]
- Guder W, Narayanan S, Wisser H, Zawta B. Samples: From the patient to the laboratory. The impact of preanalytical variables on the quality of laboratory samples. 3rd ed. Wiley-VCH Verlog GmbH & Co; Weinheim, Germany: 2003. [Google Scholar]
- Hobel CJ, Arora CP, Korst LM. Corticotrophin-releasing hormone and CRH-binding protein. Differences between patients at risk for preterm birth and hypertension. Annals of the New York Academy of Sciences. 1999;897:54–65. doi: 10.1111/j.1749-6632.1999.tb07878.x. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Culhane J. Role of psychosocial and nutritional stress on poor pregnancy outcome. Journal of Nutrition. 2003;133(5 Suppl 2):1709S–1717S. doi: 10.1093/jn/133.5.1709S. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. American Journal of Obstetrics and Gynecology. 1999;180(1 Pt 3):S257–S263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstetrics and Gynecology. 2001;97(5 Pt 1):657–663. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- Linton EA, Perkins AV, Hagan P, Poole S, Bristow AF, Tilders F, et al. Corticotrophin-releasing hormone (CRH)-binding protein interference with CRH antibody binding: Implications for direct CRH immunoassay. Journal of Endocrinology. 1995;146:45–53. doi: 10.1677/joe.0.1460045. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatric and Perinatal Epidemiology. 2001;15(Suppl 2):78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Radunovic N, Nastic D, Petkovic S, Aigner S, Berkowitz GS. Corticotropin-releasing hormone and related pituitary-adrenal axis hormones in fetal and maternal blood during the second half of pregnancy. Journal of Perinatal Medicine. 1996;24:243–251. doi: 10.1515/jpme.1996.24.3.243. [DOI] [PubMed] [Google Scholar]
- Mancuso RA, Schetter CD, Rini CM, Roesch SC, Hobel CJ. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosomatic Medicine. 2004;66:762–769. doi: 10.1097/01.psy.0000138284.70670.d5. [DOI] [PubMed] [Google Scholar]
- McGrath S, McLean M, Smith D, Bisits A, Giles W, Smith R. Maternal plasma corticotropin-releasing hormone trajectories vary depending on the cause of preterm delivery. American Journal of Obstetrics and Gynecology. 2002;186:257–260. doi: 10.1067/mob.2002.119635. [DOI] [PubMed] [Google Scholar]
- McLean M, Smith R. Corticotrophin-releasing hormone and human parturition. Reproduction. 2001;121:493–501. doi: 10.1530/rep.0.1210493. [DOI] [PubMed] [Google Scholar]
- Norwitz ER, Robinson JN, Challis JR. The control of labor. New England Journal of Medicine. 1999;341:660–666. doi: 10.1056/NEJM199908263410906. [DOI] [PubMed] [Google Scholar]
- Peninsula . General protocol for peptide radio-immunoassay. Peninsula Laboratories, Inc.; San Carlos, CA: 2006. [Google Scholar]
- Petraglia F, Genazzani AD, Aguzzoli L, Gallinelli A, de Vita D, Caruso A, et al. Pulsatile fluctuations of plasma-gonadotropin-releasing hormone and corticotropin-releasing factor levels in healthy pregnant women. Acta Obstetricia et Gynecologica Scandinavica. 1994;73:284–289. doi: 10.3109/00016349409015764. [DOI] [PubMed] [Google Scholar]
- Ruiz RJ, Fullerton J, Brown CE, Dudley DJ. Predicting risk of preterm birth: The roles of stress, clinical risk factors, and corticotropin-releasing hormone. Biological Research for Nursing. 2002;4:54–64. doi: 10.1177/1099800402004001007. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sibai B, Meis PJ, Klebanoff M, Dombrowski MP, Weiner SJ, Moawad AH, et al. Plasma CRH measurement at 16 to 20 weeks’ gestation does not predict preterm delivery in women at high-risk for preterm delivery. American Journal of Obstetrics and Gynecology. 2005;193(3 Pt 2):1181–1186. doi: 10.1016/j.ajog.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Siler-Khodr TM, Forthman G, Khodr C, Matyszczyk S, Khodr Z, Khodr G. Maternal serum corticotropin-releasing hormone at midgestation in Hispanic and White women. Obstetrics and Gynecology. 2003;101:557–564. doi: 10.1016/s0029-7844(02)03072-7. [DOI] [PubMed] [Google Scholar]
- Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regulatory Peptides. 2002;108:159–164. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Sorem KA, Smikle CB, Spencer DK, Yoder BA, Graveson MA, Siler-Khodr TM. Circulating maternal corticotropin-releasing hormone and gonadotropin-releasing hormone in normal and abnormal pregnancies. American Journal of Obstetrics and Gynecology. 1996;175(4 Pt 1):912–916. doi: 10.1016/s0002-9378(96)80024-x. [DOI] [PubMed] [Google Scholar]
- Strong EF, Kleinman KP, Gillman MW, Clark I, Emanuel RL, Majzoub JA, et al. Measuring corticotropin-releasing hormone in pregnant women—comparing blood collection protocols for epidemiological studies. Paediatric and Perinatal Epidemiology. 2006;20:67–71. doi: 10.1111/j.1365-3016.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- Torricelli M, Ignacchiti E, Giovannelli A, Merola A, Scarpetti E, Calonaci G, et al. Maternal plasma corticotrophin-releasing factor and urocortin levels in post-term pregnancies. European Journal of Endocrinology. 2006;154:281–285. doi: 10.1530/eje.1.02091. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: A prospective investigation. American Journal of Obstetrics and Gynecology. 2004;191:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]