Introduction
The neural crest comprise a group of highly migratory progenitor cells responsible for generating a wide variety of derivatives in vertebrates, including components of the nervous, musculoskeletal and cardiovascular systems (Le Douarin and Kalcheim 1999). During development, neural crest cells arise from the dorsal neural tube at all axial levels and migrate throughout the embryo, eventually contributing to virtually every vertebrate organ system. Neural crest cells from the anterior neural tube form the facial bones, most of the skull and associated mesenchymal tissue and contribute to the developing heart. Posteriorly, trunk neural crest cells give rise to the majority of the peripheral nervous system (PNS), including the neurons and glia of the dorsal root ganglia (DRG), sympathetic ganglia and Schwann cells, as well as melanocytes.
Neural crest cells are induced early in primary neurulation, prior to the closure of the neural tube, by interactions between the prospective epidermal and neural ectoderm. These interactions are mediated by members of the bone morphogenic protein (BMP), Wnt and fibroblast growth factor (FGF) families (Barembaum and Bronner-Fraser 2005; Jones and Trainor 2005; Morales et al. 2005; Raible and Ragland 2005; Sakai and Wakamatsu 2005). In response to these signals, the neural crest cells undergo an epithelial to mesenchymal transition (EMT), delaminate from the neural tube and migrate away to form their peripheral derivatives in highly precise locations (Kuriyama and Mayor 2008). In the avian embryo, trunk neural crest cells migrate along either dorsolateral or ventral routes, resulting in the formation of distinct neural crest derivatives (Teillet et al. 1987; Bronner-Fraser and Fraser 1988; Serbedzija et al. 1989; Bronner-Fraser and Fraser 1991; Frank and Sanes 1991; Fraser and Bronner-Fraser 1991; Le Douarin and Kalcheim 1999). Neural crest cells that migrate along the dorsolateral pathway pass between the dermamyotome and overlying ectoderm and differentiate into melanocytes. In contrast, neural crest cells that migrate along the ventral route travel through the anterior somite and can aggregate lateral to the neural tube to form the neurons and glial of the dorsal root ganglia (DRG), become Schwann cells along the ventral root, or continue past the ventral root and coalesce near the dorsal aorta to give rise to the sympathetic ganglia (Teillet et al. 1987; Lallier and Bronner-Fraser 1988). Thus, trunk neural crest cells are generally considered to be a heterogeneous population of progenitor cells with the potential to differentiate into sensory, glial and melanocyte fates.
While the induction of the neural crest and the pathways by which they migrate have been well studied, less understood are the molecules that mediate their subsequent aggregation to form the PNS, particularly the sensory and sympathetic ganglia. One important group of molecules implicated in both neural crest migration and ganglia formation are the cadherins. The cadherin family, whose members include both classical cadherins and the more recently discovered protocadherins, are calcium-dependent transmembrane proteins that function in cell-cell adhesion (Sano et al. 1993; Angst et al. 2001; Frank and Kemler 2002; Goodwin and Yap 2005). Classical cadherins and protocadherins mediate cell sorting and tissue histogenesis throughout development, including during the formation of the embryonic nervous system (Vleminckx and Kemler 1999; Redies 2000; Tepass et al. 2000; Gumbiner 2005; Rashid et al. 2006). In particular, a role for cadherins in neural crest cell migration and PNS formation is supported by their dynamic expression patterns in premigratory and migratory neural crest cells; furthermore, several studies have shown that regulated cadherin activity is necessary for the ability of neural crest cells to undergo an EMT and exit the dorsal neural tube (Nakagawa and Takeichi 1998; Borchers et al. 2001; Pla et al. 2001; Rangarajan et al. 2006; Shoval et al. 2007). Left unanswered by these studies are the roles cadherins play once the neural crest cells have migrated away from the neural tube and begin to coalesce to form the various PNS structures.
Here we describe the role of chicken protocadherin-1 (Pcdh1) in PNS formation. In chicken embryos, Pcdh1 is expressed in the developing DRG and sympathetic ganglia. In the DRG, Pcdh1 is predominantly found at the perimeter, where it colocalizes with the mitotically active and undifferentiated progenitor cells. Inhibiting Pcdh1 function alters the distribution of neural crest cells between the DRG and sympathetic ganglia, as well as the percentage of DRG cells that differentiate along the sensory neuron pathway. Thus, Pcdh1 likely plays important roles in mediating cell sorting and fate choices during the formation of the DRG.
Results
Pcdh1 is expressed in the trunk PNS
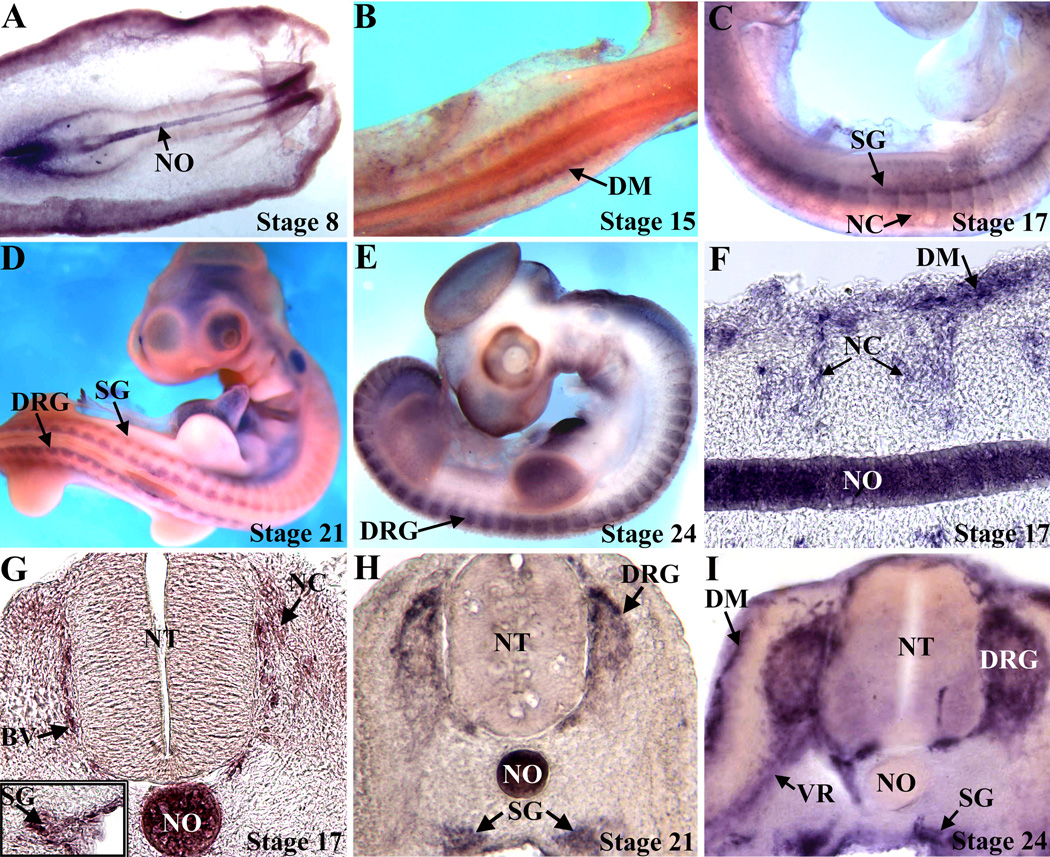
To identify protocadherins involved in neural crest migration and differentiation, a chick embryonic day 4.5 DRG library was screened, using PCR primers that recognize conserved regions found in the extracellular domains of protocadherins (Bradley et al. 1998). Sequencing of the PCR products revealed a novel protocadherin that was used to isolate a full-length cDNA. The cDNA encodes a predicted protein of 1049 amino acids and approximate molecular mass of 115 kDaltons. A comparison of the predicted protein reveals that it is a member of the δ-protocadherin subfamily (Redies et al. 2005), with 81% homology to human protocadherin-1 and 80% homology to Xenopus axial protocadherin. Blasting the cDNA against the chicken (Gallus gallus) genome reveals that the gene is located on chromosome 13 and is within a syntenic region that is orthologous to human PCDH1 on chromosome 5q and mouse Pcdh1 on chromosome 18; therefore this gene was named chicken protocadherin-1 (Pcdh1). To localize Pcdh1 expression during embryonic development, whole-mount in situ hybridization was performed. Prior to H.H. stage 17, Pcdh1 is primarily expressed in the notochord and dermamyotome (Figure 1 and data not shown). Expression in the PNS is first discernible at stage 17, with expression in migrating neural crest cells and the sympathetic ganglia. By stage 21, Pcdh1 is now expressed in the developing DRG, where the mRNA primarily localizes to the cells along the perimeter, with expression reduced in the center. At stage 24, Pcdh1 mRNA localizes to the perimeter of the DRG, sympathetic ganglia and ventral root, as well as the dermamyotome, but is now downregulated in the notochord (Figure 1). As a negative control, embryos hybridized with a sense Pcdh1 probe showed no specific stain (data not shown).
Figure 1.
Pcdh1 expression in the developing PNS. Embryos were analyzed by in situ hybridization and viewed in whole mount (A–E), or sectioned (F–I). At stage 8 (A) Pcdh1 mRNA is expressed in the notochord (NO), while by stage 15 (B), Pcdh1 is also detected in the presumptive dermamyotome (DM). Expression in the developing PNS is first detectable at stage 17 (C,F,G), as Pcdh1 mRNA is now observed in migrating neural crest cells (NC) and the sympathetic ganglia (SG), as well as in developing blood vessels (BV). By stages 21–24 (D,E,H,I) Pcdh1 is expressed in the developing dorsal root ganglia (DRG), sympathetic ganglia and presumptive Schwann cells along the ventral root (VR), with expression in the DRG highest at the perimeter. NT, neural tube.
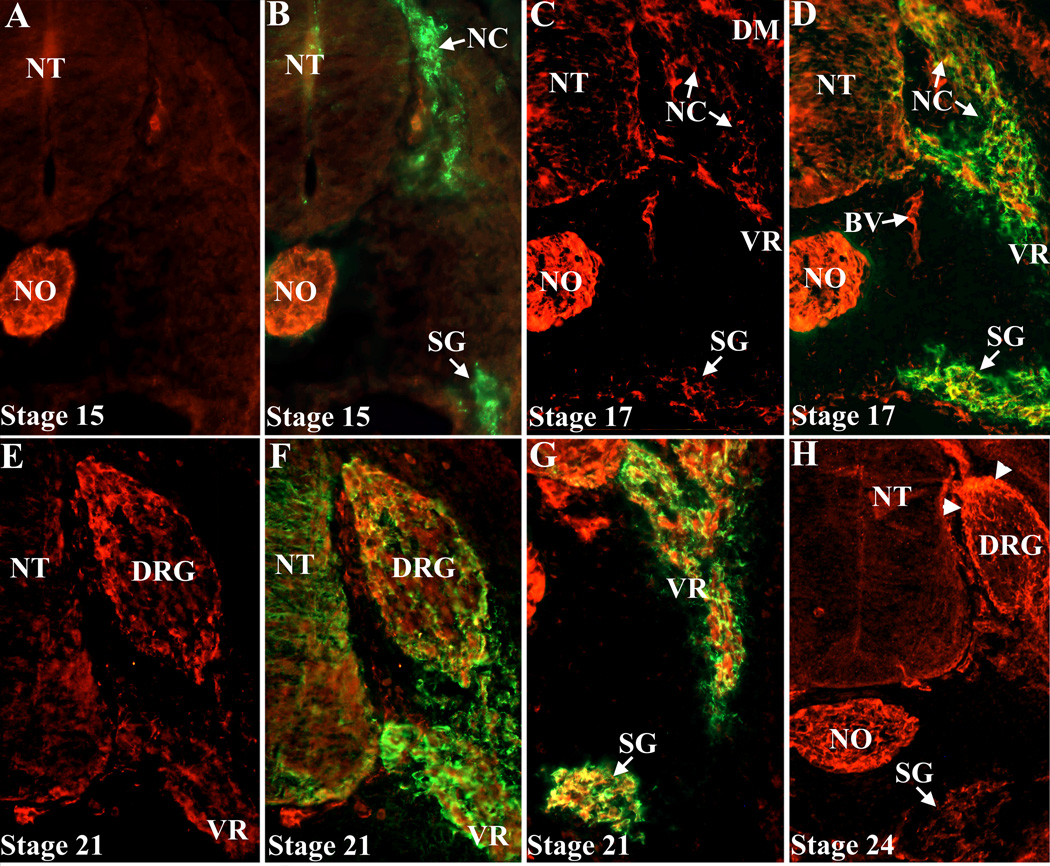
To localize Pcdh1 protein more precisely, a rabbit antiserum to the Pcdh1 extracellular domain was generated. This antiserum recognizes Pcdh1 protein in transfected tissue culture cells by both immunoblotting and immunofluorescence (data not shown). In chick embryos, prior to stage 17, immunolocalization reveals Pcdh1 protein primarily in the notochord, with no specific staining of migrating neural crest cells or their derivatives (Figure 2A and data not shown). At stage 17, Pcdh1 protein is now detectable in the developing DRG and sympathetic ganglia and by stages 21–24 Pcdh1 protein strongly localizes to the DRG perimeter and sympathetic ganglia, as well as the ventral root, consistent with the in situ results. Double labeling for Pcdh1 and the neural crest marker HNK (Bronner-Fraser 1986) reveals that the Pcdh1-positive cells are also positive for HNK, confirming Pcdh1 expression in neural crest derivatives (Figure 2). In sum, in the developing PNS, Pcdh1 expression begins at about stage 17 and by stage 21 Pcdh1 is expressed in most trunk neural crest derivatives, with the exception of melanocytes, with expression in the DRG restricted to the cells along the perimeter.
Figure 2.
Immunodetection of Pcdh1 protein in the PNS. Embryos were co-labeled with a Pcdh1 antiserum (A–H) and an antibody to the neural crest marker HNK (B,D,F,G). At stage 15 (A,B), the Pcdh1 antiserum labels the notochord (NO), but not migrating neural crest cells (NC) or sympathetic ganglia (SG). At stage 17 (C,D), Pcdh1 protein is now found in migrating neural crest cells, sympathetic ganglia and along the ventral root (VR). By stage 21–24 (E–H) Pcdh1 protein localizes to the developing DRG, as well as sympathetic ganglia and ventral root. Within the DRG, Pcdh1 protein primarily localizes to the cells along the perimeter (arrowheads in H). NT, neural tube; BV, blood vessel.
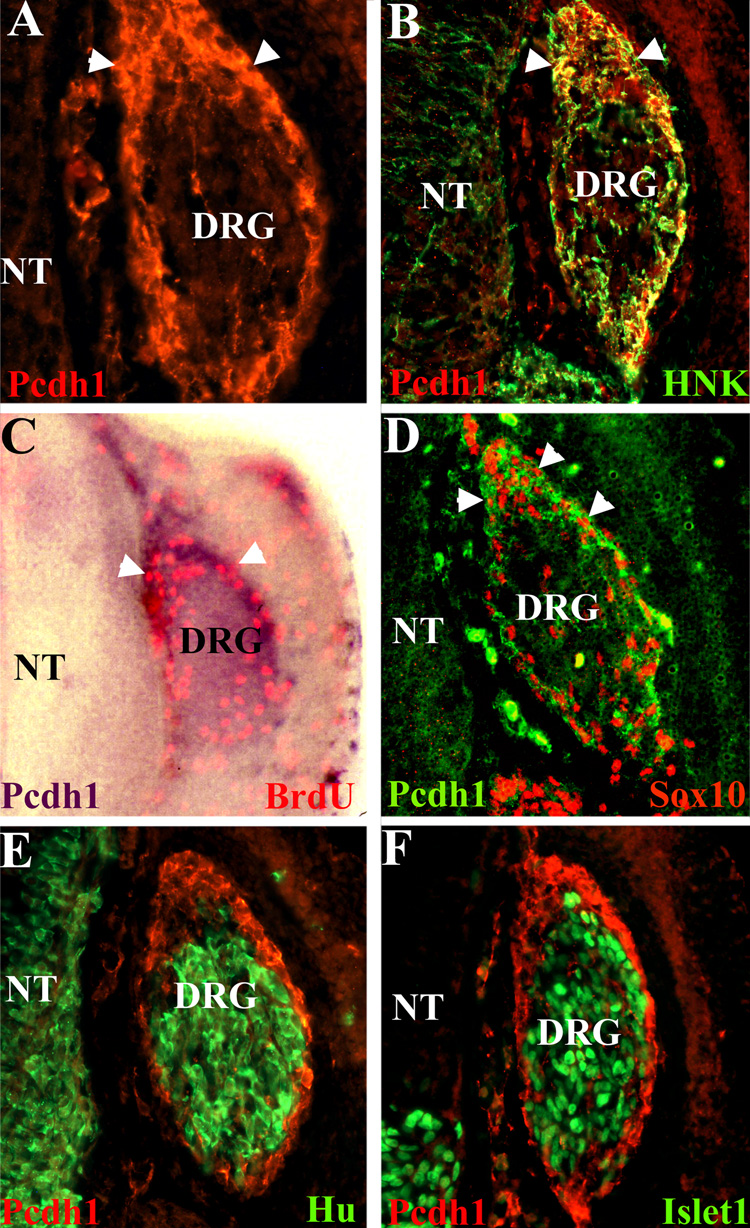
Within the DRG at stages 22–24, the cells at the perimeter are mitotically active and undifferentiated, while cells in the center are differentiating into sensory neurons (Wakamatsu et al. 2000; Nelson et al. 2002). As Pcdh1 localizes to the DRG perimeter at these stages, we next determined whether Pcdh1 colocalizes with the mitotically active cells. Embryos at stage 22 were pulse labeled with BrdU, to label cells undergoing DNA synthesis, then fixed and subjected to in situ hybridization for Pcdh1, followed by immunostaining for BrdU. As shown in Figure 3, BrdU incorporation labels a subset of cells in the DRG, mainly at the perimeter, and these cells are also positive for Pcdh1 expression. Similarly, doublelabeling for Pcdh1 and Sox10, a marker of undifferentiated cells in the DRG at stage 22 (Cheng et al. 2000), reveals that the Pcdh1- and Sox10-positive cells colocalize. As sensory neurons differentiate, they move into the interior of the DRG and begin to express neural specific markers, including Hu, a member of the Elav family of RNA binding proteins, and Islet-1, a member of the Lim homeobox family of transcription factors (Marusich et al. 1994; Avivi and Goldstein 1999; Cui and Goldstein 2000; Wakamatsu et al. 2000). To determine whether Pcdh1 is found on differentiating sensory neurons, embryos were double-labeled for Pcdh1 and either Hu or Islet-1. As shown in Figure 3E and F, Pcdh1 expression is highest in the DRG regions in which Hu and Islet-1 are not expressed, suggesting Pcdh1 expression is downregulated as sensory neurons begin to differentiate. Taken together, these results suggest that Pcdh1 specifically labels the mitotically active and undifferentiated perimeter cells and is not expressed in differentiating sensory neurons in the center of the DRG.
Figure 3.
Pcdh1 expression in the DRG colocalizes with markers of mitotically active and undifferentiated cells. A,B) Cryosections of a stage 22 DRG immunolabeled for Pcdh1(red fluorescence) or doubled labeled for Pcdh1 protein and the neural crest marker HNK (green fluorescence) reveal Pcdh1 protein is localized to the DRG dorsal pole and perimeter (arrowheads). C) Stage 22 chick embryo analyzed for BrdU incorporation (red fluorescence) and by in situ hybridization for Pcdh1 expression (purple stain), showing the mitotically active cells in the DRG are located at the perimeter, where Pcdh1 expression is highest. D) Immunostaining for Pcdh1 (green) and Sox10 (red), a marker of undifferentiated DRG cells, demonstrates colocalization in the dorsal pole and perimeter cells. E,F) Immunolocalization for Pcdh1 (red) and the neural markers Hu and Islet-1 (green) reveals that Pcdh1 is restricted from the cells undergoing neural differentiation in the DRG interior. NT, neural tube.
Pcdh1 functions in cell adhesion
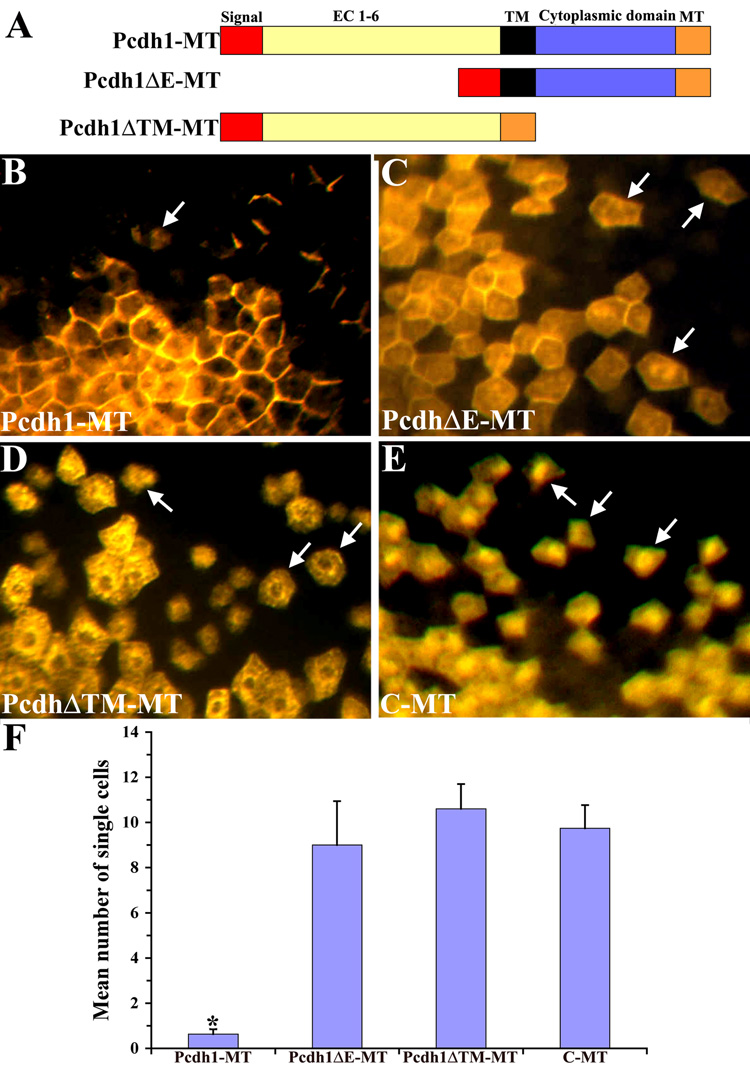
Given the known roles of cadherin and protocadherin family members in cell adhesion and tissue morphogenesis, the expression of Pcdh1 in the PNS suggested it might function as a cell adhesion molecule to promote the aggregation of neural crest cells as they reach their target tissues. However, the ability of Pcdh1 to function as a cell adhesion molecule has not previously been established. Therefore, to determine if Pcdh1 can mediate cell-cell adhesion in vivo, we utilized a cell mixing assay in Xenopus embryos (Bradley et al. 1998; Rashid et al. 2006). For this assay, frog embryos at the 16-cell stage were injected into a single dorsal blastomere with RNA encoding Pcdh1 tagged with a C-terminal myc epitope (Pcdh1-MT, Figure 4A). As a negative control embryos were injected with RNA encoding solely the myc epitope (C-MT). Injected embryos were allowed to develop until neurula stage, then fixed and immunostained for the myc epitope. The number of single-labeled cells in the ectoderm, not in contact with another labeled cell, was counted. In control embryos, the myc-stained cells undergo extensive cell mixing, with labeled cells intermingling with non-labeled cells at the borders of a patch of cells expressing the myc tag (Figure 4E). In contrast, embryos expressing Pcdh1-MT exhibited significantly fewer single labeled cells in the ectoderm, with the majority of labeled cells remaining together as a cohesive unit. These results suggest that ectopic expression of Pcdh1-MT can mediate cell adhesion in embryos, thereby suppressing the normal cell mixing that occurs in the embryonic ectoderm. In addition, Pcdh1-MT was found to localize to the cell membranes at regions of contact between two expressing cells, consistent with a role as a cell adhesion molecule (Figure 4B). To investigate the role of Pcdh1 in cell adhesion further, two mutant Pcdh1 constructs were also created: one lacking the extracellular domain (Pcdh1ΔE-MT) and a construct encoding a protein lacking both the transmembrane and cytoplasmic domains (Pcdh1ΔTM-MT), as shown in Figure 4A. Previous research has shown that protocadherin constructs lacking the extracellular domain can function as potent dominant negatives, as can constructs lacking both the transmembrane and cytoplasmic domains (Bradley et al. 1998; Kuroda et al. 2002). In the cell-mixing assay, neither deletion construct was capable of suppressing cell mixing, with the number of single-labeled cells in the ectoderm comparable to negative control embryos (Figure 4F). These results demonstrate that full-length Pcdh1 can function as an adhesion molecule in vivo and requires both the extracellular and cytoplasmic domains to promote cell adhesion.
Figure 4.
Ectopic expression of Pcdh1-MT promotes cell adhesion in a Xenopus cell mixing assay. A) Schematic diagram of the Pcdh1 constructs used in this study. The construct Pcdh1-MT encodes the full-length Pcdh1 protein. In Pcdh1ΔE-MT, the Pcdh1 extracellular domain (EC) is deleted and in Pcdh1ΔTM-MT, the transmembrane (TM) and cytoplasmic domains are deleted. All constructs are tagged with a C-terminal myc-epitope tag (MT). B–E) Xenopus embryos at the 16-cell stage were injected into a single dorsal blastomere with RNA encoding Pcdh1-MT (B), Pcdh1ΔE-MT (C), Pcdh1ΔTM-MT (D), or solely the myc-epitope (C-MT) as a control (E), then fixed at neurula stage and immunostained with the myc antibody. Arrows indicate single labeled cells not in contact with another labeled cell. F) The number of single labeled cells in the ectoderm was quantified for each construct. As compared to control embryos, embryos injected with RNA encoding Pcdh1-MT exhibited significantly fewer isolated cells, with the majority of Pcdh1-MT expressing cells remaining as a cohesive clone. In contrast, neither of the deletion constructs resulted in a decrease in the number of isolated cells, as compared to the control. * Indicates a statistically significant difference (p<0.01) from the control C-MT.
Pcdh1 is required for proper neural crest cell sorting
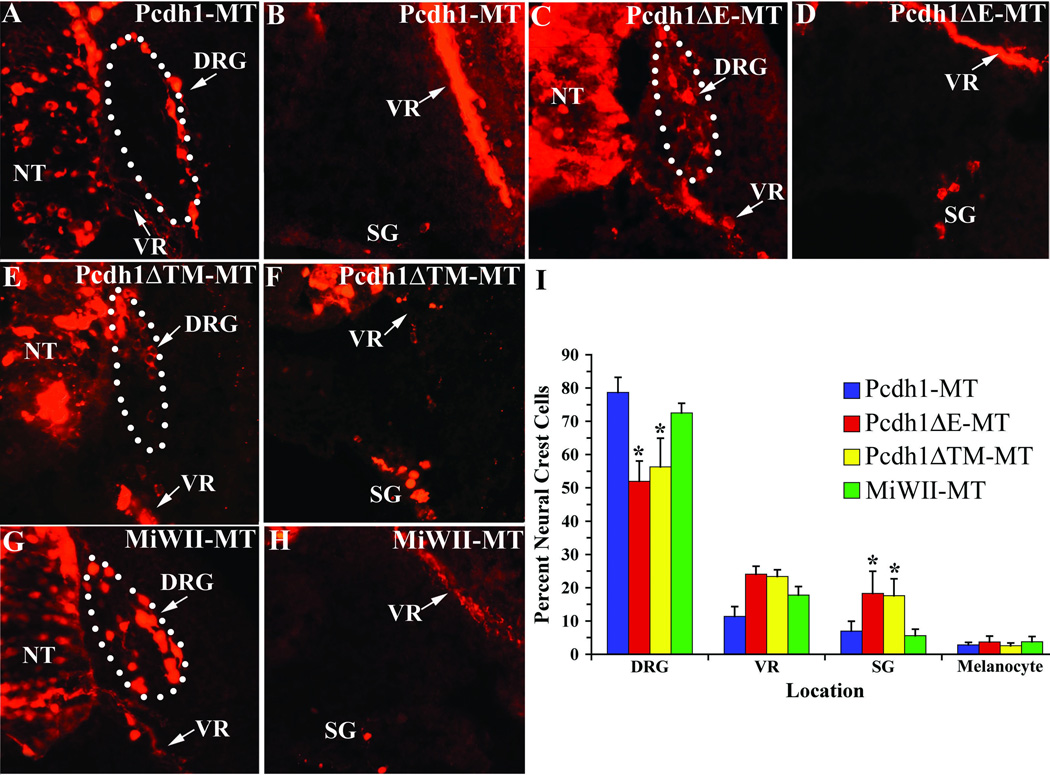
As the above experiment indicates Pcdh1-MT can function in cell adhesion in vivo, the role of Pcdh1 in neural crest cell differentiation was next examined. The expression pattern of Pcdh1 in the PNS, with highest levels observed in the developing DRG and sympathetic ganglia, suggests Pcdh1 may function to mediate the aggregation of neural crest cells during the formation of these two tissues. To test this hypothesis, we misexpressed Pcdh1 in embryos and analyzed the consequences to the localization of neural crest cells within the trunk PNS. Chicken embryos at stage 10 were subjected to in ovo electroporation with plasmid vectors encoding the various Pcdh1 constructs (Figure 4A). Embryos were allowed to develop until stage 22, then fixed and immunostained for the myc epitope and cryo-sectioned. As shown in Figure 5, all three constructs were successfully electroporated and expressed in the chick neural tube and neural crest cells. To determine if Pcdh1 is involved in the formation of particular PNS tissues, electroporated embryos were subjected to fate map analysis to identify the locations of ectopically expressing cells. The numbers of myc-positive neural crest cells in the trunk DRG, ventral root, sympathetic ganglia, and epidermis (melanocytes) were counted for each construct and expressed as a percentage of the total number of labeled trunk neural crest cells. As a negative control, embryos were electroporated with a vector that encodes solely the myc epitope (pMiWII-MT). As shown in Figure 5I, results indicate that over-expression of full-length Pcdh1-MT does not significantly alter the distribution of labeled neural crest cells, as compared to control embryos. In contrast, embryos electroporated with the deletion constructs Pcdh1ΔE-MT or Pcdh1ΔTM-MT exhibit a significant decrease in the percentage of labeled neural crest cells that localize to the DRG, with a concomitant increase in the percentage of labeled neural crest cells found in the sympathetic ganglia. These results suggest Pcdh1 may have a role in localizing and/or retaining a subset of neural crest cells to the DRG and that by disrupting Pcdh1 function, a greater percentage of neural crest cells pass through the DRG to stop at more ventral locations.
Figure 5.
Ectopic expression of the Pcdh1 deletion constructs disrupts neural crest cell sorting. A–H) Chick embryos at stage 10 were subjected to in ovo electroporation with the indicated Pcdh1 construct, then fixed at stage 22 and immunostained for the myc-epitope tag. Dotted lines outline the DRG. I) For each construct, the number of myc-positive neural crest cells in the DRG, ventral root (VR), sympathetic ganglia (SG) and epidermis (melanocytes) were quantified and expressed as percentage of the total number of myc-positive neural crest cells. While embryos ectopically expressing full-length Pcdh1-MT do not differ significantly from control embryos expressing solely the myc tag (MiWII-MT), ectopic expression of the deletion constructs Pcdh1ΔE-MT or Pcdh1ΔTM-MT results in a greater percentage of neural crest cells that migrate to the sympathetic ganglia at the expense of the DRG. * Indicates a statistically significant difference (p<0.01) from control embryos. NT, neural tube.
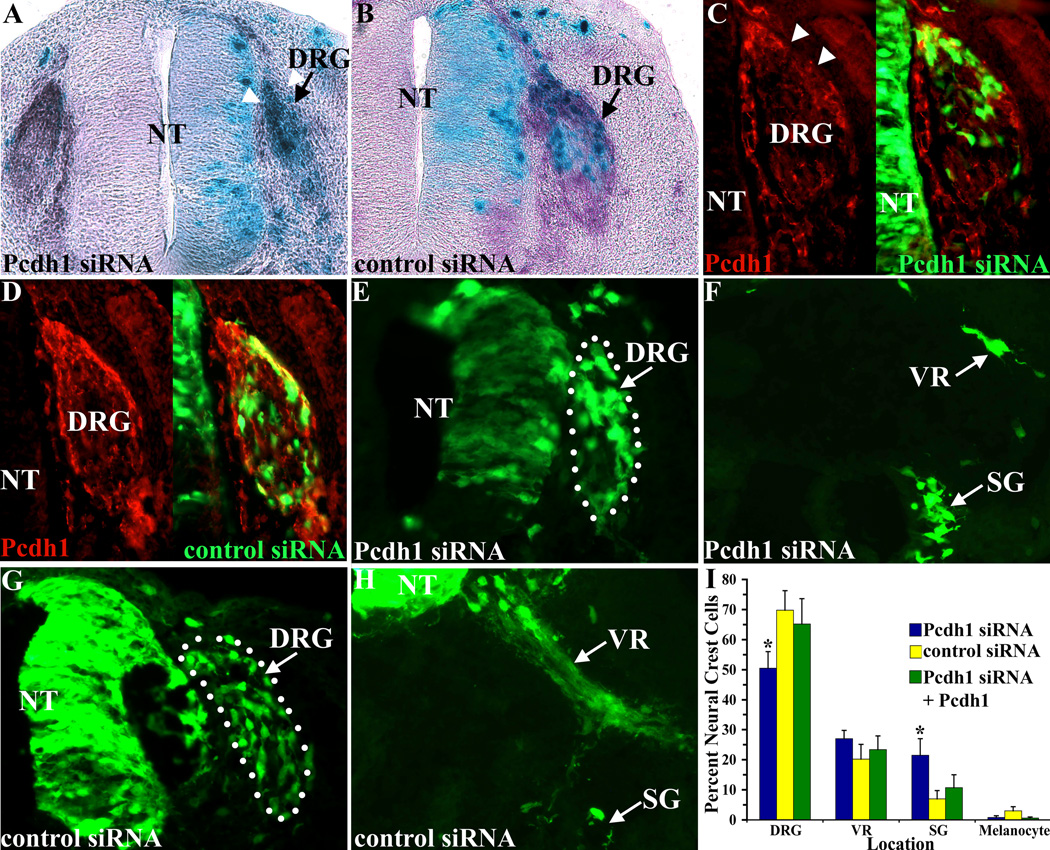
While the above experiment suggests that the mutant constructs Pcdh1ΔE-MT and Pcdh1ΔTM-MT inhibit endogenous Pcdh1, thereby acting as dominant negatives, it is possible they may inhibit other, related, protocadherins also expressed in neural crest cells. Therefore, to confirm that Pcdh1 functions to localize and/or retain a subset of neural crest cells at the DRG, Pcdh1 was inhibited at the RNA level, using RNA interference. To inhibit Pcdh1 expression, a 21-nucleotide small interfering RNA (siRNA) was selected from the Pcdh1 sequence, which did not overlap with other known genes. This Pcdh1-specific siRNA was electroporated into stage 10 chick embryos, along with a small amount of either a β-galactosidase-encoding or a GFP-encoding vector, to visualize the electroporated cells. As a negative control embryos were electroporated with a non-targeting siRNA duplex that does not recognize any known chicken genes (Dharmacon). Embryos were then harvested at stage 22 and analyzed for β-galactosidase or GFP-expressing cells in the PNS. To determine if the Pcdh1-specific siRNA is effective at suppressing endogenous Pcdh1 expression, we analyzed the electroporated embryos by situ hybridization and immunolocalization for Pcdh1. Results indicate the Pcdh1 siRNA suppresses both endogenous Pcdh1 mRNA and protein expression in the DRG. In contrast, the non-targeting control siRNA had no effect on Pcdh1 expression in embryos (Figure 6 A–D). As the Pcdh1 siRNA could inhibit endogenous Pcdh1 expression, a fate map analysis was performed to determine the locations of Pcdh1 siRNA containing cells within the PNS. Embryos were harvested at stage 22, fixed and cryosectioned, and the locations of GFP-expressing cells were determined as before. Results indicate that when Pcdh1 is inhibited, there is a significant decrease in the percentage of neural crest cells that localize to the DRG, as compared to control siRNA-injected embryos, with a concomitant increase in the percentage of neural crest cells that localize to the sympathetic ganglia (Figure 6E–I). Importantly, this shift in neural crest cells localizing to the sympathetic ganglia versus DRG can be rescued by ectopically expressing full-length Pcdh1, indicating that the siRNA specifically inhibits Pcdh1 expression. These results are consistent with results observed when Pcdh1 is inhibited using the deletion constructs (Figure 5I) and confirm Pcdh1 is required for localizing and/or retaining a subset of neural crest cells to the DRG.
Figure 6.
Inhibiting Pcdh1 mRNA expression disrupts neural crest cell sorting. A,B) Chick embryos were electroporated at stage 10 with either a Pcdh1-specific siRNA or a control, non-targeting, siRNA, together with a LacZ expression vector to localize electroporated cells (blue cells), then fixed at stage 22 and subjected to in situ hybridization for Pcdh1 expression (purple stain). The Pcdh1-specific siRNA inhibits Pcdh1 mRNA expression in the DRG on the electroporated side (arrowheads in A) while the control siRNA does not (B). C–G) Embryos electroporated with the Pcdh1-siRNA or the control siRNA, together with a GFP-expressing vector to localize electroporated cells. C, D) Sections immunostained with the Pcdh1-antiserum (red fluorescence). The Pcdh1-siRNA inhibits Pcdh1 protein expression in the DRG (arrowheads in C) while the control siRNA does not. E–H) Representative sections analyzed for the locations of GFP-expressing cells. Dotted lines denote the DRG. I) Quantification of the GFP expressing neural crest cells reveals that the Pcdh1-specific siRNA results in fewer labeled neural crest cells in the DRG, with a concomitant increase in labeled neural crest cells in the sympathetic ganglia (SG), as compared to control siRNA injected embryos. The altered cell sorting observed with the Pcdh1 siRNA was rescued by co-injecting full-length Pcdh1, indicating that the Pcdh1 siRNA specifically inhibits Pcdh1 expression. * Denotes a statistically significant difference (p<0.01) from control siRNA-electroporated embryos. NT, neural tube; VR, ventral root.
Pcdh1 localizes neural crest cells to the DRG perimeter
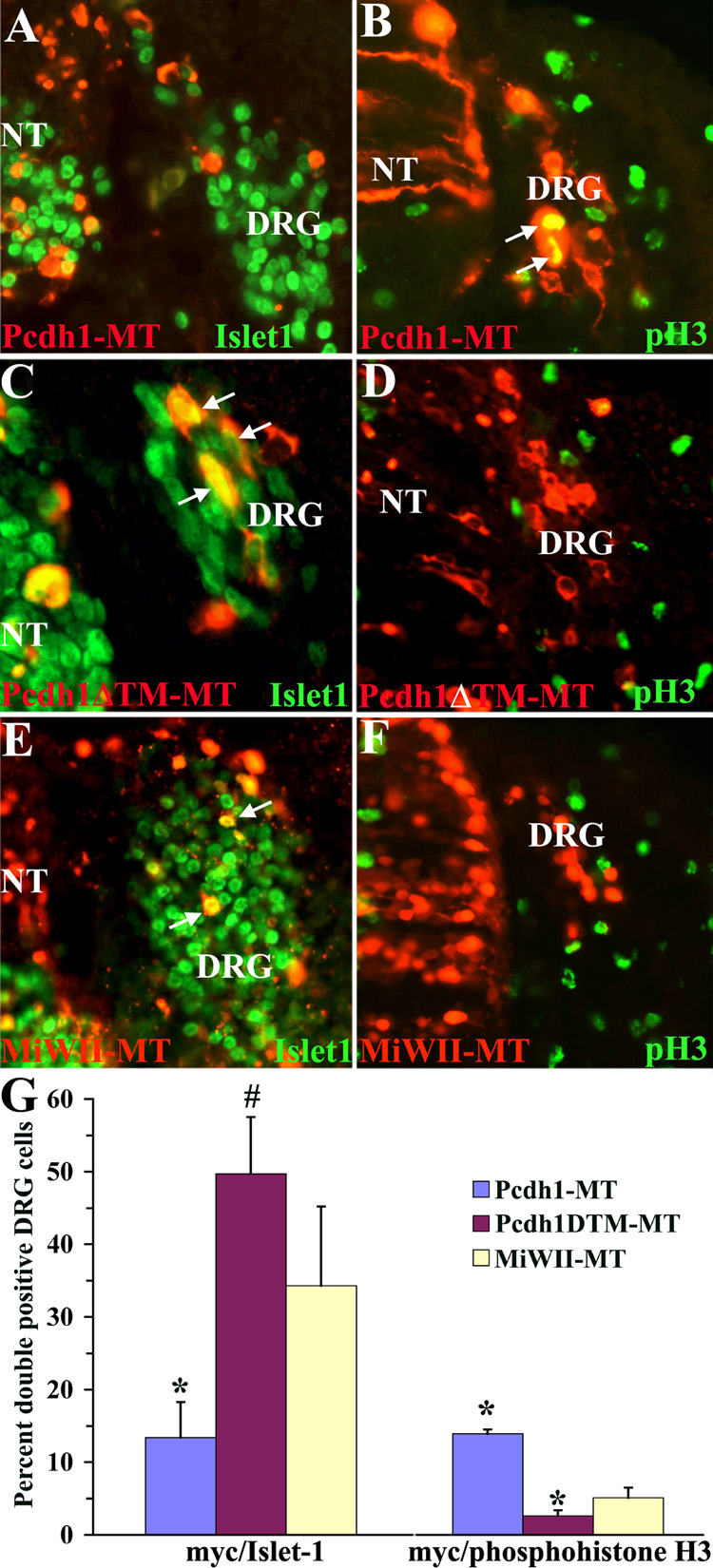
Within the DRG, Pcdh1 is highly expressed in the dorsal pole and perimeter, where it co-localizes with mitotically active cells (Figure 3). Furthermore, upon closer examination of the in ovo electroporations experiments, the location of neural crest cells overexpressing full-length Pcdh1-MT in the DRG is consistently along the perimeter (Figure 5A). In contrast, when Pcdh1ΔE-MT, Pcdh1ΔTM-MT or the Pcdh1 siRNA are ectopically expressed, this strict spatial segregation between the perimeter and interior of the DRG breaks down: neural crest cells are now found throughout the DRG (Figure 5 and Figure 6). These observations suggest a possible role for Pcdh1 in localizing neural crest cells to the margins of the DRG, where they undergo mitosis and remain undifferentiated. To test this hypothesis, we disrupted Pcdh1 in neural crest cells and analyzed whether the ectopically expressing cells in the DRG are more likely to differentiate along the sensory neuron pathway, as opposed to remaining undifferentiated and mitotically active. Embryos were subjected to in ovo electroporation with full-length Pcdh1-MT or the dominant-negative Pcdh1ΔTM-MT construct, as before, then fixed at stage 22, cryosectioned, and immunolabeled for expression of either the sensory neuron marker Islet-1 or phosphohistone H3, a marker of mitosis (Hendzel et al. 1997). The number of double-labeled cells in the DRG were counted and expressed as a percentage of the total number of myc-positive cells in the DRG. If Pcdh1 is involved in keeping neural crest cells at the perimeter, fewer Pcdh1-MT expressing cells would be expected to be Islet-1 positive, as compared to control embryos electroporated with pMiWII-MT. As shown in Figure 7, ectopic expression of full-length Pcdh1-MT does result in significantly fewer DRG cells that double-label for both myc and Islet-1, as compared to the negative control. Conversely, overexpression of Pcdh1-MT results in an increase in DRG cells that co-label for phosphohistone H3, indicating that a greater percentage of Pcdh1-MT expressing cells are undifferentiated and mitotically active. In contrast, ectopic expression of the dominant-negative construct Pcdh1ΔTM-MT results in an increase in myc-positive cells that co-label with Islet-1, and a decrease in cells that label with the phosphohistone H3 antibody, indicating that a greater percentage of Pcdh1ΔTM-MT cells have differentiated as sensory neurons.
Figure 7.
Misexpression of Pcdh1 alters the location and differentiation of neural crest cells within the DRG. A–F) Chick embryos electroporated with the Pcdh1 expression constructs and double-labeled for the myc-epitope (red fluorescence) and for either the sensory neuron marker Islet-1 (A,C,E) or the mitotic marker phosphohistone H3 (pH3 in B,D,F; green fluorescence). Arrows indicate double-labeled cells. G) Quantification of the number of double labeled cells in the DRG reveals that embryos ectopically expressing Pcdh1-MT (A,B) exhibit fewer myc/Islet-1 double positive cells and an increase in myc/phosphohistone H3 positive cells, as compared to embryos ectopically expressing the control construct MiWII-MT (E,F). In contrast, embryos expressing the deletion construct Pcdh1ΔTM-MT (C,D) exhibit an increase in myc/Islet-1 cells, and a decrease in myc/phosphohistone H3 cells. * and # denote a statistically significant difference (p<0.01 and p<0.05 respectively) from control embryos. NT, neural tube.
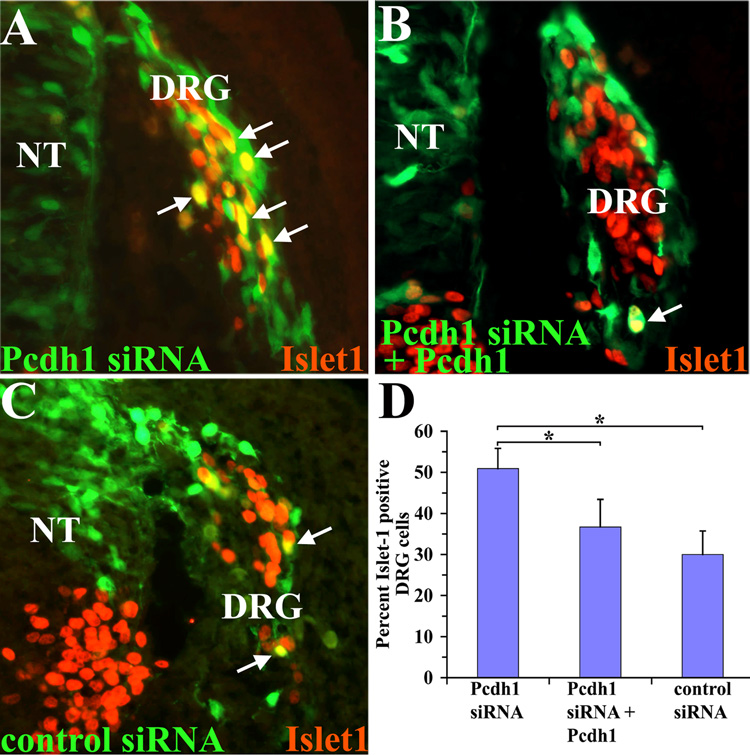
Finally, to confirm that Pcdh1 plays an important role in segregating the undifferentiated DRG cells from the cells undergoing sensory neuron differentiation, we inhibited Pcdh1 expression using the Pcdh1-specific siRNA. As shown in Figure 8, the Pcdh1-specific siRNA results in an increase in the percentage of GFP-positive cells that co-label with Islet-1, as compared to embryos electroporated with the control siRNA, similar to the results with the dominant-negative construct. Furthermore, co-injecting the Pcdh1-specific siRNA together with full-length Pcdh1 could abrogate this result, indicating that the increase in Islet-1 staining observed is specific to inhibition of Pcdh1. Taken together, these results strongly suggest that Pcdh1 normally functions to localize a subset of cells to the DRG perimeter, where they remain undifferentiated, and that by disrupting Pcdh1 those neural crest cells that still localize to the DRG are now more likely to differentiate along the sensory neuron pathway.
Figure 8.
Inhibiting Pcdh1 mRNA expression results in an increase in sensory neuron differentiation. Chick embryos electroporated with the Pcdh1-specific or control siRNA, along with a GFP-expressing vector to visualize electroporated neural crest cells (green), then fixed and immunostained for Islet-1 expression (red). Arrows indicate double-labeled cells. D) Quantification of the percentage of double-positive cells in the DRG. The Pcdh1 siRNA results in a greater percentage of GFP/Islet-1 double-positive cells in the DRG (A), as compared to the control siRNA (C), and this phenotype can be rescued by co-injecting the Pcdh1 siRNA along with a vector encoding full-length Pcdh1 (B). * Indicates a statistically significant difference (p<0.01). NT, neural tube.
Discussion
In the chicken embryo, the trunk neural crest emanate from the neural tube beginning around H.H. stage 12, in a rostral to caudal developmental gradient, with the cessation of neural crest migration by stage 22 (Teillet et al. 1987; Lallier and Bronner-Fraser 1988; Le Douarin and Kalcheim 1999). Several signaling molecules play important roles in the induction of the neural crest, as well as in their subsequent differentiation into neural and non-neural derivatives. Members of the Wnt and BMP families perform key functions in neural crest migration by directly upregulating the expression of Snail2/Slug, a zinc-finger transcription factor and one of the earliest neural crest markers (LaBonne and Bronner-Fraser 1998; Vallin et al. 2001; Sakai et al. 2005; Taneyhill and Bronner-Fraser 2005; Abu-Elmagd et al. 2006; Theriault et al. 2007). Snail2 is thought to function, in part, to mediate EMT in the prospective neural crest, allowing cells to delaminate from the dorsal neural tube and initiate migration. Disrupting Snail2 expression in chick embryos prevents neural crest migration, while in Xenopus, overexpression of Snail2 expands the neural crest domain (Nieto et al. 1994; LaBonne and Bronner-Fraser 2000; Nieto 2002; Barrallo-Gimeno and Nieto 2005; De Craene et al. 2005).
Amongst the genes directly regulated by Snail2 are members of the cadherin family of cell adhesion molecules and several studies have demonstrated important roles for cadherins during EMT (Cano et al. 2000; LaBonne and Bronner-Fraser 2000; Nieto 2002; Bolos et al. 2003; Taneyhill et al. 2007). For example, in the avian embryo, N-cadherin and cadherin-6B are both expressed in the neural folds and premigratory crest; however as the crest begins to migrate, both are downregulated. Concomitantly, the expression of cadherin-7 is upregulated in migrating neural crest cells (Nakagawa and Takeichi 1995). Overexpressing N-cadherin, cadherin-6B, cadherin-7 or cadherin-11 in the presumptive neural crest inhibits delamination, resulting in neural crest cells aggregating in the dorsal neural tube. In contrast, inhibiting cadherin-6B, or cadherin-11 function leads to premature emigration from the neural tube (Nakagawa and Takeichi 1998; Borchers et al. 2001; Coles et al. 2007; Shoval et al. 2007). Only one protocadherin has previously been shown to play a role in neural crest migration: PCNS protocadherin is expressed in the cranial neural crest in Xenopus embryos and disrupting its expression leads to a failure of the cranial neural crest to migrate (Rangarajan et al. 2006). Despite a requirement for regulated cadherin expression in the initiation of neural crest migration, functional evidence for a role for cadherin family members in mediating the aggregation of neural crest cells to form peripheral structures, particularly the DRG, has been elusive. Here we report the identification of a protocadherin family member expressed in chick embryos, Pcdh1, which plays an important role in mediating the localization and/or retention of neural crest cells within the DRG. These results provide the first functional evidence that protocadherin-mediated cell adhesion is required for cell sorting within the developing PNS.
Role of Pcdh1 in ganglia formation
In situ and immunolocalization results indicate that Pcdh1 is expressed in neural crest cells as well as most trunk PNS tissues beginning at stage 17. Pcdh1 is not detected in premigratory cells or in the earliest migrating cells (stages 12–16; Figure 1, 2). This suggests that Pcdh1 does not function in delamination or initial migration; instead Pcdh1 is likely involved in neural crest cell localization and/or aggregation after cells have left the neural tube and begun to coalesce to form the various PNS derivatives. This was confirmed by disrupting Pcdh1 function in early embryos: inhibiting endogenous Pcdh1 does not disrupt delamination, instead altering the sorting of neural crest cells within the PNS. Our results indicate that one function of Pcdh1 is to localize or retain a subpopulation of neural crest cells at the DRG. In this scenario, disrupting Pcdh1 function may inhibit the ability of neural crest cells to recognize and/or adhere to the nascent DRG, resulting in an increase in cells that now localize to more ventral locations, particularly the sympathetic ganglia.
While disrupting Pcdh1 function inhibits the localization of neural crest cells to the DRG, it does not prevent cells from localizing to the sympathetic ganglia even though both tissues express Pcdh1. Fate mapping of neural crest cells has established that the sympathetic ganglia begin to form at stage 12, from neural crest cells that migrate ventrally and condense near the dorsal aorta, while the DRG form later, around stage 17–18, although there is considerable overlap in the formation of these two structures (Serbedzija et al. 1989). Thus, one explanation for our results is that Pcdh1 plays a role in the temporal restriction of neural crest cell fate. The initial expression of Pcdh1 in the developing PNS, beginning around stage 17, corresponds to the stages at which DRG cells are beginning to coalesce, but after the primary sympathetic ganglia have formed. Thus, if Pcdh1 is required during the aggregation of neural crest cells to form the DRG, then inhibiting Pcdh1 could cause a subset of cells normally retained in the DRG to now sort to the sympathetic ganglia, which previously formed independent of Pcdh1. We cannot rule out a role for Pcdh1 in the sympathetic ganglia later in development, as the cells re-sort to give rise to the primary sympathetic ganglia chain (Kasemeier-Kulesa et al. 2005; Kasemeier-Kulesa et al. 2006).
While inhibiting Pcdh1 results in fewer labeled neural crest cells that localize to the DRG, over-expression of full-length Pcdh1 results neither in the failure of neural crest cell emigration, due to cells aggregating in the neural tube, nor in a significant increase in labeled neural crest cells that localize to the DRG (Figure 5I). It is possible that Pcdh1-mediated cell adhesion requires a cellular cofactor that is also temporally expressed, which is under investigation.
A correlation between the neural crest cells that migrate to the DRG versus the sympathetic ganglia has been previously established. By grafting rostral somite tissue into embryos, Goldstein and Kalcheim demonstrated an increase in DRG size at the expense of the sympathetic ganglia (Goldstein and Kalcheim 1991). Furthermore, time-lapse confocal imaging of migrating neural crest cells in vivo demonstrated that some neural crest cells that have migrated ventral to the DRG can contribute to the sympathetic ganglia or can reorient themselves and migrate back to the DRG (Kasemeier-Kulesa et al. 2005). Therefore, another potential explanation for our results is that Pcdh1 may be required to mediate migration arrest in the DRG for this subpopulation of cells, and the absence of Pcdh1 results in these neural crest cells defaulting to the sympathetic ganglia.
Pcdh1 and sensory neuron differentiation
While the DRG first begin to coalesce around stage 17, neurogenesis in the DRG occurs in two overlapping waves; the first wave beginning around stage 20 and lasting until stage 25/26 and the second wave beginning at stage 25 and lasting until approximately stage 30 (Pannese 1974; Carr and Simpson 1978). In the immature DRG, the cells at the periphery express Sox10 and Notch, which are thought to maintain these cells in an undifferentiated and mitotically active state. As cells differentiate into neurons they segregate to the center of the DRG and begin to express neural specific markers (Wakamatsu et al. 2000; Nelson et al. 2002). After neurogenesis is complete, the cells at the perimeter will give rise to satellite glia (Carr and Simpson 1978; Wakamatsu et al. 2000; Hanani 2005). Thus while the mature DRG contains a heterogeneous population of sensory neurons and glia, the immature DRG, at stages 22–24 (the stages examined in this study), is composed mostly of a core of differentiating sensory neurons surrounded by mitotically active progenitor cells at the periphery.
In addition to promoting the localization and/or retention of neural crest cells in the DRG, we show that Pcdh1 plays an important role in segregating mitotically active DRG cells from differentiating sensory neurons. It this regard it is possible that Pcdh1 functions downstream of Notch and Sox10 to retain the undifferentiated and mitotic cells at the perimeter. In addition, recent research has indicated the existence of a late migratory wave of neural crest cells (stages 19–26) that specifically localize to the DRG perimeter, where they remain mitotically active progenitor cells for the second wave of neurogenesis (George et al. 2007). It is conceivable that these are the cells that express Pcdh1; furthermore, Pcdh1 may function both to target this subpopulation of cells to the DRG perimeter as well as keep them at the perimeter where they remain mitotically active.
Protocadherins and cell adhesion
Several studies have examined the role of δ-protocadherin family members in early embryogenesis, and while it is clear that δ-protocadherins play important roles in cell sorting and tissue histogenesis, the mechanism by which they act is less well understood (Bradley et al. 1998; Kim et al. 1998; Kim et al. 2000; Kuroda et al. 2002; Rangarajan et al. 2006; Rashid et al. 2006; Aamar and Dawid 2008). For example, in frogs, axial protocadherin is found in the notochord, whereas paraxial protocadherin (PAPC) is expressed in the presomitic mesoderm and this differential expression is required for the proper histogenesis of the notochord and somites, respectively. The expression of Pcdh1 in the notochord may suggest a similar role in the differentiation of the dorsal mesoderm in chick embryos, although this has not yet been examined. It is important to note that while PAPC plays an important role in mesodermal cell sorting, it does not mediate cell adhesion directly. Instead, PAPC expression in Xenopus blastomeres decreases the adhesive activity of the classical cadherin, C-cadherin, and this leads to cell sorting based on differential activity of C-cadherin (Chen and Gumbiner 2006). We are currently investigating if Pcdh1 functions directly in cell adhesion or indirectly via downregulating the activity of a classical cadherin. Xenopus blastomeres that ectopically express Pcdh1 do not show reduced adhesion to an E-cadherin substrate (data not shown). Since C-cadherin, which can form heterophilic adhesive interactions with E-cadherin (Niessen and Gumbiner 2002), is the predominant classical cadherin in early Xenopus embryos, this indicates that Pcdh1 expression does not downregulate the activity of C-cadherin. Furthermore, this implies that the results from our cell mixing assay, in which Pcdh1-expressing cells remain together as a cohesive unit (Figure 4), are not due to reduced C-cadherin activity in the blastomeres, suggestive of a direct role for Pcdh1 in cell adhesion. However, at this time, we cannot rule out whether Pcdh1 functions indirectly to alter the activity of other cell adhesion molecules that are expressed in the developing PNS.
Cadherins and PNS formation
Several lines of evidence suggest Pcdh1 likely functions in concert with other cell adhesion molecules to mediate the sorting of neural crest cells during PNS formation. Firstly, when Pcdh1 is inhibited, some labeled neural crest cells are still found in the DRG, indicating Pcdh1 is not necessary for the ability of all neural crest cells to localize to the DRG. Secondly, while Pcdh1 is expressed in presumptive Schwann cells (Figure 1,Figure 2 and data not shown), it is likely not required for neural crest cells to localize to the ventral root, since the percentage of cells found at this site is not significantly altered when Pcdh1 is disrupted. Thirdly, Pcdh1 is not required for the formation of the primary sympathetic ganglia, as previously discussed. Finally, when Pcdh1 is disrupted, neither an increase in cells that localize to the epidermis, to become melanocytes, nor an increase in neural crest cells that localize aberrantly outside of the ventral pathway is observed. Altogether, these results indicate Pcdh1 is not the sole adhesion molecule involved in the sorting of neural crest cells along the ventral path. In support of this hypothesis, cadherins-6 and -7 are upregulated in neural crest cells that reside along the dorsal and ventral roots and may function to target cells to these locations (Akitaya and Bronner-Fraser 1992; Nakagawa and Takeichi 1995; Inoue et al. 1997). Neural crest cells that migrate to the DRG re-express N-cadherin and it is conceivable that Pcdh1 functions in concert with N-cadherin to sort undifferentiated cells at the perimeter from differentiating sensory neurons in the interior (Akitaya and Bronner-Fraser 1992). N-cadherin is also required for the proper formation of the sympathetic ganglia (Kasemeier-Kulesa et al. 2006). Thus, the complement of adhesion molecules expressed likely determines both the emigration and sorting of neural crest cells and it is probable that both classical cadherins and protocadherins play important roles at multiple steps during PNS formation.
Experimental Procedures
Isolation of Pcdh1 and expression constructs
Degenerate oligonucleotides corresponding to conserved regions in protocadherin extracellular domains (Bradley et al. 1998) were used as PCR primers to amplify protocadherin sequences from a chicken E4.5 DRG cDNA library (gift of F. Lefcort). A novel 350 bp PCR product was chosen to rescreen the E4.5 DRG library, resulting in the isolation of a full-length cDNA encoding Pcdh1 (GenBank Accession number AY676328). Full-length Pcdh1 was cloned into pCS2-MT (Turner and Weintraub 1994), then subcloned into pMiwII (Araki and Nakamura 1999) to create Pcdh1-MT, for use in ovo electroporations. To create Pcdh1ΔE-MT, a PCR product encoding the transmembrane and cytoplasmic domains of Pcdh1-MT was ligated to the signal sequence of N-cadherin and subcloned into pMiwII. The construct Pcdh1ΔTM-MT encodes the entire extracellular domain of Pcdh1, fused to a C-terminal myc-epitope, subcloned into pMiwII.
In situ hybridization and immunofluorescence
Fertilized white leghorn chicken eggs (Spafas) were incubated at 38°C in a rocking incubator (Kuhl). Embryos were dissected and staged according to Hamburger and Hamilton (Hamburger and Hamilton 1951). In situ hybridizations were performed according to protocols (Wilkinson and Nieto 1993; Nieto et al. 1996), using a digoxygenin-labeled riboprobe corresponding to the entire Pcdh1 coding region. Stained embryos were viewed in wholemount, then embedded in OCT (Tissue-Tek) and cryosectioned. The Pcdh1 antiserum was generated by cloning a PCR product, corresponding to amino acids 397–560 of the Pcdh1 extracellular domain, into the pQE vector (Qiagen). Recombinant protein was purified on Ni-NTA-agarose (Qiagen) and used to immunize rabbits. For immuno-labeling experiments, chick embryos were cryosectioned, then sections were rehydrated in PBS, blocked in 1% goat serum in PBS and immunostained with antibodies to the myc epitope (DSHB or Santa Cruz Biotechnology), Islet-1 (DSHB), Hu (Marusich et al. 1994), Sox10 (Maka et al. 2005), HNK (Becton Dickinson) or phosphohistone H3 (Upstate Biotechnology) followed by an Alexafluor 488- or 568-conjugated secondary antibody (Molecular Probes). BrdU incorporation was as previously published (Nelson et al. 2002). Images were obtained using a Zeiss AxioCam Hrc digital camera and Axiovision 3.1 software.
Ectopic expression and RNA interference
Synthesis and injection of mRNA into Xenopus embryos and cell mixing assays were performed as described (Bradley et al. 1998; Rashid et al. 2006). In ovo electroporations of plasmids and siRNA were performed as described (Muramatsu et al. 1997; Swartz et al. 2001). Fertilized chicken eggs were incubated at 38°C until Hamburger-Hamilton (HH) stage 10. All plasmids were diluted to 0.2 µg/µl in PBS. The Pcdh1-specific siRNA targets the sequence CCAACUCGCUGAAGGUACAUU and was synthesized by Dharmacon, as was a non-targeting control siRNA. The siRNA was diluted to 2 µg/µl and mixed with either 0.2 µg/µl of a LacZ-expressing vector or a GFP-expressing vector (pMES) (Swartz et al. 2001), to visualize electroporated cells. Rescue experiments were performed using 2 µg/µl Pcdh1-specific siRNA plus 0.2 µg/µl of a construct encoding full-length Pcdh1 subcloned into pMES. Electroporations consisted of five 23-volt pulses using an ECM830 electroporator (Genetronics, Inc). Embryos were incubated until stage 22 and then fixed in 4% paraformaldehyde and processed for immunofluorescence.
Fate mapping
For cell fate analysis, every third cryosection from the trunk region (between the limb buds) was examined using a Nikon Microphot-FXA and the numbers of myc- or GFP-positive cells located in the DRG, ventral root, sympathetic ganglia and epidermis (melanocytes) were tallied. To control for differences in electroporation and expression of the constructs, the numbers of myc- or GFP-positive cells in each tissue was expressed as a percentage of the total number of ectopic expressing cells in each embryo. To identify sensory neurons or mitotic cells, cryosections were labeled for either Islet-1 or phosphohistone H3, respectively, and the number of double positive cells counted and expressed as a percentage of the total number of myc-positive (or GFP-positive) cells in the DRG. At least 8 embryos were analyzed for each construct, with each embryo having at least 100 labeled cells, and average cell counts determined. Statistical significance was determined by student’s t-test.
Acknowledgments
We thank Dana Rashid, Haley Dunkel and Anne Rusoff for technical assistance and Frances. Lefcort for reagents and advice. This study was supported by a grant from NCRR COBRE (RR15583).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aamar E, Dawid IB. Protocadherin-18a has a role in cell adhesion, behavior and migration in zebrafish development. Dev. Biol. 2008;318:335–346. doi: 10.1016/j.ydbio.2008.03.040. [DOI] [PubMed] [Google Scholar]
- Abu-Elmagd M, Garcia-Morales C, Wheeler GN. Frizzled7 mediates canonical Wnt signaling in neural crest induction. Dev. Biol. 2006;298:285–298. doi: 10.1016/j.ydbio.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Akitaya T, Bronner-Fraser M. Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev. Dyn. 1992;194:12–20. doi: 10.1002/aja.1001940103. [DOI] [PubMed] [Google Scholar]
- Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J. Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- Araki I, Nakamura H. Engrailed defines the position of dorsal di-mesencephalic boundary by repressing diencephalic fate. Development. 1999;126:5127–5135. doi: 10.1242/dev.126.22.5127. [DOI] [PubMed] [Google Scholar]
- Avivi C, Goldstein RS. Differential expression of Islet-1 in neural crest derived ganglia: Islet-1+ dorsal root ganglion cells are post-mitotic and Islet-1+ and Islet-1+ sympathetic ganglion cells are still cycling. Dev. Brain Res. 1999;115:89–92. doi: 10.1016/s0165-3806(99)00054-1. [DOI] [PubMed] [Google Scholar]
- Barembaum M, Bronner-Fraser M. Early steps in neural crest specification. Semin. Cell Dev. Biol. 2005;16:642–646. doi: 10.1016/j.semcdb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA. The Snail genes are inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: comparison with Snail and E47 repressors. J. Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Borchers A, David R, Wedlich D. Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development. 2001;128:3049–3060. doi: 10.1242/dev.128.16.3049. [DOI] [PubMed] [Google Scholar]
- Bradley RS, Espeseth A, Kintner C. NF-protocadherin, a novel member of the cadherin superfamily, is required for Xenopus ectodermal differentiation. Curr. Biol. 1998;8:325–334. doi: 10.1016/s0960-9822(98)70132-0. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK1. Dev. Biol. 1986;115:44–55. doi: 10.1016/0012-1606(86)90226-5. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Fraser SE. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature. 1988;335:161–164. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Fraser SE. Cell lineage analysis of the avian neural crest. Development Suppl. 1991;2:17–22. [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Carr VM, Simpson SB. Proliferative and degenerative events in the early development of the chick dorsal root ganglia. I. Normal Development. J. Comp. Neurol. 1978;182:727–740. doi: 10.1002/cne.901820410. [DOI] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulated C-cadherin adhesion activity. J. Cell Biol. 2006;174:301–313. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Cheung M, Abu-Elmagd MM, Orme A, Scotting PJ. Chick Sox10, a transcription factor expressed in both early neural crest cells and central nervous system. Dev. Brain Res. 2000;121:233–241. doi: 10.1016/s0165-3806(00)00049-3. [DOI] [PubMed] [Google Scholar]
- Coles EG, Taneyhill LA, Bronner-Fraser M. A critical role for Cadherin6B in regulating avian neural crest emigration. Dev. Biol. 2007;312:533–544. doi: 10.1016/j.ydbio.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Goldstein RS. Early markers of neuronal differentiation in DRG: Islet-1 expression precedes that of Hu. Dev. Brain Res. 2000;121:209–212. doi: 10.1016/s0165-3806(00)00034-1. [DOI] [PubMed] [Google Scholar]
- De Craene B, van Roy F, Berx G. Unraveling signalling cascades for the Snail family of transcription factors. Cell. Signal. 2005;17:535–547. doi: 10.1016/j.cellsig.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Frank E, Sanes JR. Lineage of neurons and glia in chick dorsal root ganglia: analysis in ovo with a recombinant retrovirus. Development. 1991;111:895–908. doi: 10.1242/dev.111.4.895. [DOI] [PubMed] [Google Scholar]
- Frank M, Kemler R. Protocadherins. Curr. Opin. Cell Biol. 2002;14:557–562. doi: 10.1016/s0955-0674(02)00365-4. [DOI] [PubMed] [Google Scholar]
- Fraser SE, Bronner-Fraser M. Migrating neural crest cells in the trunk of the avian embryo are multipotent. Development. 1991;112:913–920. doi: 10.1242/dev.112.4.913. [DOI] [PubMed] [Google Scholar]
- George L, Chaverra M, Todd V, Lansford R, Lefcort F. Nociceptive sensory neurons derive from contralaterally-migrating, fate-restricted neural crest cells. Nature Neurosci. 2007;10:1287–1293. doi: 10.1038/nn1962. [DOI] [PubMed] [Google Scholar]
- Goldstein RS, Kalcheim C. Normal segmentation and size of the primary sympathetic ganglia depend upon the alternation of rostrocaudal properties of the somites. Development. 1991;112:327–334. doi: 10.1242/dev.112.1.327. [DOI] [PubMed] [Google Scholar]
- Goodwin M, Yap AS. Classical cadherin adhesion molecules: coordinating cell adhesion, signaling and the cytoskeleton. J. Mol. Med. 2005;35:839–844. doi: 10.1007/s10735-004-1833-2. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res. Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Inoue T, Chisaka O, Matsunami H, Takeichi M. Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev. Biol. 1997;183:183–194. doi: 10.1006/dbio.1996.8501. [DOI] [PubMed] [Google Scholar]
- Jones NC, Trainor PA. Roles of morphogens in neural crest determination. J. Neurobiol. 2005;64:388–404. doi: 10.1002/neu.20162. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Bradley R, Pasquale EB, Lefcort F, Kulesa PM. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development. 2006;133:4839–4847. doi: 10.1242/dev.02662. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Kulesa PM, Lefcort F. Imaging neural crest cell dynamics during formation of dorsal root ganglia and sympathetic ganglia. Development. 2005;132:235–245. doi: 10.1242/dev.01553. [DOI] [PubMed] [Google Scholar]
- Kim SH, Jen WC, DeRobertis EM, Kintner C. The protocadherin PAPC establishes segmental boundaries during somitogenesis in Xenopus embryos. Curr. Biol. 2000;10:821–830. doi: 10.1016/s0960-9822(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Kim SH, Yamamoto A, Bouwmeester T, Agius E, Robertis EM. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 1998;125(23):4681–4690. doi: 10.1242/dev.125.23.4681. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Mayor R. Molecular analysis of neural crest migration. Philos. Trans. R Soc. Lond. B Biol. Sci. 2008;363:1349–1362. doi: 10.1098/rstb.2007.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda H, Inui M, Sugimoto K, Hayata T, Asashima M. Axial protocadherin is a mediator of prenotochord cell sorting in Xenopus. Dev. Biol. 2002;244:267–277. doi: 10.1006/dbio.2002.0589. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Developmental. 1998;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Snail-related transcriptional repressors are required in Xenopus for both the induction of the neural crest and its subsequent migration. Dev. Biol. 2000;221:195–205. doi: 10.1006/dbio.2000.9609. [DOI] [PubMed] [Google Scholar]
- Lallier TE, Bronner-Fraser M. A spatial and temporal analysis of dorsal root ganglia and sympathetic ganglion formation in the avian embryo. Dev. Biol. 1988;127:99–112. doi: 10.1016/0012-1606(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. Cambridge University Press; 1999. [Google Scholar]
- Maka M, Stolt CC, Wegner M. Identification of Sox8 as a modifier gene in a mouse model of Hirschsprung disease reveals underlying molecular defect. Dev. Biol. 2005;277:155–169. doi: 10.1016/j.ydbio.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J. Neurobiol. 1994;25:143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- Morales AV, Barbas JA, Nieto MA. How to become neural crest: from segregation to delamination. Semin. Cell Dev. Biol. 2005;16:655–662. doi: 10.1016/j.semcdb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Mizutani Y, Ohmori Y, Okumura J. Comparison of three nonviral transfection methods for foreign gene expression in early chicken embryos in ovo. Biochem. Biophys. Res. Comm. 1997;230:376–380. doi: 10.1006/bbrc.1996.5882. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Develop. 1995;121(5):1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Develop. 1998;125(15):2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Matsuhashi S, Lefcort F. Restricted neural epidermal growth factor-like like 2 (NELL2) expression during muscle and neuronal differentiation. Mech. Dev. 2002;119:S11–S19. doi: 10.1016/s0925-4773(03)00084-4. S.1. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Gumbiner BM. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J. Cell Biol. 2002;156:389–399. doi: 10.1083/jcb.200108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA. The Snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 1996;51:219–235. doi: 10.1016/s0091-679x(08)60630-5. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Pannese E. The histogenesis of the spinal ganglia. Adv. Anat. Embryol. Cell Biol. 1974;47:7–97. doi: 10.1007/978-3-662-10338-8_2. [DOI] [PubMed] [Google Scholar]
- Pla P, Moore R, Morali OG, Grille S, Martinozzi S, Delmas V, Larue L. Cadherins in neural crest cell development and transformation. J. Cell. Physiology. 2001;189:121–132. doi: 10.1002/jcp.10008. [DOI] [PubMed] [Google Scholar]
- Raible DW, Ragland JW. Reiterated Wnt and BMP signals in neural crest development. Semin. Cell Dev. Biol. 2005;16:673–682. doi: 10.1016/j.semcdb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Rangarajan J, Luo T, Sargent TD. PCNS: A novel protocadherin required for cranial neural crest migration and somite morphogenesis in Xenopus. Dev. Biol. 2006;295:206–218. doi: 10.1016/j.ydbio.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Rashid D, Newell K, Shama L, Bradley R. A requirement for NF-protocadherin and TAF1/Set in cell adhesion and neural tube formation. Dev. Biol. 2006;291:170–181. doi: 10.1016/j.ydbio.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Redies C. Cadherins in the central nervous system. Prog, Neurobiol. 2000;61:611–648. doi: 10.1016/s0301-0082(99)00070-2. [DOI] [PubMed] [Google Scholar]
- Redies C, Vanhalst K, van Roy F. δ-protocadherins: unique structures and functions. Cell. Mol. Life Sci. 2005;62:2840–2852. doi: 10.1007/s00018-005-5320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D, Tanaka Y, Endo Y, Osumi N, Okamoto H, Wakamatsu Y. Regulation of slug transcription in embryonic ectoderm by beta-catenin-Lef/Tcf and BMP-Smad signaling. Dev. Growth Differ. 2005;47:471–482. doi: 10.1111/j.1440-169X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- Sakai D, Wakamatsu Y. Regulatory mechanisms for neural crest formation. Cells Tissues Organs. 2005;179:24–35. doi: 10.1159/000084506. [DOI] [PubMed] [Google Scholar]
- Sano K, Tanihara H, Heimark RL, Obata S, Davidson M, St. John T, Taketani S, Suzuki S. Protocadherins: a large family of cadherin-related molecules in central nervous system. EMBO J. 1993;12:2249–2256. doi: 10.1002/j.1460-2075.1993.tb05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. A vital dye analysis of the timing and pathways of avian trunk neural crest migration. Development. 1989;106:809–816. doi: 10.1242/dev.106.4.809. [DOI] [PubMed] [Google Scholar]
- Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development. 2007;134:491–501. doi: 10.1242/dev.02742. [DOI] [PubMed] [Google Scholar]
- Swartz ME, Eberhart J, Mastick GS, Krull CE. Sparking new frontiers: using in vivo electroporation for genetic manipulations. Dev. Biol. 2001;233:13–21. doi: 10.1006/dbio.2001.0181. [DOI] [PubMed] [Google Scholar]
- Swartz ME, Eberhart J, Pasquale EB, Krull CE. EphA4/ephrin-A5 interactions in muscle precursor cell migration in the avian forelimb. Development. 2001;128:4669–4680. doi: 10.1242/dev.128.23.4669. [DOI] [PubMed] [Google Scholar]
- Taneyhill LA, Bronner-Fraser M. Dynamic alterations in gene expression after wnt-mediated induction of avian neural crest. Mol. Bio. Cell. 2005;16:5283–5293. doi: 10.1091/mbc.E05-03-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneyhill LA, Coles EG, Bronner-Fraser M. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development. 2007;134:1481–1490. doi: 10.1242/dev.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teillet M, Kalcheim C, Le Douarin NM. Formation of the dorsal root ganglia in the avian embryo: segmental origin and migratory behavior of neural crest progenitor cells. Dev. Biol. 1987;120:329–347. doi: 10.1016/0012-1606(87)90236-3. [DOI] [PubMed] [Google Scholar]
- Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nat. Rev. Mol. Cell Biol. 2000;1:91–100. doi: 10.1038/35040042. [DOI] [PubMed] [Google Scholar]
- Theriault BL, Shepherd TG, Mujoomdar ML, Nachtigal MW. BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007;28:1153–1162. doi: 10.1093/carcin/bgm015. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of acheate-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, Broders F. Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. J. Biol. Chem. 2001;276:30350–30358. doi: 10.1074/jbc.M103167200. [DOI] [PubMed] [Google Scholar]
- Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays. 1999;21:211–220. doi: 10.1002/(SICI)1521-1878(199903)21:3<211::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Maynard TM, Weston JA. Fate determination of neural crest cells by NOTCH-mediated lateral inhibition and asymmetrical cell division during gangliogenesis. Development. 2000;127:2811–2821. doi: 10.1242/dev.127.13.2811. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]