Abstract
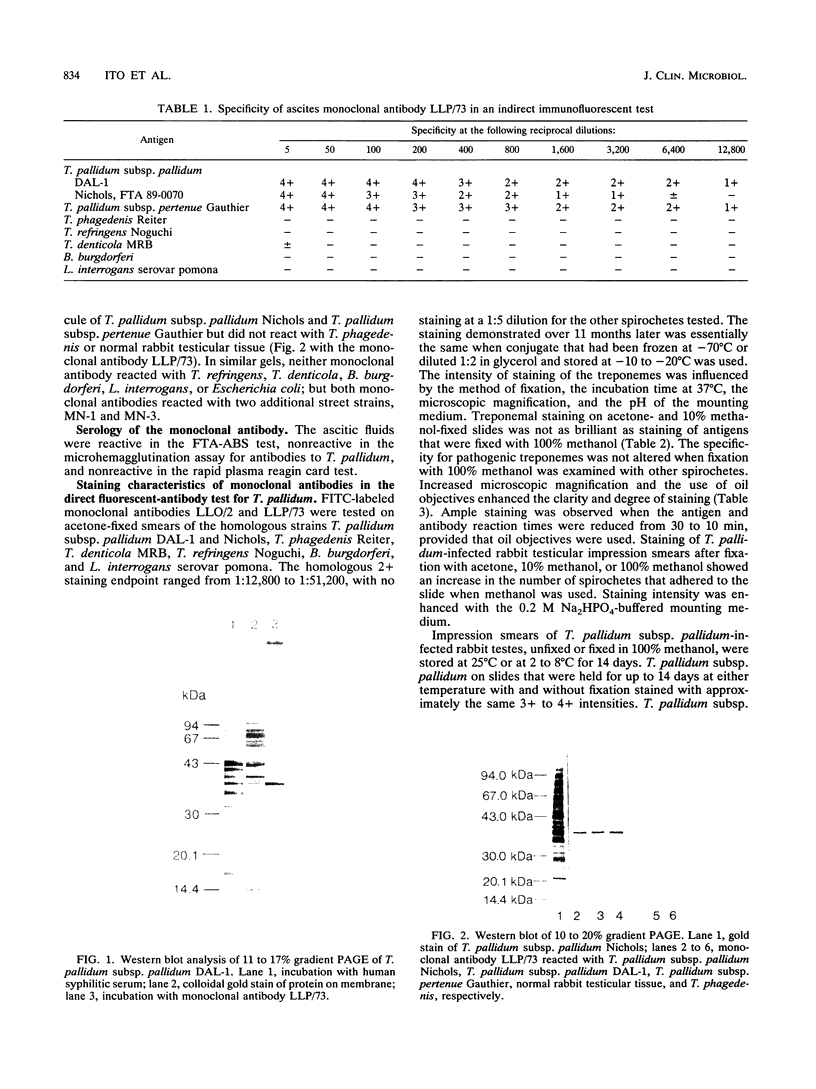
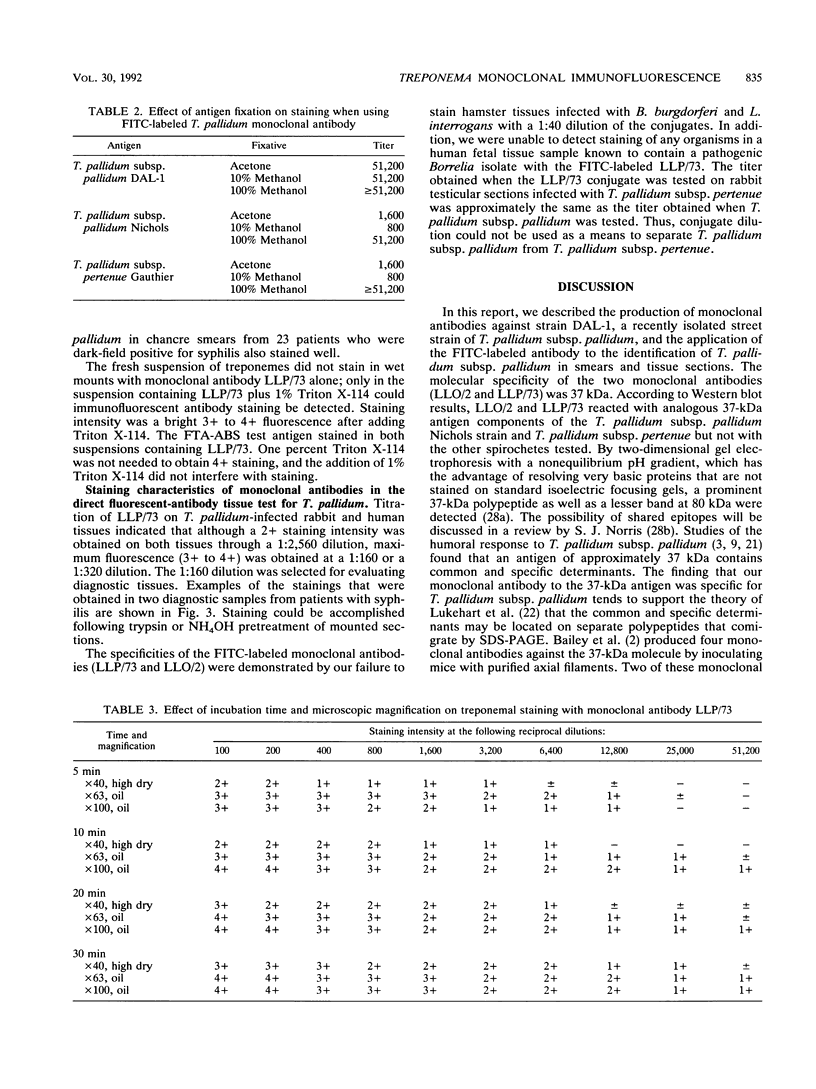
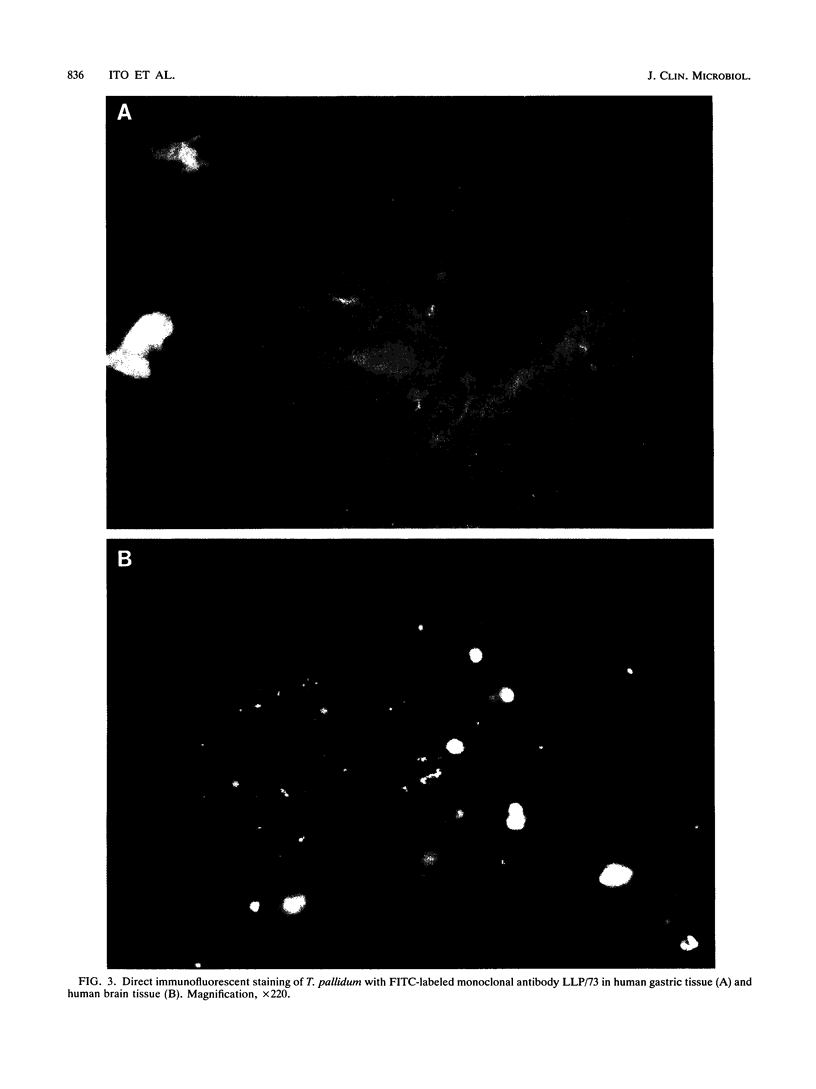
Two hybrid cell lines which produced mouse monoclonal antibody to the DAL-1 street strain of Treponema pallidum subsp. pallidum were established. These monoclonal antibodies strongly reacted with T. pallidum subsp. pallidum (Nichols strain, DAL-1, and two other street strains, strains MN-1 and MN-3) and T. pallidum subsp. pertenue by indirect microimmunofluorescent antibody and enzyme-linked immunosorbent assay techniques, but they did not react with normal rabbit testicular tissue. These monoclonal antibodies did not react with nonpathogenic treponemes, such as T. phagedenis Reiter, T. denticola MRB, T. refringens Noguchi, or other spirochetes, such as Borrelia burgdorferi and Leptospira interrogans serovar pomona in microimmunofluorescent antibody smear slides or in Western blots (immunoblots). While unlabeled antibodies are useful for investigating the antigenic structures of T. pallidum, we labeled these monoclonal antibodies with fluorescein isothiocyanate and used them for diagnosing syphilis by direct staining of lesion exudate or T. pallidum subsp. pallidum in formalin-fixed tissues from patients suspected of having syphilis. Both monoclonal antibodies were directed against antigens of T. pallidum subsp. pallidum with a molecular weight of 37,000 as determined by the Western blotting technique.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey M. J., Cockayne A., Penn C. W. Monoclonal antibodies directed against surface-associated polypeptides of Treponema pallidum define a biologically active antigen. J Gen Microbiol. 1987 Jul;133(7):1793–1803. doi: 10.1099/00221287-133-7-1793. [DOI] [PubMed] [Google Scholar]
- Bailey M. J., Cockayne A., Penn C. W. Production of murine monoclonal antibodies to the major axial filament polypeptide of Treponema pallidum. J Gen Microbiol. 1987 Jul;133(7):1805–1813. doi: 10.1099/00221287-133-7-1805. [DOI] [PubMed] [Google Scholar]
- Baker-Zander S. A., Hook E. W., 3rd, Bonin P., Handsfield H. H., Lukehart S. A. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis in humans. J Infect Dis. 1985 Feb;151(2):264–272. doi: 10.1093/infdis/151.2.264. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Hanff P. A., Fehniger T. E., Miller J. N., Lovett M. A. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J Immunol. 1982 Sep;129(3):1287–1291. [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Hicks C. B., Benson P. M., Lupton G. P., Tramont E. C. Seronegative secondary syphilis in a patient infected with the human immunodeficiency virus (HIV) with Kaposi sarcoma. A diagnostic dilemma. Ann Intern Med. 1987 Oct;107(4):492–495. doi: 10.7326/0003-4819-107-4-492. [DOI] [PubMed] [Google Scholar]
- Hunter E. F., McKinney R. M., Maddison S. E., Cruce D. D. Double-staining procedure for the fluorescent treponemal antibody absorption (FTA-ABS) test. Br J Vener Dis. 1979 Apr;55(2):105–108. doi: 10.1136/sti.55.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito F., Hunter E. F., George R. W., Swisher B. L., Larsen S. A. Specific immunofluorescence staining of Treponema pallidum in smears and tissues. J Clin Microbiol. 1991 Mar;29(3):444–448. doi: 10.1128/jcm.29.3.444-448.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. A., Marchitto K. S., Miller J. N., Norgard M. V. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols). J Exp Med. 1984 Nov 1;160(5):1404–1420. doi: 10.1084/jem.160.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr The detection of Treponema Pallidum by a rapid, direct fluorescent antibody darkfield (DFATP) procedure. Health Lab Sci. 1970 Jan;7(1):34–41. [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Gubish E. R., Jr Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J Immunol. 1982 Aug;129(2):833–838. [PubMed] [Google Scholar]
- Lukehart S. A., Tam M. R., Hom J., Baker-Zander S. A., Holmes K. K., Nowinski R. C. Characterization of monoclonal antibodies to Treponema pallidum. J Immunol. 1985 Jan;134(1):585–592. [PubMed] [Google Scholar]
- Marchitto K. S., Jones S. A., Schell R. F., Holmans P. L., Norgard M. V. Monoclonal antibody analysis of specific antigenic similarities among pathogenic Treponema pallidum subspecies. Infect Immun. 1984 Sep;45(3):660–666. doi: 10.1128/iai.45.3.660-666.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchitto K. S., Kindt T. J., Norgard M. V. Monoclonal antibodies directed against major histocompatibility complex antigens bind to the surface of Treponema pallidum isolated from infected rabbits or humans. Cell Immunol. 1986 Sep;101(2):633–642. doi: 10.1016/0008-8749(86)90173-5. [DOI] [PubMed] [Google Scholar]
- Marchitto K. S., Selland-Grossling C. K., Norgard M. V. Molecular specificities of monoclonal antibodies directed against virulent Treponema pallidum. Infect Immun. 1986 Jan;51(1):168–176. doi: 10.1128/iai.51.1.168-176.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney R., Thacker L., Hebert G. A. Conjugation methods in immunofluorescence. J Dent Res. 1976 Jan;55:A38–A44. doi: 10.1177/002203457605500117011. [DOI] [PubMed] [Google Scholar]
- Moskophidis M., Müller F. Molecular characterization of glycoprotein antigens on surface of Treponema pallidum: comparison with nonpathogenic Treponema phagedenis biotype Reiter. Infect Immun. 1984 Dec;46(3):867–869. doi: 10.1128/iai.46.3.867-869.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis M., Müller F. Monoclonal antibodies to immunodominant surface-exposed protein antigens of Treponema pallidum. Eur J Clin Microbiol. 1985 Oct;4(5):473–477. doi: 10.1007/BF02014427. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Kettman J. R., Miller J. N., Norgard M. V. Murine monoclonal antibodies specific for virulent Treponema pallidum (Nichols). Infect Immun. 1982 Jun;36(3):1076–1085. doi: 10.1128/iai.36.3.1076-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. M., Folds J. D. Development of monoclonal antibodies that recognize Treponema pallidum. Infect Immun. 1983 Aug;41(2):844–847. doi: 10.1128/iai.41.2.844-847.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter A. L., Lucas J. B., Price E. V., Falcone V. H. Treatment for early syphilis and reactivity of serologic tests. JAMA. 1972 Jul 31;221(5):471–476. [PubMed] [Google Scholar]
- Stoenner H. G., Dodd T., Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982 Nov 1;156(5):1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg R. W., Baseman J. B. Comparison of major protein antigens and protein profiles of Treponema pallidum and Treponema pertenue. Infect Immun. 1983 Nov;42(2):623–627. doi: 10.1128/iai.42.2.623-627.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg R. W., Morrison-Plummer J., Baseman J. B. Monoclonal antibodies to Treponema pallidum: recognition of a major polypeptide antigen. Genitourin Med. 1985 Feb;61(1):1–6. doi: 10.1136/sti.61.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel G. D., Jr, Sánchez P. J., Peters M. T., Harstad T. W., Potter L. L., Norgard M. V. Identification of Treponema pallidum in amniotic fluid and fetal blood from pregnancies complicated by congenital syphilis. Obstet Gynecol. 1991 Nov;78(5 Pt 2):890–895. [PubMed] [Google Scholar]
- van de Donk H. J., van Embden J. D., de Jong A., van Olderen M. F., Osterhaus A. D. Monoclonal antibodies to Treponema pallidum. Dev Biol Stand. 1984;57:107–111. [PubMed] [Google Scholar]






