The terminology used in clinical trials means something to researchers and trial sponsors, but what does it mean for you? More importantly, how should you use it to evaluate a therapy or to make coverage decisions?
Abstract
Oncology endpoints are an essential component of cancer trials, but often they are confusing, making it difficult to evaluate cancer therapies based on trial data. As more oncology agents hit the market and as indications expand for existing products, familiarity with these endpoints is critical for payers when making coverage decisions.
Payer management of oncology is in flux, as evolving Medicare coverage and payment initiatives continue to provide private health plans with potential new direction in managing the cost of cancer treatments. These changes are taking place against a background of new oncologic product introductions, rapid expansions of indications for existing therapies, and controversies over reimbursement methodologies. Management of and payment for oncologic agents is further complicated by the lack of data examining the impact of reimbursement policies on oncology outcomes.
When determining coverage policies, payers would do well to seek input from experts who can interpret the significance of oncology clinical trial data, says co-author Allan Jay Kogan, MD, medical director of Great-West Healthcare, a national MCO. Only then, he says, can decisions be made that meet the needs of all stakeholders.
As payers seek to keep pace with groundbreaking changes in the oncology arena, it is critical that they have a solid understanding of how oncology clinical trial endpoints can or should be used to guide decisions about the care that patients receive. This article explains the most commonly used endpoints in cancer trials, and summarizes the U.S. Food and Drug Administration’s assessment of their clinical utility and applicability to review by payers.
UNDERSTANDING CANCER TRIAL ENDPOINTS
Ideally, when reviewing an oncology agent for coverage, a payer should augment the review of safety and efficacy data with a thorough cost/benefit analysis, weighing the costs of the agent against the benefits to payers, healthcare providers, and patients. Such an analysis, however, often is not possible because of a paucity of supporting data. Payers, therefore, should have a clear understanding of oncology clinical trial endpoints to make accurate decisions about the value of various treatments.
According to the FDA’s Guidance for Industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics (FDA 2007), several endpoints are commonly used to measure the efficacy of oncology agents in clinical trials (Table 1, page 25). These endpoints are discussed below.
TABLE 1.
A comparison of important clinical approval endpoints
| Endpoint | Regulatory evidence | Study design | Advantages | Disadvantages |
|---|---|---|---|---|
| Overall survival | Clinical benefit for regular approval |
|
|
|
| Symptom endpoints (patient- reported outcomes) | Clinical benefit for regular approval |
|
|
|
| Disease-free survival | Surrogate for accelerated approval or regular approval* |
|
|
|
| Objective response rate | Surrogate for accelerated approval or regular approval* |
|
|
|
| Complete response | Surrogate for accelerated approval or regular approval* |
|
|
|
| Progression- free survival (includes all deaths) or time to progression (deaths before progression censored) | Surrogate for accelerated approval or regular approval* |
|
|
|
Adequacy as a surrogate endpoint for accelerated approval or regular approval is highly dependent upon other factors, such as effect size, effect duration, and benefits of other available therapy. Source: FDA 2007
Calculating survival benefit
When reviewing cancer treatments, the key benefit for all stakeholders is the ability to extend survival.
The two most common methods of calculating survival rates are the Kaplan-Meier method and life table analysis. The Kaplan-Meier method is preferred because survival is calculated using each event (patient death) as an interval; life table analysis, by contrast, uses a fixed-time interval.
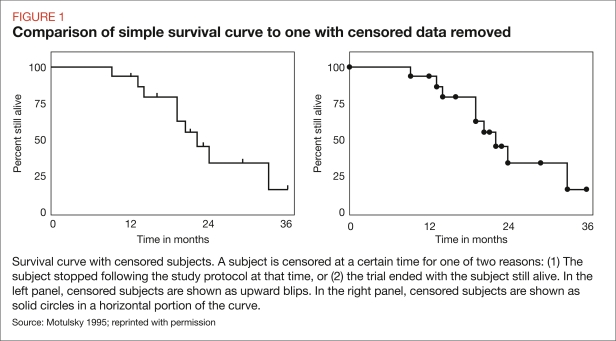
With Kaplan-Meier, survival is recalculated every time any patient dies. Survival is calculated by dividing the number of patients alive at the end of a day by the number alive at the beginning of that day. Censored patients (those who were alive at the end of the trial but not followed for the entire length of the trial) are excluded from both the numerator and the denominator. To calculate the number of patients who survive from day 0 until a predetermined measurement point (such as the end of the study), the number of patients who survive day 1 is multiplied by the number of patients who survive day 2, and this calculation is done for each succeeding day included in the study. Figure 1 shows a simple survival curve with censored data compared with a curve without censored data.
FIGURE 1.
Comparison of simple survival curve to one with censored data removed
Two terms that are frequently confused when evaluating survival data are median survival time and mean survival time. Understanding the nuances of each of these metrics is crucial if payers are to be able to assess how efficacious an oncologic agent may be.
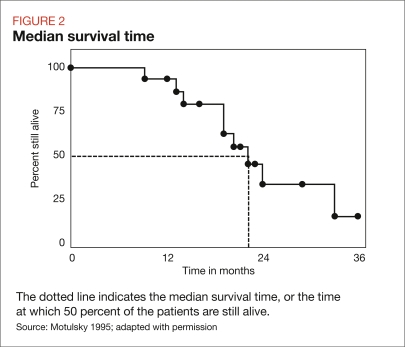
Median survival time is the point at which half of the patients are alive and half are dead (Figure 2, next page). Mean survival time is the point at which individuals in the study stayed alive divided by the length of the study, also referred to as the area under the curve. Mean survival time includes data from censored patients, so the median survival time is less likely to be skewed.
FIGURE 2.
Median survival time
Overall survival
Overall survival (OS) represents the time from clinical trial randomization until death from any cause, and is measured in the intent-to-treat population. OS remains the preferred endpoint for clinical trials as it has the greatest clinical relevance to patients (FDA 2007, Gill 2006). For payers, OS provides the most objective measurement of how efficacious an oncology agent is.
An endpoint such as disease-free survival may give patients access to effective therapy sooner — potentially saving lives while reducing long-term costs.
Overall survival is an endpoint that takes time to evaluate in full, requiring relatively large and lengthy clinical trials. It also includes non-cancer deaths, particularly in larger or older populations, that may skew the study (FDA 2007).With disease states such as metastatic breast cancer, where chemotherapy regimens are often rotated, it can be difficult to determine which regimen contributed to OS. Moreover, when first approved, a product may not have OS data. Payers must be vigilant in evaluating the data from post-launch trials to accurately assess OS benefit.
Disease-free survival
The FDA defines disease-free survival (DFS) is defined as “the time from randomization until recurrence of tumor or death from any cause” (FDA 2007). According to the FDA, “Although overall survival is a conventional endpoint for most adjuvant settings, DFS can be an important endpoint in situations where survival may be prolonged, making a survival endpoint impractical.” (FDA 2007). DFS needs to be evaluated carefully — a patient’s quality of life (QOL) in the period of extended survival is an important consideration for payers, providers, and patients alike (Gill 2006).
DFS often is used as a surrogate for OS in which recurrence of the disease represents a major reason for death in the treated population. In such cases, therapies used to treat cancer recurrencemay prolong survival but are unlikely to result in a cure. DFS is particularly appropriate when the interval between recurrence and death is lengthy and would otherwise require a longer follow-up for evaluation of OS (Gill 2006).
Recurrences between episodes of breast cancer, for instance, may be measured in years. As breast cancer is the second-leading cause of cancer-related deaths in women (ACS 2008), recent research has focused on potential cures. Between 20 and 30 percent of all metastatic breast cancer cases overexpress a protein called HER2. Patients with HER2 overexpressing tumors generally have faster tumor growth, a greater chance of relapse, and a reduced chance of long-term survival (Vogel 2005, Neyt 2006).
Trastuzumab (Herceptin), a humanized monoclonal antibody that targets the HER2 receptor, is approved for the treatment of HER2 overexpressing breast cancer (Neyt 2006). The Herceptin Adjuvant (HERA) clinical trial measured DFS as the primary endpoint; other endpoints included OS, time to recurrence, time to distant recurrence, overall safety, and cardiac safety (Piccart-Gebhart 2005). The HERA trial had an external independent data monitoring committee, the primary role of which was to monitor safety data and advise the trial’s steering committee about the release of trial results (HERA 2006). A planned interim efficacy analysis was published in 2005 and included 475 events (Piccart-Gebhart 2005). OS results had not yet reached statistical significance at the time of publication, underscoring the need for a surrogate endpoint, such as DFS. The trial results suggest that trastuzumab significantly improved DFS (unadjusted hazard ratio of the trastuzumab group versus control was 0.54, which corresponded to an absolute benefit in DFS of 8.4 percentage points at 2 years) and reduced the risk of distant metastases with a low risk of cardiac events (Piccart-Gebhart 2005).
CASE STUDY: Comparing aromatase inhibitors with tamoxifen for early-stage breast cancer
Even though breast cancer mortality is decreasing, its incidence is increasing (Cianfrocca 2005), raising the concern that breast cancer is evolving into a chronic condition for which expensive treatments may continue indefinitely. For payers, this has heightened the importance of ensuring that patients identified with early-stage breast cancer receive the most efficacious care.
Treatment for early-stage breast cancer has been studied extensively, and the current standard of care is “…to administer adjuvant systemic therapy to patients with node-positive and high-risk, node-negative breast cancer” (Cianfrocca 2005). But which systemic therapy should be used? For the purposes of this case study, we focus on aromatase inhibitors (AIs) versus tamoxifen.
Comparison of the few head-to-head clinical trials evaluating AIs versus tamoxifen shows a favorable absolute difference for AIs in rates of disease-free survival and overall survival, with a slightly reduced rate of endometrial cancer and a higher rate of osteoporosis (Table 2).
Tamoxifen is considered the standard-of-care adjuvant therapy for patients with high-risk breast cancer; therefore, it provides a good comparator for evaluating efficacy and safety of agents within this class.
For payers, the key question is whether the difference in cost of the AIs over tamoxifen is justified by the data. Patients and providers need to focus on whether the increased efficacy of AIs compared with tamoxifen is offset by the tradeoffs in adverse events.
Time to progression of symptoms is a direct measure of clinical benefit rather than subjective factors, and can be useful in cancers with low response rates.
Gill (2006) has discussed the interim results in the context of the appropriateness of using DFS as a surrogate endpoint in early-stage breast cancer. Although the authors believe this trial provided proof of concept that DFS may be an acceptable primary endpoint for adjuvant trials employing targeted therapies, they also think that a formal pooled analysis of clinical trials using DFS as a surrogate endpoint in adjuvant breast cancer treatment would be valuable.
Surrogacy in cancer trials requires that the endpoint used predicts overall survival (Fleming 1996). DFS has been validated for adjuvant chemotherapy for colo-rectal cancer (Sargent 2005), and strong evidence exists for its validity in early-stage breast and non-resected, non-small cell lung cancer (Gill 2006).
The FDA has accepted DFS as an endpoint for drug approval in adjuvant breast cancer hormonal therapy, adjuvant colon cancer, and adjuvant cytotoxic breast cancer therapy (FDA 2007).
The advantages of accessing effective therapies in the adjuvant setting, where the treatment goal is a cure, cannot be underestimated. An endpoint such as DFS may allow comparison of the effectiveness of therapies in a class while waiting for data on OS to develop. When DFS is employed as a surrogate endpoint, payers need to weigh the potential benefits in improved QOL and decreased costs against the risk of assessment bias in trial results.
Tumor response
The National Cancer Institute (NCI) defines response rate as the “percentage of patients whose cancer shrinks or disappears after treatment” (NCI 2007). Two types of response rates are commonly used to measure tumor response to treatment in oncology clinical trials: objective response rate (ORR) and complete response (CR) rate (also known as pathologic complete response) (FDA 2007).
The FDA defines ORR as the “proportion of patients with a tumor size reduction of a predefined amount and for a minimum period of time” (FDA 2007). NCI defines CR as the “disappearance of all signs of cancer in response to treatment.” Although CR is preferred over ORR, very few drugs produce high rates of CR (FDA 2004).
The use of response rate as an endpoint, especially when considering the mechanisms of action of newer cancer agents, presents several problems. Aside from the well-documented difficulties concerning the variability of tumor measurement (Carey 2005), tumor response evaluation rests upon the theory that because anticancer agents kill cancer cells, if an agent is effective, tumor size will decrease.
Unfortunately, there is not necessarily a correlation between tumor size and a patient’s overall survival. Newer targeted therapies do not work in the same cytotoxic way as older agents, and these newer agents are more likely to inhibit tumor growth rather than to decrease tumor size. It is possible for a treatment to convey clinical benefit while not creating tumor regression (Yu 2007). Although response rate may be an appropriate tool to identify agents for continued evaluation, it is probably inappropriate for determining efficacy for drug approval (Michaelis 2006).
Evaluating surrogate endpoints
Overall survival can be expensive and time consuming to calculate. Clinical trials, therefore, often use other endpoints to demonstrate a drug’s efficacy. Surrogate endpoints can accelerate the evaluation of new therapies, allowing patients to have access to, and benefit from, these therapies sooner (Gill 2006, Fleming 1996, Molenberghs 2000). According to Gill (2006), a surrogate endpoint “is selected based on a biologic rationale and may be employed when the primary endpoint of interest is difficult or expensive to measure and when an alternative, more accessible end point is sufficiently well correlated with the primary to justify its use as a substitute.” But how do you determine if the surrogate endpoint is “sufficiently well correlated?” Often, this is an area of confusion for providers and payers. Payers must understand these surrogate endpoints when making coverage decisions, and should be able to determine when their use is appropriate and how these data can be used to compare a product’s efficacy with similar treatments.
Colorectal cancer is the third-leading cause of cancer-related deaths and, in terms of incidence, is the third most common cancer (ACS 2008). If found early enough, surgery is the primary treatment and often results in a cure. According to the Cancer Trends Progress Report on Colorectal Cancer (NCI 2005), when the condition is metastatic, the recommended treatment is adjuvant chemotherapy. Unfortunately, mortality still remains high with advanced metastatic colorectal cancer (Ries 2000), but recent research shows that the addition of an epidermal growth factor receptor inhibitor to chemotherapy can be helpful (Van Cutsem 2006).
Meyerhardt (2006) studied the efficacy of cetuximab (Erbitux) therapy in 24 patients with metastatic colorectal cancer previously treated with either gefitinib (Iressa) or erlotinib (Tarceva) and standard chemotherapy. None of the patients had a partial or CR as defined by the trial protocols, but 72 percent had stable disease, and progression-free survival (PFS) was 5.1 months for all patients. Those patients who had documented disease progression while receiving either gefitinib or erlotinib had 6 months of PFS (Meyerhardt 2006).
Although cetuximab is more likely to inhibit tumor growth than cause tumor regression, it does appear to convey some clinical benefit in this difficult-to-treat population. It would be hard for payers to make coverage decisions based solely on the response data.
Payers should understand cancer trial endpoints and their clinical relevance, while allowing for individual variations that may affect patient care.
Time to progression of symptoms
Time to progression (TTP) is the time from randomization until objective tumor progression. TTP of symptoms is a direct measure of clinical benefit rather than of subjective factors. It is difficult to differentiate symptoms resulting from tumor progression from those resulting from drug toxicity. If the measures used to compare efficacy among drugs are not carefully evaluated, a drug that is less toxic may appear to be more efficacious (FDA 2007). TTP can be a useful measurement in cancers that have low partial or CR rates.
Taxanes have recently reemerged as chemotherapy agents with significant treatment potential for patients with metastatic breast cancer. Nabholtz (2003) studied the efficacy of docetaxel (Taxotere), a taxane, and doxorubicin (Adriamycin) compared with doxorubicin and cyclophosphamide (Cytoxan) as first-line chemotherapy for metastatic breast cancer patients. The primary endpoint was TTP of symptoms, and a secondary endpoint was time-to-treatment failure. Although both TTP of symptoms and time-to-treatment failure were shown to be significantly longer with docetaxel and doxorubicin, overall survival was comparable in both arms (Nabholtz 2003).
Simply evaluating TTP of symptoms and time-to-treatment failure may lead to the presumption that one treatment is superior to another, but further data would be needed to come to this conclusion. OS rates may indicate that the docetaxel and doxorubicin regimen had a lower rate of toxic effects than the doxorubicin and cyclophosphamide arm that led to superior TTP of symptoms and time-to-treatment failure rates. To reach a conclusion about the efficacy of these therapeutic regimens, payers need a detailed analysis of adverse events in both clinical arms, as well as an evaluation of information from other trials of these agents.
Progression-free survival
PFS differs from TTP in that PFS represents the time from randomization until objective tumor progression or until death occurs. This method is typically preferred over TTP of symptoms as a regulatory endpoint because in a TTP analysis, the deaths are censored (FDA 2007).
In rare cases, such as metastatic breast cancer, PFS may be preferable to OS. Patients with metastatic breast cancer frequently rotate through many different chemotherapy regimens; as such, the value of OS may be limited because it is difficult to tell which regimen contributed to OS and which did not.
Although PFS can demonstrate direct patient benefit, the trial should have a control arm and be blinded to minimize bias (FDA 2007). PFS may have more value in phase 2 trials, where the purpose is to determine effective agents that are worthy of more in-depth study (Michaelis 2006). Payers should be extremely cautious when making coverage decisions in which PFS data are the only data available.
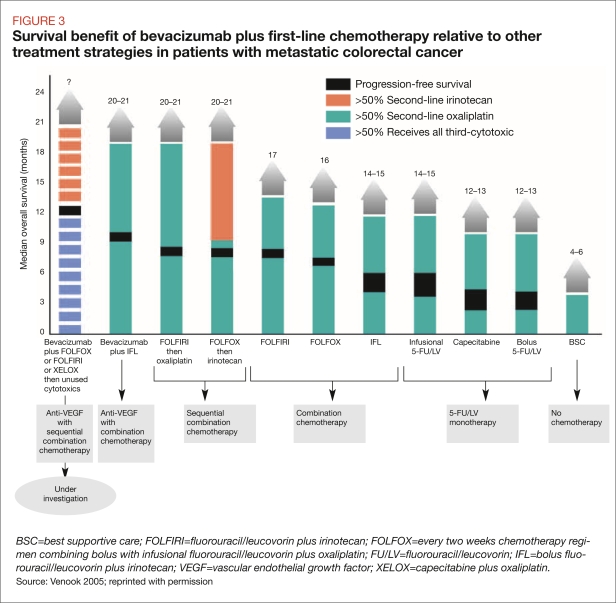
The use of PFS is appropriate in select situations, however. For example, PFS is often used as an endpoint in diseases with very low survival rates, such as advanced metastatic colorectal cancer (FDA 2007) In these cases, PFS may be helpful in comparing treatment regimens. Venook (2005) recently assessed several different agents used to treat metastatic colorectal cancer. Although multiple endpoints were used in the clinical trials that were compared, PFS provided a useful comparator across different trial designs and study populations (Figure 3). In the absence of other data, payers can use PFS to find the context within which other treatments can be used for the same cancer.
FIGURE 3.
Survival benefit of bevacizumab plus first-line chemotherapy relative to other treatment strategies in patients with metastatic colorectal cancer
SUGGESTIONS FOR PAYERS
The careful evaluation of clinical studies, based on an understanding of cancer trial endpoints, is crucial to the effective management of oncology agents in the managed care context.
Payers must not only understand the many oncology clinical trial endpoints and their clinical relevance, they also must allow for individual variations that may be necessary in the care of the patient with cancer. Expert reviews of the available data, therefore, would be useful in placing into perspective the value of the various cancer therapies available.
Oncology remains a specialty in which treatment regimens are carefully tailored to individual patients. Therefore, conversations with experts knowledgeable about trial endpoints would be valuable in gaining and disseminating knowledge of which products show clinical value. Communication with and among physicians also will increase expertise across the field about novel treatments.
Understanding cancer trial endpoints also can improve the effectiveness of disease management programs. Care coordinators should be educated on the pros and cons of each treatment and its potential effects on a patient’s QOL, as well as the emotional and financial effects of therapies on the patient’s family. Case and disease managers who understand the nuances of clinical trial language will be better prepared to assist patients and their families in making critical treatment decisions.
In some cases, external reviews or appeals may be necessary for approval or coverage of a treatment that may not be well understood or well known. Payers, therefore, should seek the opinions of independent oncologists to inform plan management about a patient’s treatment, and, if possible, to expedite treatment delivery to the patient.
As more oncology agents become available and as the management of oncology treatments changes, all stakeholders must clearly communicate their needs and intent. Understanding the merit and application of different oncology endpoints will support sensible coverage decisions.
TABLE 2.
Comparing aromatase inhibitors with tamoxifen
| Absolute difference | Adverse events | ||||||
|---|---|---|---|---|---|---|---|
| Drug | DFS | OS | Hot flashes | Endometrial cancer | Thromboembolic events | Bone fracture/ osteoporosis | Trial |
| Anastrozole vs. tamoxifen | 2.4% | 0.3% | 36% | 0.2%* | 4.5%* | 11%† | ATAC, AIs started immediately postsurgery; 3,215 patients in the anastrozole arm, 3,116 in the tamoxifen arm |
| Letrozole vs. tamoxifen | 1.9% | 0.7% | 34% | 0.2% | 2.0%* | 5.8%* | BIG 01-198, AIs started immediately postsurgery; 4,003 patients in the letrozole arm, 4,007 in the tamoxifen arm |
| Exemestane vs. tamoxifen | 3.5% | 0.6% | 42% | NR | 1.9%* | 7.4%* | IES, AIs started after 2 to 3 yrs of tamoxifen; 2,362 patients in the exemestane arm, 2,372 in the tamoxifen arm |
AI=aromatase inhibitors; ATAC=arimidex, tamoxifen alone or in combination; BIG=Breast International Group; DFS=disease-free survival; IES=International Exemestane Study; NR=not reported; OS=overall survival.
Difference statistically significant in favor of AI.
Difference statistically significant in favor of tamoxifen.
Table should not be used to compare AIs, as trial designs differed. These agents’ comparisons to tamoxifen are valid only in the context of each individual trial.
Sources: Nordman 2005, Masakazu 2006
Footnotes
Disclosures
Allan Jay Kogan, MD, received an honorarium from Genentech for authorship of this article. Melinda Haren, RN, reports no conflicts of interest with respect to the content of this article.
REFERENCES
- American Cancer Society (ACS) Cancer Facts & Figures 2008. Atlanta: ACS; 2008. [Google Scholar]
- Carey L, Metzger R, Dees EC, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst. 2005;97:1137–1142. doi: 10.1093/jnci/dji206. [DOI] [PubMed] [Google Scholar]
- Cianfrocca M, Gradishar WJ. Controversies in the therapy of early stage breast cancer. Oncologist. 2005;10:766–779. doi: 10.1634/theoncologist.10-10-766. [DOI] [PubMed] [Google Scholar]
- FDA (U.S. Food and Drug Administration) FDA Public Workshop on Clinical Trial Endpoints in Prostate Cancer, June 21–22, 2004, Bethesda, Md, Summary. [Accessed April 7, 2008]. « http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4095B1_03_03-FDA-Tab3.pdf.».
- FDA. Guidance for Industry. Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. May, 2007. [Accessed April 7, 2008]. « http://www.fda.gov/CBER/gdlns/7478fnl/clintrialend.htm».
- Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? . Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- Gill S, Sargent D. End points for adjuvant therapy trials: has the time come to accept disease-free survival as a surrogate end point for overall survival? . Oncologist. 2006;11:624–629. doi: 10.1634/theoncologist.11-6-624. [DOI] [PubMed] [Google Scholar]
- HERA Study Team. BIG 1-01/BO 16348 – HERA - Herceptin® (H) Given for One Year Following Completion of Adjuvant Chemotherapy Significantly Improves Disease-free Survival (DFS) in Early Breast Cancer (BC) with HER2-Overexpression: results of the Interim Analysis of the Breast International Group (BIG) [Accessed April 7, 2008];Breast International Group. 2006 8(1):1–4. « http://www.breastinternationalgroup.org/downloads/archive/1ebf9d8d-2413-43f6-9b9c-2b708a79f5ba.pdf». [Google Scholar]
- Masakazu T, Yuichi I. Hormone therapy: from basic science to clinical science: Who benefits from hormone therapy? . Breast Cancer. 2006;13:117–122. [Google Scholar]
- Meyerhardt JA, Heseltine D, Ogino S, et al. Efficacy of cetuximab after treatment with oral epidermal growth factor receptor tyrosine kinase inhibitor-based chemotherapy in metastatic colorectal cancer. Clin Colorectal Cancer. 2006;6(1):59–65. doi: 10.3816/CCC.2006.n.022. [DOI] [PubMed] [Google Scholar]
- Michaelis LC, Ratain MJ. Measuring response in a post-RECIST world: from black and white to shades of grey. Nat Rev Cancer. 2006;6:409–414. doi: 10.1038/nrc1883. [DOI] [PubMed] [Google Scholar]
- Molenberghs G, Burzykowski D, Renard D, Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1:49–67. doi: 10.1093/biostatistics/1.1.49. [DOI] [PubMed] [Google Scholar]
- Motulsky H. Intuitive Biostatistics. New York: Oxford University Press; 1995. [Accessed April 7, 2008]. Survival curves. « http://www.graphpad.com/www/book/survive.htm». [Google Scholar]
- Nabholtz JM, Falkson C, Campos D, et al. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol. 2003;21:968–975. doi: 10.1200/JCO.2003.04.040. [DOI] [PubMed] [Google Scholar]
- NCI (National Cancer Institute) A Snapshot of Breast Cancer. 2007. [Accessed April 7, 2008]. « http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_010808/page9».
- NCI (National Cancer Institute) Cancer Trends Progress Report-2005 Update: Colorectal Cancer Treatment. [Accessed April 7, 2008]. « http://progressreport.cancer.gov/doc_detail.asp?pid=1&did=2005&chid=24&coid=224&mid=».
- Neyt M, Albrecht J, Cocquyt V. An economic evaluation of herceptin in adjuvant setting: the Breast Cancer International Research Group 006 trial. Ann Oncol. 2006;17:381–390. doi: 10.1093/annonc/mdj101. [DOI] [PubMed] [Google Scholar]
- Nordman IC, Spillane AJ, Hamilton AL. The aromatase inhibitors in early breast cancer: who, when, and why? . Med J Aust. 2005;183(1):24–27. doi: 10.5694/j.1326-5377.2005.tb06882.x. [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973–1997, with special section on colorectal cancer. Cancer. 2000;88:2398–2424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E. Challenges in the use of epidermal growth factor receptor inhibitors in colorectal cancer. Oncologist. 2006;11:1010–1017. doi: 10.1634/theoncologist.11-9-1010. [DOI] [PubMed] [Google Scholar]
- Venook A. Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist. 2005;10:250–261. doi: 10.1634/theoncologist.10-4-250. [DOI] [PubMed] [Google Scholar]
- Vogel CL, Tan-Chiu E. Trastuzumab plus chemotherapy: convincing survival benefit or not? . J Clin Oncol. 2005;23:4247–4250. doi: 10.1200/JCO.2005.12.903. [DOI] [PubMed] [Google Scholar]
- Yu RX, Holmgren E. Endpoints for agents that slow tumor growth. Contemp Clin Trials. 2007;28(1):18–24. doi: 10.1016/j.cct.2006.05.011. [DOI] [PubMed] [Google Scholar]