Abstract
Background
Schizophrenia symptoms can be conceptualized in terms of a breakdown of a balance between (a) activating, retrieving and matching stored representations to incoming information (semantic memory-based processing), and (b) fully integrating activated semantic representations with one another and with other types of representations to form a gestalt representation of meaning (semantic integration). Semantic memory-based processes are relatively more dependent on inferior frontal and temporal cortices, while more demanding integrative processes additionally recruit the DLPFC and sometimes parietal cortices. We used fMRI to determine whether the modulation of temporal/inferior frontal cortices and the DLPFC can be neuroanatomically dissociated in schizophrenia, as semantic integration demands increase. Integration demands were manipulated by varying the nature (concrete versus abstract) and the congruity (incongruous versus congruous) of words within sentences.
Methods
Sixteen right-handed schizophrenia patients and sixteen healthy volunteers, matched on age and parental socio-economic status, underwent event-related fMRI scanning while they read sentences. BOLD effects were contrasted to words within sentences that were (a) concrete versus abstract, and (b) semantically incongruous versus congruous with their preceding contexts.
Results
In both contrasts, large networks mediating the activation and retrieval of verbal and imagistic representations were normally modulated in patients. However, unlike controls, patients failed to recruit the DLPFC, medial frontal and parietal cortices to incongruous (relative to congruous) sentences, and failed to recruit the DLPFC to concrete (relative to abstract) sentences.
Conclusions
As meaning is built from language, schizophrenia patients demonstrate a neuroanatomical dissociation in the modulation of temporal/inferior frontal cortices and the DLPFC.
Keywords: schizophrenia, semantic, language, fMRI, context, prefrontal cortex, DLPFC, temporal cortex
Introduction
Deriving an accurate representation of meaning requires us to strike a fine balance between two types of semantic processes: (a) activating, retrieving and matching stored semantic information with incoming material (semantic memory-based processes), and (b) fully integrating activated semantic representations with one another and with other types of activated representations to derive a gestalt meaning (semantic integration). Both mechanisms are used to construct the meanings of words and whole sentences. Semantic activation, retrieval and matching are thought to be most reliant on temporal and inferior frontal cortices, while more demanding semantic integrative processes additionally engage more superior dorsolateral prefrontal cortices (DLPFC), sometimes together with parietal cortices. This study used fMRI to demonstrate a neuroanatomical dissociation in the modulation of temporal/inferior frontal cortices and the DLPFC in schizophrenia, as meaning is built from language.
Encountering all types of words leads to the retrieval and activation of stored lexico-semantic representations, reflected by activity within left-lateralized temporal and inferior frontal cortices (1, 2). In addition, words with concrete meanings activate ‘imagistic’ representations to a greater degree than abstract words (3). This is reflected by widespread activity, distributed across bilateral ventromedial temporal, orbitofrontal and occipito-parietal cortices, known to subserve perceptual processing of real-world objects (4–6). Therefore, to derive a full representation of concrete word meaning, activated verbal and imagistic perceptual semantic representations must be integrated and, in some cases, undergo additional manipulation, such as mental imagery (7, 8). These additional demands of integrating and manipulating the meaning of concrete, relative to abstract, words may be reflected by the increased recruitment of the DLPFC to concrete words (4, 5).
Comprehending the meaning of whole sentences also engages semantic memory-based processes in which incoming relationships between content words are matched against relationships that are prestored within semantic memory (9, 10) – operations that are again mediated by inferior frontal, and sometimes temporal, cortices (11–13). In addition, integrative processes are engaged, whereby activated semantic and syntactic representations are combined to determine ‘who does what to whom’ in a sentence. This semantic-syntactic integrative activity is also thought to be mediated within left-lateralized inferior frontal and temporal cortices (14, 15). However, when integration demands are particularly high, additional regions, including the DLPFC and parietal cortices, are recruited (13).
In schizophrenia, there is evidence from semantic priming studies that patients’ automatic activation of lexico-semantic representations is normal and, in thought-disordered patients, even increased ((16–20); reviewed in (21, 22)). Behavioral studies also suggest that many aspects of semantic memory organization are normal in schizophrenia (23), and that patients can successfully retrieve semantic information, so long as appropriate semantic cues are provided (24, 25). Neuroanatomically, activity within the inferior frontal cortex is generally preserved during deep semantic encoding (26, 27), and functional connectivity between temporal and inferior frontal cortices may be increased (28). Abnormal increases in activity within temporal-occipital cortices have been reported in patients when processing indirectly related (versus unrelated) word-pairs (29), during semantic (versus shallow) verbal encoding (27), and when completing sentences given highly predictable contexts (versus reading the same single word) (30).
In contrast to this normal or increased semantic memory-based activity in schizophrenia, evidence from behavioral and event-related potential (ERP) studies suggests that patients are relatively impaired when required to integrate activated semantic representations with one another or with other types of activated representations. There is evidence for such impairments at the level of both words and whole sentences. For example, under non-automatic experimental conditions, patients fail to fully integrate semantic representations of prime and target words, leading to relatively reduced semantic priming effects ((31); reviewed in (21)). And, during sentence comprehension, patients show abnormally reduced electrophysiological responses when semantic-syntactic integration demands are increased (32–36)1.
Despite this behavioral and ERP evidence for a dissociation between (a) preserved or increased activity in association with semantic memory-based processing, and (b) decreased activity with increased integration demands in schizophrenia, there have been no attempts to determine how this dissociation plays out at a neuroanatomical level. In paradigms probing the maintenance and use of contextual information using simple non-verbal stimuli, clear functional dissociations between activity within the DLPFC and other regions have been described in schizophrenia (37–39). The goal of this study was to determine whether patients show such neuroanatomical dissociations when semantic integration demands are increased, as meaning is built from language. We aimed to dissociate activity within temporal-inferior frontal networks from activity within the DLPFC in schizophrenia in two ways: first, by manipulating the concreteness of individual words within sentences, and second, by manipulating the semantic congruity of a sentence-final word with its preceding context. These factors – Concreteness and Congruity – were fully crossed, such that each participant viewed sentences composed of concrete and abstract words, in which the final words were either congruous or incongruous with their preceding stems (Table 1; see (40) for a similar design).
Table 1.
| Sentence type and examples | No. of content words per sentence | Cloze probability y of congruous sentences | Frequency of content words per sentence | Concreteness rating of all content words, per sentence | Concreteness rating of sentence-final words | Min and Max Concreteness Rating Final word |
|---|---|---|---|---|---|---|
|
ConcreteCongruous: During the rain storm he carried a large golf umbrella. Incongruous: The twenty dairy cows are kept in the red bench. |
5.48 ± 0.96 |
0.74 ± 0.19 |
138.22± 107.89 |
5.43 ± 0.42 |
6.30 ± 0.42 |
5.00, 6.89 |
|
Abstract: Congruous: Although she strove for perfection she continued to make mistakes. Incongruous: Her outer expressions were completely blank and revealed no equality. |
5.48 ± 1.06 |
0.69 ± 0.21 |
162.57 ± 145.95 |
3.64 ± 0.47 |
2.94 ± 0.70 |
1.22, 4.78 |
| P Value | 0.974 | 0.103 | 0.144 | < 1 E -20 | < 1 E -20 |
Means are shown with standard deviations in brackets. In the examples, critical sentence-final words are underlined.
Values for cloze probability and concreteness ratings (on a scale of 1–7) were derived in normative studies ratings in two separate groups of healthy participants who did not participate in the fMRI experiment. Frequencies (per million) were taken from Frances & Kucera (75).
We predicted that, in both contrasts (concrete versus abstract and incongruous versus congruous sentences), patients would show normal activation, retrieval and matching of verbal and perceptual semantic representations, reflected by normal modulation of occipito-temporal and inferior frontal cortices (26, 27). Based on our previous fMRI study using semantically related word-pairs (29), we also considered the possibility that patients would show inappropriate increases in activity within inferior temporal and fusiform cortices to the congruous sentences, which contained more semantically related words than the incongruous sentences. Critically, based on behavioral and ERP findings at the level of words (21, 22, 31) and sentences (32–36), we predicted that patients would be relatively impaired in semantic integrative processes, and that this would be reflected by a failure to recruit the DLPFC to concrete (versus abstract) and to incongruous (versus congruous) sentences.
Methods
Materials
Two-hundred-and-forty 10-word congruous sentences, half primarily containing concrete words and half containing abstract words, matched on frequency and number of letters, were constructed (Table 1). The sentences were divided into two counterbalanced lists. Incongruous abstract and concrete sentences were generated by pseudo-randomizing the final words of the congruous abstract and concrete sentences respectively. Each list contained 60 sentences in each sentence type. Although each of the 240 sentence stems and final words appeared only once per list, across lists, they each appeared in both the congruous and incongruous sentences.
Participants
Sixteen patients meeting DSM-IV criteria for schizophrenia (41) (confirmed using the SCID (42)), receiving stable doses of atypical antipsychotics, were recruited from the Lindemann Mental Health Center, Boston. Sixteen demographically-matched volunteers on no medication, without histories of psychiatric disorders (42), were recruited by advertisement. All participants were native, primarily monolingual English speakers who had not learned any other language before age five. All were right-handed (43, 44), without histories of head trauma, neurological disorder, substance abuse within six months, or substance dependence. Written consent was obtained following the guidelines of the Partners Healthcare Institutional Review Board. Clinical assessments were carried out within two weeks of scanning. Demographic and clinical data are summarized in Table 2.
Table 2.
Demographic and psychopathological data of healthy controls and patients with schizophrenia
| Subject Group |
||
|---|---|---|
| Parameter | Controls (n=16) |
Patients (n=16) |
| Gender (M/F) | 11/5 | 12/4 |
| Race (C/AA) | 12/4 | 13/3 |
| Age (years) | 44.4 (5.9) | 45.9 (8.0) |
| Hollingshead Index | 3.0 (1.3) | 3.2 (1.1) |
| Premorbid verbal IQ | 116.2 (9.4) | 105 (14.2) |
| CPZ equivalent | - | 395 (223) |
| Duration of illness (years) | - | 20.3 (7.7) |
| PANSS positive (total) | 10.8 (6.1) | |
| PANSS negative (total) | 16.9 (8.4) | |
Patients and controls matched closely in gender and race/ethnicity distributions and there was no significant difference between the groups in age (p = 0.55). The patient and control groups showed no significant difference on parental socioeconomic status (p =0.5), as determined by Hollingshead Index scores (76), although patients had a lower premorbid IQ (p < 0.03) as assessed by the North American Adult Reading Test 77 (NAART).
Abbreviations: M = Male; F = Female; C = Caucasian; AA = African-American; CPZ = chlorpromazine; PANSS: Positive and Negative Syndrome Scale. (78.)
Stimulus presentation and task
A typical 8-sec trial is depicted in Figure 1. Participants decided whether or not each sentence made sense by pressing one of two buttons on a response box (using their left hand, with fingers counterbalanced). Sentence trials were pseudo-randomly presented amongst fixation trials (20%) in which subjects fixated on a "+" (for variable durations: 1975–17975sec), allowing efficient deconvolution of the hemodynamic response (45).
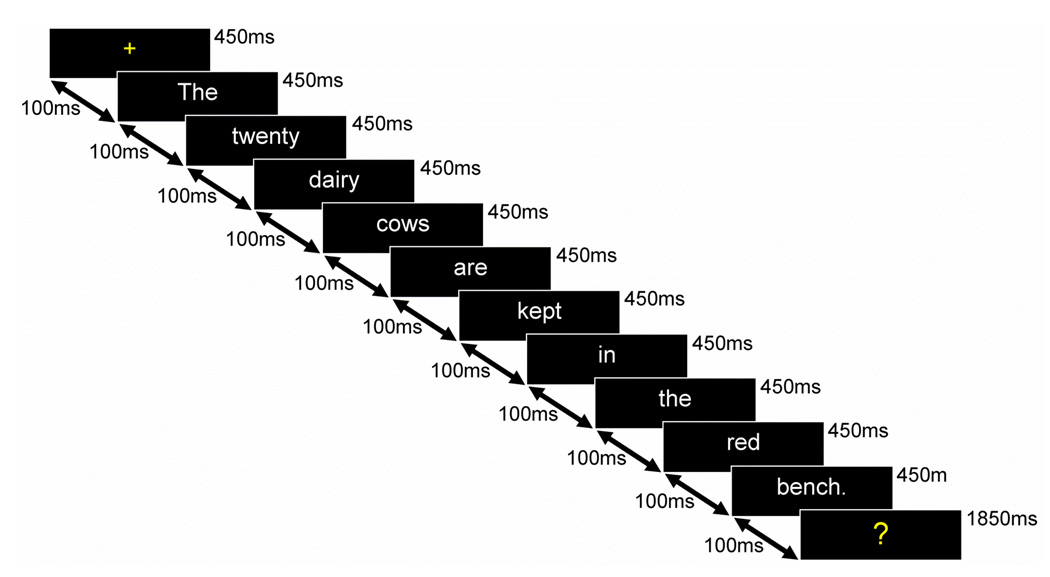
Figure 1. Depiction of a single trial.
Each trial began with a centered yellow fixation followed by each word (450msec, ISI: 100msec). The sentence-final word was followed by a response cue (“?”), giving subjects 1850msec (ISI: 100msec) to respond before the next trial began. The sentence shown is a concrete incongruous sentence. See Table 1 for examples of sentences presented in the other three conditions.
MRI Data acquisition
Imaging took place on a 3 Tesla MR scanner (Siemens Trio). Participants underwent two high-resolution 3D structural scans (spoiled GRASS sequence; 128 sagittal slices, 1.33mm thickness, TR: 2530 ms, TE: 3.3msec, flip angle: 7 degrees, bandwidth: 199 Hz, in-plane resolution: 1 × 1.33 mm), followed by T1-weighted anatomic images (30 slices, 3mm thickness, skip 1 mm) and a T2-weighted image acquired in plane with the functional images to assist in manual registration of functional and structural data.
During functional scanning, each participant viewed sentences in one list, divided over six functional runs, each lasting 414sec during which T2*-weighted echoplanar (EP) images were acquired (30 slices, 203 images/slice, 3mm thickness, skip 1mm, in-plane resolution of 3.125 mm, 30\grad axially), using a gradient echo sequence (TR: 2sec; TE: 30 msec; flip angle: 90\grad).
Behavioral data analysis
Percentages of errors and reaction times (RTs) to each sentence type (collapsed across items) were entered into 2 × 2 × 2 repeated-measures ANOVAs with Group (patients versus controls) as a between-subject factor and Concreteness (concrete versus abstract) and Congruity (incongruous versus congruous) as within-subject factors. Analyses were repeated after logarithmic transformation and revealed the same pattern of findings unless otherwise noted. Alpha was set to 0.05.
MRI Data analysis
Following motion correction, each participant’s two high-resolution structural scans were averaged to increase the signal:noise. The resulting volume was reconstructed using semi-automatic procedures (FreeSurfer) to yield a model of each individual’s cortical surface (46–49). To average functional data across subjects (see below), each subject's cortical surface was morphed/registered to an average spherical surface representation that optimally aligned sulcal and gyral features across subjects (49, 50).
Functional images were motion corrected using the AFNI alogarithm (51, 52). There was no significant difference in the total (vector) translation between controls (mean ± SD: 3.1 ± 1.1mm; range: 1.7–5.8mm) and patients (mean ± SD: 2.6 ± 0.8mm; range: 1–3.8mm), p = 0.15. Following spatial smoothing (3-D Gaussian filter: 6mm FWHM) and intensity normalization, the functional images were analyzed with a General Linear Model (GLM) using the FreeSurfer Functional Analysis Stream. The HRF for each condition was modeled using three components, each constituting a canonical HRF (53), convolved with a box-car of an appropriate length. The first component, modeled as a single regressor, lasted from the onset of the trial until the onset of the critical sentence-final word (5500 msec). The second component lasted from the onset of the critical sentence-final word until the onset of the “?” (550 msec) and was modeled separately for each of the four sentence types. The third component lasted from the offset of the critical word until the end of the trial (i.e. the decision: 1950 msec) and was again modeled as a single regressor. Mean offset and linear trend regressors were also included to remove low-frequency drift.
These GLM parameter estimates were resampled onto each individual’s inflated cortical surface, iteratively smoothed (equivalent to a 3D kernel of approx. 8.5mm FWHM), and resampled onto the average cortical spherical representation. The regression weights of the second canonical HRF component (capturing the hemodynamic response to the sentence-final word) were used to construct statistical maps using a 2 × 2 × 2 mixed model with Group (patient versus controls) as a between-subjects factor and Concreteness (concrete versus abstract) and Congruity (incongruous versus congruous) as within-subjects factors. Only ‘highest order’ effects are reported, i.e. clusters reported as showing main effects for a particular factor are those that failed to reach significance on any interactions involving that factor, and clusters reported as showing two-way interactions failed to show three-way interactions.
To correct for multiple comparisons, clusters covering at least 300mm2, with a corrected threshold for rejection of the null hypothesis of p < 0.05, were identified on the basis of a Monte-Carlo simulation (54). Within-group maps were generated to examine sources of effects; clusters overlapping with those showing main effects or interactions are also reported at both cluster-level significance and at a less conservative uncorrected threshold (p < 0.01).
Maps were generated using all trials. Analyses were repeated using only correctly-answered trials and showed the same pattern of findings.
Results
Behavioral data
As shown in Table 3 and Table 4, both groups were more accurate and faster to judge the acceptability of the concrete than the abstract sentences, but these differences were greater in patients than controls (Group by Concreteness interactions). There were no overall differences in accuracy or RTs of acceptability judgments between the incongruous and congruous sentences (no main effects of Congruity), and this pattern did not differ between groups (no Group by Congruity interactions). Concreteness by Congruity interactions, however, reflected participants’ relatively more accurate and faster judgments of abstract congruous than abstract incongruous sentences, but no such difference was observed for concrete sentences. This pattern did not differ between groups (no Group by Concreteness by Congruity interactions). As expected, patients were generally less accurate and slower than controls in judging all sentences (main effects of Group).
Table 3.
| Controls | Patients | Controls | Patients | |
|---|---|---|---|---|
| Concrete | Abstract | |||
| Congruous % errors | 3.1 (2.5) | 10.9 (8.6) | 3.1 (3.6) | 15.5 (14.3) |
| Incongruous % errors | 2.8 (3.3) | 8.2 (7.7) | 10.7 (8.3) | 20.8 (9.9) |
| Congruous (RTs) | 937.3 (152.2) | 1130.1 (159.2) | 972.9 (151.5) | 1137.2 (141.3) |
| Incongruous (RTs) | 902.6 (182.7) | 1105.6 (165.8) | 1016.1 (163.3) | 1150.9 (121.3) |
Mean percentage of errors and reaction times across the four sentence types, with standard deviations in brackets. Note that an error to a congruous sentence is a false negative (classifying it as incongruous) and an error to an incongruous sentence is a false positive (classifying it as congruous).
Table 4.
Behavioral data
| Accuracy | Reaction times | |||||
|---|---|---|---|---|---|---|
| DOF | F | p | DOF | F | p | |
| Group × Congruity × Concreteness | 1, 30 | 0.003 | p=.96 | 1, 30 | 1.52 | p=.22 |
| Congruity × Concreteness | 1, 30 | 24.39 | p<.0001**** | 1, 30 | 13.05 | p<.001*** |
| Group × Congruity | 1, 30 | 0.96 | p=.33 | 1, 30 | 0.11 | p=.74 |
| Group × Concreteness | 1, 30 | 8.49 | p<.01** | 1, 30 | 6.49 | p<.05* |
| Concreteness | 1, 30 | 64.51 | p<.0001**** | 1, 30 | 28.19 | p<.001*** |
| Congruity | 1, 30 | 4.20 | p<.05* | 1, 30 | 0.001 | p=.97 |
p<.05
p<.01
p<.001
p<.0001
Note: For reaction time data, the Group × Concreteness interaction did not remain significant when analyzed using a log transform or when correct responses only were examined.
fMRI data
Concreteness: main effects and interactions
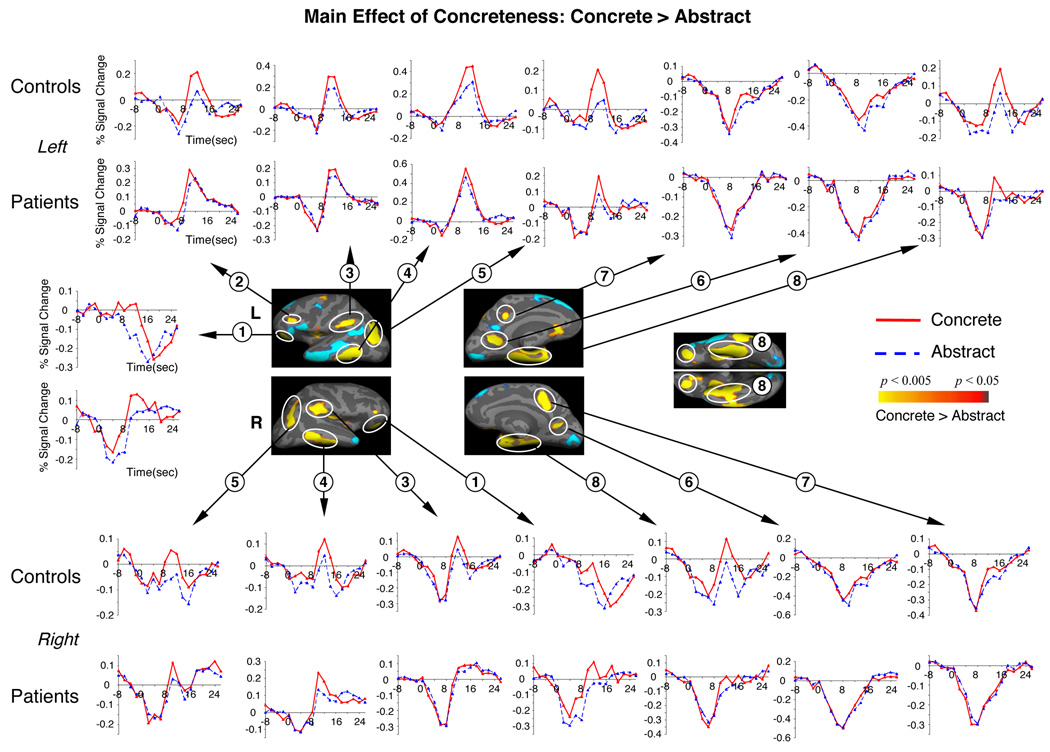
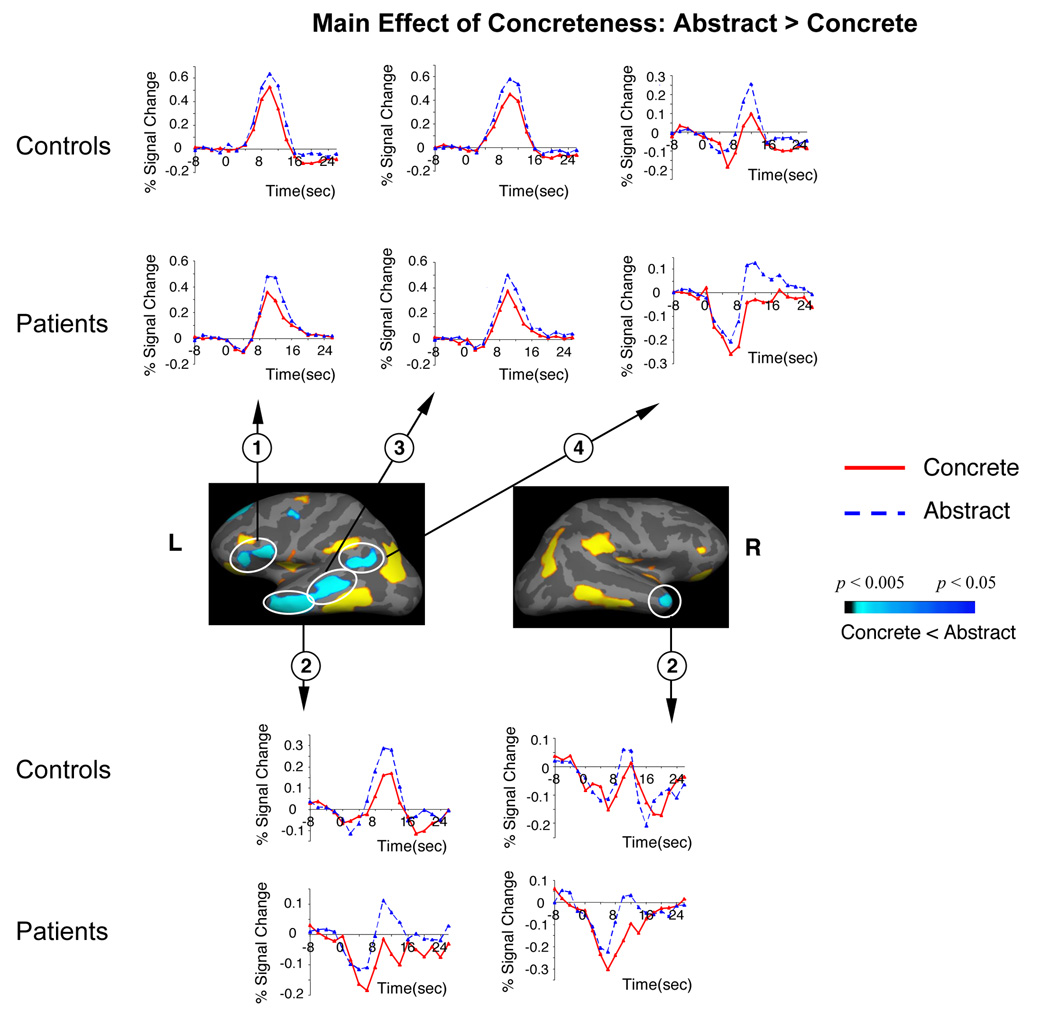
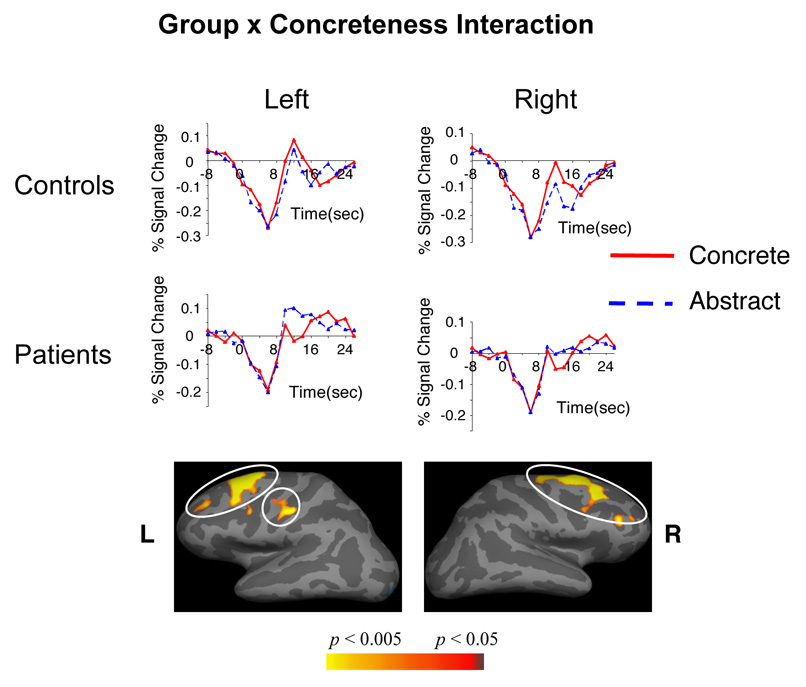
In both groups, sentence-final concrete words were associated with significantly more activity than sentence-final abstract words across bilateral orbitofrontal, inferior/ventral/medial temporal cortices, and occipito-parietal cortices (main effects of Concreteness, Table 5A, Figure 2). Abstract sentence-final words, however, were associated with more activity within a left-lateralized superior/middle temporal and inferior frontal network (Table 5B, Figure 3). Within bilateral superior/middle prefrontal cortices, there were Group by Concreteness interactions (Table 6, Figure 4). This area encompassed Brodmann Areas 8/9/46, and is henceforth referred to as the DLPFC; here, controls showed relatively more activity but patients showed relatively less activity to concrete (versus abstract) sentence-final words. These interactions remained significant when premorbid IQ (that differed slightly between the two groups, Table 2) was entered as a potentially confounding covariate, and when analyses were repeated using a subset of the patients and controls (n=12 in each group) matched on premorbid IQ.
Table 5.
Main effects across all participants: Concrete sentences versus abstract sentences
| No. | Region | Lat. | BA | Area mm2 | Tal. x, y, z | p | Area mm2 | Tal. x, y, z | p | Area mm2 | Tal. x, y, z | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Table 5A: CONCRETE VERSUS ABSTRACT | Main effect of Concreteness: Concrete > Abstract | Controls: Concrete > Abstract | Patients: Concrete > Abstract | |||||||||
| 1 | Orbito frontal cortex | L | 11 | 800 | −21 24 −13 | 2.2e−6 | 775 | −21 25 −11 | .00049 | 368 | −21 24 −11 | .00046 |
| R | 1040 | 20 26 −15 | 1.8e−7 | 592 | 19 28 −19 | .00017 | 526 | 21 29 −18 | 8.1e−5 | |||
| 2 | Inferior frontal sulcus | L | 46/45 | 429 | −36 34 11 | .0002 | 799 | −38 36 10 | .00012 | NS | ||
| R | 451 | 39 40 −4 | .0001 | 781 | 40 38 7 | .00024 | NS | |||||
| 3 | Supramar ginal gyrus/Postcentr al gyrus | L | 40/1 | 930 | −54 −26 36 | 5.0e−6 | 781 | −53 −32 37 | .00047 | 1320 | −60 −23 21 | .00063 |
| R | 1471 | 57 −21 31 | 5.4e−5 | 1035 | 55 −26 27 | .00037 | 357 | 60 −19 33 | .00096 | |||
| 4 | Middle/Inferior temporal gyrus (posterior) | L | 21 | 1700 | −53 −42 −5 | 4.07e−9 | 1490 | −54 −47 2 | 6.9e−5 | 958 | −54 −44 1 | .00032 |
| R | 1650 | 58 −37 −2 | 9.33e−5 | 309 | 55 − 47 3 | .00135 | 520 | 56 −42 −1 | .00157 | |||
| 5 | Angular gyrus/Middle occipital gyrus | L | 39/19 | 1670 | −37 −65 32 | 2.0e−7 | 1382 | −39 −72 31 | .00076 | 90 | −46 −77 30 | 6.3e−6 |
| R | 1483 | 46 −67 30 | .0003 | 1278 | 46 −68 30 | .00313 | NS | |||||
| 6 | Precuneus | L | 31 | 579 | −12 −60 16 | 2.0e−5 | 246 | −12 −60 15 | ^.00631 | 327 | −10 −60 15 | .00245 |
| 7 | Post. Cingulated gyrus | L | 31/23 | 334 | −11 −32 35 | 5.0e−5 | 523 | −12 −31 36 | .00032 | NS | ||
| R | 803 | 12 −34 37 | .0002 | 916 | 12 −34 42 | 4.6e−5 | NS | |||||
| 8 | Parahippo campal gyrus/Medial fusiform | L | 36 | 3000 | −27 −25 −20 | .0009 | 1600 | −26 −27 − 20 | .0015 | 2229 | −27 −29 − 16 | .00182 |
| R | 2000 | 25 −25 −19 | .0005 | 890 | 22 −8 −28 | .0046 | 968 | 28 −26 −18 | .00479 | |||
| Table 5B. ABSTRACT > CONCRETE | Main effect of Concreteness: Abstract > Concrete | Controls: Abstract > Concrete | Patients: Abstract > Concrete | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Inferior frontal gyrus | L | 47/45 | 999 | −45 31 −4 | 1.8ei−5 | 465 | −43 31 −4 | .0004 | 988 | −48 35 2 | .01259 | |
| 2 | Anterior lateral temporal cortex (middle temporal & temporal pole) | L | 21/20 | 1576 | −50 −10 −15 | 1.6e−7 | 851 | −57 −6 −19 | .00013 | 1361 | −51 −1 −23 | .00372 | |
| R | 21/38 | 360 | 43 14 −27 | 3.2e−5 | 276 | 48 7 −28 | ^.00324 | 334 | 40 14 −28 | .00174 | |||
| 3 | Mid-portion of superior temporal sulcus and middle temporal gyrus | L | 22/21 | 1100 | −53 −33 1 | 6.3e−6 | 320 | −56 −41 6 | .00126 | 1221 | −50 −40 4 | .00065 | |
| 4 | Inferior parietal lobule and Posterior superior temporal sulcus | L | 40/22 | 910 | −40 −48 31 | 3.0e−6 | 497 | −38 −52 32 | .00083 | 970 | −39 −50 34 | .00129 | |
| 5 | Cuneus | L | 18 | 491 | −9 −93 9 | 1.5e5 | 943 | −5 −93 4 | .00087 | 1230 | −9 −92 8 | .00186 | |
| R | 911 | 4 −85 21 | .00032 | 800 | 6 −90 11 | .00056 | 561 | 10 −83 2 | 0.005 | ||||
No. corresponds directly to cluster labels in Figure 2 (for Table 5A) and Figure 3 (for Table 5B) When clusters or Brodmann Areas (BAs) span over more than one region, both regions/BAs are indicated, separated by a hash sign. Lat.: Laterality. Tal. (Talairach) coordinates. All clusters indicated in p column reached cluster-level significance, p < 0.05 corrected across the whole cortex, except for those marked with ^ that reached a voxel-wise significance of p < 0.05, uncorrected across the cortex. NS: non-significant.
Figure 2. Cortical statistical maps comparing the hemodynamic response to concrete and abstract sentence-final words, across patients and controls (main effects of Concreteness in the absence of any interactions involving Group).
Yellow-red: more activity to concrete than to abstract words. Blue: more activity to abstract than concrete words (shown again in Figure 3). These maps were generated by contrasting the second component of the HDR to each sentence type (lasting 550msec, from the onset of the critical sentence-final word until the onset of the “?”). They are therefore likely to reflect differences in neurocognitive activity at the point of sentence-final word, as well as during decision making (see (11) and (13) for Discussion). Hemodynamic time courses show activity in patient and control groups within each of these clusters at each TR. On the time axes, zero refers to the onset of the trial as a whole. The critical events – the sentence-final words – began 4950msec into the trial. All clusters circled are significant at a cluster-level p < 0.05. Cluster numbers correspond directly to the regions reported in Table 5A.
Figure 3. Cortical statistical maps comparing the hemodynamic response to concrete and abstract sentence-final words, across patients and controls (main effects of Concreteness in the absence of any interactions involving Group).
Blue: more activity to abstract than to concrete words. Hemodynamic time courses show activity in patient and control groups within each of these clusters at each TR (on the time axes, zero refers to the onset of the whole trial). All clusters circled are significant at a cluster-level p < 0.05. Cluster numbers correspond directly to the regions reported in Table 5B.
Table 6.
Group by Concreteness Interactions: Differences between the patient and control groups in hemodynamic modulation to concrete versus abstract sentences.
| No. | Region | Lat. | BA | Area mm2 | Tal. x, y, z | P | Area mm2 | Tal. x, y, z | p | Area mm2 | Tal. x, y, z | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group by Concreteness Interactions | Controls: Concrete > Abstract | Patients: Abstract > Concrete | ||||||||||
| 1 | Superior and middle frontal gyrus | L | 8/9/46 | 2450 | −21 24 47 | 2.7e−5 | 1364 | −18 23 50 | .00021 | 3800 | −17 47 27 | 6.0e−5 |
| R | 2300 | 31 25 40 | .0007 | 1631 | 26 7 41 | .00071 | 2151 | 41 27 21 | .00132 | |||
| 2 | Post central gyrus | L | 1/2 | 740 | −45 −16 34 | .0006 | 728 | −44 −16 34 | .00015 | NS | ||
| 3 | Precuneus | L | 7 | 649 | −2 −62 41 | .0007 | NS | 921 | −8 −68 43 | 6.3e−5 | ||
No. corresponds directly to cluster labels in Figure 4. When clusters or Brodmann Areas (BAs) span over more than one region, both regions/BAs are indicated, separated by a hash sign. Lat.: Laterality. Tal. (Talairach) coordinates. All clusters indicated in p column reached cluster-level significance of p < 0.05, corrected across the whole cortex, except for those marked with ^ that reached a voxel-wise significance of p < 0.05, uncorrected across the cortex. NS: non-significant.
Figure 4. Group by Concreteness interactions: areas showing different patterns of modulation between patients and controls in comparing concrete and abstract sentence-final words.
Hemodynamic time courses show activity in patient and control groups within each of these clusters at each TR (on the time axes, zero refers to the onset of the whole trial). All clusters circled are significant at a cluster-level p < 0.05. Cluster numbers correspond directly to the regions reported in Table 6.
Analyses were repeated using a two-component model in which the first component (modeled separately for the four conditions) lasted the duration of the entire sentence until the onset of the “?”. This analysis yielded the same pattern of findings, demonstrating that these findings could be generalized to all content words of the sentences (rather than just the sentence-final word).
Congruity: main effects and interactions
Across all participants, bilateral inferior frontal cortices were recruited to the incongruous relative to congruous sentence-final words (main effects of Congruity, Table 7, Figure 5). In addition, a network that included bilateral DLPFC (BA 8/9), the left inferior parietal lobule, medial frontal (including anterior cingulate) cortices, as well as the right middle temporal cortex and bilateral anterior fusiform gyri, showed significant Group by Congruity interactions. In most of these regions (except for the right middle temporal and fusiform cortex), controls showed more activity to incongruous (versus congruous) sentence-final words, and in all these regions, patients showed relatively less activity to incongruous (versus congruous) sentence-final words (Table 8, Figure 6). All these interactions remained significant when premorbid IQ was entered as a covariate and when analyses were repeated using the IQ-matched subset of patients and controls.
Table 7.
Main effects across all participants: Incongruous versus Congruous sentences
| No. | Region | Lat. | BA | Area mm2 | Tal. x, y, z | p | Area mm2 | Tal. x, y, z | p | Area mm2 | Tal. x, y, z | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INCONGRUOUS > NORMAL | Main effect of Congruity Incongruous > Congruous | Controls: Incongruous > Normal | Patients: Incongruous > Normal | |||||||||
| 1 | Inferior frontal gyrus | L | 45 | 362 | −49 25 8 | .00065 | 380 | −50 32 10 | .00159 | NS | ||
| R | 1250 | 49 22 10 | .00018 | 1782 | 49 36 8 | .00081 | NS | |||||
| 2 | Precentral gyrus (inferior) | L | 6 | 527 | −43 3 12 | .00021 | 752 | −44 3 14 | .00129 | NS | ||
No. corresponds directly to cluster labels in Figure 5. When clusters or Brodmann Areas (BAs) span over more than one region, both regions/BAs are indicated, separated by a hash sign. Lat.: Laterality. Tal. (Talairach) coordinates. All clusters indicated in p column reached cluster-level significance, p < 0.05 corrected across the whole cortex, except for those marked with ^ that reached a voxel-wise significance of p < 0.05, uncorrected across the cortex. NS: non-significant.
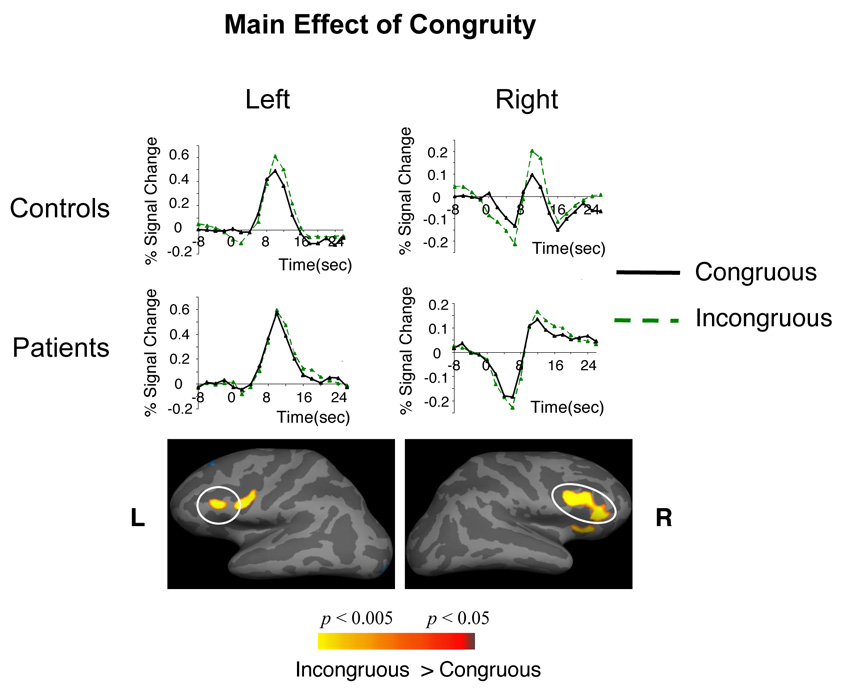
Figure 5. Cortical statistical maps comparing the hemodynamic response to incongruous and congruous sentence-final words, across patients and controls (main effects of Congruity in the absence of any interactions involving Group).
Hemodynamic time courses show activity in patient and control groups within each of these clusters at each TR (on the time axes, zero refers to the onset of the whole trial). All clusters circled are significant at a cluster-level p < 0.05. Cluster numbers correspond directly to the regions reported in Table 7.
Table 8.
Group by Congruity Interaction: Differences between the patient and control groups in hemodynamic modulation to incongruous versus congruous sentences.
| No. | Region | Lat. | BA | Area mm2 | Tal. x, y, z | p | Area mm2 | Tal. x, y, z | p | Area mm2 | Tal. x, y, z | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group by Congruity Interactions | Controls: Incongruous > Congruous | Patients: Congruous > Incongruous | ||||||||||
| 1 | Middle/superior frontal gyrus | L | 8/9 | 2100 | −24 26 36 | .00513 | 182 | −19 49 20 | ^.0234 | 2633 | −16 24 41 | .00120 |
| R | 1650 | 21 40 30 | .00324 | 408 | 13 53 20 | .00058 | 1021 | 21 36 40 | .00063 | |||
| 2 | Inferior parietal lobule | L | 40 | 1291 | −50 −46 47 | .002 | 278 | −45 −43 41 | ^.00229 | 2111 | −45 −52 36 | 2.5e−5 |
| 3 | Middle temporal gyrus/inferior temporal sulcus | R | 21 | 311 | 58 −33 −9 | .001 | NS | 177 | 57 −32 −12 | ^.001 | ||
| 4 | Fusiform gyrus (anterior) | L | 20 | 380 | −49 −21 −27 | .0006 | 72 | −42 −18 −26 | ^.0245 | 425 | −49 −26 −22 | .00102 |
| R | 395 | 40 −12 −29 | .0006 | NS | 510 | 37 −10 −32 | .001 | |||||
| 5 | Precuenus | L | 7 | 1400 | −2 −56 42 | .00178 | 428 | −11 −71 37 | .00047 | 1287 | −4 −62 30 | 6.0e−5 |
| R | 380 | 6 −68 40 | .00501 | 649 | 13 −63 36 | .00031 | 1033 | 6 −61 27 | 6.46e−5 | |||
| 6 | Ant. cingulated gyrus and medial prefrontal cortex | L | 24/32/9/33 | 3200 | −4 25 −5 | .00126 | 608 | −8 16 33 | .00417 | 2798 | −8 31 −11 | .00115 |
| R | 335 | 7 23 24 | .00013 | 359 | 11 23 22 | 1.2e−5 | 597 | 7 26 −9 | .00316 | |||
No. corresponds directly to cluster labels in Figure 6. When clusters or Brodmann Areas (BAs) span over more than one region, both regions/BAs are indicated, separated by a hash sign. Lat.: Laterality. Tal. (Talairach) coordinates. All clusters indicated in p column reached cluster-level significance of p < 0.05, corrected across the whole cortex, except for those marked with ^ that reached a voxel-wise significance of p < 0.05, uncorrected across the cortex. NS: non-significant.
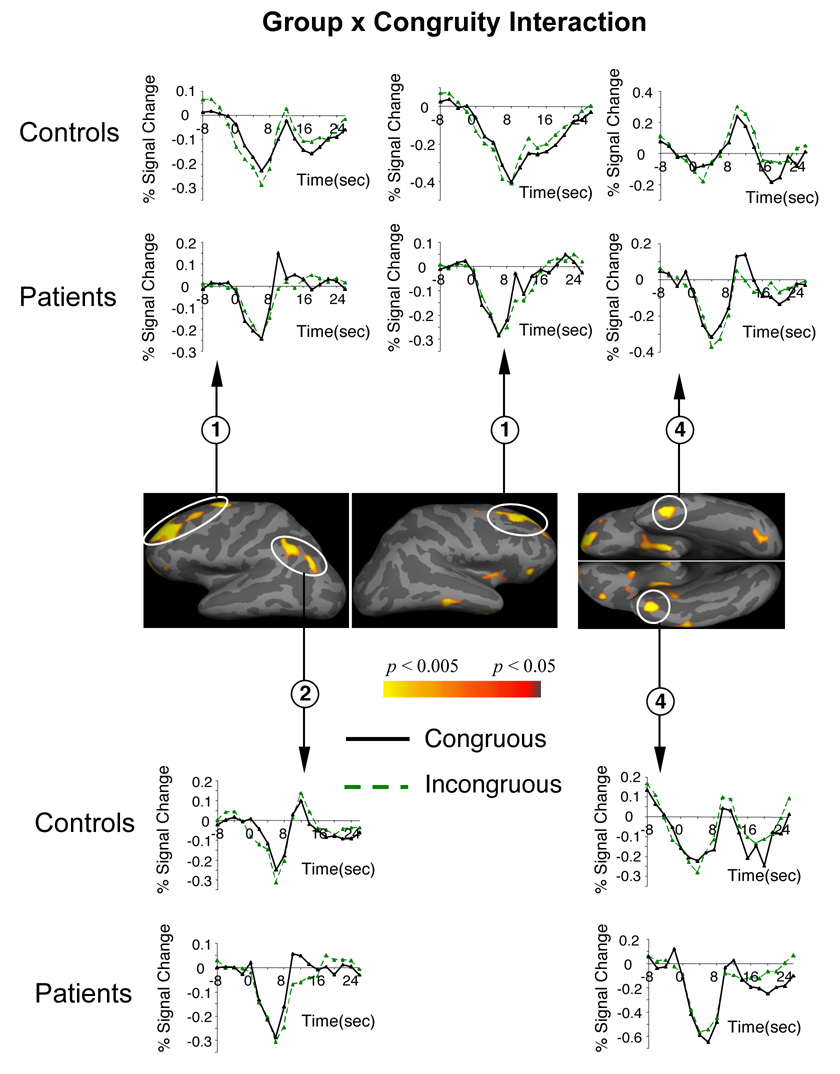
Figure 6. Group by Congruity interactions: areas showing different patterns of modulation between patients and controls in comparing incongruous and congruous sentence-final words.
Hemodynamic time courses show activity in patient and control groups within each of these clusters at each TR (on the time axes, zero refers to the onset of the whole trial).
All clusters circled are significant at a cluster-level p < 0.05. Cluster numbers correspond directly to the regions reported in Table 8.
There was an interaction between Congruity and Concreteness within bilateral medial superior frontal cortices, due to increases in activity to abstract incongruous (versus congruous) sentence-final words, but no such differences in comparing concrete incongruous and congruous sentence-final words, across all participants (Table 9). There were no interactions between Group, Concreteness and Congruity.
Table 9.
Concreteness by Congruity interactions
| Region | Lat. | BA | Area mm2 | Tal. x, y, z | p | Area mm2 | Tal. x, y, z | p | Area mm2 | Tal. x, y, z | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concreteness by Congruity | Concrete: Incongruous vs congruous | Abstract: incongruous vs congruous | ||||||||||
| Superior frontal gyrus (medial) | L | 8/32 | 398 | −8 18 46 | 0.00015 | NS | 737 | −8 18 46 | −0.0000 67 | |||
| R | 476 | 7 23 34 | 0.00035 | NS | 1008 | 8 23 38 | − 0.0000 58 | |||||
All clusters indicated in p column reached cluster-level significance of p < 0.05, corrected across the whole cortex. NS: non-significant.
Correlations with clinical features
As described above, Group interacted independently with (a) Concreteness and (b) Congruity within bilateral superior DLPFC. Within the patient group, the difference in hemodynamic activity between concrete and abstract words within the left DLPFC inversely correlated with both total positive symptoms (Spearman's rho: -0.6, p < 0.04) and total negative symptoms (Spearman's rho: -0.6, p < 0.038), and, within the right DLPFC, inversely correlated with patients’ difficulty in abstract thinking (Spearman's rho: -0.6, p < 0.02). The difference in hemodynamic activity between incongruous and congruous sentences within the left or right DLPFC did not correlate with any symptoms. Medication dosage did not correlate with differences in hemodynamic activity within the DLPFC for either the concrete versus abstract or the incongruous versus congruous comparisons, ps > 0.5.
Discussion
We investigated the neural underpinnings of building meaning from language in schizophrenia by contrasting words within sentences that were (a) concrete versus abstract, and (b) semantically incongruous versus congruous with their preceding contexts. In both these contrasts, widespread temporal-occipital and inferior frontal cortices showed similar patterns of modulation in patients and controls. However, whereas controls recruited bilateral DLPFC (and parietal cortices) to incongruous (versus congruous) sentences, and recruited bilateral DLPFC to concrete (versus abstract) sentences, patients showed the opposite pattern of modulation in both contrasts.
Neuroanatomical dissociations in effects of Concreteness
Both patient and control groups showed more activity to concrete, relative to abstract, sentences within a large, bilateral network distributed across ventromedial temporal, occipitoparietal and orbitofrontal cortices. This replicates previous findings in healthy individuals using single words presented outside a sentence context (4, 5, 55). This network is likely to have reflected the activation of non-verbal ‘imagistic’ representations of concrete words (3) – representations of percepts and affordances that are represented by such words (6, 56). Also, consistent with previous findings in healthy individuals (4, 5, 57), both groups showed more activity to abstract, relative to concrete, sentences words within a more localized left-lateralized network, distributed across inferior frontal and lateral temporal cortices. This suggests that both groups accessed and retrieved verbal representations of abstract words. In both groups, the superior behavioral performance (fewer errors and shorter RTs) in judging the acceptability of concrete, relative to abstract, sentences may have arisen either because participants’ implicit access to imagistic representations of concrete words facilitated processing, or because the increased lexico-semantic retrieval demands of abstract words slowed processing (58).
Controls and patients, however, showed strikingly different patterns of hemodynamic modulation within bilateral superior DLPFC. In controls, the increased DLPFC recruitment to concrete, relative to abstract, sentences is again consistent with previous studies in healthy individuals using words outside a sentence context (4, 5). Its activity may have reflected the demands of integrating verbal and non-verbal representations of meaning that are activated by concrete words (see (59) for evidence for a role of the DLPFC in relational binding), as well as further elaborative semantic processing, such as imagery, of the concrete sentences (7, 8). Such additional integrative and elaborative semantic activity to concrete, relative to abstract, words may contribute to their superior recall in memory paradigms (3) (and, indeed, activity within the DLPFC during encoding predicts the success of later recognizing words that are cued by their corresponding pictures (60)).
In patients, we suggest that the failure to recruit the DLPFC to concrete, relative to abstract, sentences reflected a failure to fully integrate activated verbal and non-verbal representations. Rather, the increased activity within this region to abstract, relative to concrete, sentences may have reflected patients’ relative inefficiency in processing abstract concepts whose meanings are unsupported by imagistic representations. Consistent with this interpretation, patients performed worse than controls in judging the acceptability of the abstract relative to the concrete sentences (Group by Concreteness interactions in accuracy and, less robustly, in RTs) and, within the patient group, the degree of abnormal modulation within the right DLPFC correlated with patients’ clinically-assessed difficulties with abstract thinking.
Neuroanatomical dissociations in effects of Congruity
Both patient and control groups showed increases in activity to all incongruous, relative to all congruous, sentences within bilateral inferior frontal cortices2. Such increases are likely to have reflected increased semantic memory-based processing, i.e. participants’ increased and prolonged efforts to retrieve and match stored semantic material with the semantic relationships between incoming content words within incongruous, relative to congruous, sentences (11–13, 61, 62).
Controls also showed increased recruitment of bilateral superior DLPFC, the left inferior parietal lobule and bilateral medial frontal and medial parietal cortices to the incongruous, relative to the congruous, sentence-final words – a network that we have previously reported as more active to semantic anomalies when semantic-syntactic integration demands are particularly high (13). Although the functional roles of each of these regions are debated (see (13) for a discussion of alternative hypotheses), additional recruitment of this network in the present study can be broadly interpreted as reflecting the increased semantic-syntactic integration and reanalysis demands required to integrate sentence-final incongruities3.
Patients failed to recruit this DLPFC/medial frontal/parietal network to the incongruous sentence-final words (indeed, in some of these regions, activity fell below that to the congruous sentences). We suggest that this failure reflects an impairment in patients’ engagement of additional integrative and reanalysis processes to the sentence-final incongruities – an interpretation that is consistent with ERP studies reporting a normal modulation of waveforms in schizophrenia to semantic incongruities that are detectable through semantic memory-based processing (33, 63, 64), but reduced modulation of components when there are additional demands to integrate semantic and syntactic information ((33–36, 65) and see footnote 1). This interpretation is also consistent with findings of an fMRI study that reported abnormally reduced activity within the DLPFC as patients produced syntactically more complex (versus less complex) sentences (66) – a situation where demands for integrating word meaning and syntactic structure are again increased.
Patients were generally successful in judging the incongruous sentences as unacceptable and the congruous sentences as acceptable. We suggest that their relatively intact behavioral performance was driven by normal semantic memory-based retrieval and matching processes, mediated within inferior frontal cortices; sentence-final words of the incongruous sentences were generally less semantically related to their preceding content words than those of the congruous sentences, explaining why semantic memory-based mechanisms would lead to successful behavioral judgments4. Indeed, there was some indirect evidence that implicit semantic memory-based activity within temporal cortices may have even been increased in patients: consistent with previous studies demonstrating inappropriate recruitment of temporal cortices to indirect and predictable semantic relationships (29, 30), patients showed abnormally increased activity within right-sided middle/inferior temporal and fusiform regions to the congruous, relative to incongruous, sentences. This may have reflected some hyperactivity to semantically-associated congruous sentence-final words. This interpretation, however, remains speculative and future experiments directly manipulating semantic association within sentences should explore this hypothesis further.
Open questions and Conclusions
An open question is whether patients’ abnormal pattern of activity in both contrasts (concrete versus abstract, and incongruous versus congruous sentences) reflected a single neurocognitive deficit. The absence of a three-way interaction between Group, Concreteness and Congruity argues against this hypothesis5. Moreover, whereas reduced activity to concrete (versus abstract) words within the left DLPFC correlated with positive and negative symptom severity (although not medication dosage) within the patient group, this was not true of the contrast between incongruous and congruous sentences, possibly because semantic-syntactic integration and reanalysis was relatively less dependent on this single region.
Nonetheless, it is still possible that an abnormality in a more basic cognitive operation contributed independently to the abnormal modulation of activity seen in each contrast. One obvious candidate for such an operation is the maintenance and/or manipulation of information within working memory – a process long known to be impaired (67), correlated with language comprehension deficits (68, 69), and associated with abnormal DLPFC modulation (37–39, 70, 71) in schizophrenia. There is evidence that working memory operations subserved by the DLPFC play a role in relational binding between activated representations of single concepts (72), providing a mechanism by which a working memory deficit in schizophrenia could contribute the abnormal DLPFC modulation to concrete (versus abstract) words. And, although there has been debate as to whether integration processes during sentence comprehension are language-specific (73), recent evidence in healthy individuals suggests that they may engage at least some domain-general working memory mechanisms (74). Future studies should determine whether DLPFC dysfunction in schizophrenia, indexed using general working memory paradigms, predicts DLPFC dysfunction during the construction of higher-order meaning.
In conclusion, as meaning is built from language in schizophrenia, large networks mediating the retrieval and activation of verbal and imagistic representations are spared (and some may even show increased activity in response to semantically associated material). However, when demands for integrating multiple different activated representations together are increased, patients fail to recruit the DLPFC (and sometimes also medial frontal and lateral/medial parietal cortices). In healthy individuals, semantic memory-based and integrative mechanisms both contribute to language processing and are highly interactive (10). We suggest that abnormalities in schizophrenia are best conceived of as a disturbance in the balance between these mechanisms. As meaning is built from language, patients may be relatively more dependent on semantic memory-based processes at the expense of integrative processes (10). This imbalance may contribute to intrusions of internal semantic representations at the expense of forming accurate representations of meaning of the external world in schizophrenia.
Supplementary Material
Acknowledgments
This research was supported by NIMH (R01 MH071635) and the Institute for Mental Illness and Neuroscience Discovery (MIND). Gina R Kuperberg was also supported by a NARSAD (with the Sidney Baer Trust) and a Claflin Distinguished Scholars Award from Mass. General Hospital. We thank Jordana Cotton, Karin Blais and Alexis Coty for their help with data acquisition, Christine Portal, Kaila Norman and Daphne Holt for help with patient recruitment and assessment, Arim Choi for help with analysis, and Tatiana Sitnikova, Mante Nieuwland and Tali Ditman for their helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
Dr. Goff has received honoraria or research support over the past year from Pfizer Inc., Cephalon, Xenoport, Dainippon Sumitomo, Janssen Pharmaceuticals, Solvay/Wyeth, Eli Lilly, BristolMyerSquibb, Verusmed, Letters and Science, Primedia, SG Cowen, Vista Research, Organon, Proteus, NIH, NARSAD, Sidney Baer Foundation, DOE and Vanda Pharmaceuticals. All other authors reported no biomedical financial interests or potential conflicts of interest.
Such increased semantic-syntactic integration demands may occur when a dominant meaning of a homograph contradicts the incongruous meaning of the entire sentence context (34), when a word is semantically associated with a previous word but the entire context dictates an incongruous meaning (33), or when an incongruous word occurs at the end of a sentence (35, 36) where, during ‘wrap-up’ of final sentence meaning, comprehenders will generally make additional attempts to make sense of a sentence. Note that, depending on the type of word that is to be integrated and its position in the sentence, the abnormally attenuated electrophysiological response in schizophrenia may manifest either as a reduction of the N400 effect (34–36), or of the later P600 effect (33).
In the RT data, there was no main effect of Congruity. This may be because, to reduce the neural effects of the motor response, participants were asked to delay their button presses until the “?” cue appeared after the sentences. Thus, potential RT differences between the incongruous and congruous sentences at the point of the sentence-final word may not have been detected. There was, however, an interaction between Congruity and Concreteness due to longer RTs to incongruous than congruous abstract sentences. Abstract sentences were generally harder to comprehend than concrete sentences (reflected by the main effect of Concreteness), and participants’ acceptability decisions at the point of incongruous abstract sentence-final words are therefore more likely to have occurred after the “?” appeared.
Activity within the left inferior frontal cortex is also likely to mediate semantic-syntactic integration processes in both congruous and incongruous sentences; our assumption here is that more superior frontal and parietal cortices are additionally engaged when integration demands are particularly high on semantically anomalous sentence-final words.
Although such a semantic memory-based mechanism would be successful in interpreting the types of sentences used in this study, such a strategy would be inadequate in other situations where semantic-syntactic integration and reanalysis is necessary for successful interpretation. For example, a pure semantic memory-based analysis would fail to determine that an anomalous sentences containing semantically associated content words (e.g. “At breakfast the eggs would eat…”) is implausible. Indeed, there is some evidence that patients’ accuracy in judging the acceptability of such sentences is selectively impaired (32, 33).
The presence of an interaction between Congruity and Concreteness in the medial superior frontal gyrus, mirroring the behavioral interaction, suggests that some common neurocognitive mechanism may have been engaged in these two contrasts, but this did not differ between patients and controls.
References
- 1.Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg RF, Perfetti CA, Fiez JA, Schneider W. Selective retrieval of abstract semantic knowledge in left prefrontal cortex. J Neurosci. 2007;27(14):3790–3798. doi: 10.1523/JNEUROSCI.2381-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paivio A. Mental Representations. Oxford: Clarendon Press; 1986. [Google Scholar]
- 4.Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage. 2005;27(1):188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA. Distinct brain systems for processing concrete and abstract concepts. J Cogn Neurosci. 2005;17(6):905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- 6.Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11(2):194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 7.Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2(9):635–642. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- 8.West WC, Holcomb PJ. Imaginal, semantic, and surface-level processing of concrete and abstract words: an electrophysiological investigation. J Cogn Neurosci. 2000;12(6):1024–1037. doi: 10.1162/08989290051137558. [DOI] [PubMed] [Google Scholar]
- 9.Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci. 2000;4(12):463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- 10.Kuperberg GR. Neural mechanisms of language comprehension: Challenges to syntax. Brain Res, Special Issue: Mysteries of Meaning. 2007;1146:23–49. doi: 10.1016/j.brainres.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 11.Kuperberg GR, Holcomb PJ, Sitnikova T, Greve D, Dale AM, Caplan D. Distinct patterns of neural modulation during the processing of conceptual and syntactic anomalies. Journal of Cognitive Neuroscience. 2003;15(2):272–293. doi: 10.1162/089892903321208204. [DOI] [PubMed] [Google Scholar]
- 12.Hagoort P, Hald L, Bastiaansen M, Petersson KM. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304(5669):438–441. doi: 10.1126/science.1095455. [DOI] [PubMed] [Google Scholar]
- 13.Kuperberg GR, Sitnikova T, Lakshmanan BM. Neuroanatomical distinctions within the semantic system during sentence comprehension: Evidence from functional magnetic resonance imaging. Neuroimage. 2008;40(1):367–388. doi: 10.1016/j.neuroimage.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen E, West WC, Waters G, Caplan D. Determinants of BOLD signal correlates of processing object-extracted relative clauses. Cortex. 2006;42(4):591–604. doi: 10.1016/s0010-9452(08)70397-6. [DOI] [PubMed] [Google Scholar]
- 15.Caplan D, Chen E, Waters G. Task-dependent and task-independent neurovascular responses to syntactic processing. Cortex. doi: 10.1016/j.cortex.2006.06.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manschreck TC, Maher BA, Milavetz JJ, Ames D, Weisstein CC, Schneyer ML. Semantic priming in thought disordered schizophrenic patients. Schizophrenia Research. 1988;1:61–66. doi: 10.1016/0920-9964(88)90041-2. [DOI] [PubMed] [Google Scholar]
- 17.Spitzer M, Weisker I, Winter M, Maier S, Hermle L, Maher BA. Semantic and phonological priming in schizophrenia. Journal of Abnormal Psychology. 1994;103:485–494. doi: 10.1037//0021-843x.103.3.485. [DOI] [PubMed] [Google Scholar]
- 18.Mathalon DH, Faustman WO. Ford JM. N400 and automatic semantic processing abnormalities in patients with schizophrenia. Archives of General Psychiatry. 2002;59(7):641–648. doi: 10.1001/archpsyc.59.7.641. [DOI] [PubMed] [Google Scholar]
- 19.Kreher DA, Holcomb PJ, Goff D, Kuperberg GR. Increased neural semantic priming in schizophrenic thought disorder: evidence from event-related potentials. Schizophrenia Bulletin. In press. Advance Access published September 28, 2007. [Google Scholar]
- 20.Moritz S, Woodward TS, Kuppers D, Lausen A, Schickel M. Increased automatic spreading of activation in thought-disordered schizophrenic patients. Schizophrenia Research. 2002;59(2–3):181–186. doi: 10.1016/s0920-9964(01)00337-1. [DOI] [PubMed] [Google Scholar]
- 21.Kuperberg GR, Ditman T, Kreher DA, Goldberg T. Approaches to understanding language dysfunction in neuropsychiatric disorders: Insights from the study of schizophrenia. In: Wood S, Allen N, Pantelis C, editors. Handbook of Neuropsychology of Mental Illness. Cambridge University Press; 2008. [Google Scholar]
- 22.Kuperberg GR, Kreher DA, Ditman T. What can event-related potentials tell us about language, and perhaps even thought, in schizophrenia? International Journal of Psychophysiology. Special Issue on Language and Psychophysiology. doi: 10.1016/j.ijpsycho.2009.09.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elvevag B, Heit E, Storms G, Goldberg T. Category content and structure in schizophrenia: an evaluation using the instantiation principle. Neuropsychology. 2005;19(3):371–380. doi: 10.1037/0894-4105.19.3.371. [DOI] [PubMed] [Google Scholar]
- 24.Paul BM, Elvevag B, Bokat CE, Weinberger DR, Goldberg TE. Levels of processing effects on recognition memory in patients with schizophrenia. Schizophr Res. 2005;74(1):101–110. doi: 10.1016/j.schres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Ragland JD, Moelter ST, McGrath C, et al. Levels-of-processing effect on word recognition in schizophrenia. Biol Psychiatry. 2003;54(11):1154–1161. doi: 10.1016/s0006-3223(03)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biol Psychiatry. 2005;58(1):47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ragland JD, Gur RC, Valdez JN, et al. Levels-of-Processing Effect on frontotemporal function in schizophrenia during word encoding and recognition. American Journal of Psychiatry. 2005;162(10):1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf DH, Gur RC, Valdez JN, et al. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Res. 2007;154(3):221–232. doi: 10.1016/j.pscychresns.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuperberg G, Deckersbach T, Holt D, Goff D, West WC. Increased temporal and prefrontal activity to semantic associations in schizophrenia. Archives of General Psychiatry. 2007;64:138–151. doi: 10.1001/archpsyc.64.2.138. [DOI] [PubMed] [Google Scholar]
- 30.Kircher TT, Bulimore ET, Brammer MJ, et al. Differential activation of temporal cortex during sentence completion in schizophrenic patients with and without formal thought disorder. Schizophrenia Research. 2001;50(1–2):27–40. doi: 10.1016/s0920-9964(00)00042-6. [DOI] [PubMed] [Google Scholar]
- 31.Barch DM, Cohen JD, Servan-Schreiber D, Steingard S, Steinhauer S, van Kammen D. Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. Journal of Abnormal Psychology. 1996;105:592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- 32.Kuperberg GR, Kreher DA, Goff D, McGuire PK, David AS. Building up linguistic context in schizophrenia: evidence from self-paced reading. Neuropsychology. 2006;20(4):442–452. doi: 10.1037/0894-4105.20.4.442. [DOI] [PubMed] [Google Scholar]
- 33.Kuperberg GR, Sitnikova T, Goff D, Holcomb PJ. Making sense of sentences in schizophrenia: electrophysiological evidence for abnormal interactions between semantic and syntactic processing. Journal of Abnorm Psychol. 2006;115(2):243–256. doi: 10.1037/0021-843X.115.2.251. [DOI] [PubMed] [Google Scholar]
- 34.Sitnikova T, Salisbury DF, Kuperberg G, Holocomb PI. Electrophysiological insights into language processing in schizophrenia. Psychophysiology. 2002;39(6):851–860. doi: 10.1111/1469-8986.3960851. [DOI] [PubMed] [Google Scholar]
- 35.Adams J, Faux SF, Nestor PG, et al. ERP abnormalities during semantic processing in schizophrenia. Schizophr Res. 1993;10(3):247–257. doi: 10.1016/0920-9964(93)90059-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell PF, Andrews S, Fox AM, Catts SV, Ward PB, McConaghy N. Active and passive attention in schizophrenia: An ERP study of information processing in a linguistic task. Biological Psychiatry. 1991;32:101–124. doi: 10.1016/0301-0511(91)90004-z. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald AW, 3rd, Carter CS, Kerns JG, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162(3):475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 38.Holmes AJ, MacDonald A, 3rd, Carter CS, Barch DM, Andrew Stenger V, Cohen JD. Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophr Res. 2005;76(2–3):199–206. doi: 10.1016/j.schres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald AW, 3rd, Carter CS. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J Abnorm Psychol. 2003;112(4):689–697. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- 40.Holcomb PJ, Kounios J, Anderson JE, West WC. Dual-coding, context-availability, and concreteness effects in sentence comprehension: an electrophysiological investigation. J Exp Psychol Learn Mem Cogn. 1999;25(3):721–742. doi: 10.1037//0278-7393.25.3.721. [DOI] [PubMed] [Google Scholar]
- 41.DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th Rev ed. American Psychiatric Press: Washington, DC; 1990. [Google Scholar]
- 42.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID) I: History, rationale and description. Archives of General Psychiatry. 1992;49:642–649. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 43.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 44.White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14(2):261–264. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- 45.Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9(16):3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- 46.Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 47.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 48.Fischl B, Liu A, Dale AM. Automated Manifold Surgery: Constructing Geometrically Accurate and Topologically Correct Models of the Human Cerebral Cortex. IEEE Transactions on Medical Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 49.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 50.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance Medicine. 1999;42(6):1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 52.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 53.Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 54.Doherty CP, West WC, Dilley LC, Shattuck-Hufnagel S, Caplan D. Question/statement judgments: an fMRI study of intonation processing. Human Brain Mapping. 2004;23(2):85–98. doi: 10.1002/hbm.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiebach CJ, Friederici AD. Processing concrete words: fMRI evidence against a specific right-hemisphere involvement. Neuropsychologia. 2004;42(1):62–70. doi: 10.1016/s0028-3932(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 56.Barsalou LW. Perceptual symbol systems. Behav Brain Sci. 1999;22(4):577–609. doi: 10.1017/s0140525x99002149. discussion 10–60. [DOI] [PubMed] [Google Scholar]
- 57.Noppeney U, Price CJ. Retrieval of abstract semantics. Neuroimage. 2004;22(1):164–170. doi: 10.1016/j.neuroimage.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Kroll JF, Merve JS. Lexical access for concrete and abstract words. Journal of Experimental Psychology Learning, Memory and Cognition. 1986;12:92–107. [Google Scholar]
- 59.Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci. 2007;27(20):5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park H, Rugg MD. The Relationship between Study Processing and the Effects of Cue Congruency at Retrieval: fMRI Support for Transfer Appropriate Processing. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm130. [DOI] [PubMed] [Google Scholar]
- 61.Beeman M, Friedman RB, Grafman J, Perez E, Diamond S, Lindsay MB. Summation priming and coarse coding in the right hemisphere. J Cogn Neurosci. 1994;6:26–45. doi: 10.1162/jocn.1994.6.1.26. [DOI] [PubMed] [Google Scholar]
- 62.Faust M, Lavidor M. Semantically convergent and semantically divergent priming in the cerebral hemispheres: lexical decision and semantic judgment. Cogn Brain Res. 2003;17(3):585–597. doi: 10.1016/s0926-6410(03)00172-1. [DOI] [PubMed] [Google Scholar]
- 63.Niznikiewicz MA, O'Donnell BF, Nestor PG, et al. ERP assessment of visual and auditory language processing in schizophrenia. Journal of Abnormal Psychology. 1997;106:85–94. doi: 10.1037//0021-843x.106.1.85. [DOI] [PubMed] [Google Scholar]
- 64.Ruchsow M, Trippel N, Groen G, Spitzer M, Kiefer M. Semantic and syntactic processes during sentence comprehension in patients with schizophrenia: evidence from event-related potentials. Schizophr Res. 2003;64(2–3):147–156. doi: 10.1016/s0920-9964(02)00482-6. [DOI] [PubMed] [Google Scholar]
- 65.Ohta K, Uchiyama M, Matsushima E, Toru M. An event-related potential study in schizophrenia using Japanese sentences. Schizophr Res. 1999;40(2):159–170. doi: 10.1016/s0920-9964(99)00048-1. [DOI] [PubMed] [Google Scholar]
- 66.Kircher TT, Oh TM, Brammer MJ, McGuire PK. Neural correlates of syntax production in schizophrenia. Br J Psychiatry. 2005;186:209–214. doi: 10.1192/bjp.186.3.209. [DOI] [PubMed] [Google Scholar]
- 67.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 68.Bagner DM, Melinder MR, Barch DM. Language comprehension and working memory language comprehension and working memory deficits in patients with schizophrenia. Schizophr Res. 2003;60(2–3):299–309. doi: 10.1016/s0920-9964(02)00280-3. [DOI] [PubMed] [Google Scholar]
- 69.Condray R, Steinhauer SR, van Kammen DP, Kasparek A. Working memory capacity predicts language comprehension in schizophrenic patients. Schizophr Res. 1996;20(1–2):1–13. doi: 10.1016/0920-9964(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 70.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60(2–3):285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 71.Barch DM, Sheline YI, Csernansky JG, Snyder AZ. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry. 2003;53(5):376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- 72.Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26(3):916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caplan D, Waters GS. Verbal working memory and sentence comprehension. Behav Brain Sci. 1999;22(1):77–94. doi: 10.1017/s0140525x99001788. discussion 5–126. [DOI] [PubMed] [Google Scholar]
- 74.Fedorenko E, Gibson E, Rohde D. The nature of working memory capacity in sentence comprehension. Journal of Memory and Language. 2006;54:541–553. [Google Scholar]
- 75.Kucera H, Francis WN. Computational Analysis of Present Day American English. 1967 [Google Scholar]
- 76.Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- 77.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychologist. 1989:129–136. [Google Scholar]
- 78.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(12):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.