Abstract
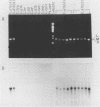
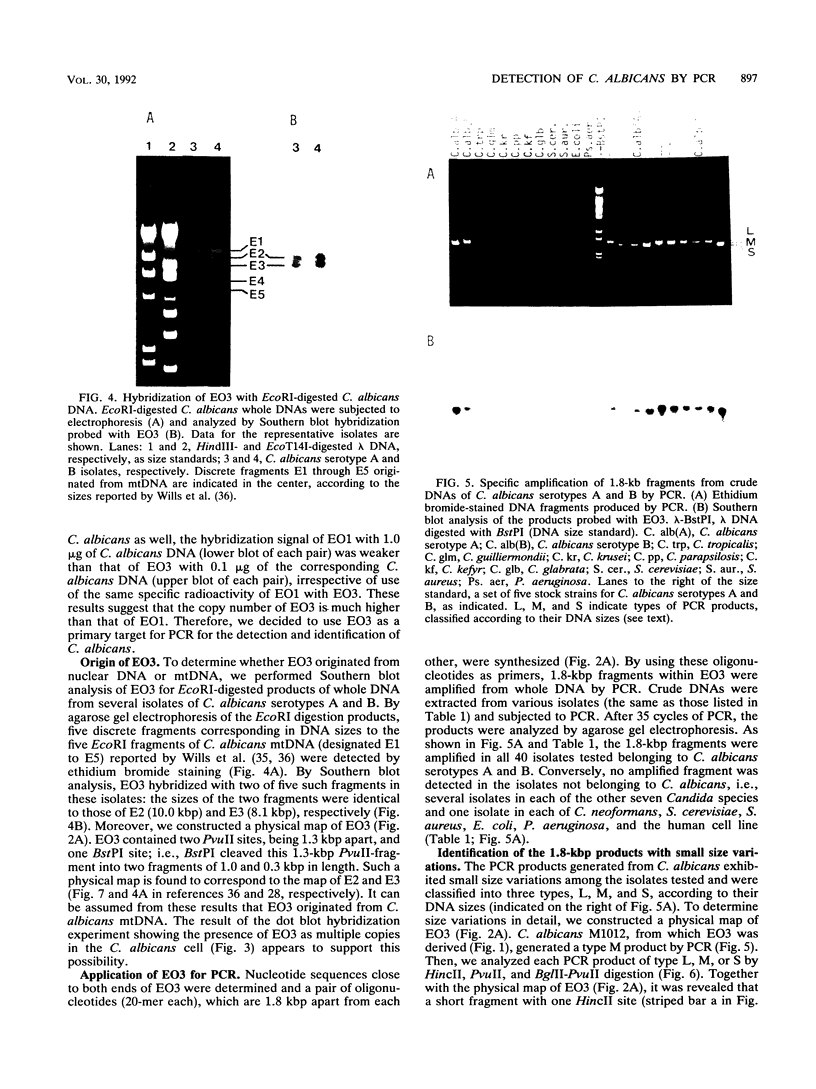
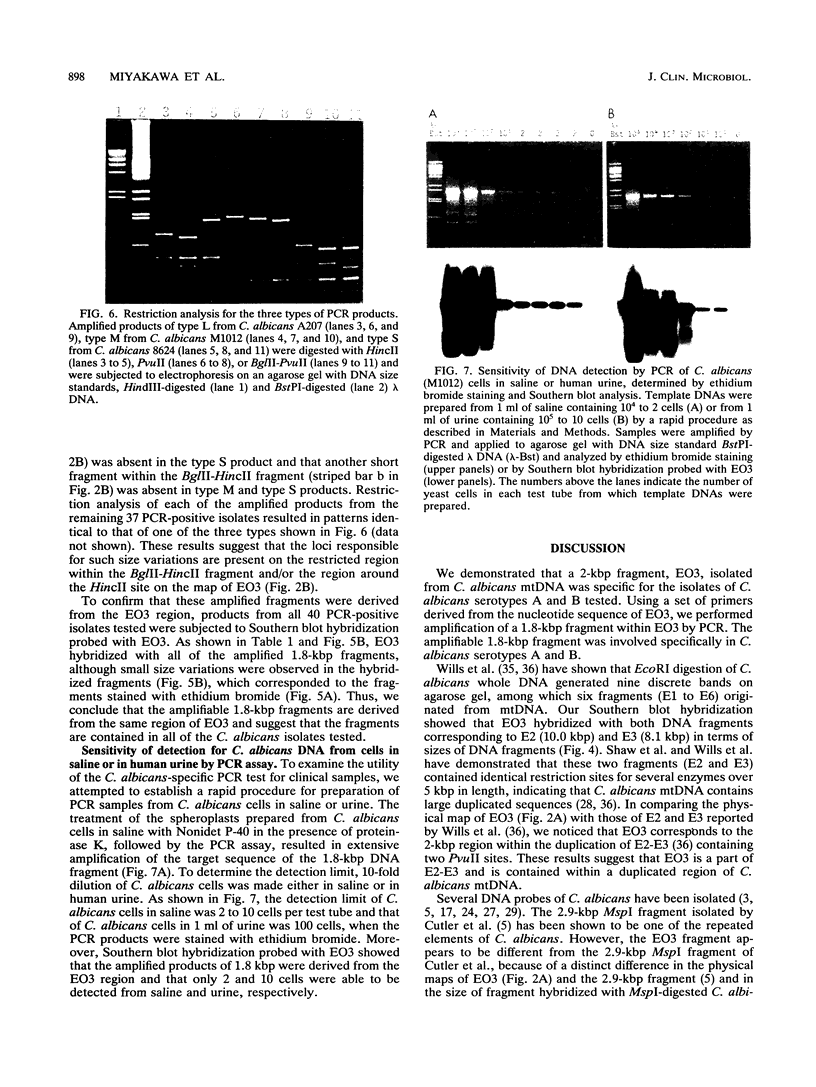
A 2-kbp DNA fragment, EO3, that was present in multiple copies in the Candida albicans genome was isolated for use in developing a detection method for C. albicans by polymerase chain reaction (PCR). Dot blot hybridization revealed that EO3 was specific for the 40 isolates of C. albicans serotypes A and B used. Using a set of primers (20-mer each) derived from the nucleotide sequence of EO3, we performed specific amplification of a 1.8-kbp DNA fragment within EO3 by PCR. All 40 isolates belonging to C. albicans serotypes A and B contained amplifiable 1.8-bkp fragments, although the DNA of the amplified products exhibited small variations in size, yielding three different fragment groups. Southern blot hybridization probed with EO3 showed that these 1.8-kbp fragments were derived from the EO3 region. Conversely, the 1.8-kbp fragment was not amplified from 38 isolates belonging to seven other medically important Candida species or from isolates of Cryptococcus neoformans, Saccharomyces cerevisiae, various bacteria, and a human cell line. The detection limit of the PCR assay for C. albicans with the EO3 fragment was shown to be approximately 2 to 10 cells and 100 cells in saline and human urine, respectively, by ethidium bromide staining and 2 and 10 cells, respectively, by Southern blot analysis. In addition, EO3 was assumed to originate from mitochondrial DNA on the basis of the results of its characterizations. These results indicate that the PCR system using the 1.8-kbp fragment as a target is a reliable method for identifying C. albicans isolates, thereby suggesting its potentials for specific and sensitive detection of C. albicans in samples from patients with candidiasis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D., Trombley C., Cimolai N. Assessment of the BACTEC NR660 blood culture system for the detection of bacteremia in young children. J Clin Microbiol. 1989 Apr;27(4):721–723. doi: 10.1128/jcm.27.4.721-723.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougnoux M. E., Hill C., Moissenet D., Feuilhade de Chauvin M., Bonnay M., Vicens-Sprauel I., Pietri F., McNeil M., Kaufman L., Dupouy-Camet J. Comparison of antibody, antigen, and metabolite assays for hospitalized patients with disseminated or peripheral candidiasis. J Clin Microbiol. 1990 May;28(5):905–909. doi: 10.1128/jcm.28.5.905-909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung L. L., Hudson J. B. Development of DNA probes for Candida albicans. Diagn Microbiol Infect Dis. 1988 Jul;10(3):171–179. doi: 10.1016/0732-8893(88)90037-5. [DOI] [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- Cutler J. E., Glee P. M., Horn H. L. Candida albicans- and Candida stellatoidea-specific DNA fragment. J Clin Microbiol. 1988 Sep;26(9):1720–1724. doi: 10.1128/jcm.26.9.1720-1724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. C., Mobley H. L., Wade J. C. The use of a DNA probe for epidemiological studies of candidiasis in immunocompromised hosts. J Infect Dis. 1989 Mar;159(3):488–494. doi: 10.1093/infdis/159.3.488. [DOI] [PubMed] [Google Scholar]
- Hermans P. W., Schuitema A. R., Van Soolingen D., Verstynen C. P., Bik E. M., Thole J. E., Kolk A. H., van Embden J. D. Specific detection of Mycobacterium tuberculosis complex strains by polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1204–1213. doi: 10.1128/jcm.28.6.1204-1213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya K., Miyakawa Y., Fujihara H., Suzuki M., Soe G., Fukazawa Y. Immunologic significance of diverse specificity of monoclonal antibodies against mannans of Candida albicans. J Immunol. 1989 Nov 15;143(10):3353–3358. [PubMed] [Google Scholar]
- Kagaya K., Yamada T., Miyakawa Y., Fukazawa Y., Saito S. Characterization of pathogenic constituents of Cryptococcus neoformans strains. Microbiol Immunol. 1985;29(6):517–532. doi: 10.1111/j.1348-0421.1985.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Magee B. B., D'Souza T. M., Magee P. T. Strain and species identification by restriction fragment length polymorphisms in the ribosomal DNA repeat of Candida species. J Bacteriol. 1987 Apr;169(4):1639–1643. doi: 10.1128/jb.169.4.1639-1643.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. M., Lasker B. A., Riggsby W. S. Molecular probe for identification of medically important Candida species and Torulopsis glabrata. J Clin Microbiol. 1987 Mar;25(3):563–566. doi: 10.1128/jcm.25.3.563-566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa Y., Kagaya K., Fukazawa Y., Soe G. Production and characterization of agglutinating monoclonal antibodies against predominant antigenic factors for Candida albicans. J Clin Microbiol. 1986 May;23(5):881–886. doi: 10.1128/jcm.23.5.881-886.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa Y., Kagaya K., Kuribayashi T., Suzuki M., Fukazawa Y. Isolation and chemical and biological characterization of antigenic mutants of Candida albicans serotype A. Yeast. 1989 Apr;5(Spec No):S225–S229. [PubMed] [Google Scholar]
- Murray P. R., Spizzo A. W., Niles A. C. Clinical comparison of the recoveries of bloodstream pathogens in Septi-Chek brain heart infusion broth with saponin, Septi-Chek tryptic soy broth, and the isolator lysis-centrifugation system. J Clin Microbiol. 1991 May;29(5):901–905. doi: 10.1128/jcm.29.5.901-905.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivo P. D., McManus E. J., Riggsby W. S., Jones J. M. Mitochondrial DNA polymorphism in Candida albicans. J Infect Dis. 1987 Jul;156(1):214–215. doi: 10.1093/infdis/156.1.214. [DOI] [PubMed] [Google Scholar]
- Restrepo B. I., Barbour A. G. Cloning of 18S and 25S rDNAs from the pathogenic fungus Cryptococcus neoformans. J Bacteriol. 1989 Oct;171(10):5596–5600. doi: 10.1128/jb.171.10.5596-5600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhu C., McEachern M. J., Rustchenko-Bulgac E. P., Schmid J., Soll D. R., Hicks J. B. Telomeric and dispersed repeat sequences in Candida yeasts and their use in strain identification. J Bacteriol. 1991 Jan;173(2):842–850. doi: 10.1128/jb.173.2.842-850.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. A Candida albicans dispersed, repeated gene family and its epidemiologic applications. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1452–1456. doi: 10.1073/pnas.85.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. A., Troutman W. B., Lasker B. A., Mason M. M., Riggsby W. S. Characterization of the inverted duplication in the mitochondrial DNA of Candida albicans. J Bacteriol. 1989 Nov;171(11):6353–6356. doi: 10.1128/jb.171.11.6353-6356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Staebell M., Langtimm C., Pfaller M., Hicks J., Rao T. V. Multiple Candida strains in the course of a single systemic infection. J Clin Microbiol. 1988 Aug;26(8):1448–1459. doi: 10.1128/jcm.26.8.1448-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Insertion of a genetic marker into the ribosomal DNA of yeast. Plasmid. 1979 Oct;2(4):536–554. doi: 10.1016/0147-619x(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Torres-Bauzá L. J., Riggsby W. S. Protoplasts from yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1980 Aug;119(2):341–349. doi: 10.1099/00221287-119-2-341. [DOI] [PubMed] [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990 Aug;172(8):4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Lasker B. A., Sirotkin K., Riggsby W. S. Repetitive DNA of Candida albicans: nuclear and mitochondrial components. J Bacteriol. 1984 Mar;157(3):918–924. doi: 10.1128/jb.157.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Troutman W. B., Riggsby W. S. Circular mitochondrial genome of Candida albicans contains a large inverted duplication. J Bacteriol. 1985 Oct;164(1):7–13. doi: 10.1128/jb.164.1.7-13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]