Abstract
The proteolytic activation of pro-matrix metalloproteinase (MMP)-9 by conversion of the 92-kDa precursor into an 82-kDa active form has been observed in chronic wounds, tumor metastasis, and many inflammation-associated diseases, yet the mechanistic pathway to control this process has not been identified. In this report, we show that the massive expression and activation of MMP-9 in skin tissue from patients with chronically unhealed wounds could be reconstituted in vitro with cultured normal human skin by stimulation with transforming growth factor-β and tumor necrosis factor (TNF)-α. We dissected the mechanistic pathway for TNF-α induced activation of pro-MMP-9 in human skin. We found that proteolytic activation of pro-MMP-9 was mediated by a tissue-associated chymotrypsin-like proteinase, designated here as pro-MMP-9 activator (pM9A). This unidentified activator specifically converted pro-MMP-9 but not pro-MMP-2, another member of the gelatinase family. The tissue-bound pM9A was steadily expressed and not regulated by TNF-α, which indicated that the cytokine-mediated activation of pro-MMP-9 might be regulated at the inhibitor level. Indeed, the skin constantly secreted tissue inhibitor of metalloproteinase-1 at the basal state. TNF-α, but not transforming growth factor-β, down-regulated this inhibitor. The TNF-α-mediated activation of pro-MMP-9 was tightly associated with down-regulation of tissue inhibitor of metalloproteinase-1 in a dose-dependent manner. To establish this linkage, we demonstrate that the recombinant tissue inhibitor of metalloproteinase-1 could block the activation of pro-MMP-9 by either the intact skin or skin fractions. Thus, these studies suggest a novel regulation for the proteolytic activation of MMP-9 in human tissue, which is mediated by tissue-bound activator and controlled by down-regulation of a specific inhibitor.
Matrix metalloproteinases (MMPs)1 are essential for remodeling of the extracellular matrix in physiologic and pathologic conditions (1, 2). Within the MMP family, MMP-9 (gelatinase B; EC 3.4.24.35) is particularly important, because it has a documented role in many human diseases. Like most MMPs, MMP-9 is secreted as a latent zymogen that is maintained by the interaction between a cysteine in the N-terminal pro-domain and the active-site zinc atom. Proteolytic cleavage of the pro-domain is a common control mechanism for MMP activation, which triggers a conformational change of the enzyme and is termed the “cysteine switch” (3). The conversion of pro-MMP-9 with an apparent molecular mass of 92-kDa to the 82-kDa active MMP-9 has been commonly observed in the pathogenesis of many diseases (4).
Metastasis is a multistep process, including detachment of cancer cells from a primary site, invasion into surrounding tissue through breakdown matrix, spreading through circulation, and proliferation in distant organs. Breakdown of the basement membrane zone (BMZ) is necessary to allow malignant cells to migrate from the primary location. MMP-9 is not normally expressed in developed tissues, yet it is highly expressed in many cancers (5–7). During the early phase of breast cancer, only pro-MMP-9 is increased. As the cancer stage increases, evidenced by skin invasion or lymphovascular permeation, active MMP-9 is found (8). Similarly, sequential expression and activation of pro-MMP-9 has been observed in liver metastasis (9). An interesting pattern of MMP-9 expression has also been documented in wound healing. In the normal repair of acute trauma or burn wound, pro-MMP-9 is transiently expressed and declines with the progress of healing (10, 11). On the other hand, persistent expression of a larger amount of MMP-9, especially the active 82-kDa MMP-9, has been repeatedly documented in chronic wounds (12–14). Given the proteolytic function of MMP-9 in the digestion of BMZ components such as type IV collagen, the pathogenesis of these diseases could be due to the activity of MMP-9.
A critical unanswered question regarding MMP-associated disease pathogenesis is the identification of specific factors controlling the expression and activation of the proteinase. MMP activities are regulated at multiple levels from their expression to activation by cleavage of the inhibitory domain as well as blockage by tissue inhibitors. Although the proteolytic activation of MMP-9 has been well documented in many diseases, the mechanism for the proteolytic conversion is not established. In this report, we show for the first time that the increased expression and activation of MMP-9 found in patients with chronic wounds could be reconstituted in cultured normal human skin by treatment with specific cytokines. Furthermore, we found that activation of pro-MMP-9 was mediated by a tissue-associated chymotrypsin-like proteinase. The TNF-α-mediated activation of pro-MMP-9 is controlled by down-regulation of the MMP inhibitor, TIMP-1. These findings provide a novel model to explain the MMP-9 activation in the pathogenesis of chronic wounds and tumor metastasis through the action of specific proinflammatory cytokines.
EXPERIMENTAL PROCEDURES
Materials and Reagents
Cytokines were purchased from R & D Systems (Minneapolis, MN). The antibodies against TIMP-1 (MAB13437) and the purified recombinant TIMP-1 protein (CC3328) were purchased from Chemicon International (Temecula, CA). The antibody for mast cell chymase (MS-1217) was from NeoMarkers (Fremont, CA). Aprotinin, L-1-chloro-3-(4-tosylamido)-7-amino-2-heptanone-HCl (TLCK), and L-1-chloro-3-(4-tosylamido)-4-phenyl-2-butanone (TPCK) were purchased from Roche Molecular Biochemicals. MMP-3 inhibitor II (N-isobutyl-n-(4-methoxyphenylsulfonyl)-glycylhydroxamic acid) was from Calbiochem. The Immobilon-P was purchased from Millipore Corp. (Bedford, MA). ECL was purchased from Amersham Biosciences. The gelatin was from Sigma. Gelatin-Sepharose 4B was purchased from Amersham Biosciences AB (Uppsala, Sweden).
Organ Culture and Cytokine Stimulation of Human Skin
Normal human skin was obtained as discarded tissue from patients undergoing reconstructive or aesthetic surgery (University of Southern California Internal Review Board no. 999061). The full thickness skin was decontaminated by incubation in DMEM containing 2× antibiotic (200 units/ml penicillin G sodium, 200 units/ml streptomycin sulfate, and 0.5 mg/ml amphotericin B) at 4 °C overnight before all subsequent procedures. Then the skin was cut into squares of equal sizes with 0.5 cm in each side and incubated in DMEM at 37 °C with 5% CO2 for 6 h. To decrease the effects of endogenous soluble factors in the skin induced by the harvesting process, the medium was changed three times during the 6-h incubation. Finally, the explant was floated in 2 ml of DMEM with specific cytokines and was maintained at 37 °C with 5% CO2.
Preparation and Culture of Human Dermal Fibroblasts and Keratinocytes
Dermal fibroblasts and keratinocytes were isolated from normal full thickness or partial thickness human skin (15, 16). The isolated fibroblasts were cultivated in DMEM containing 10% fetal bovine serum and with penicillin (100 units/ml) and streptomycin sulfate (100 µg/ml). The keratinocytes were cultivated with complete keratinocyte growth medium. Before exposure to cytokines, the medium was replaced with serum- and antibiotic-free DMEM for fibroblasts and keratinocyte basal medium for keratinocytes.
Preparation of Pro-MMP-9
The transformed human keratinocytes (kindly provided by Dr. David T. Woodley at USC) were grown in keratinocyte growth medium to confluence. The cells were treated by 2 ng/ml TNF-α in keratinocyte basal medium for 72 h in standard culture condition. In this condition, most of the gelatinase secreted in the medium is the 92-kDa pro-MMP-9. The conditioned medium from 20 10-cm dishes was collected and cleared by centrifugation at 4000 × g. The conditioned medium was then passed to a 5-ml gelatin-Sepharose 4B column followed by washing with 400 mm NaCl, 0.5% Triton X-100 in 50 mm Tris, pH 7.5. The bound gelatinase was eluted by 6 m urea followed by dialysis against buffer containing 100 mm NaCl and 50 mm Tris at pH 7.5. The identity of pro-MMP-9 was confirmed by Western blot with antibody against MMP-9 as reported previously (17).
Preparation of Pro-MMP-2
Human dermal fibroblasts were grown as monolayers in DMEM with 10% fetal bovine serum. To generate pro-MMP-2, the medium was replaced by serum-free DMEM. After culturing for 74 h, the conditioned medium was cleared by centrifugation. The gelatinase was purified by gelatin-Sepharose 4B as described above. In this preparation, most of the gelatinase is pro-MMP-2 (18). The identity was confirmed by Western blot with antibody against MMP-2.
Assay of Tumor Necrosis Factor from Skin Biopsies
6-mm punch biopsies were taken from each patient at the site of an unhealed burn wound, healed wound, and normal skin (University of Southern California Internal Review Board no. 999061). The skin biopsies were briefly washed by phosphate-buffered saline and immersed in 0.5 ml of DMEM with proper antibiotics. The tissues were incubated at 37 °C with 5% CO2 for 18 h. The conditioned medium and tissue were stored at −80 °C before assay. Bioactivity of TNF-α was determined as previously described (15, 19).
Extraction of Pro-MMP-9 Activator (pM9A) from Human Skin
The skin tissue was minced and ground in liquid nitrogen by a mechanic miller. The skin powder was washed by NT buffer containing 100 mm NaCl and 50 mm Tris at pH 7.5. Then the tissue powder was extracted by 2.5% Triton X-100, 2 m NaCl, and 6 m urea, respectively. The extracts were subjected to dialysis against NT buffer, and the insoluble fractions were washed using the same buffer.
pM9A Activity Assay
For each assay, 80 µl of tissue extracts were incubated with 15 µl of pro-MMP-9 together with 5 µl of 100 mm CaCl2. The reaction was carried at 37 °C for 16 h followed by gelatinolytic zymogram analysis. For the inhibition experiments, 2.5 µl of inhibitors and 2.5 µl of 200 mm CaCl2 were added to the system before incubation.
Gelatinolytic Zymogram
The conditioned medium was mixed with SDS-PAGE sample buffer in the absence of reducing agent and electro-phoresed in 10% polyacrylamide gel containing 0.1% (w/v) gelatin. Electrophoresis was carried out at 4 °C with 120 V for 16 h. After electrophoresis, SDS in the gel was removed by incubation with 2.5% Triton X-100. Gelatinolytic activities were developed in buffer containing 5 mm CaCl2, 150 mm NaCl, and 50 mm Tris at pH 7.5 for 16 h at 37 °C. The gelatinolytic activities were visualized by staining with Coomassie Blue R-250.
Western Blot
The conditioned medium or skin extracts were resolved by reducing SDS-PAGE (15%). The protein was transferred to Immobilon-P (Millipore Corp.). The membrane was exposed to antibodies against human TIMP-1 or mast cell chymase followed by blot with peroxidase-conjugated secondary antibodies, which were subsequently detected by enhanced chemiluminescence.
RESULTS
The Excessive Expression and Activation of MMP-9 in the Tissue from Patients with Chronic Unhealed Wounds Can Be Reconstituted by Cultured Human Skin with Specific Cytokines
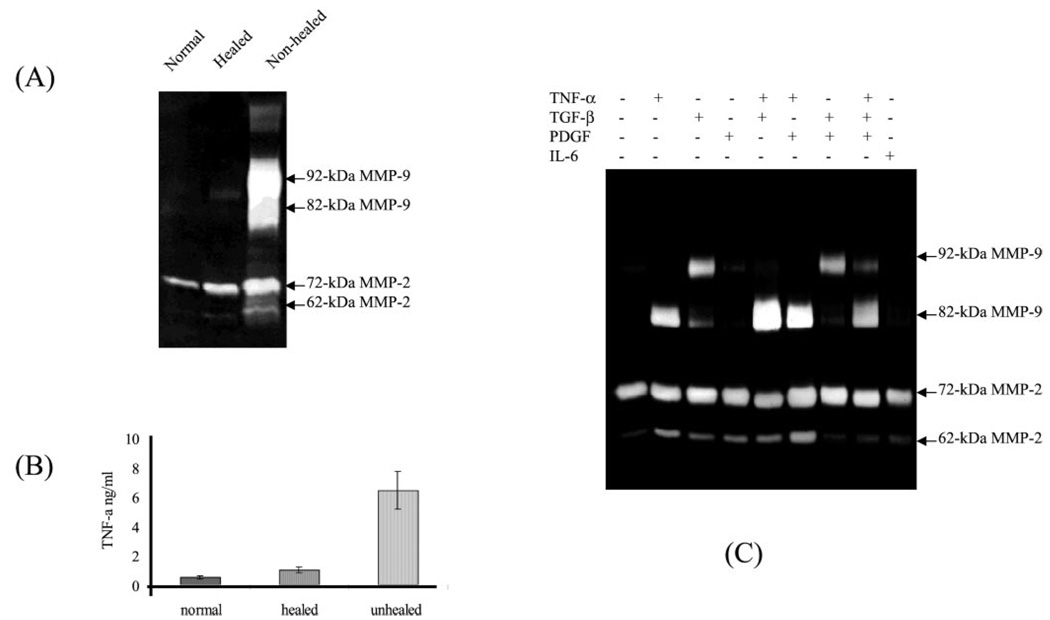
Massive expression and activation of MMP-9 have been extensively reported in the tissue fluid of chronic wounds (12–14). To find the causal factors that induce expression and activation of MMP-9, we measured the gelatinase profile and cytokine concentration from the tissue of patients’ unhealed wounds, and then we attempted to reconstitute the results in vitro by using cultured normal human skin. Here we show a typical gelatinase profile from a patient with a chronic wound. Biopsies from the unhealed wound, healed wound, and the surrounding normal skin were taken from a 19-year-old patient 60 days after partial graft loss during treatment for a thermal burn. The unhealed wound was a small area of graft loss (<2 cm2) that would normally be expected to heal within 2–3 weeks. The biopsies were immersed in DMEM with antibiotics and incubated at culture condition for 6 h. This short term culture was designed to allow the diffusion of the soluble factors produced by the tissue. The conditioned medium was resolved by a gelatinolytic zymogram. As shown in Fig. 1, substantial gelatinase activities with apparent molecular masses of 92 and 82 kDa, representing the pro-MMP-9 and active MMP-9, respectively, were found. Conversely, little gelatinase activities were secreted from the normal skin and healed wound.
FIG. 1. The expression and activation of pro-MMP-9 in chronic patients can be reconstituted by cultured human skin with specific cytokines.
A, biopsies from unhealed wound, healed wound, and surrounding normal skin were taken from a 19-year-old patient 60 days after partial graft loss during treatment for a thermal burn. The biopsies were immersed in 0.5 ml of DMEM with antibiotics and incubated for 6 h. The conditioned medium was resolved by gelatinolytic zymogram. B, TNF-α level in biopsies from normal skin and healed and chronic wounds was measured as described under “Experimental Procedures.” C, the cytokine-induced expression and activation of MMP-9 by human skin. Normal human skin was cultured in 2 ml of DMEM with cytokines as indicated for 64 h. The gelatinase activities from the conditioned medium were analyzed by gelatin zymogram.
To identify the potential factors that cause the expression and activation of MMPs in human skin, we initially tested a panel of cytokines that have been found elevated in chronic wounds and at metastatic tumor sites. Single or combinations of cytokines were added to normal human full thickness skin floating in DMEM (1 ng/ml for TGF-β and 10 ng/ml for the other cytokines). After culture for 64 h, the conditioned media were resolved by zymogram. As shown in Fig. 1, among this panel of cytokines only TGF-β induces the expression of 92-kDa pro-MMP-9, and only TNF-α promotes the formation of 82-kDa MMP-9. The TNF-α -mediated formation of 82-kDa MMP-9 is sequentially processed by induction of the 92-kDa form followed by conversion to the 82-kDa form (17). As expected, combination of TNF-α with TGF-β leads to greater expression and activation of MMP-9 than either factor alone. Other cytokines, such as platelet-derived growth factor, interleukin-6, and interleukin-8 (data not shown), were without effect alone or in combination. The identity of 92- and 82-kDa MMP-9 were confirmed by Western blot as demonstrated in our previous report (17). To confirm this result, we measured the cytokine levels produced by biopsies of chronically unhealed wound, healed wound, and normal skin tissues. In a comparison of unhealed, healed, and normal skin in nine patients, the TNF-α level was found to be significantly higher in chronic wound tissue (Fig. 1). The concentration of TNF-α found in chronic wound tissue (6 ± 2 ng/ml) is comparable with the cytokine concentration necessary to promote the proteolytic conversion of pro-MMP-9 in normal skin. Taken together, we identified TGF-β and TNF-α as causal factors for induction and activation of MMP-9, respectively in chronic wounds. These results indicate that the induction and activation of MMP-9 observed in chronic wounds and tumors are probably mediated through these cytokines.
Human Skin Steadily Expresses the Pro-MMP-9 Activator, Which Is Not Regulated by TNF-α
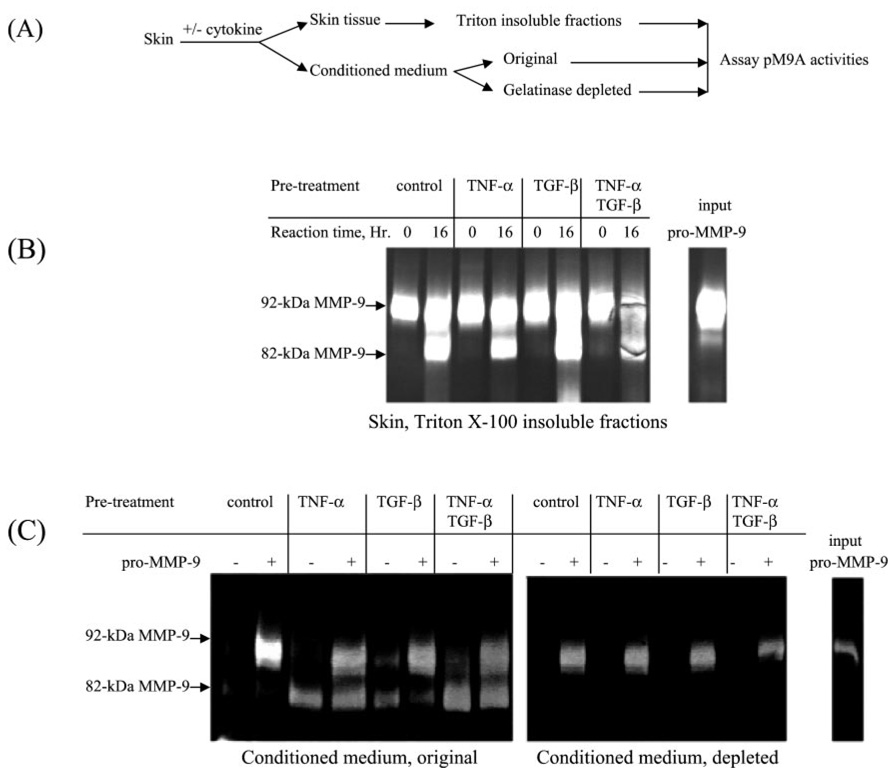
Initially, we thought that the TNF-α-mediated activation of pro-MMP-9 might be regulated through the increase of pM9A activity, perhaps through an increase in synthesis of pM9A. To address this question, we performed the following two experiments. In the first experiment, we examined whether pM9A activity was enhanced by cytokines. Normal human skin was stimulated with TNF-α and TGF-β individually or simultaneously. After culturing for 64 h, the conditioned medium and skin tissue were separated. The skin tissues were washed by phosphate-buffered saline and ground in liquid nitrogen. The tissue powder was then extracted by 2% Triton X-100 for 16 h, and the resulting insoluble fractions were further washed to remove the detergent. To assay pM9A activities, purified pro-MMP-9 was incubated with the skin fractions for 16 h followed by zymogram analysis. As shown in Fig. 2A, 92-kDa pro-MMP-9 was converted to the 82-kDa form by the tissue fractions under all conditions. Notably, TNF-α did not increase the pM9A activity.
FIG. 2. Human skin-associated pM9A is not regulated by TNF-α.
A, a scheme for the experiment design. Normal human skin was stimulated by TNF-α and TGF-β individually or simultaneously. After culture for 64 h, the conditioned medium and skin tissue were separated. B, the skin tissues were washed and then ground in liquid nitrogen. The resulting tissue powder was then extracted by 2% Triton X-100 followed by separation into soluble and insoluble fractions. To assay pM9A activities, purified pro-MMP-9 was incubated with these insoluble fractions followed by zymogram analysis. C, measurement of the pM9A activities in the conditioned medium. The original conditioned medium and the gelatinase-depleted medium were incubated with or without purified pro-MMP-9 followed by zymogram analysis.
We also tested whether pM9A activity was secreted into the conditioned medium. We measured the pM9A activities in the original conditioned medium as well as the conditioned medium that had been depleted of gelatinase in order to lower the background. In the untreated conditioned media, there was cytokine-induced MMP-9 that came from the organ culture, and the exogenous added pro-MMP-9 was not converted (Fig. 2B). Similarly, as shown in Fig. 2C, the added pro-MMP-9 failed to be converted into the 82-kDa form in the depleted conditioned media. Thus, either the tissue-bound pM9A is not secreted or there is a specific inhibitor that is also secreted with the activator.
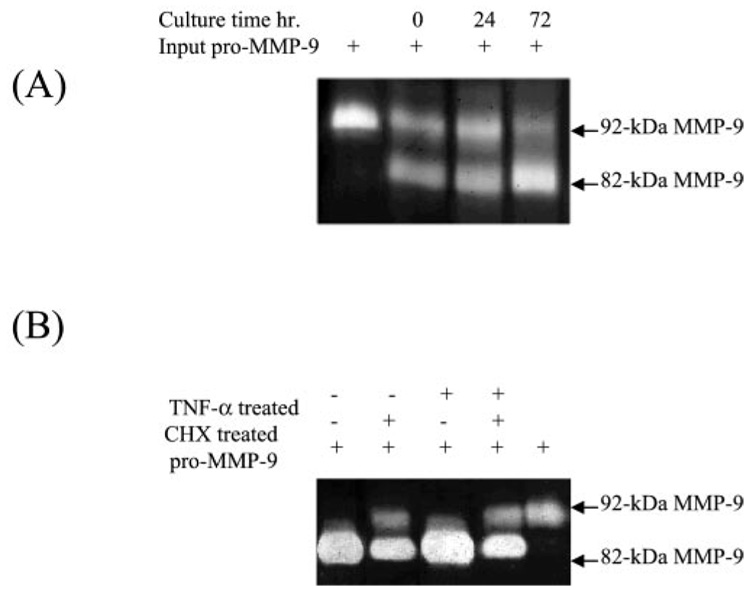
Because the organ culture technique utilized in our experiments might lead to the expression of pM9A, we performed a time course experiment. Normal human skin was cut into small pieces. Some were immediately frozen in liquid nitrogen, as day 0 samples. Other pieces were cultured in DMEM for 24 and 72 h. Then the skin tissues were ground, followed by Triton X-100 extraction. Purified pro-MMP-9 was added to these tissue fractions for pM9A assay. The data show that the tissue-associated pM9A activity is clearly detectable in the day 0 sample and increased slightly during the subsequent 3-day culturing (Fig. 3A).
FIG. 3. The pM9A is steadily expressed in human skin.
A, normal human skin was cut into small pieces, and some were immediately stored at −80 °C and regarded as day 0 samples. Others were cultured in DMEM for an additional 24 or 72 h. Tissue samples were ground, followed by Triton X-100 extraction. The pM9A activities were assayed by incubation with purified pro-MMP-9 followed by zymogram analysis. B, cycloheximide inhibited the expression of pM9A by human skin. Normal human skin was cultured in DMEM with or without cycloheximide (40 µg/ml) and with or without TNF-α (10 ng/ml) for 46 h. The tissue-bound pM9A activities were assayed by incubation with pro-MMP-9.
We then asked whether de novo protein synthesis in the skin was required for the expression of pM9A. Normal human skin was cultured in DMEM with or without TNF-α (10 ng/ml) in the presence or absence of cycloheximide (40 µg/ml). Then skin tissue was extracted with Triton X-100, and the insoluble fractions were assayed for pM9A activities by incubation with purified pro-MMP-9. The reaction was resolved by zymogram (Fig. 3B). The results show that cycloheximide treatment decreases the pM9A activities in the skin tissue. Again, TNF-α did not alter the cycloheximide-induced inhibition. The remaining pM9A activities from the cycloheximide-treated skin were probably derived from the skin prior to the treatment. Thus, pM9A is constitutively synthesized in skin, and its level is not regulated by TNF-α.
Tissue-associated pM9A Is a Chymotrypsin-like Proteinase
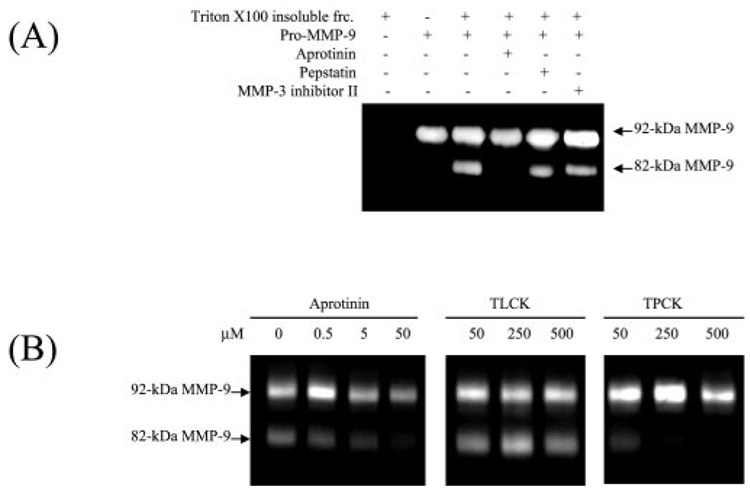
We then characterized this unidentified proteinase by its sensitivity to various proteinase inhibitors. The Triton-insoluble fractions of human skin and purified pro-MMP-9 were incubated with pepstatin (aspartic proteinase inhibitor), aprotinin (serine proteinase inhibitor), and MMP-3 inhibitor II. The results show that aprotinin but not other inhibitors can block the skin tissue-mediated conversion of pro-MMP-9. This indicates that pM9A is a serine proteinase (Fig. 4A). We further investigated the inhibition specificity of pM9A through a pair of serine proteinase inhibitors, TLCK, a specific inhibitor for trypsin-like proteinase and TPCK, a specific inhibitor for chymotrypsin-like proteinase. The results show that the tissue-associated pM9A activity is inhibited by TPCK at 50 µm and completely blocked by the inhibitor at 250 µm, which is within the normal concentration to block typical chymotrypsin (Fig. 4B). In contrast, TLCK has no inhibitory effect, even at 500 µm. Thus, the tissue-associated pM9A represents an as yet unidentified chymotrypsin-like proteinase.
FIG. 4. Tissue-associated pM9A is a chymotrypsin-like proteinase.
A, the Triton-insoluble fractions of human skin were incubated with purified pro-MMP-9 together with pepstatin (100 µm), aprotinin (50 µm), and MMP-3 inhibitor II (3 µm). After a 16-h incubation, the reaction products were resolved by zymogram. B, the tissue-bound pM9A is inhibited by TPCK, a specific inhibitor for chymotrypsin-like proteinase but not TLCK, the inhibitor for trypsin-like proteinase. The inhibitors at the indicated concentration were incubated with the Triton X-100 fractions followed by zymogram analysis.
Extraction of the Tissue-associated pM9A
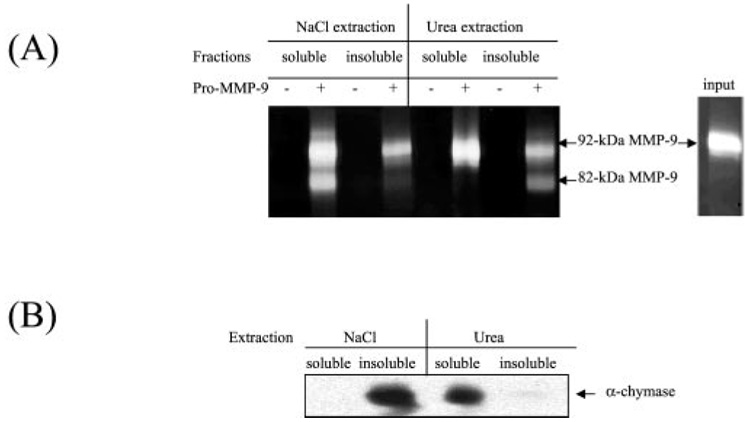
Our next aim was to extract pM9A from the skin tissue for identification. For this purpose, a serial extraction was performed. As shown previously, Triton X-100 was inefficient to extract the pM9A even at quite high concentration (17). We then tried salt extraction. The full thickness skin was ground in liquid nitrogen followed by extraction with either 2 m NaCl or 6 m urea. The extracts were subsequently dialyzed and assayed for pM9A activities by incubation with purified pro-MMP-9. As shown in Fig. 5A, 2 m NaCl efficiently extracted pM9A activity, whereas urea at 6 m failed to do so. In analysis of the debris, 2 m NaCl removed almost all pM9A activities, leaving no significant pro-MMP9-converting activity in the insoluble fraction. Conversely, most pM9A activity remained in the urea-insoluble fractions. In addition, the pM9A could also be extracted by SDS and retained its activity (data not shown). We conclude that the failure of extraction of pM9A by either urea or Triton X-100 was not due to denaturation or inactivation of pM9A but rather the incapability of these agents to dissociate the proteinase from the skin tissue.
FIG. 5. Extraction of the tissue-associated pM9A and mast cell chymase.
A, the full thickness skin was ground in liquid nitrogen followed by extraction with either 2 m NaCl or 6 m urea. The soluble fractions were subsequently dialyzed, and the insoluble fractions were washed. The fractions were assayed for pM9A activities by incubation with pro-MMP-9. B, the fractions were analyzed for the mast cell α-chymase by Western blot.
Human Pro-MMP-9 Activation Is Probably Not through the Mast Cell Chymase
Mast cells play important roles in skin inflammation by secretion of histamine and proteinases, including a chymotrypsin-like proteinase, α-chymase. Whether α-chymase can convert pro-MMP-9 is a subject of debate. Mast cell α-chymase prepared from dog mastocytoma cells was shown to be unable to activate human pro-MMP-9 (20), whereas another group showed that the dog mast cell chymase could activate the dog pro-MMP-9 by cleaving at the Phe88-Gln89 and Phe91-Glu92 (21). To clarify whether the human skin mast α-chymase is pM9A, we compared the extraction behavior of α-chymase versus pM9A activities. Results show that NaCl at 2 m could extract pM9A activity but failed to extract the mast cell chymase as measured by Western blot (Fig. 5B). Conversely, urea at 6 m failed to extract pM9A activity but could extract α-chymase. Thus, these two lines of evidence indicate that the mast cell α-chymase is not likely to be pM9A in human skin.
The Cytokine-induced MMP-3 Is Not Sufficient to Activate Pro-MMP-9
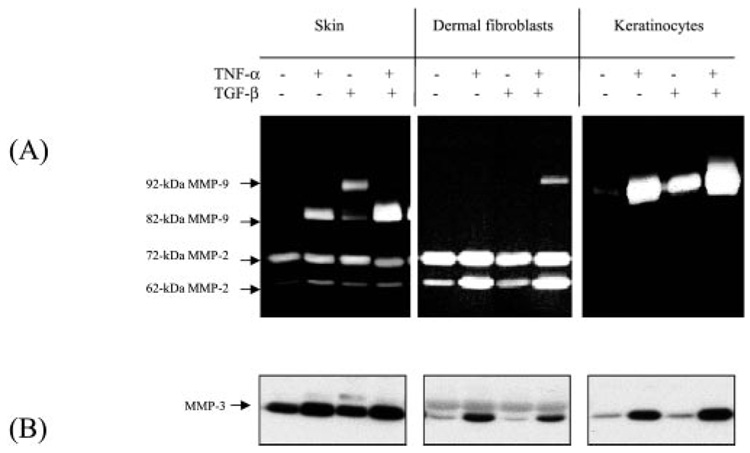
Based on in vitro constitution experiments, MMP-3 has been previously suggested as a potential activator for pro-MMP-9 (22). However, in the mice with homozygous knockout of MMP-3 (MMP-3−/−), the activation of MMP-9 was found to be normal, which suggests an MMP-3-independent pathway (23). To test this possibility, we examined whether TNF-α can regulate MMP-3. Normal human skin and primary dermal fibroblasts and keratinocytes were stimulated by TNF-α and TGF-β After culture for 64 h, the conditioned medium was analyzed by zymogram for MMP-9 activation and Western blot for MMP-3 protein (Fig. 6). As expected, the 92-kDa MMP-9 was induced by TGF-β, and the active 82-kDa was generated by TNF-α stimulation in human skin, whereas pro-MMP-9 was induced but no activation was observed in either dermal fibroblasts or epidermal keratinocytes. A small amount of MMP-3 was found expressed by human skin at basal state, and the protein level was significantly increased by TNF-α stimulation. Similarly, MMP-3 protein was induced by TNF-α in dermal fibroblasts and keratinocytes, where no activation of pro-MMP-9 was found. In addition, most of the MMP-3 is secreted as a soluble factor, whereas pM9A is tissue-bound. As shown in Fig. 4A, the specific inhibitor for MMP-3 failed to inhibit the activation of pro-MMP-9. All of these results suggest that expression of MMP-3 is not sufficient to activate pro-MMP-9.
FIG. 6. The cytokine-induced MMP-3 is not sufficient to activate pro-MMP-9.
Normal human skin, primary dermal fibroblasts embedded in collagen matrix, and keratinocytes on monolayers were stimulated by TNF-α and TGF-β individually or combined. After culture for 64 h, the conditioned media were collected. A, conditioned media were analyzed by zymogram for MMP-9 induction and activation. B, MMP-3 protein in the conditioned medium was analyzed by Western blot.
Specificity of pM9A
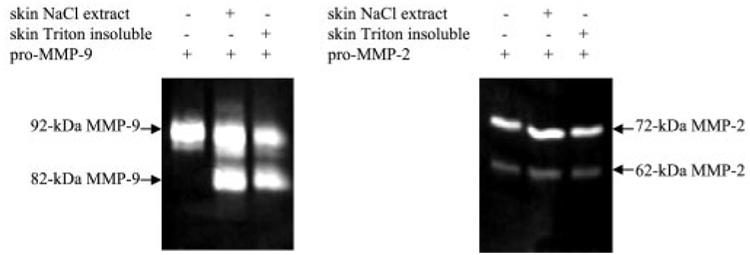
The specificity of pM9A as an activator was determined by testing its ability to activate MMP-2, another member of the gelatinase family. Pro-MMP-2 activation is generally thought to be mediated through the membrane type MMP with the participation of TIMP-2 (24). We asked whether the tissue-associated pM9A could also activate pro-MMP-2. The Triton X-100-insoluble and NaCl-soluble fractions from normal human skin were incubated with purified pro-MMP-2 and pro-MMP-9, respectively, and the products were resolved by zymogram. As shown, pro-MMP-9 but not the pro-MMP-2 is converted by the skin fractions (Fig. 7). Note that some 62-kDa active MMP-2 was derived from the original preparation, and that amount was not increased by incubation with the skin fractions, whereas most of the pro-MMP-9 was converted to the 82-kDa form. Therefore, pM9A is specific for pro-MMP-9 activation.
FIG. 7. Specificity of pM9A.
The Triton X-100-insoluble and NaCl-soluble fractions from normal human skin were incubated with purified pro-MMP-2 or pro-MMP-9. The products were resolved by zymogram. Some active 62-kDa MMP-2 was present in the preparation, and that amount was not increased by incubation with the skin fractions.
Correlation of TNF-α-mediated Down-regulation of TIMP-1 with the Cytokine-induced Activation of Pro-MMP-9
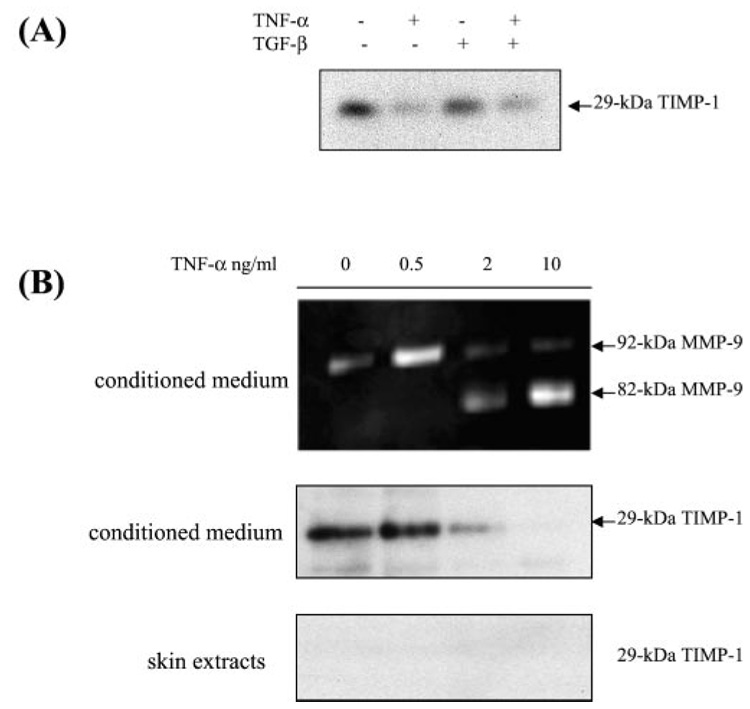
As shown in Fig. 2 and Fig. 3, pM9A activity is steadily expressed in human skin, and TNF-α seemingly has no additional regulatory effect on it. On the other hand, TNF-α can induce the conversion of pro-MMP-9. Since the activator was not regulated by TNF-α, we hypothesized that the regulation might be at the inhibitor level. An important hint came from the effects of TGF-β, as shown in Fig. 1. TGF-β induced expression of pro-MMP-9 in normal skin but has little effect on conversion of pro-MMP-9. However, the TGF-β-primed skin was capable of proteolytically processing the pro-MMP-9 once the soluble factors were removed (Fig. 2). This suggests that skin secrets a pM9A inhibitor and that TNF-α, but not TGF-β, may down-regulate it, resulting in the activation of pro-MMP-9. Initially, we considered TIMP-1, because it has been demonstrated that TIMP-1 can form a complex with pro-MMP-9 through their carboxyl terminus (25, 26). Specifically, we determined whether TNF-α could regulate TIMP-1. The conditioned medium from skin stimulated by TNF-α and TGF-β was resolved by Western blot with monoclonal antibody against human TIMP-1. As expected, TIMP-1 protein was steadily expressed at the basal state, and the protein level was decreased by TNF-α. TGF-β alone or in combination with TNF-α had no additional effect on the decrease of TIMP-1 (Fig. 8A). This profile of TNF-α -mediated down-regulation of TIMP-1 correlates well with the cytokine-mediated conversion of pro-MMP-9 as shown in Fig. 6. In addition, like the putative pro-MMP-9 activation inhibitor, TIMP-1 is mostly distributed as a secreted factor. To further characterize this correlation, we examined the effects of increasing concentrations of TNF-α on both the activation of pro-MMP-9 and TIMP-1 protein level. Normal human skin was stimulated with a range of concentrations of TNF-α for 64 h, and the conditioned medium was analyzed for MMP-9 activation and TIMP-1 protein. As shown in Fig. 8B, TNF-α caused a dose-dependent activation of pro-MMP-9 with efficient action at 2 nm. The TIMP-1 protein level was simultaneously down-regulated by TNF-α. Importantly, the cytokine-mediated activation of pro-MMP9 and down-regulation of TIMP-1 occurred at similar concentrations. To determine the specificity of this result, we also tested for TIMP-2, the inhibitor for MMP-2, and found that TNF-α has no effect on its expression (data not shown). Thus, the experimental evidence indicates that TNF-α-mediated down-regulation of TIMP-1 may participate in the conversion of pro-MMP-9.
FIG. 8. Correlation of TNF-α-mediated down-regulation of TIMP-1 with the cytokine-induced activation of pro-MMP-9 by human skin.
A, normal human skin was cultured in DMEM with TNF-α and TGF-β as indicated. The conditioned medium was resolved by Western blot with monoclonal antibody against human TIMP-1. B, normal human skin was cultured in DMEM with TNF-α at the indicated concentration. After culturing for 64 h, the conditioned medium was subjected to zymogram analysis. The conditioned medium and SDS-extracted skin tissue were resolved by Western blot for TIMP-1.
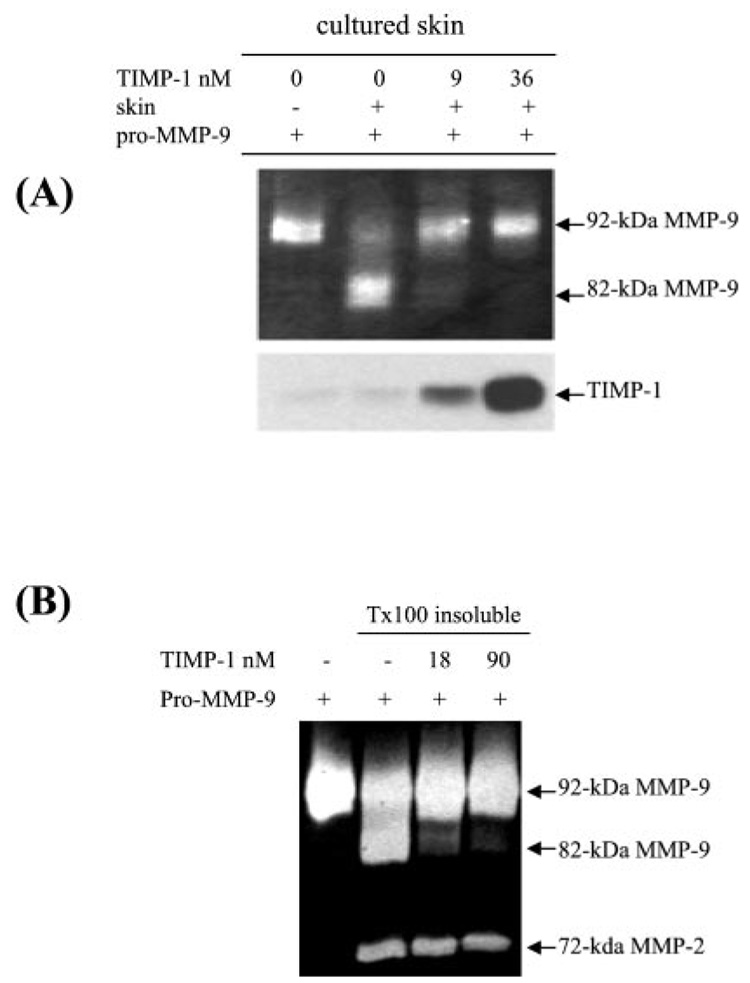
Recombinant TIMP-1 Blocks the Tissue-mediated Activation of Pro-MMP-9
To confirm the role of TIMP-1 in the activation of pro-MMP-9, we tested whether the purified recombinant TIMP-1 could inhibit the conversion of pro-MMP-9. To simulate the environment in which activation of pro-MMP-9 was initially observed, we added the TIMP-1 protein directly to the skin culture together with purified pro-MMP-9 as substrate. As shown in Fig. 9A, the skin-mediated activation of pro-MMP-9 was blocked by TIMP-1. At 9 nm, most of the activation was blocked (Fig. 9A). Further, Triton X-100-insoluble fractions were incubated with human TIMP-1 together with pro-MMP-9 as substrate. As expected, the TIMP-1 blocked the tissue-bound pM9A-mediated activation of pro-MMP-9 (Fig. 9B). These results confirm our hypothesis that TIMP-1 blocks pro-MMP-9 activation and support our conclusion that TNF-α-induced pro-MMP-9 activation is mediated by down-regulation of TIMP-1.
FIG. 9. Recombinant TIMP-1 blocks pM9A activity.
A, normal human skin was incubated in DMEM with purified pro-MMP-9 as substrate together with the bovine TIMP-1 protein at the indicated concentration. After incubation for 16 h, the product was resolved by zymogram and Western blot. B, normal human skin was extracted by 2% Triton X-100 and the insoluble fractions were incubated with pro-MMP-9 and the recombinant human TIMP-1 at the indicated concentration. After 16 h, the reaction was resolved by zymogram.
DISCUSSION
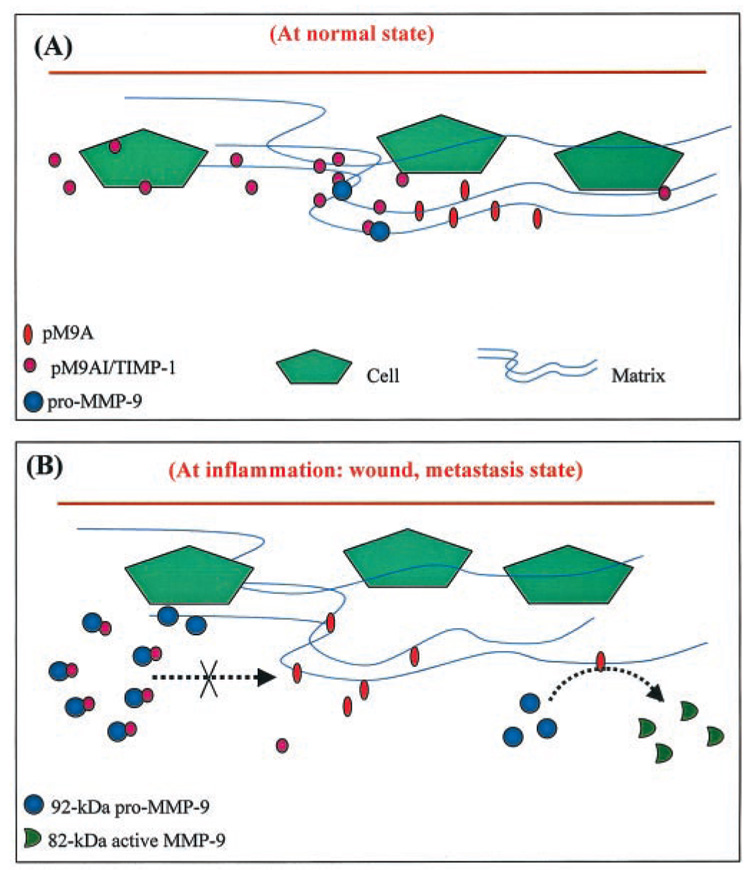
In this paper we present for the first time a novel model for the proteolytic activation of pro-MMP-9 in human skin. As shown schematically in Fig. 10, multiple factors, including specific cytokines, a tissue-secreted inhibitor, and a tissue-bound proteinase participate in the regulation of pro-MMP-9 activation in human skin. In quiescent tissue, very little MMP-9 is expressed, and the proteinase inhibitor, TIMP-1, is constantly expressed. During any inflammatory process, TGF-β induces the expression of pro-MMP-9. The MMP retains its latency by forming a complex with TIMP-1, which prevents its access to the tissue-associated activator, pM9A. When TNF-α is also present, it down-regulates TIMP-1. Then pro-MMP-9 can be converted by the tissue-associated chymotrypsin-like pM9A. We believe that this model can be extended to the pathogenesis of many inflammation-associated human diseases. Because these cytokines are present in significant concentrations in diseases such as chronic wounds (27–29) and metastatic cancer (30–33), we believe that these cytokines may be the causal factors for MMP-9 expression and activation in these conditions and may ultimately lead to basement membrane remodeling.
FIG. 10. A proposed model for pro-MMP-9 activation in human tissue.
Based on the evidence presented here and reported previously, we propose a model to explain the induction and activation of pro-MMP-9 in human tissue. A, at basal state, MMP-9 is not expressed in most developed tissues. Conversely, the inhibitor pM9AI, presumably TIMP-1, is constitutively expressed and secreted. B, during inflammation, including wound repair and tumor metastasis, TGF-β induces the expression of pro-MMP-9. The pro-MMP-9 forms a complex with TIMP-1, and that prevents access to the pM9A, the tissue-associated chymotrypsin-like proteinase. When TNF-α is present, it down-regulates the pM9AI/TIMP-1 and thereafter releases pro-MMP-9. The free pro-MMP-9 is converted into 82-kDa enzyme through the tissue-associated pM9A.
MMP-9 has been implicated in the pathogenesis of several different types of diseases. A major biological function for MMP-9 has been suggested in the remodeling of the BMZ of the skin, which is based on its substrate preference for type IV collagen, the major component in BMZ and type VII collagen, which anchors the BMZ to the dermis (34). The association between nonhealing wounds and MMP-9 is thought to be related to a breakdown of the matrix upon which keratinocytes migrate to reepithelialize the tissue. The best evidence for a role for MMP-9 in BMZ remodeling comes from the study of a skin disease called bullous pemphigoid (BP), which is characterized by the separation of the epidermis from the dermis at the BMZ and is caused by autoantibodies and complements (35). BP has been reconstituted in a mouse model through injection of antibodies against BP180, a transmembrane protein anchoring the basal keratinocytes to BMZ (36). The linkage of MMP-9 to BP has been established by showing the in vitro cleavage of BP180 (37). Conclusive evidence for this model comes from the MMP-9-deficient mice, which are resistant to experimentally induced bullous pemphigoid (38).
Our model proposes two distinct components in the biochemical control of MMP-9 activation. One is the tissue-associated chymotrypsin-like proteinase, the unidentified pM9A. We have characterized much about this enzyme, although its specific identity is not yet determined. Based on the inhibition by TPCK and other inhibitors for chymotrypsin,2 pM9A is a chymotrypsin-like proteinase. The tight tissue/cell association of pM9A suggests interesting possibilities about its nature. We found that ionized salt such as NaCl can easily dissociate pM9A from tissue, while the nonionized components such as urea and Triton X-100 failed to do so. This suggests that pM9A may bind to a charged component in skin, which is likely to be an extracellular matrix. The inhibition of aprotinin on pM9A also suggests a potential way to purify this protein through the inhibitor-conjugated chromatography. We anticipate that the initial biochemical characterization of pM9A presented here will provide the basis for the purification of this enzyme in the future. Under “Results,” we provide compelling evidence showing that candidate proteinases for the pM9A, MMP-3 and mast cell chymase, are not likely to be pM9A. We considered mast cell α-chymase as a candidate for pM9A because mast cells are strongly associated with skin inflammation. At the basal state, this chymase is located intracellularly in granules, and it is released by IgE-mediated stimulation. The best evidence to rule out this chymase as pM9A comes from the extraction experiment; NaCl can extract pM9A but not the chymase, and conversely, urea can extract the chymase but not pM9A. Two other groups have studied dog mast cell chymase on MMP-9 activation. These studies gave contradictory conclusions (20, 21, 39). We believe that the difference may be due to the nature of substrates used in those experiments. Taken together, the work presented in this paper indicates that the mast cell α-chymase is not likely to be the pM9A. Based on in vitro reconstitution experiments, MMP-3 was shown to convert pro-MMP-9 (40). In this study, we tested the role of MMP-3 in pro-MMP-9 activation in human skin and provide a clear finding that expression of MMP-3 is not sufficient for MMP-9 activation. In addition, the inhibitor for MMP-3 does not affect the skin-mediated pro-MMP-9 activation. Finally, MMP-3 and pM9A are distributed differently; MMP-3 is secreted, and pM9A is tissue-bound. Although we believe that we have excluded these two candidates as pM9A, the final conclusive evidence must wait until the molecular identification of pM9A, which will be the subject of our future work.
Initially, we assumed that TNF-α-mediated activation of pro-MMP-9 by human skin was through induction of its activator. To our surprise, the pM9A activities are steadily expressed and not regulated by the cytokines. This led us to a notion that TNF-α-mediated activation of pro-MMP-9 is probably governed at the inhibitor level. We believe that we have identified this inhibitor as TIMP-1. The most compelling single piece of evidence for a pM9A inhibitor comes from the effects of TGF-β. In the organ explant experiments, TGF-β potently induces pro-MMP-9 but fails to promote its activation. However, once the conditioned medium was removed from the explant tissue, the remaining skin tissue could activate pro-MMP-9. This indicates that the skin secretes an inhibitor, which in turn prevents the tissue-associated pM9A from processing pro-MMP-9. This putative pM9A inhibitor should satisfy the following criteria. First, it should be expressed at basal state and not down-regulated by TGF-β Second, it must be down-regulated or sequestrated by TNF-α. Third, it must be secreted as a soluble factor. Fourth, down-regulation of the pM9A inhibitor should be linked to the activation of pro-MMP-9, and finally, the accumulation of the inhibitor should block the activation of pro-MMP-9. We provide evidence in this report that TIMP-1 meets all of these demands: 1) TIMP-1 is steadily expressed by skin; 2) most of TIMP-1 is secreted as a soluble factor; 3) TNF-α down-regulates TIMP-1, and this down-regulation correlates with activation of pro-MMP-9 in a dose-dependent manner; and 4) when exogenous TIMP-1 is added to skin explant culture, it blocks the activation of pro-MMP-9. However, whether TIMP-1 is the only inhibitor regulated by TNF-α is currently unknown to us. This possibility will be examined by depletion of TIMP-1 from the conditioned medium and testing whether the pM9A inhibitor function is also eliminated. An additional question we have not yet addressed is the nature of TNF-α-induced down-regulation of TIMP-1; it could be achieved at the level of transcriptional suppression or at the post-transcriptional level such as protein degradation. The cytokine-induced down-regulation of TIMP-1 is unlikely to occur through the sequestration or translocation of the protein, because we measured both the secreted and tissue-associated fractions, and the total amount of the inhibitor is down-regulated.
The findings we have reported here show that multiple factors, including specific cytokine, tissue-bound proteinase, and soluble factor, together participate in the activation of pro-MMP-9 in human skin. This complexity, with the simultaneous involvement of multiple factors, may explain why others failed to observe MMP-9 activation when utilizing the homogenous cell culture. In fact, we also failed to measure the proteolytic activation by culturing the keratinocytes and dermal fibroblasts (17). This is noteworthy in comparison with the activation of pro-MMP-2, which has been demonstrated to involve the membrane type MMP, MT1-MMP (24). A comparison of the primary sequences between pro-MMP-9 and pro-MMP-2 shows that they are different at their N-terminal regions, where the presumed activation cleavage sites are located. This also indicates that there is a different mechanism for the activation of these two gelatinases, allowing for differences in control and regulation.
Finally, we believe that the model we have presented here may be extended to other human tissues besides skin. This model may also provide targets for pathophysiological responses in many inflammation-associated diseases. Blocking the potential linkage between inflammation and tumor metastasis by preventing the induction and activation of MMP-9 may prevent BMZ breakdown, limiting tumor cell migration. Acute liver failure induced by viral hepatitis, alcohol, or other hepatotoxic drugs has been reconstituted by injection of TNF-α into mice (41). In these TNF-α-treated mice, MMP-9 was massively induced and proteolytically activated in the liver. However, the cytokine-induced liver failure could be prevented by either knockout of mmp genes or administration of MMP inhibitor. Thus, TNF-α-regulated factors such as TIMP-1 and the pM9A, as we show in this article, may also participate in the liver failure. Taken together, identification of the factors involved in MMP-9 expression and activation in human tissue may provide useful targets for potential therapeutic treatment of the diseases that involve matrix degradation.
Acknowledgments
We gratefully acknowledge the determination of TNF levels in tissue biopsies by Dr. Dan Remick (University of Michigan). We thank Dr. Ronald A. Kohanski (Mount Sinai School of Medicine) for valuable comments and editing of this manuscript. We thank Dr. Susan Downey (USC) for supplying the human skin.
Footnotes
This work was supported in part by the Plastic Surgery Education Society and the Wound Healing Foundation (to Y. P. H.) and National Institutes of Health Grant GM 50967 (to W. L. G.).
The abbreviations used are: MMP, matrix metalloproteinase; BMZ, basement membrane zone; TLCK, L-1-chloro-3-(4-tosylamido)-7-amino-2-heptanone-HCl; TPCK, L-1-chloro-3-(4-tosylamido)-4-phenyl-2-butanone; DMEM, Dulbecco’s modified Eagle’s medium; TNF, tumor necrosis factor; TGF, transforming growth factor; pM9A, pro-MMP-9 activator; TIMP, tissue inhibitor of metalloproteinase; BP, bullous pemphigoid.
Y.-P. Han, Y.-D. Nien, and W. L. Garner, unpublished data.
REFERENCES
- 1.Werb Z. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 2.Parks WC. Wound Repair Regen. 1999;7:423–432. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 3.Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Proc. Natl. Acad. Sci. U. S. A. 1990;87:364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Crit. Rev. Oral Biol. Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 5.Sier CF, Kubben FJ, Ganesh S, Heerding MM, Griffioen G, Hanemaaijer R, van Krieken JH, Lamers CB, Verspaget HW. Br. J. Cancer. 1996;74:413–417. doi: 10.1038/bjc.1996.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood M, Fudge K, Mohler JL, Frost AR, Garcia F, Wang M, Stearns ME. Clin. Exp. Metastasis. 1997;15:246–258. doi: 10.1023/a:1018421431388. [DOI] [PubMed] [Google Scholar]
- 7.Zucker S, Wieman J, Lysik RM, Imhof B, Nagase H, Ramamurthy N, Liotta LA, Golub LM. Invasion Metastasis. 1989;9:167–181. [PubMed] [Google Scholar]
- 8.Rha SY, Kim JH, Roh JK, Lee KS, Min JS, Kim BS, Chung HC. Breast Cancer Res. Treat. 1997;43:175–181. doi: 10.1023/a:1005701231871. [DOI] [PubMed] [Google Scholar]
- 9.Zeng ZS, Guillem JG. Br. J. Cancer. 1998;78:349–353. doi: 10.1038/bjc.1998.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarlton JF, Vickery CJ, Leaper DJ, Bailey AJ. Br. J. Dermatol. 1997;137:506–516. doi: 10.1111/j.1365-2133.1997.tb03779.x. [DOI] [PubMed] [Google Scholar]
- 11.Young PK, Grinnell F. J. Invest. Dermatol. 1994;103:660–664. doi: 10.1111/1523-1747.ep12398424. [DOI] [PubMed] [Google Scholar]
- 12.Wysocki AB, Staiano-Coico L, Grinnell F. J. Invest. Dermatol. 1993;101:64–68. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 13.Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Wound Repair Regen. 1999;7:154–165. doi: 10.1046/j.1524-475x.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- 14.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. J. Invest. Dermatol. 1996;107:743–748. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 15.Garner WL, Karmiol S, Rodriguez JL, Smith DJ, Jr, Phan SH. J. Invest. Dermatol. 1993;101:875–879. doi: 10.1111/1523-1747.ep12371710. [DOI] [PubMed] [Google Scholar]
- 16.Tuan TL, Keller LC, Sun D, Nimni ME, Cheung D. J. Cell Sci. 1994;107:2285–2289. doi: 10.1242/jcs.107.8.2285. [DOI] [PubMed] [Google Scholar]
- 17.Han YP, Tuan TL, Hughes MW, Wu H, Garner WL. J. Biol. Chem. 2001;273:22341–22350. doi: 10.1074/jbc.M010839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han YP, Tuan TL, Wu H, Hughes M, Garner WL. J. Cell Sci. 2001;114:131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remick DG, DeForge LE, Sullivan JF, Showell HJ. Immunol. Invest. 1992;21:321–327. doi: 10.3109/08820139209069371. [DOI] [PubMed] [Google Scholar]
- 20.Lees M, Taylor DJ, Woolley DE. Eur. J. Biochem. 1994;223:171–177. doi: 10.1111/j.1432-1033.1994.tb18980.x. [DOI] [PubMed] [Google Scholar]
- 21.Fang KC, Raymond WW, Blount JL, Caughey GH. J. Biol. Chem. 1997;272:25628–25635. doi: 10.1074/jbc.272.41.25628. [DOI] [PubMed] [Google Scholar]
- 22.Ogata Y, Enghild JJ, Nagase H. J. Biol. Chem. 1992;267:3581–3584. [PubMed] [Google Scholar]
- 23.Lijnen HR, Silence J, Van Hoef B, Collen D. Blood. 1998;91:2045–2053. [PubMed] [Google Scholar]
- 24.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm SM, Collier IE, Marmer BL, Eisen AZ, Grant GA, Goldberg GI. J. Biol. Chem. 1989;264:17213–17221. [PubMed] [Google Scholar]
- 26.O’Connell JP, Willenbrock F, Docherty AJ, Eaton D, Murphy G. J. Biol. Chem. 1994;269:14967–14973. [PubMed] [Google Scholar]
- 27.Stadelmann WK, Digenis AG, Tobin GR. Am. J. Surg. 1998;176:26S–38S. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 28.Cooney R, Iocono J, Maish G, Smith JS, Ehrlich P. J. Trauma. 1997;42:415–420. doi: 10.1097/00005373-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Wound Repair Regen. 2000;8:13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 30.Kurtzman SH, Anderson KH, Wang Y, Miller LJ, Renna M, Stankus M, Lindquist RR, Barrows G, Kreutzer DL. Oncol. Rep. 1999;6:65–70. doi: 10.3892/or.6.1.65. [DOI] [PubMed] [Google Scholar]
- 31.Giavazzi R, Garofalo A, Bani MR, Abbate M, Ghezzi P, Boraschi D, Mantovani A, Dejana E. Cancer Res. 1990;50:4771–4775. [PubMed] [Google Scholar]
- 32.Nash MA, Ferrandina G, Gordinier M, Loercher A, Freedman RS. Endocr. Relat. Cancer. 1999;6:93–107. doi: 10.1677/erc.0.0060093. [DOI] [PubMed] [Google Scholar]
- 33.Kleer CG, van Golen KL, Merajver SD. Breast Cancer Res. 2000;2:423–429. doi: 10.1186/bcr89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matrisian LM. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 35.Jordon RE, Kawana S, Fritz KA. J. Invest. Dermatol. 1985;85:72s–78s. doi: 10.1111/1523-1747.ep12275497. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, Giudice GJ. J. Clin. Invest. 1993;92:2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahle-Backdahl M, Inoue M, Guidice GJ, Parks WC. J. Clin. Invest. 1994;93:2022–2030. doi: 10.1172/JCI117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, Senior RM. J. Exp. Med. 1998;188:475–482. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang KC, Raymond WW, Lazarus SC, Caughey GH. J. Clin. Invest. 1996;97:1589–1596. doi: 10.1172/JCI118583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogata Y, Itoh Y, Nagase H. J. Biol. Chem. 1995;270:18506–18511. doi: 10.1074/jbc.270.31.18506. [DOI] [PubMed] [Google Scholar]
- 41.Wielockx B, Lannoy K, Shapiro SD, Itoh T, Itohara S, Vandekerckhove J, Libert C. Nat. Med. 2001;7:1202–1208. doi: 10.1038/nm1101-1202. [DOI] [PubMed] [Google Scholar]